Background: The role of nitric oxide (NO) in pain regulation remains controversial. It is unclear how NO affects spinal synaptic transmission.
Results: NO increases glycine release but inhibits glutamate release in the spinal cord through distinct mechanisms.
Conclusion: NO inhibits nociceptive transmissions at the spinal level.
Significance: To learn how NO controls spinal synaptic transmission is critical for understanding the role of NO in pain processing.
Keywords: Calcium Channels, Cyclic GMP (cGMP), Nitric Oxide, Nitrosylation, Synaptic Plasticity
Abstract
Nitric oxide (NO) is involved in many physiological functions, but its role in pain signaling remains uncertain. Surprisingly, little is known about how endogenous NO affects excitatory and inhibitory synaptic transmission at the spinal level. Here we determined how NO affects excitatory and inhibitory synaptic inputs to dorsal horn neurons using whole-cell recordings in rat spinal cord slices. The NO precursor l-arginine or the NO donor SNAP significantly increased the frequency of glycinergic spontaneous and miniature inhibitory postsynaptic currents (IPSCs) of lamina II neurons. However, neither l-arginine nor SNAP had any effect on GABAergic IPSCs. l-arginine and SNAP significantly reduced the amplitude of monosynaptic excitatory postsynaptic currents (EPSCs) evoked from the dorsal root with an increase in paired-pulse ratio. Inhibition of the soluble guanylyl cyclase abolished the effect of l-arginine on glycinergic IPSCs but not on evoked monosynaptic EPSCs. Also, inhibition of protein kinase G blocked the increase in glycinergic sIPSCs by the cGMP analog 8-bromo-cGMP. The inhibitory effects of l-arginine on evoked EPSCs and high voltage-activated Ca2+ channels expressed in HEK293 cells and dorsal root ganglion neurons were abolished by blocking the S-nitrosylation reaction with N-ethylmaleimide. Intrathecal injection of l-arginine and SNAP significantly increased mechanical nociceptive thresholds. Our findings suggest that spinal endogenous NO enhances inhibitory glycinergic input to dorsal horn neurons through sGC-cGMP-protein kinase G. Furthermore, NO reduces glutamate release from primary afferent terminals through S-nitrosylation of voltage-activated Ca2+ channels. Both of these actions probably contribute to inhibition of nociceptive transmission by NO at the spinal level.
Introduction
Nitric oxide (NO) is freely diffusible across the cell membranes and is synthesized by the nitric-oxide synthase (NOS)2 from l-arginine and different cofactors. The three NOS isoforms, including neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS), have distinct structures and functions (1, 2). The diverse effects of NO is commonly mediated through increased cGMP production upon activation of NO-sensitive soluble guanylyl cyclase (sGC), S-nitrosylation, tyrosine nitration, and the interaction with superoxide to form peroxynitrite (3–5). The spinal dorsal horn is a critical site for nociceptive transmission and modulation. Although both nNOS and sGC are present in the superficial dorsal horn (6–8), their functions in the control of synaptic transmission in the spinal dorsal horn remain unknown.
The precise role of NO in nociceptive transmission at the spinal level is still controversial. Some studies suggest that spinal NO is involved in the potentiation of nociception. For example, mechanical hypersensitivity induced by nerve injury or tissue inflammation is reduced by intrathecal administration of nNOS inhibitors and in nNOS-knock-out mice (6, 9, 10). Also, sGC-knock-out mice show reduced nociceptive responses to tissue inflammation or nerve injury, but their responses to acute pain are not affected (11). In contrast, other studies have shown that spinal NO plays a role in the inhibition of nociceptive processing. In this regard, intrathecal administration of l-arginine increases the mechanical nociceptive withdrawal threshold in rats (12). Furthermore, spinally administered NO donors predominantly reduce the firing activity of spinal dorsal horn neurons and inhibition of spinal NOS or sGC increases the activity of nociceptive dorsal horn neurons (13–15). The discrepancy regarding the complex function of NO in nociceptive processing may result from the use of different animal models of pain and the levels of NO produced at the spinal level in different studies. For instance, it has been shown that intrathecal injection of low doses of l-arginine or NO donors reduce nociception, but l-arginine or NO donors at the high doses potentiates nociceptive responses to formalin injection or nerve injury (16, 17). In addition, it should be noted that in eNOS-, nNOS-, or iNOS-knock-out mice, an increase in the expression of other NOS isoforms in the spinal cord has been reported (18, 19). This compensatory up-regulation of other NOS subtypes in specific NOS isoform-knock-out mice further confounds the interpretation of the results. Strikingly, little is known about how endogenous NO affects excitatory and inhibitory synaptic input to spinal dorsal horn neurons.
Therefore, in the present study, we determined the role of NO in the control of glutamatergic excitatory and GABAergic and glycinergic inhibitory synaptic input to spinal dorsal horn neurons. We also investigated the downstream signaling mechanisms involved in NO actions on spinal synaptic transmission. Our results indicate that NO enhances inhibitory glycinergic input to dorsal horn neurons through sGC-cGMP-protein kinase G signaling. Furthermore, NO attenuates glutamate release from primary afferent terminals through S-nitrosylation of high voltage-activated Ca2+ channels (HVACCs). Our findings provide new insights into the underlying cellular and signaling mechanisms of NO in the inhibition of nociceptive transmission at the spinal level.
EXPERIMENTAL PROCEDURES
Animals
Male Sprague-Dawley rats (8 weeks old; Harlan, Indianapolis, IN) were used in this study. All the surgical operation and experimental protocols were approved by the Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center and conformed to the NIH guidelines on the ethical use of animals.
Spinal Cord Slice Preparation
Rats were anesthetized with 2% isoflurane in O2, and laminectomy was performed to rapidly remove the lumbar segment of the spinal cord. The rats were then killed with 5% isoflurane. The lumbar spinal cord segments were immediately placed in ice-cold sucrose artificial cerebrospinal fluid saturated with 95% O2 and 5% CO2. The sucrose artificial cerebrospinal fluid contained (mm) sucrose, 234; KCl, 3.6; MgCl2, 1.2; CaCl2, 2.5; NaH2PO4, 1.2; glucose, 12.0; and NaHCO3, 25.0. The spinal tissue block was placed in a gelatin block and glued onto the stage of a vibratome (Technical Product International, St. Louis, MO). Transverse spinal cord slices were cut to 400 μm in ice-cold sucrose artificial cerebrospinal fluid and then preincubated in Krebs solution oxygenated with 95% O2 and 5% CO2 at 34 °C for at least 1 h before being transferred to the recording chamber. The Krebs solution contained (in mm) NaCl, 117.0; KCl, 3.6; MgCl2, 1.2; CaCl2, 2.5; NaH2PO4, 1.2; glucose, 11.0; and NaHCO3, 25.0.
Recordings of Synaptic Activity
Whole-cell voltage-clamp recordings of postsynaptic currents have been described in our previous studies (20, 21). Neurons in the lamina II in the spinal cord slice were visually identified using differential interference contrast/infrared illumination on a fixed-stage microscope (BX51WI, Olympus, Tokyo, Japan). The impedance of the pipette electrode was 4–7 MΩ when it was filled with the internal solution. The internal pipette solution for recording inhibitory postsynaptic currents (IPSCs) contained (in mm) Cs2SO4, 110; KCl, 5; MgCl2, 2.0; CaCl2, 0.5; Hepes, 5.0; EGTA, 5.0; ATP-Mg, 5.0; Na-GTP, 0.5; and QX314 10 with pH 7.2–7.4 adjusted by 1 m CsOH (290–320 mOsm). The internal pipette solution for recording excitatory postsynaptic currents (EPSCs) contained (in mm) K-gluconate 135; KCl, 5; MgCl2, 2.0; CaCl2, 0.5; Hepes, 5.0; EGTA, 5.0; ATP-Mg, 5.0; Na-GTP, 0.5; QX314 10; adjusted to pH 7.2–7.4 with 1 m KOH (290–320 mOsm). QX314 was added to the internal solution to suppress the action potential generation from the recorded cells. The slice was placed in a glass-bottomed chamber and was continuously perfused with Krebs solution at 5.0 ml/min at 34 °C. Recordings of postsynaptic currents began 5–6 min after whole-cell access was established and the current reached a steady state. The input resistance was monitored, and the recording was abandoned if it changed by more than 15%.
