Abstract
Tau is a microtubule-associated protein that accumulates in at least 15 different neurodegenerative disorders, which are collectively referred to as tauopathies. In these diseases, tau is often hyperphosphorylated and found in aggregates, including paired helical filaments, neurofibrillary tangles and other abnormal oligomers. Tau aggregates are associated with neuron loss and cognitive decline, which suggests that this protein can somehow evade normal quality control allowing it to aberrantly accumulate and become proteotoxic. Consistent with this idea, recent studies have shown that molecular chaperones, such as heat shock protein 70 and heat shock protein 90, counteract tau accumulation and neurodegeneration in disease models. These molecular chaperones are major components of the protein quality control systems and they are specifically involved in the decision to retain or degrade many proteins, including tau and its modified variants. Thus, one potential way to treat tauopathies might be to either accelerate interactions of abnormal tau with these quality control factors or tip the balance of triage towards tau degradation. In this review, we summarize recent findings and suggest models for therapeutic intervention.
Tauopathies are a family of neurodegenerative disorders characterized by the appearance of aggregates of the microtubule-associating protein, tau. These diseases include Alzheimer’s disease (AD), the most common neurodegenerative disorder, as well as devastating diseases such as frontotemporal dementia with parkinsonism linked to chromosome 17 and progressive supranuclear palsy [1–5]. In these diseases, tau is found in aggregates termed paired helical filaments [6,7], which assemble into the neurofibrillary tangles that were originally described as ‘senile’ plaques in the neurons of AD patients [8].
Numerous observations have converged on a model in which tau aggregation is important for clinical symptoms. For example, tau pathology closely correlates to neuron loss and cognitive deficits [9,10]. Furthermore, the post-translationally modified forms of tau (e.g., hyperphosphorylated and/or proteolyzed) that are enriched in paired helical filaments and neurofibrillary tangles are also more prone to self-assemble in vitro [11]. Finally, fronto-temporal dementia with parkinsonism linked to chromosome 17 is directly linked to point mutations that make tau more aggregation-prone. Together, these observations have led to the hypothesis that aggregation and abnormal accumulation of tau aggregates are significant contributing factors in tauopathies.
Tau is a cytosolic protein that is abundantly expressed in neurons and found in at least 13 splice isoforms in the brain [12,13]. Its major cellular function is to stabilize microtubules and this activity has been found to be essential for axonal transport [14]. Tau is a member of a class of intrinsically disordered proteins, whose free structures are believed to be best represented by an ensemble of possible orientations with weak preference for any specific structural motif [15–18]. However, tau is likely to adopt local structure when bound to microtubules. This interaction occurs through the microtubule-binding repeats of tau, with the 3R and 4R splice isoforms having either three or four repeats, respectively. Consistent with the importance of this domain, mutations in the microtubule-binding repeats have been found to weaken tau binding, reducing microtubule stability and sometimes leading to neuron loss [19,20].
Phosphorylation of tau by the kinases GSK3β, Cdk5 and MARK2 is a major regulator of its microtubule interactions [21–24]. GSK3β is a proline-directed serine/threonine kinase involved in many signaling pathways, including signaling downstream of wnt, insulin and many G-protein-coupled receptors [25]. Cdk5 is another serine/threonine kinase involved in multiple pathways, including NMDA receptor and growth factor signaling. Cdk5 exists in two complexes in post-mitotic neurons, a prosurvival complex with p35 (Cdk5–p35) and an apoptotic complex with p25 (Cdk5–p25), the latter of which has stronger kinase activity [22,26,27]. Together, GSK3β and Cdk5 are thought to be major kinases of tau in the brain [28]. Importantly, MARK2-based phosphorylation of tau is accelerated by the priming activity of either Cdk5 or GSK3β [29], suggesting that tau phosphorylation involves a series of ordered kinase events. In general, phosphorylation of tau reduces its affinity for microtubules [30], while dephosphorylation via enzymes such as PP2A and PP5 restores binding [30,31]. This reversible cycle of association and dissociation is a normal cellular process that facilitates axonal transport [30–33]. However, hyperphosphorylated forms of tau are more prone to aggregate, which might decrease their solubility and remove them from normal cycling [34]. Furthermore, proteolytic processing of tau, by caspases, calpains and other enzymes, can significantly accelerate hyperphosphorylation and facilitate aggregation [35]. Thus, tauopathies might be considered as involving an imbalance in the normal processing of tau, which affects its microtubule binding, aggregation propensity, phosphorylation status and, ultimately, its turnover.
Current therapies for tauopathies
There are no cures for any tauopathy. Neuroprotective agents, such as acetylcholin-esterase inhibitors and NMDA antagonists, have been approved for use in the clinic, based on their ability to slow the rate of cognitive decline in patients with moderate to severe AD (reviewed in [36]). However, long-term strategies for tauopathies will likely need to focus on impacting the underlying, disease-causing accumulation of modified and aggregated tau (reviewed in [37,38]). For example, because of the importance of phosphorylation, there are a number of kinase inhibitors being explored as therapeutics for tauopathies [39]. Whether this strategy will be able to improve cognition without adverse effects on other cellular processes remains to be determined. Nevertheless, some studies targeting kinases have demonstrated promising early efficacy in patients (reviewed in [40]). In addition to kinase inhibitors, compounds that directly block the aggregation of tau are also being explored as potential therapeutics [41]. These efforts have produced early-stage molecules of multiple different chemical classes, including rhodanine-based inhibitors, phenylthiazolyl-hydrazides, N-phenylamines, anthraquinones, benzothiazoles, phenothiazines and polyphenols [41]. However, recent studies suggest that intermediate, soluble oligomers of tau might best correlate with disease, suggesting that any anti-aggregation strategy will have to selectively reduce the levels of these structures [42–44].
Another possible way to treat tauopathies may be to manipulate the quality control pathways that regulate tau turnover. This hypothesis is based on the idea that tauopathies result, in part, from a failure of neurons to properly recognize and remove hyperphosphorylated and/or aggregated tau. All proteins, including tau, are subject to extensive regulation by the cellular quality control pathways, which carefully control the balance between protein expression and turnover to maintain healthy protein homeostasis (or proteostasis). Interestingly, tau clearance is known to be impaired in the aging brain [45], supporting the idea that diminished quality control might be conducive to certain tauopathies, such as AD, which are linked to aging. Based on this idea, one might imagine that pharmacologically accelerating tau degradation could be a possible strategy to relieve tauopathies. Furthermore, recent evidence suggests that tau is essential for the neurotoxicity of amyloid-β, providing a possible link between these classic AD targets and suggesting that reductions in tau levels might be important via multiple, beneficial mechanisms [46–48]. In this review, we will discuss some recent advances in this direction and discuss the strengths and challenges of this strategy. However, it is likely useful to first introduce the structure, function and ‘drugability’ of some of the key components of the quality control pathways: the molecular chaperones.
The molecular chaperones heat shock protein 70 & heat shock protein 90
Molecular chaperones are abundant and highly conserved proteins that assume an important role in protein quality control [49,50]. Several members of the chaperone family are upregulated in response to stress and, thus, these factors have been termed heat shock proteins (Hsps). The expression of Hsps is regulated by heat shock factor 1 (HSF1), which, under stress conditions, becomes associated with heat shock elements to elevate the transcription of Hsps and other proteins [51]. Each class of Hsp is named according to its molecular weight; for example, Hsp27, Hsp70 and Hsp90. These proteins are thought to serve individual functions, while collectively they monitor most aspects of protein synthesis, folding, trafficking and assembly of multiprotein complexes (reviewed in [52]). Importantly, the Hsps are also critical at the end of a protein’s life, as they facilitate turnover by the proteasome system and the clearance of proteotoxic aggregates by autophagy [53]. Even under normal conditions, as much as 30% of newly synthesized polypeptides are degraded [54], suggesting that all proteins are in equilibrium between folding and degradation; a balance that is maintained, in part, by the Hsp molecular chaperones.