Postsynaptic currents were recorded using an amplifier (MultiClamp700A, Axon Instruments, Foster City, CA) at a holding potential of 0 mV for inhibitory postsynaptic currents (IPSCs) and −60 mV for excitatory postsynaptic currents (EPSCs). Signals were filtered at 1–2 kHz, digitized at 10 kHz, and stored in a computer. In dorsal spinal cord, EPSCs are mediated by synaptic glutamate release because EPSCs of dorsal horn neurons are abolished by a glutamate AMPA receptor antagonist, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX). On the other hand, IPSCs are typically mediated by both glycine and GABA in the spinal cord (20, 22). To record GABA-mediated IPSCs, spontaneous IPSCs (sIPSCs) were recorded in the presence of 2 μm strychnine, a glycine receptor antagonist. Conversely, glycinergic sIPSCs were isolated by using 10 μm bicuculline, a GABAA receptor blocker. Miniature EPSCs (mEPSCs) and IPSCs (mIPSCs) were recorded in the presence of 1 μm tetrodotoxin (TTX). In some neurons, EPSCs of lamina II neurons were evoked by electrical stimulation (0.2 ms, 0.6–0.8 mA, and 0.1 Hz) through a bipolar electrode placed on the dorsal root to stimulate both Aδ- and C-fibers (21, 22).
Strychnine, CNQX, 2-(4-carboxyphenyl)-4,4,5,5-tetramethyl-imidazoline-l-oxyl-3-oxide potassium (carboxy-PTIO), and bicuculline were obtained from Sigma-Aldrich. TTX and QX314 were purchased from Alomone Laboratories (Jerusalem, Israel). 8-Bromo-cGMP, Rp-8-Br-PET-cGMPS, and 1,2-trifluoromethylphenyl imidazole (TRIM) were purchased from Tocris Bioscience (Ellisville, MO). 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) and S-nitroso-N-acetylpenicillamine (SNAP) were obtained from Ascent Scientific (Princeton, NJ). TRIM and ODQ stock solutions were first dissolved in dimethyl sulfoxide and were then diluted in artificial cerebrospinal fluid contained to the final concentration in the in vitro experiment.
Dissociation of Dorsal Root Ganglion Neurons
Acute dissociation of dorsal root ganglion (DRG) neurons and electrophysiological recordings of voltage-activated calcium channels (VACCs) have been described in detail in our previous studies (23, 24). In brief, rats were anesthetized with 2–3% isoflurane and then rapidly decapitated. The lumbar L4-L6 DRGs were quickly dissected out, and neurons were dissociated enzymatically. The cell suspension was subsequently plated onto a 35-mm culture dish containing poly-l-lysine pre-coated coverslips and incubated in 5% CO2 at 37 °C for 1 h. The neurons were then kept in the incubator for at least another hr before they were used for electrophysiological recordings.
Electrophysiological Recordings of Voltage-activated Calcium Channels in DRG Neurons and G1A1 Cells
Whole-cell patch clamp recordings were performed using an EPC-10 amplifier (HEKA Instruments, Lambrecht, Germany). Barium (Ba2+) was used as the charge carrier to record voltage-activated Ca2+ channel (VACC) currents in G1A1 cell lines or small (cell diameter < 30 μm) DRG neurons. Electrodes (2–3 MΩ) were filled with pipette solution (in mm): 120 CsCl, 1 MgCl2, 10 HEPES, 10 EGTA, 2 Mg-ATP, and 0.1 Na-GTP (pH 7.2 adjusted with CsOH, osmolarity 300 mOsm). The extracellular solution consisted of (in mm) 140 tetraethylammonium chloride, 2 MgCl2, 3 Cl2, 10 glucose, and 10 HEPES (pH 7.4; osmolarity, 320 mOsm). The cell membrane capacitance and series resistance were electronically compensated. Signals were filtered at 1 kHz, digitized at 10 kHz, and acquired using the Pulse software program. All experiments were performed at ∼25 °C.
Intrathecal Catheterization and Behavioral Testing of Mechanical Nociception
After the rats were anesthetized with isoflurane, the PE-10 catheters were inserted through an incision in the cisternal membrane and advanced 8 caudal so that the tip of each catheter was positioned at the lumbar spinal level (25). The catheters were externalized to the back of the neck and sutured to the musculature and skin at the incision site. After a 5–7-day recovery, the rats were used for the behavioral testing. Drugs for intrathecal injections were dissolved in normal saline except TRIM and SNAP, which were first dissolved in dimethyl sulfoxide and were then diluted in saline to the final concentration. Drugs were administered in a volume of 5 μl followed by a 10 μl flush with normal saline.
Nociceptive mechanical thresholds were measured with the Randall-Selitto test using an Ugo Basil Analgesimeter (Varese, Italy). The test was performed by applying a noxious pressure to the hindpaw. By pressing a pedal that activated a motor, the force increased at a constant rate on a linear scale. When the animal displayed pain by withdrawal of the paw or vocalization, the pedal was immediately released, and the nociceptive pain threshold was read on a scale. The cutoff of 400 g was used to avoid potential tissue injury (25). Both hindpaws were tested in each rat, and the mean value was used as the withdrawal threshold in response to the noxious pressure.
Data Analysis
Data are presented as means ± S.E. In general, 2–3 neurons were recorded from each rat, and at least 4 rats were used for each protocol. Spontaneous and miniature IPSCs (sIPSCs and mIPSCs, respectively) and EPSCs (sEPSCs and mEPSCs, respectively) were analyzed offline using a peak detection program (MiniAnalysis, Synaptosoft Inc., Decatur, GA). Detection of events was accomplished by setting a threshold above the noise level. The sIPSCs, mIPSCs, sEPSCs, and mEPSCs were detected by the fast rise time of the signal over an amplitude threshold above the background noise (20–22). The cumulative probability of the amplitude and inter-event interval (inversely related to the frequency) of sIPSCs and sEPSCs was compared using the Kolmogorov-Smirnov test, which estimates the probability that two distributions are similar. The amplitude of evoked EPSCs was analyzed using Clampfit (Axon Instruments). The treatment effects on IPSCs, EPSCs, VACC currents, and the paw withdrawal threshold were determined using either Student's t test or one-way analysis of variance followed by Dunnett's or Tukey's post hoc test. p < 0.05 was considered to be statistically significant.
RESULTS
NO Potentiates Synaptic Glycine Release in the Spinal Dorsal Horn
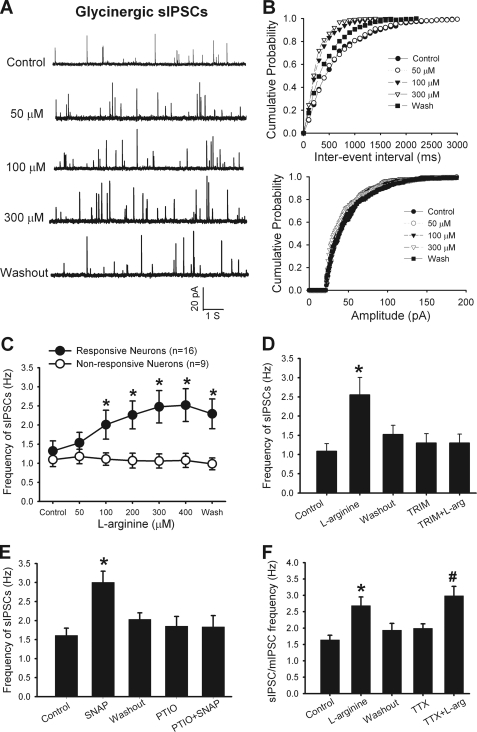
To determine the role of endogenous NO in the control of glycinergic input to dorsal horn neurons, we tested the effect of the NO precursor l-arginine on glycinergic sIPSCs of lamina II neurons. Bath application of l-arginine at 100 to 400 μm rapidly increased the frequency, but not the amplitude, of glycinergic sIPSCs in 16 of 25 (64%) neurons in a concentration-dependent manner (Fig. 1, A–C). l-Arginine had no significant effect on glycinergic sIPSCS in the remaining 9 neurons (Fig. 1C).