Heat shock protein 70
Heat shock protein 70 is a major component in protein quality control and, as discussed below, a key factor in tau turnover. There are multiple, highly conserved Hsp70s in humans and members of this family of chaperones are abundant in all major organelles and the cytosol. Each Hsp70 is composed of a nucleotide-binding domain (NBD) and a substrate-binding domain (SBD) [55]. The NBD contains two lobes comprising a deep nucleotide-binding cleft that binds ATP. The SBD can be further divided into β-sandwich and α-helical lid subdomains, the latter of which contains a motif characterized by the amino acid EEVD that binds to tetratricopeptide repeat (TPR) domains. The NBD hydrolyzes ATP, while the SBD binds to hydrophobic regions of polypeptides via the β-sandwich subdomain [56]. It is thought that Hsp70 promiscuously binds to many hydrophobic polypeptides [57,58], including regions of tau adjacent to the microtubule-binding repeats [59]. A flexible linker connects the NBD and SBD and these domains appear to communicate via allostery. For example, with ATP bound to the NBD, the SBD has a weaker binding affinity for substrate polypeptides. The intrinsic ATPase activity of Hsp70 is very weak (~0.2 nmol/μg/min) [60] and, under physiological conditions, it is regulated by co-chaperones, including J-proteins and nucleotide exchange factors (NEFs). Briefly, J-proteins cause a conformational change in Hsp70s that accelerates ATP hydrolysis [61], while NEFs facilitate ADP release [62]. Finally, TPR domain-containing proteins are co-chaperones that bind to the EEVD motif and help dictate the fate of substrates bound to Hsp70. Thus, Hsp70s are part of a multiprotein complex that utilizes coordinated ATPase activity and multiple co-chaperone partners to shape interactions with misfolded substrates.
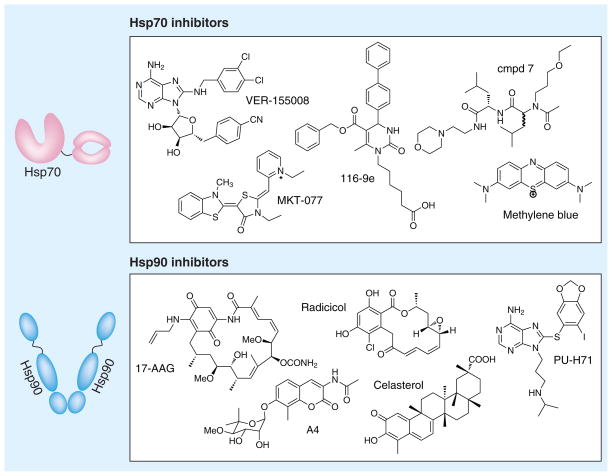
Heat shock protein 70 has been the subject of recent efforts to develop chemical inhibitors [52,63] and a number of high throughput screening efforts have been reported [60,64–67]. Drug development on this emerging target is still in its early stages, with only a few potent and/or selective inhibitors described [68]. Most of the currently available chemical probes target the ATPase activity of Hsp70, through either competitive or noncompetitive allosteric mechanisms (Figure 1). For example, VER-155008 is an ATP-competitive compound developed by structure-guided design [69,70]. However, there is an indirect relationship between ATP turnover and chaperone functions [71], suggesting that other aspects of Hsp70 function might also be worth exploring for drug discovery. For example, our groups have identified compounds that operate at protein–protein interfaces to disrupt interactions with co-chaperones, such as 116-9e [65,72,73], while other inhibitors target the SBD to block binding to substrates, such as compound 7 [74,75]. As might be expected given the diverse mechanisms of these compounds, known Hsp70 inhibitors represent a variety of chemical classes, including dihydropyrimidines, adenosine analogs, polyamines and others (Figure 1) [52,63]. Moreover, many of these inhibitors, including methylene blue and MKT-077, have poorly understood mechanisms.
Figure 1. Hsp70, Hsp90 and the chemical structures of select inhibitors.
Hsp: Heat shock protein.
Heat shock protein 90
Heat shock protein 90 has three domains: a 25-kDa N-terminal domain connected by a flexible, charged linker to the 40-kDa middle domain and 12-kDa C-terminal domain [76–83]. These three domains contain an ATP-binding pocket, a substrate peptide-binding site, and a TPR domain-binding EEVD motif, respectively. Recent structural studies have suggested that Hsp90 functions as a homodimer in which the C-terminal domains of two Hsp90 molecules are in contact at the bottom of the ‘V-shaped’ open conformer. As in the case of Hsp70, co-chaperones of Hsp90, such as Aha1, cdc37 and TPR domain-containing proteins, regulate its ATPase activity and control its conformational transitions (reviewed in [84]). Hsp90 is thought to have a more restricted set of substrates than Hsp70, but these clients include a number of important kinases and signaling proteins.
Owing to its client pool, Hsp90 has become an established anticancer target, with a number of selective and potent inhibitors available [85,86]. Work on Hsp90 inhibitors benefited from the early discovery of the natural product, geldanamycin, which competes with ATP and induces destabilization of Hsp90-bound proteins [87]. Since this discovery, a number of high-affinity analogs, such as 17-AAG, and alternative synthetic scaffolds, including radicicol and PU-H71, have been reported (Figure 1) [85,88]. These compounds bind in either the N-terminal ATP-binding site (e.g., 17-AAG, radicicol and PU-H71) [89] or C-terminal dimerization domain (e.g., novobiocin and A4) [90,91], and they show great promise as both anticancer compounds and research tools for understanding Hsp90 biology. Several Hsp90 inhibitors are currently in clinical trials, all of which target the N-terminal domain [92]. More recently, there has also been interest in developing compounds, such as celastrol (Figure 1), that selectively disrupt association of co-chaperones with Hsp90 as an alternative way to control chaperone activity [93–96].
Heat shock protein 27
Heat shock protein 27 is a member of the conserved, small heat shock protein family. These proteins are ATP-independent chaperones that undergo homo-oligomerization in response to stress [97,98]. Small heat shock proteins have been imaged by electron microscopy and these studies have revealed that some of these oligomers are ordered polyhedrons [99]. Furthermore, gel filtration and centrifugation studies have suggested that Hsp27 exists as an ensemble of oligomers, which range in size from dimers to approximately 32 mers or greater. Binding of susceptible substrates to the surface of these Hsp27 oligomers is thought to limit stress-induced aggregation and, thus, this chaperone is considered a ‘holdase’. To allow substrate release, Hsp27 oligomerization is reversible; a process that is regulated, at least in part, by phosphorylation. Like Hsp90 and Hsp70, Hsp27 plays important prosurvival roles; however, no drug-like molecules have been reported that target its activities or protein–protein interactions.
The Hsp70–Hsp90 chaperone cycle
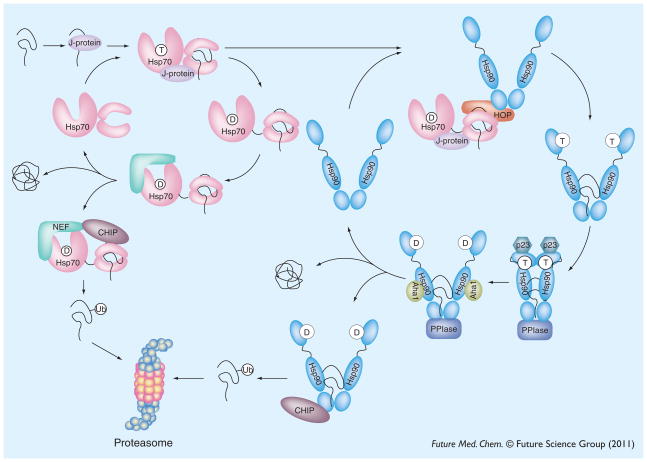