FIGURE 1.
NO increases glycinergic inhibitory input to spinal dorsal horn neurons. A, representative recordings show the effects of 50–300 μm of l-arginine on glycinergic sIPSCs of a lamina II neuron. B, cumulative plot analysis shows the effects of 50–300 μm l-arginine on the distribution of the inter-event interval and amplitude of glycinergic sIPSCs in the same neuron in A. C, summary data show the differential effects of l-arginine on the frequency of glycinergic sIPSCs in 2 groups of lamina II neurons. D, group data show the effects of 300 μm l-arginine on the glycinergic sIPSC frequency before and during application of 100 μm TRIM in 13 neurons. E, summary data show the effects of 100 μm SNAP on the glycinergic sIPSC frequency before and during application of 3 μm carboxy-PTIO in 11 neurons. F, group data show that the effects of 300 μm of l-arginine on the frequency of glycinergic sIPSCs and mIPSCs (recorded in the presence of 1 μm of TTX) in 8 lamina II neurons. *, p < 0.05 compared with the baseline control. #, p < 0.05 compared with TTX alone.
To determine whether the effect of l-arginine on glycinergic sIPSCs is mediated through nNOS, TRIM, a selective nNOS inhibitor (26), was used. Bath application of 100 μm TRIM alone for 6 min had no significant effect on glycinergic sIPSCs. In 13 neurons that initially responded to 300 μm l-arginine, repeated application of l-arginine failed to significantly increase the frequency of glycinergic sIPSCs in the presence of TRIM (Fig. 1D).
We also examined the effects of NO on glycinergic sIPSCs in spinal dorsal horn neurons. SNAP, an NO donor, and carboxy-PTIO (27), a highly specific NO scavenger, were used. Bath application of 100 μm SNAP significantly increased the frequency, but not the amplitude, of glycinergic sIPSCs in 6 of 10 neurons tested (Fig. 1E). After testing the initial effect of SNAP, 3 μm carboxy-PTIO was perfused for 6 min before SNAP was applied again. Carboxy-PTIO alone had no significant effect on glycinergic sIPSCs. SNAP failed to significantly affect the frequency of glycinergic sIPSCs in the presence of carboxy-PTIO (Fig. 1E).
We next determined if NO increases glycine release from presynaptic terminals in the spinal cord. In all 8 lamina II neurons tested, subsequent application of 300 μm l-arginine still significantly increased the frequency of glycinergic mIPSCs in the presence of 1 μm TTX (Fig. 1F). These data suggest that spinal NO acts at presynaptic terminals to potentiate glycine release in the majority of dorsal horn neurons.
NO Does Not Affect Synaptic GABAergic Input to Spinal Dorsal Horn Neurons
Because immunocytochemical labeling experiments suggest that nNOS is present on some GABAergic interneurons in the spinal dorsal horn (28, 29), we determined whether NO affects GABAergic input to lamina II neurons. Bath application of 50 to 300 μm l-arginine had no significant effect on the frequency or amplitude of GABAergic sIPSCs in all 9 neurons tested (Fig. 2, A–C). Furthermore, bath application of 100 μm SNAP did not significantly alter GABAergic sIPSCs in another 9 lamina II neurons (Fig. 2D).
FIGURE 2.
Lack of an effect of NO on GABAergic input to spinal dorsal horn neurons. A, original traces of GABAergic sIPSCs during control, application of 300 μm l-arginine, and washout. B, cumulative plot analysis of GABAergic sIPSCs of the same neuron showing the distribution of the inter-event interval and amplitude of GABAergic sIPSCs before and during l-arginine application. C, group data show that 300 μm l-arginine had no effect on the frequency of GABAergic sIPSCs in 9 lamina II neurons. D, summary data show lack of an effect of 100 μm SNAP on the frequency of GABAergic sIPSCs in another 9 neurons.
NO Enhances Synaptic Glycine Release to Dorsal Horn Neurons through sGC-cGMP-Protein Kinase G
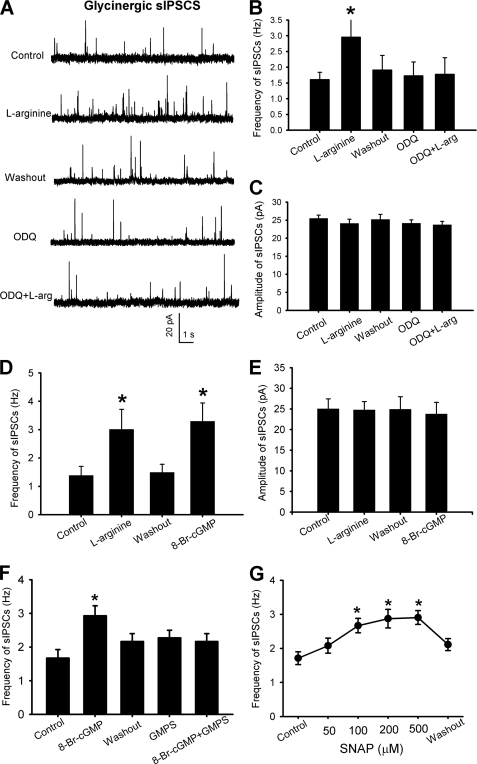
To determine if NO stimulates synaptic glycine release through sGC-cGMP signaling, a specific sGC inhibitor, ODQ (30, 31), was used. In 7 neurons initially responded to 300 μm l-arginine, bath application of 10 μm ODQ for 6 min completely blocked the stimulatory effect of l-arginine on the frequency of glycinergic sIPSCs (Fig. 3, A–C).
FIGURE 3.
NO potentiates glycinergic input to spinal dorsal horn neurons through sGC-cGMP-protein kinase G. A, representative traces of glycinergic sIPSCS during control and bath application of 300 μm l-arginine, and l-arginine plus 10 μm ODQ in a lamina II neuron. B, group data show the effect of l-arginine on the frequency of glycinergic sIPSCs with and without 10 μm ODQ in 7 lamina II neurons. C, group data show a lack of effects of 300 μm l-arginine and 10 μm ODQ on the amplitude of glycinergic sIPSCs in 7 neurons. D, summary data show the effects of 300 μm l-arginine and 8-bromo-cGMP (8-Br-cGMP, 60 μm) on the frequency (left) and amplitude (right) of glycinergic sIPSCs in 6 lamina II neurons. F, group data show that inhibition of protein kinase G with Rp-8-Br-PET-cGMPS (GMPS, 1 μm) blocked the stimulatory effect of 8-Br-cGMP on the glycinergic sIPSC frequency in 7 neurons. G, summary data show the concentration-dependent effect of SNAP on the frequency of glycinergic sIPSCs in 6 lamina II neurons. *, p < 0.05 compared with the baseline control.
We next determined the effect of a membrane-permeable cGMP analog, 8-bromo-cGMP (32), on synaptic glycine release to dorsal horn neurons. In lamina II neurons in which 300 μm l-arginine increased the frequency of glycinergic sIPSCs, subsequent application of 60 μm 8-bromo-cGMP also significantly increased the frequency, not the amplitude, of glycinergic sIPSCs (n = 6, Fig. 3, D and E). The stimulating effect of 8-bromo-cGMP on glycinergic IPSCs was not altered in the presence of ODQ.