During protein quality control, Hsp70, Hsp90 and Hsp27 (and their co-chaperones) often work in concert. If prolonged misfolding is detected, the chaperones shuttle the protein to a degradation endpoint, such as the proteasome or autophagy. This general model arises, in large part, from a series of seminal experiments on the Hsp70/Hsp90 chaperone system and how it regulates the nuclear hormone receptors [100,101]. In this model, a J-protein (also known as Hsp40) is thought to facilitate high-affinity substrate binding to Hsp70 by coordinating substrate delivery and ATP turnover (Figure 2) [60]. The action of an NEF then releases the substrate [49], which can either achieve its proper folding outcome, re-enter the Hsp70-based ATPase cycle, or be fated for subsequent steps in quality control. In these latter cases, an Hsp70-bound substrate can be transferred to Hsp90 via Hsp70/Hsp90 organizing protein (HOP) [102,103]. Alternatively, either Hsp70 or Hsp90 can recruit the ubiquitin E3 ligase, C-terminal Hsp70 interacting protein (CHIP), to degrade the bound substrate [104]. During this triage process, certain substrates are known to require additional co-chaperones at distinct steps. For example, the co-chaperones cdc37, a peptidyl-prolyl cis-trans isomerase (PPIase) family member, and p23 are all critical for the transfer of kinases to Hsp90 and maturation of the active protein [76,105]. In addition, Hsp90’s hydrolysis of ATP, which is stimulated by Aha1, facilitates polypeptide release [82,106] and transfer to CHIP or other E3 ligases [107,108]. Thus, protein quality control is thought to involve a series of ‘checkpoint’ steps that monitor substrate folding and enact triage decisions. These steps are defined by ATP/ADP cycling, changes in tertiary and quaternary structure, and fluctuation in the identity of associated co-chaperones.
Figure 2. Model of heat shock protein 70–heat shock protein 90 cycling.
Unfolded or misfolded substrates enter the chaperone cycle by engaging in interactions with J-proteins, which recruit Hsp70 family chaperones. The ATPase cycle of Hsp70 can result in substrate folding, triage through the proteasome or ‘hand-off’ to the Hsp90 system. The ATPase cycle of Hsp90 refines folding and stabilizes the active form of many kinases and transcription factors. In addition, the Hsp90 cycle makes triage decisions, through CHIP-mediated ubiquitination and degradation.
CHIP: C-terminal Hsp70 interacting protein; HOP: Hsp70/Hsp90 organizing protein; Hsp: Heat shock protein; NEF: Nucleotide exchange factor; PPIase: Peptidyl-prolyl cis-trans isomerase.
Although both Hsp70 and Hsp90 can promote degradation of client proteins, it has recently been shown that, functionally, the Hsp70 complex often dominates triage decisions [85,107,109]. Thus, Hsp70 may function as a primary checkpoint prior to entering the Hsp90-based complex for maturation. In this model, it may be helpful to simplify protein quality control as occuring in three phases: J-protein-directed recruitment; primary folding and triage in the Hsp70 complex; and secondary folding and function in the Hsp90 system (Figure 2). In theory, any of these steps might contain suitable drug targets for tauopathies.
Tau regulation by molecular chaperones
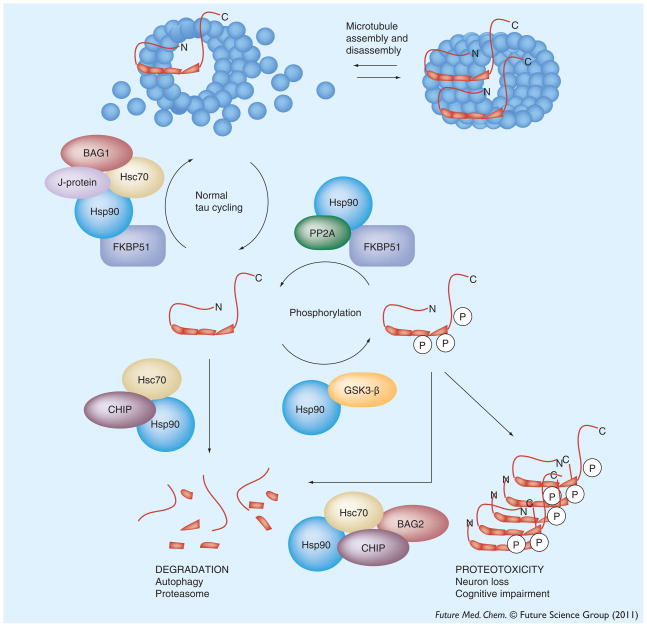
Tau cycling on/off microtubules and its proteolytic turnover are assisted by molecular chaperones and, thus, these proteins play an important role in its normal homeostasis [110,111]. However, under potentially proteotoxic conditions, the post-translational modifications or mutations that damage tau’s affinity for microtubules and favor its aggregation are thought to generate a molecular ‘danger signal’ that specifically alerts the quality control system [112,113]. As discussed above, this system then ‘decides’ to retain or degrade that substrate. In the case of abnormal tau, imbalances in this decision appear to allow accumulation, proteotoxicity and neuron loss (Figure 3). Here, we will review the evidence suggesting an essential ‘decision-making’ role for molecular chaperones in tau quality control and tauopathies.