We then determined whether protein kinase G is a downstream effector of the stimulating effect of cGMP on synaptic glycine release to dorsal horn neurons. In 7 lamina II neurons, initial bath application of 60 μm 8-bromo-cGMP significantly increased the frequency of glycinergic IPSCs. However, in the presence of 1 μm Rp-8-Br-PET-cGMPS, a specific and membrane-permeable protein kinase G inhibitor (33), subsequent application of 8-bromo-cGMP failed to significantly change the frequency of glycinergic IPSCs in these 7 neurons (Fig. 3F). Additionally, bath application of 50–500 μm SNAP increased the frequency of glycinergic IPSCs in a concentration-dependent manner in 6 lamina II neurons (Fig. 3G). Thus, the sGC-cGMP-protein kinase G signaling cascade mediates the potentiating effect of NO on synaptic glycine release to spinal cord horn neurons.
NO Inhibits Glutamatergic Synaptic Input to Spinal Dorsal Horn Neurons
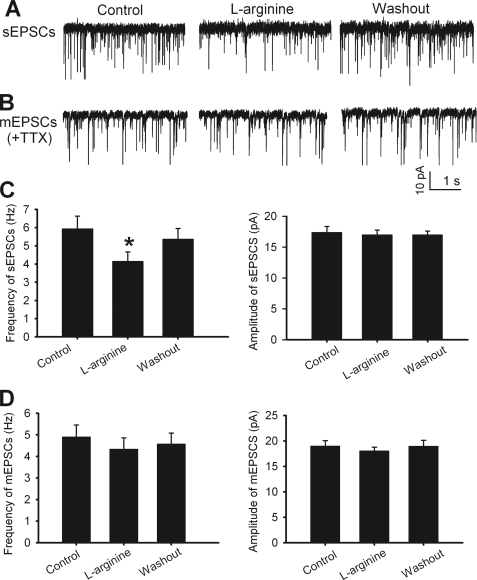
We first determined the role of NO in the control of spontaneous and quantal release of glutamate to dorsal horn neurons. In all 9 lamina II neurons tested, 300 μm l-arginine significantly decreased the frequency, but not the amplitude, of sEPSCs (Fig. 4, A and C). However, in another 11 neurons tested, l-arginine had no significant effect on the frequency or amplitude of mEPSCs recorded in the presence of 1 μm TTX (Fig. 4, B and D).
FIGURE 4.
NO inhibits glutamatergic input to spinal dorsal horn neurons. A, original recordings of glutamatergic sEPSCs of a lamina II neuron during control, bath application of 300 μm l-arginine, and washout. B, representative traces of mEPSCs of a lamina II neuron during control, l-arginine application, and washout. C, group data show the effect of 300 μm l-arginine on the frequency (left) and amplitude (right) of sEPSCs in 9 neurons. D, summary data show lack of an effect of l-arginine on the frequency (left) and amplitude (right) of mEPSCs in 11 lamina II neurons. *, p < 0.05 compared with the baseline control.
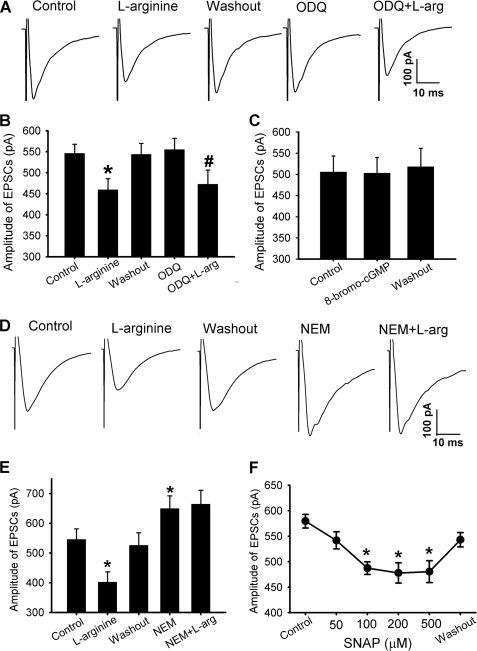
To determine the role of NO in the control of glutamate release from primary afferent terminals, we tested the effect of l-arginine on glutamatergic EPSCs evoked from the dorsal root. The evoked EPSCs were considered monosynaptic if the latency was constant after electrical stimulation at 0.2 Hz and if no conduction failure or increased latency occurred when stimulation frequency was increased to 20 Hz (21, 22). l-Arginine (300 μm) significantly reduced the amplitude of evoked monosynaptic EPSCs in 13 of 16 (81.3%) neurons tested. In these 13 neurons, TRIM (100 μm) alone had no significant effect on the amplitude of evoked EPSCs. Subsequent application of l-arginine failed to significantly change the amplitude of evoked EPSCs in the presence of TRIM (Fig. 5, A and B). In another 10 lamina II neurons, SNAP (100 μm) also significantly inhibited the amplitude of evoked monosynaptic EPSCs of 8 (80%) neurons. In the presence of 3 μm carboxy-PTIO, SNAP had no significant effect on the amplitude of evoked EPSCs in these 8 neurons (Fig. 5C).
FIGURE 5.
NO attenuates glutamatergic transmission between dorsal horn neurons and primary afferents. A, original tracings of monosynaptic EPSCs of a lamina II neuron evoked by dorsal root stimulation during control, application of 300 μm l-arginine, and application of 100 μm TRIM plus l-arginine. B, group data show the effects of l-arginine on the amplitude of evoked monosynaptic EPSCs before and during application of 100 μm TRIM in 13 neurons. C, summary data show the effects of 100 μm SNAP on the amplitude of evoked monosynaptic EPSCs before and during application of 3 μm carboxy-PTIO in 8 lamina II neurons. D, representative recordings show the effect of 300 μm l-arginine on monosynaptic EPSCs of a lamina II neuron evoked by paired-pulse stimulation of the dorsal root. E, group data show that l-arginine significantly increased the paired-pulse ratio of evoked EPSCs in 11 lamina II neurons. F, summary data show the inhibitory effect of 300 μm l-arginine on the amplitude of monosynaptic EPSCs of 10 neurons before and during application of 2 μm strychnine. *, p < 0.05 compared with the baseline control. #, p < 0.05 compared with strychnine alone.
Next, to determine whether NO alters evoked EPSCs presynaptically, a paired-pulse paradigm was used in which two stimuli were delivered with an inter-stimulus interval of 50 ms (21). The paired-pulse ratio was calculated as the peak amplitude of the second evoked EPSC divided by the first evoked EPSC. Application of 300 μm l-arginine reduced the amplitude of both evoked EPSCs in 11 lamina II neurons. However, l-arginine inhibited the first evoked EPSCs more than the second evoked EPSCs, resulting in an increase in the paired-pulse ratio (Fig. 5, D and E).
Because l-arginine potentiated the synaptic release of glycine to dorsal horn neurons, the released glycine may diffuse to the presynaptic terminals and affect glutamatergic transmission through activation of presynaptic glycine receptors (34, 35). To examine this possibility, the selective glycine receptor antagonist strychnine was applied to the bath after confirming the initial effect of l-arginine in 10 lamina II neurons. In the presence of 2 μm strychnine, 300 μm l-arginine still significantly inhibited the amplitude of evoked monosynaptic EPSCs of all neurons (Fig. 5F). Collectively, these data suggest that NO inhibits glutamatergic synaptic transmission between primary afferents and second-order dorsal horn neurons and that this action is independent of the NO potentiating effect on synaptic glycine release.
NO Reduces Glutamate Release from Primary Afferent Terminals through S-Nitrosylation
We then determined whether the sGC-cGMP signaling pathway plays a role in the inhibitory effect of 300 μm l-arginine on glutamate release from primary afferent terminals. In 8 lamina II neurons in which initial application of l-arginine inhibited the amplitude of monosynaptic evoked EPSCs, bath application of 10 μm ODQ had no significant effect on the amplitude of evoked EPSCs. Also, ODQ did not significantly alter the inhibitory effect of 300 μm l-arginine on the amplitude of evoked EPSCs in these 8 neurons (Fig. 6, A and B). In addition, bath application of 60 μm 8-bromo-cGMP had no significant effect on the amplitude of evoked monosynaptic EPSCs in another 9 neurons (Fig. 6C).
FIGURE 6.
NO reduces glutamatergic transmission between dorsal horn neurons and primary afferents through S-nitrosylation. A, original traces of monosynaptic EPSCs of a lamina II neuron evoked from dorsal root stimulation during control, application of 300 μm l-arginine, and application of 10 μm ODQ plus l-arginine. B, summary data show ODQ did not alter the inhibitory effect of l-arginine on evoked EPSCs in 8 lamina II neurons. C, group data show a lack of an effect of 60 μm 8-bromo-cGMP on the amplitude of evoked EPSCs in 9 lamina II neurons. D, original recordings of evoked monosynaptic EPSCs of a lamina II neuron during control, application of 300 μm l-arginine, and application of 100 μm NEM plus l-arginine. E, summary data show that NEM blocked the inhibitory effect of l-arginine on evoked EPSCs in 7 lamina II neurons. F, group data show that SNAP inhibited the amplitude of evoked EPSCs in 6 lamina II neurons in a concentration-dependent manner. *, p < 0.05 compared with the baseline control. #, p < 0.05 compared with ODQ alone.
Because we found no evidence that sGC-cGMP is involved in the NO effect on synaptic glutamate release to dorsal horn neurons, we next determined whether S-nitrosylation plays a role in the inhibitory effect of NO on glutamate release from primary afferents. N-ethylmaleimide (NEM), a specific alkylating agent of cysteine sulfhydryls, covalently modifies protein sulfhydryl groups thereby preventing subsequent S-nitrosylation of proteins (36, 37). Bath application of 100 μm NEM alone caused a significant increase in the amplitude of evoked monosynaptic EPSCs of 7 lamina II neurons. In the presence of NEM, l-arginine failed to significantly change the amplitude of evoked EPSCs of these 7 neurons (Fig. 6, D and E). In another 6 lamina II neurons, bath application of 50–500 μm SNAP inhibited the amplitude of monosynaptic EPSCs evoked from the dorsal root (Fig. 6F). Thus, these results suggest that NO inhibits glutamate release from primary afferent terminals through S-nitrosylation of presynaptic proteins.
NO Inhibits High Voltage-activated Ca2+ Channels in DRG Neurons and G1A1 Cell Lines through S-Nitrosylation
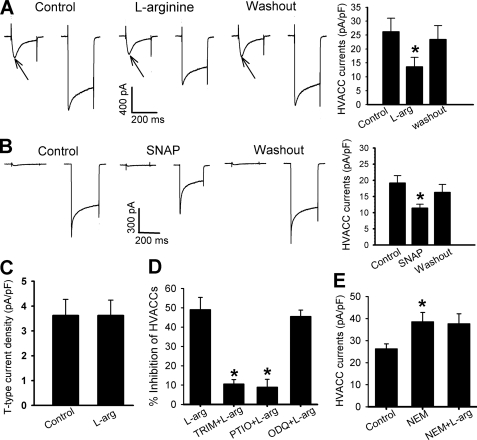
It is unclear whether NO targets presynaptic HVACCs to decrease glutamate release in the spinal dorsal horn. Because we found that NO inhibited evoked glutamate release from primary afferent terminals through S-nitrosylation, we reasoned whether NO can inhibit HVACCs in DRG neurons through S-nitrosylation. To test this hypothesis, we first examined the effects of l-arginine or SNAP on the activity of T-type VACCs and HVACCs in small DRG neurons. Neurons were voltage-clamped at −90 mV and depolarized to −45 mV for 200 ms (T-type VACCs) followed by a pulse from −90 to 0 mV for 200 ms (HVACCs) at 1-s intervals. l-arginine (300 μm, n = 13) and SNAP (100 μm, n = 9) caused a large decrease in the HVACC currents in DRG neurons (Fig. 7, A and B). l-Arginine had no effects on T-type VACC currents in all 6 neurons tested (Fig. 7C).
FIGURE 7.
NO inhibits HVACC currents in DRG neurons through S-nitrosylation. A, representative traces and group data show that 300 μm l-arginine reduced HVACC currents in 13 DRG neurons. Presence of T-type current is indicated by arrows. B, original current traces and summary data show that 100 μm SNAP inhibited HVACC currents in 9 DRG neurons. C, group data show the lack of effect of 300 μm l-arginine on T-type currents in 6 DRG neurons. D, summary data show that pretreatment with 100 μm TRIM (n = 11) or 3 μm carboxy-PTIO (n = 9) blocked the inhibitory effect of 300 μm l-arginine on HVACCs in DRG neurons. Note that pretreatment with 10 μm ODQ failed to alter the inhibitory effect of 300 μm l-arginine on HVACC currents in 9 DRG neurons. E, summary data show that 300 μm l-arginine failed to inhibit HVACC currents in the presence of 100 μm NEM in 9 DRG neurons. *, p < 0.05 compared with the baseline control or l-arginine alone.
The inhibitory effect of l-arginine on HVACC currents was blocked by pretreatment with 100 μm TRIM for 10 min (n = 11). Similarly, 300 μm l-arginine failed to affect HVACC currents in the presence of 3 μm carboxyl-PTIO (n = 9, Fig. 7D). However, 10 μm ODQ did not significantly affect the inhibitory effect of l-arginine on HVACC currents (n = 9, Fig. 7D).
We next investigated whether S-nitrosylation is involved in the inhibitory effect of NO on HVACCs in DRG neurons. Bath application of 100 μm NEM alone for 10 min significantly increased the current amplitude of HVACCs in 9 neurons. However, 300 μm l-arginine had no inhibitory effect on HVACC currents in the presence of NEM (Fig. 7E).
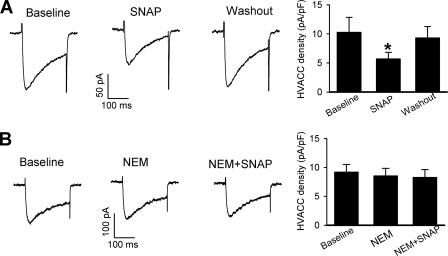
To provide more direct evidence that NO inhibits HVACCs through S-nitrosylation, we examined the effect of SNAP on HVACCs using G1A1 cells, a HEK 293 subclone stably expressing N-type (Cav2.2, β1b, and α2δ subunits) HVACCs (38). To record HVACC currents, cells were voltage-clamped at −90 mV and depolarized to 0 mV for 200 ms. In 9 cells tested, 100 μm SNAP significantly inhibited the current density (Fig. 8A). Pretreatment with 100 μm NEM completely blocked the inhibitory effect of SNAP on N-type HVACCs (n = 6, Fig. 8B).
FIGURE 8.
NO inhibits N-type HVACC currents in G1A1 cells through S-nitrosylation. A, representative traces and group data show that 100 μm SNAP reduced N-type HVACC currents in 9 A1G1 cells. B, original current traces and summary data show that pretreatment with 100 μm NEM abolished the inhibitory effect of 100 μm SNAP on N-type HVACCs in 6 G1A1 cells. *, p < 0.05 compared with the baseline control.