Figure 3. Overview of tau quality control by molecular chaperones.
From the microtubule-bound pool, tau might be released in a form that is capable of re-binding microtubule (termed ‘cycling tau’). Alternatively, hyperphosphorylated and aggregation-prone tau might accumulate. Chaperones are involved in all stages of cycling tau, in mediating phosphorylation and in making triage decisions.
BAG: Bcl2-associated anthanogene; CHIP: C-terminal Hsp70 interacting protein; FKBP51: FK506-binding protein 51 kDa; Hsp: Heat shock protein.
Tau & Hsp70
Hsp70 has been shown to both stabilize binding of tau to microtubules [114] and promote its degradation in combination with CHIP [115,116]. In addition, recent work has demonstrated that Hsc70, the constitutive cytosolic form of Hsp70s, also dynamically regulates the association of tau with microtubules [112]. These activities arise via direct binding of Hsp70s to the microtubule binding repeats [59]. This central role for Hsp70 family members in regulating tau cycling and triage suggests possible ways of tipping the balance. In fact, recent work from our group has shown that inhibition of the ATPase activity of Hsp70/Hsc70 promotes proteasomal degradation of tau; whereas activation results in tau accumulation [117]. Furthermore, one of these inhibitors was able to reduce phospho-tau levels and improve cognition in a transgenic mouse model [44]. Interestingly, these effects were observed without apparent toxicity, induction of HSF1 activity or adverse health effects on the animals, providing preliminary evidence that targeting Hsp70 may be a surprisingly viable pharmacological approach. However, many critical questions remain. For example, what is the molecular mechanism(s) by which inhibition of Hsp70 leads to tau degradation? How does Hsp70 selectively recognize abnormal tau from a pool of normal cycling tau? Speculative models to account for these interesting observations are elaborated upon in the last section of this review.
Tau & Hsp90
Hsp90 was also shown to increase association of tau with microtubules [114]; however, its binding is not well characterized and it is not known whether this is a direct or indirect process. For example, it has recently been shown that Hsp90 promotes tau’s phosphorylation by its ability to stabilize GSK3β [118]. These data may suggest that Hsp90 allows accumulation of pathogenic tau species. However, this issue is more complicated, as other work has shown that chemical inhibition of Hsp90 by 17-AAG and other inhibitors reduces cellular levels of two phospho-tau species, pS202/T205 and pS396/S404, both of which are relevant to AD pathogenesis [119]. In response to the inhibitors, tau clearance occurs via CHIP-mediated, proteasomal degradation [119–121]. In addition, other mutant forms of tau have also been particularly susceptible to Hsp90 inhibition, while wild-type tau is not [122]. Interestingly, it was recently found that reducing the levels of Akt, another client of the Hsp90/CHIP complex, facilitates tau degradation [123], suggesting a synchronized balance between competing Hsp90 substrates that may be driven, in part, by their relative abundance or susceptibility to Hsp90 binding.
Together, multiple studies suggest that Hsp90 regulates the stability of both phospho- and mutant-tau. However, a greater effort needs to be made to elucidate the mechanisms driving the apparent preservation of aggregation-prone isoforms. Potential mechanisms and research avenues will be discussed in more detail later, but one interesting idea comes from studies implicating Hsp90 as a driving force in evolution via its ability to preserve mutant proteins [124]. If Hsp90 can buffer against mutations, whether deleterious or beneficial, then this suggests that it might be adopting a similar role in tau pathogenesis. In other words, Hsp90 inhibition may be able to ‘de-evolve’ the chaperone system to clear proteotoxic tau species.