NO Attenuates Mechanical Nociception at the Spinal Level
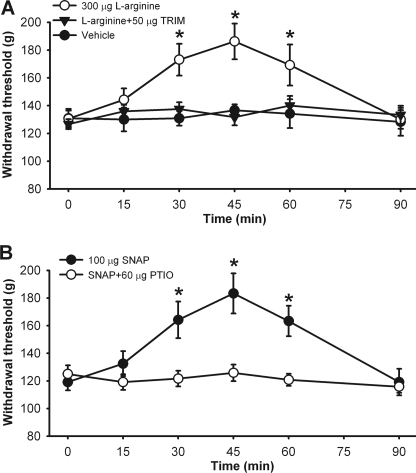
Because our findings presented above suggest that NO inhibits nociceptive transmission in the spinal cord, we determined the possible antinociceptive effect of NO at the spinal level in rats. Intrathecal injection of 300 μg l-arginine significantly increased the paw withdrawal threshold in response to a noxious pressure stimulus. The antinociceptive effect of l-arginine reached maximal at 45 min and lasted for about 60 min (n = 12 rats, Fig. 9A). Intrathecal pretreatment with 50 μg of TRIM blocked the effect of l-arginine on the paw withdrawal threshold (n = 7 rats, Fig. 9A).
FIGURE 9.
NO reduces mechanical nociception at the spinal level. A, time course of the effects of intrathecal injection of vehicle (n = 7), 300 μg l-arginine (n = 12), and 50 μg TRIM plus 300 μg l-arginine (n = 7) on the paw withdrawal threshold in response to the noxious pressure stimulus in rats. B, time course of the effects of intrathecal injection of 100 μg of SNAP (n = 7) and 100 μg of SNAP plus 60 μg of carboxy-PTIO (n = 7) on the paw withdrawal threshold in rats. *, p < 0.05 compared with the baseline control (time 0).
Intrathecal injection of 100 μg of SNAP also significantly increased the paw withdrawal threshold in 7 rats tested. Pretreatment with intrathecal injection of 60 μg carboxy-PTIO abolished the effect of SNAP on the nociceptive threshold (n = 7 rats, Fig. 9B). Intrathecal administration of TRIM or carboxy-PTIO alone has no effects on the baseline withdrawal threshold in rats, as we reported previously (25). There were no sedation, agitation, and impaired motor function (judged by ambulation behavior) observed after intrathecal administration of l-arginine or SNAP.
DISCUSSION
In this study, we determined systemically the role of endogenous NO in the regulation of excitatory and inhibitory synaptic transmission in the spinal dorsal horn. Previous studies using various nociceptive tests in animal models suggest that NO is either pronociceptive or antinociceptive (9, 10, 12, 16, 17). The discrepancy may result from the use of different pain models, the amount of NO produced locally, and the specific CNS sites involved. For example, NO-cGMP inhibits dorsal horn neuronal activity at the spinal level but excites spinal dorsal horn neurons at the supraspinal level (15, 39). Also, while low concentrations of NO inhibit NMDA receptor activity (40, 41), high concentrations of NO stimulate TRPV1 and TRPA1 receptors (42). Yet, little is known about how endogenous NO regulates excitatory and inhibitory synaptic transmission at the spinal level. GABA and glycine are the two predominant inhibitory neurotransmitters in the spinal cord. Blocking GABAA or glycine receptors in the spinal cord induces pain hypersensitivity in rats (43, 44). In the present study, we found that both the NO precursor l-arginine and the NO donor SNAP significantly increased the frequency of glycinergic sIPSCs and mIPSCs in the majority of lamina II neurons. The effects of l-arginine and SNAP on glycinergic sIPSCs were blocked by the nNOS inhibitor TRIM and the NO scavenger carboxy-PTIO, respectively. Of note, l-arginine and SNAP had similar effects on glycinergic sIPSCs and mIPSCs, suggesting that NO can potentiate glycine release from presynaptic terminals of interneurons in the spinal dorsal horn. Thus, our findings indicate that NO potentiates glycinergic input to spinal dorsal horn neurons to attenuate nociceptive transmission.
Immunocytochemical labeling shows that NOS-positive terminals in lamina II are largely GABA immunoreactive in rats (29), and nNOS is present in 14% GAD67-positive neurons in the spinal dorsal horn of mice (28, 45). Although these reports suggest that NO may be produced by some GABAergic interneurons and terminals in the spinal cord, we obtained no evidence showing that NO is involved in the control of synaptic GABA release to dorsal horn neurons. We found that neither l-arginine nor SNAP had any significant effect on GABAergic sIPSCs in all lamina II neurons tested in our study. It has been reported that GABA- and glycine-like immunoreactivities are often colocalized in the spinal dorsal horn (46, 47). However, functional studies have failed to substantiate the hypothesis that GABA and glycine are co-released from the same synaptic terminal in the superficial dorsal horn. This is because direct paired recordings of dorsal horn neurons show that the IPSCs evoked from a single presynaptic terminal are mediated by either GABAA or glycine receptors but not by both (48, 49). Furthermore, we have shown that muscarinic receptor subtypes and group II and III metabotropic glutamate receptors are involved in the differential control of GABAergic and glycinergic input to dorsal horn neurons (50–54). Of note, it has been shown that NO regulates synaptic glycine, but not GABA, release to sympathetic preganglionic neurons in the lateral spinal cord (55).
Glutamate is an excitatory neurotransmitter critically involved in nociceptive transmission in the spinal dorsal horn. In this study, we found that l-arginine and SNAP significantly inhibited the frequency of glutamatergic sEPSCs of lamina II neurons. l-Arginine and SNAP also consistently inhibited glutamatergic EPSCs evoked from primary afferents in most lamina II neurons. The inhibitory effects of l-arginine and SNAP on evoked monosynaptic EPSCs were blocked by TRIM and carboxy-PTIO, respectively. Because the inhibitory effect of l-arginine on evoked EPSCs is associated with an increase in the paired-pulse ratio, our data suggest that NO acts on the presynaptic site to inhibit glutamate release from primary afferents. Consistent with this notion, we found that l-arginine and SNAP significantly inhibited HVACC currents in dissociated DRG neurons. HVACCs are inhibited in the presence of TTX, which can explain why NO failed to affect mEPSCs. In addition, we observed that the glycine receptor antagonist strychnine had no effect on inhibition of evoked EPSCs by l-arginine. Thus, it is unlikely that the inhibitory effect of NO on synaptic glutamate release to dorsal horn neurons is secondary to increased glycine release and stimulation of presynaptic glycine receptors (35). Our findings strongly suggest that NO attenuates synaptic glutamate release by inhibition of HVACCs at primary afferent terminals.
Another salient finding of our study is that NO potentiates glycinergic input and inhibits glutamatergic synaptic transmission in the spinal dorsal horn through distinct signaling pathways. We found that the specific sGC inhibitor ODQ abolished the potentiating effect of l-arginine on the frequency of glycinergic sIPSCs, and the membrane-permeable cGMP analog 8-bromo-cGMP mimicked the potentiating effect of NO on glycinergic sIPSCs. In addition, we found that inhibition of the protein kinase G activity with Rp-8-Br-PET-cGMPS abolished the increase in the frequency of glycinergic IPSCs by 8-bromo-cGMP, suggesting that protein kinase G is a downstream mechanism involved in the synaptic release of glycine by an increase in NO and sGC activity in the spinal cord. Currently, little is know about the protein kinase G substrates and synaptic vesicle proteins phosphorylated by protein kinase G at the nerve terminals, which needs to be addressed in future studies. Our study provides novel evidence that NO stimulates glycinergic interneurons in the dorsal horn through sGC-cGMP-protein kinase G signaling. sGC is present in some interneurons in the spinal dorsal horn (7). Whereas nNOS rarely colocalizes with sGC, nNOS-positive structures are often apposed to sGC-positive structures (7), which suggests that endogenous NO that increases sGC activity and glycine release may be produced in adjacent neurons. Our electrophysiological data indicate that sGC is present only in a subpopulation of glycinergic neurons and terminals in the spinal dorsal horn.
In contrast, we found that sGC-cGMP signaling is not involved in the regulation of glutamate release by NO in the spinal dorsal horn. We observed that ODQ did not alter the inhibitory effect of l-arginine on evoked glutamatergic EPSCs. Also, 8-bromo-cGMP had no effect on monosynaptic EPSCs of dorsal horn neurons evoked by primary afferent stimulation. The lack of a role of sGC-cGMP in the inhibitory effect of NO on glutamate release from primary afferents could be due to the fact that sGC is not expressed in DRG neurons (11) and the primary afferent terminals in the superficial dorsal horn (7). NO can inhibit HVACCs in cardiomyocytes through S-nitrosylation (66). We observed that NO inhibited evoked EPSCs and sEPSCs but had no effect on mEPSCs, suggesting that HVACCs on the primary afferent terminals may be the target of S-nitrosylation by NO. NEM covalently modifies sulfhydryl groups and prevents S-nitrosylation (36, 37). We found that NEM abolished the inhibitory effects of l-arginine on evoked EPSCs of lamina II neurons and HVACC currents in DRG neurons. Additionally, NEM completely blocked the inhibitory effect of SNAP on N-type HVACCs expressed in G1A1 cells, thus providing further direct evidence that NO inhibits HVACCs through S-nitrosylation. NEM has been reported to be capable of inactivating inhibitory G proteins (56, 57). Removal of tonic G protein inhibition of HVACCs (58, 59) could explain why NEM alone increased the amplitude of evoked EPSCs of lamina II neurons and HVACC currents in DRG neurons observed in our study. Because NEM alone had no effect on HVACC currents in G1A1 cells, it suggests that NEM does not affect HVACCs directly. Therefore, our findings suggest that spinal NO primarily inhibits glutamate release through S-nitrosylation of HVACCs at primary afferent terminals.
In addition to its inhibitory effect on HVACCs shown in our study, NO can inhibit NMDA receptor currents in recombinant systems (60, 61) and in spinal lamina II neurons (41). Because both HVACCs and NMDA receptors are critically involved in nociceptive transmission, it seems difficult to explain the proposed pronociceptive role of NO at the spinal level. Of note, systemic use of NO or NO donors has been shown to reduce pain intensity caused by sickle cell crisis or diabetic neuropathy in patients (62, 63). We found in the present study that intrathecal administration of l-arginine or SNAP in rats significantly increased the nociceptive mechanical thresholds. Pretreatment with intrathecal TRIM and carboxy-PTIO blocked the antinociceptive effect of l-arginine and SNAP, respectively. In the central nervous system, nNOS is located at the postsynaptic density and is closely linked to NMDA receptors (64, 65). Hence, NMDA receptor activation could lead to increased NO production and release, and NO can diffuse to the presynaptic site to reduce glutamate release from primary afferent terminals. By reducing glutamatergic transmission, NO could serve as a feedback regulator to attenuate nociceptive transmission at the spinal level during painful conditions. We found that SNAP consistently increased the frequency of glycinergic sIPSCs and reduced the amplitude of evoked EPSCs of dorsal horn neurons in a concentration-dependent manner. These data suggest that the pro- and antinociceptive effects of NO are less likely dependent on different NO concentrations at the spinal level.
In summary, our study provides important new evidence that spinal NO potentiates inhibitory glycinergic input but reduces glutamatergic synaptic transmission between primary afferents and dorsal horn neurons through distinct signaling mechanisms. The opposing effects of NO on glutamatergic and glycinergic synaptic transmission could contribute to the antinociceptive effect of NO at the spinal level. This new information is important for our understanding of the synaptic actions underlying the antinociceptive effect of NO at the spinal level.
Acknowledgment
We thank Dr. Heidi Hamm (Vanderbilt University) for providing G1A1 cells used for this study.
This work was supported, in whole or in part, by National Institutes of Health Grants GM064830 and NS073935 and the N. G. and Helen T. Hawkins Endowment (to H.-L. P.).
- NOS
- nitric-oxide synthase
- sIPSCs
- spontaneous inhibitory postsynaptic currents
- mIPSCs
- miniature inhibitory postsynaptic currents
- EPSCs
- excitatory postsynaptic currents
- mEPSCs
- miniature inhibitory postsynaptic currents
- sEPSCs
- spontaneous excitatory postsynaptic currents
- CNQX
- 6-cyano-7-nitroquinoxaline-2,3-dione
- DRG
- dorsal root ganglion
- nNOS
- neuronal nitric-oxide synthase
- HVACCs
- high voltage-activated calcium channels
- ODQ
- 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- sGC
- soluble guanylyl cyclase
- SNAP
- S-nitroso-N-acetylpenicillamine
- TRIM
- 1,2-trifluoromethylphenyl imidazole
- VACCs
- voltage-activated calcium channels
- TTX
- tetrodotoxin.
REFERENCES
- 1. Stuehr D. J. (1997) Annu. Rev. Pharmacol. Toxicol. 37, 339–359 [DOI] [PubMed] [Google Scholar]
- 2. Lipton S. A., Singel D. J., Stamler J. S. (1994) Prog. Brain Res. 103, 359–364 [DOI] [PubMed] [Google Scholar]
- 3. Rudkouskaya A., Sim V., Shah A. A., Feustel P. J., Jourd'heuil D., Mongin A. A. (2010) Free Radic. Biol. Med. 49, 757–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feil R., Hartmann J., Luo C., Wolfsgruber W., Schilling K., Feil S., Barski J. J., Meyer M., Konnerth A., De Zeeuw C. I., Hofmann F. (2003) J. Cell Biol. 163, 295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gow A. J., Farkouh C. R., Munson D. A., Posencheg M. A., Ischiropoulos H. (2004) Am. J. Physiol. Lung Cell Mol. Physiol. 287, L262–268 [DOI] [PubMed] [Google Scholar]
- 6. Guan Y., Yaster M., Raja S. N., Tao Y. X. (2007) Mol. Pain 3, 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ding J. D., Weinberg R. J. (2006) J. Comp. Neurol. 495, 668–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Terenghi G., Riveros-Moreno V., Hudson L. D., Ibrahim N. B., Polak J. M. (1993) J. Neurolog Sci. 118, 34–37 [DOI] [PubMed] [Google Scholar]
- 9. Tanabe M., Nagatani Y., Saitoh K., Takasu K., Ono H. (2009) Neuropharmacology 56, 702–708 [DOI] [PubMed] [Google Scholar]
- 10. Chu Y. C., Guan Y., Skinner J., Raja S. N., Johns R. A., Tao Y. X. (2005) Pain 119, 113–123 [DOI] [PubMed] [Google Scholar]
- 11. Schmidtko A., Gao W., König P., Heine S., Motterlini R., Ruth P., Schlossmann J., Koesling D., Niederberger E., Tegeder I., Friebe A., Geisslinger G. (2008) J. Neurosci. 28, 8568–8576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhuo M., Meller S. T., Gebhart G. F. (1993) Pain 54, 71–78 [DOI] [PubMed] [Google Scholar]