Tau & small heat shock proteins
Hsp27 has emerged as a potential target for tau regulation based on early findings that it preferentially binds to phosphorylated and hyperphosphorylated tau and promotes their clearance [125,126]. However, astrocyte-derived Hsp27 has been shown to promote tau accumulation and Hsp27 associates with tau tangles in a mouse model [127,128], suggesting a more complex relationship. Recently, our group demonstrated that viral delivery of wild-type Hsp27 into the brains of tau-transgenic mice reduced tau levels and rescued long-term potentiation deficits. Conversely, delivery of a mock-phosphorylated mutant Hsp27 caused increased tau levels [129]. This study suggests that Hsp27 may need to cycle between phosphorylated and de-phosphorylated states to promote tau clearance, perhaps explaining the apparent contradictions observed previously. Until more is known about the structure and biology of Hsp27, and why certain multimeric states are necessary for its chaperoning abilities, it will likely remain an elusive target.
Tau & Bcl2-associated anthanogenes
Proteins containing Bcl2-associated anthanogene (BAG) domains, such as BAG1 and BAG2, have been found to function as NEFs for Hsp70s [62]. BAG1 is upregulated in the hippocampus of AD patients [130], where it associates with tau and increases tau levels in cooperation with Hsp70 [131]. BAG1 silencing decreases tau levels, consistent with a critical role for this co-chaperone in protecting tau from degradation. However, another related BAG family member, BAG2, interacts with Hsp70 and tau but, unlike BAG1, assists clearance of phosphorylated tau [132]. Thus, it appears that different BAG family members can have distinct and dramatic impacts on the fate of Hsp70-bound tau. Moreover, these studies suggest that pharmacologically enhancing BAG2 or inhibiting BAG1 might be expected to decrease tau levels. However, the best way to leverage this information is not yet clear.
Tau & Hsp110
Recent work has defined Hsp110 as another NEF for Hsp70s [133,134]. This co-chaperone is of interest in tauopathies because Hsp110 knockout mice show an age-dependent accumulation of phosphorylated tau in the hippocampus [135]. As little is known about direct binding of Hsp110 to tau or its specific effects on substrate selection by Hsp70s, it is not yet clear how these findings might be translated to therapeutics.
Tau & FK506-binding protein 51 kDa
Recently, the co-chaperone FK506-binding protein 51 kDa (FKBP51) has been implicated as a modulator of tau binding to microtubules. FKBP51 contains a PPIase domain as well as a TPR domain; thus it can be recruited to the Hsp90 system where it catalyzes isomerization of proline residues in chaperone-bound substrates. In relation to tau biology, FKBP51 enhances the association of Hsp90 with tau, co-localizes with tau in murine neurons, co-immunoprecipitates with tau in AD tissue samples and increases with age in an AD mouse model [136]. These interactions may be functionally important because silencing FKBP51 reduces tau and phosphorylated-tau levels [136]. In addition, the PPIase activity of FKBP51 was necessary for tau-related microtubule formation in a Xenopus oocyte model, suggesting that this co-chaperone is not only interacting with tau but that its enzymatic activity is important. Finally, the FKBP51–Hsp90 complex has been proposed to be responsible for the interaction of tau with phosphatases, helping to restore binding to microtubules [137]. Together, these studies suggest that an Hsp90–FKBP51 complex assists normal cycling of tau via multiple mechanisms. Moreover, since high-affinity inhibitors of FKBPs (e.g., FK506) are well known, these findings suggest important avenues for therapeutic intervention [138].