- 13. Pehl U., Schmid H. A. (1997) Neuroscience 77, 563–573 [DOI] [PubMed] [Google Scholar]
- 14. Hoheisel U., Unger T., Mense S. (2005) Pain 117, 358–367 [DOI] [PubMed] [Google Scholar]
- 15. Hoheisel U., Unger T., Mense S. (2000) Pain 88, 249–257 [DOI] [PubMed] [Google Scholar]
- 16. Li K., Qi W. X. (2010) Neurosci. Bull. 26, 211–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sousa A. M., Prado W. A. (2001) Brain Res. 897, 9–19 [DOI] [PubMed] [Google Scholar]
- 18. Boettger M. K., Uceyler N., Zelenka M., Schmitt A., Reif A., Chen Y., Sommer C. (2007) Eur. J. Pain 11, 810–818 [DOI] [PubMed] [Google Scholar]
- 19. Tao F., Tao Y. X., Mao P., Zhao C., Li D., Liaw W. J., Raja S. N., Johns R. A. (2003) Neuroscience 120, 847–854 [DOI] [PubMed] [Google Scholar]
- 20. Pan Y. Z., Pan H. L. (2004) J. Neurophysiol. 91, 2413–2421 [DOI] [PubMed] [Google Scholar]
- 21. Zhou H. Y., Chen S. R., Chen H., Pan H. L. (2010) J. Neurosci. 30, 4460–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li D. P., Chen S. R., Pan Y. Z., Levey A. I., Pan H. L. (2002) J. Physiol. 543, 807–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu Z. Z., Pan H. L. (2004) Neurosci. Lett. 368, 96–101 [DOI] [PubMed] [Google Scholar]
- 24. Wu Z. Z., Chen S. R., Pan H. L. (2005) J. Biol. Chem. 280, 18142–18151 [DOI] [PubMed] [Google Scholar]
- 25. Chen S. R., Pan H. L. (2003) Anesthesiology 98, 217–222 [DOI] [PubMed] [Google Scholar]
- 26. Handy R. L., Harb H. L., Wallace P., Gaffen Z., Whitehead K. J., Moore P. K. (1996) Br J. Pharmacol. 119, 423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Akaike T., Yoshida M., Miyamoto Y., Sato K., Kohno M., Sasamoto K., Miyazaki K., Ueda S., Maeda H. (1993) Biochemistry 32, 827–832 [DOI] [PubMed] [Google Scholar]
- 28. Heinke B., Ruscheweyh R., Forsthuber L., Wunderbaldinger G., Sandkühler J. (2004) J. Physiol. 560, 249–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Valtschanoff J. G., Weinberg R. J., Rustioni A., Schmidt H. H. (1992) Neurosci. Lett. 148, 6–10 [DOI] [PubMed] [Google Scholar]
- 30. Zhao Y., Brandish P. E., DiValentin M., Schelvis J. P., Babcock G. T., Marletta M. A. (2000) Biochemistry 39, 10848–10854 [DOI] [PubMed] [Google Scholar]
- 31. Li D. P., Chen S. R., Pan H. L. (2002) J. Neurophysiol. 88, 2664–2674 [DOI] [PubMed] [Google Scholar]
- 32. Sekhar K. R., Hatchett R. J., Shabb J. B., Wolfe L., Francis S. H., Wells J. N., Jastorff B., Butt E., Chakinala M. M., Corbin J. D. (1992) Mol. Pharmacol. 42, 103–108 [PubMed] [Google Scholar]
- 33. Butt E., Pöhler D., Genieser H. G., Huggins J. P., Bucher B. (1995) Br J. Pharmacol. 116, 3110–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jeong H. J., Jang I. S., Moorhouse A. J., Akaike N. (2003) J. Physiol. 550, 373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee E. A., Cho J. H., Choi I. S., Nakamura M., Park H. M., Lee J. J., Lee M. G., Choi B. J., Jang I. S. (2009) J. Neurochem. 109, 275–286 [DOI] [PubMed] [Google Scholar]
- 36. Bolotina V. M., Najibi S., Palacino J. J., Pagano P. J., Cohen R. A. (1994) Nature 368, 850–853 [DOI] [PubMed] [Google Scholar]
- 37. Broillet M. C., Firestein S. (1996) Neuron 16, 377–385 [DOI] [PubMed] [Google Scholar]
- 38. McDavid S., Currie K. P. (2006) J. Neurosci. 26, 13373–13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hoheisel U., Sander B., Mense S. (1995) Neurosci. Lett. 188, 143–146 [DOI] [PubMed] [Google Scholar]
- 40. Manzoni O., Prezeau L., Marin P., Deshager S., Bockaert J., Fagni L. (1992) Neuron 8, 653–662 [DOI] [PubMed] [Google Scholar]
- 41. Nicholson R., Spanswick D., Lee K. (2004) Neurosci. Lett. 359, 180–184 [DOI] [PubMed] [Google Scholar]
- 42. Miyamoto T., Dubin A. E., Petrus M. J., Patapoutian A. (2009) PLoS One 4, e7596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yaksh T. L. (1989) Pain 37, 111–123 [DOI] [PubMed] [Google Scholar]
- 44. Sorkin L. S., Puig S., Jones D. L. (1998) Pain 77, 181–190 [DOI] [PubMed] [Google Scholar]
- 45. Bernardi P. S., Valtschanoff J. G., Weinberg R. J., Schmidt H. H., Rustioni A. (1995) J. Neurosci. 15, 1363–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Todd A. J. (1996) Eur. J. Neurosci. 8, 2492–2498 [DOI] [PubMed] [Google Scholar]
- 47. Todd A. J., Sullivan A. C. (1990) J. Comp. Neurol. 296, 496–505 [DOI] [PubMed] [Google Scholar]
- 48. Lu Y., Perl E. R. (2003) J. Neurosci. 23, 8752–8758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Santos S. F., Rebelo S., Derkach V. A., Safronov B. V. (2007) J. Physiol. 581, 241–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang H. M., Li D. P., Chen S. R., Pan H. L. (2005) J. Pharmacol Exp. Ther. 313, 697–704 [DOI] [PubMed] [Google Scholar]
- 51. Wang X. L., Zhang H. M., Li D. P., Chen S. R., Pan H. L. (2006) J. Physiol. 571, 403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhou H. Y., Zhang H. M., Chen S. R., Pan H. L. (2007) J. Neurophysiol. 97, 871–882 [DOI] [PubMed] [Google Scholar]
- 53. Zhou H. Y., Chen S. R., Chen H., Pan H. L. (2008) J. Pharmacol Exp. Ther. 327, 375–382 [DOI] [PubMed] [Google Scholar]
- 54. Zhou H. Y., Chen S. R., Chen H., Pan H. L. (2011) J. Pharmacol Exp. Ther. 336, 254–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wu S. Y., Dun N. J. (1996) J. Physiol. 495, 479–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ueda H., Misawa H., Katada T., Ui M., Takagi H., Satoh M. (1990) J. Neurochem. 54, 841–848 [DOI] [PubMed] [Google Scholar]
- 57. Winslow J. W., Bradley J. D., Smith J. A., Neer E. J. (1987) J. Biol. Chem. 262, 4501–4507 [PubMed] [Google Scholar]
- 58. Jeong S. W., Ikeda S. R. (1998) Neuron 21, 1201–1212 [DOI] [PubMed] [Google Scholar]
- 59. Ikeda S. R. (1996) Nature 380, 255–258 [DOI] [PubMed] [Google Scholar]
- 60. Lei S. Z., Pan Z. H., Aggarwal S. K., Chen H. S., Hartman J., Sucher N. J., Lipton S. A. (1992) Neuron 8, 1087–1099 [DOI] [PubMed] [Google Scholar]
- 61. Aizenman E., Potthoff W. K. (1999) Br. J. Pharmacol. 126, 296–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Head C. A., Swerdlow P., McDade W. A., Joshi R. M., Ikuta T., Cooper M. L., Eckman J. R. (2010) Am. J. Hematol. 85, 800–802 [DOI] [PubMed] [Google Scholar]
- 63. Yuen K. C., Baker N. R., Rayman G. (2002) Diabetes Care 25, 1699–1703 [DOI] [PubMed] [Google Scholar]
- 64. Christopherson K. S., Hillier B. J., Lim W. A., Bredt D. S. (1999) J. Biol. Chem. 274, 27467–27473 [DOI] [PubMed] [Google Scholar]
- 65. Valtschanoff J. G., Weinberg R. J. (2001) J. Neurosci. 21, 1211–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sun J., Picht E., Ginsburg K. S., Bers D. M., Steenbergen C., Murphy E. (2006) Circ. Res. 98, 403–411 [DOI] [PubMed] [Google Scholar]