Models for tau quality control & future opportunities for drug discovery
Together, these genetic and pharmacological studies suggest that targeting molecular chaperones may be a way to reduce phosphorylated tau levels and treat tauopathies. In fact, this idea has been shown for Hsp70 and Hsp90 inhibitors in model systems [44,117,120,121]. However, there are many open questions about the best way to proceed on the path to therapeutics. How do you develop drugs that reduce abnormal tau levels without impacting global proteostasis? Is it possible to selectively induce a BAG2–Hsp70 or CHIP–Hsp90 complex to accelerate tau degradation? In general, the field has a limited understanding of how normal triage decisions are made by the Hsp70/Hsp90 chaperone systems. How are substrates normally selected? What structural transitions lead to normal substrate turnover? What features of a substrate are important for communicating its failed folding to the chaperones? Here, we will briefly provide some plausible models that are consistent with the available findings. These discussions, while admittedly oversimplified, are intended to act as a framework for guiding future therapeutic efforts.
The role of ATP hydrolysis & substrate dwell time
From a medicinal chemist’s perspective, targeting the ATP-binding site of either Hsp70 or Hsp90 has significant advantages, because the nucleotide-binding pockets are deep, well-defined clefts (for a recent, in-depth review of this concept see [68]). Moreover, as mentioned previously, inhibitors of the ATPase activities of either Hsp70 or Hsp90 have been found to reduce tau levels, supporting the general promise of this approach [44,117,120,121]. However, the mechanisms that correlate changes in nucleotide state with substrate degradation are not clear. In general, it is thought that inhibition of ATP cycling will lead to release of the substrate to the degradation pathways because of the allostery between nucleotide state and substrate-binding affinity. However, what is the structure and composition of the early degradation complex? How does it differ from the folding or retention complex? We propose that if we better understood the molecular and structural underpinnings of this triage decision, we might be able to develop therapeutic strategies that best target abnormal tau for turnover.
One potential model linking ATPase inhibitors to changes in tau fate is based on the relative partitioning of substrates onto the Hsp70/Hsp90 chaperones [139]. As discussed earlier, both of these chaperones hydrolyze ATP and use this cycling to regulate their affinity for substrates. Thus, substrate ‘dwell time’ in the chaperone-bound state will depend, in part, on nucleotide turnover rate and the relative abundance of the ATP- and ADP-bound forms [140]. It seems possible that folding-competent proteins, on average, might be more transiently associated with the Hsp70–Hsp90 complex, while misfolded substrates could be ‘stalled’ and have increased dwell time. In this model, accumulation of an Hsp70–substrate complex (either via treatment with chemical inhibitors or because of intrinsic properties of the substrate) might allow enough time for a degradation factor (e.g., CHIP) to bind and facilitate polyubiquitination. This idea is supported by the findings that inhibitors of Hsp70’s ATPase activity favor degradation of tau, while activators (which would presumably promote ATP cycling) led to net retention [73,117]. Similarly, AdaSGC, which inhibits J-protein-stimulated Hsp70 ATPase activity, promotes escape of ΔF508 cystic fibrosis transmembrane regulator from ER-associated degradation [141]. For studies on Hsp90 inhibitors, this model also has some support. For example, Hsp90 inhibitors cause degradation of tau and many cancer-related substrates [85]. Moreover, these compounds were found to prolong binding of Hsp90 to a model substrate, which was sufficient to promote its degradation [142]. Together, these studies suggest that degradation factors, such as CHIP, might be particularly recruited to chaperones in a ‘stalled’ conformation that is enforced by prolonged ATP/ADP binding or another, yet uncharacterized, structural signal. This ‘degradation cue’ seems likely to be additionally impacted by other factors, including the context of the client, availability of co-chaperones and, possibly, the organelle environment. The fact that Hsp70 inhibitors reduce tau levels without affecting other likely Hsp70 substrates, such as α-synuclein or TDP-43, generally supports the idea that substrates are actively involved in dictating their own fate [117]. Direct measurements of substrate dwell times in cells will likely be required to clarify the impact of this variable on quality control decisions.
Selecting abnormal tau for degradation
The context-sensitive activity of the chaperone systems naturally gives rise to a model in which they can ‘sense’ molecular features of their substrates, such as aggregation propensity. This model is consistent with the data that hyperphosphorylated tau appears to be specifically selected for degradation by some chaperone machines, such as the Hsp90–FKBP51 complex, without effects on normal tau [132,136]. How does this selectivity arise? This question is important because any future therapy will likely need to reduce abnormal tau levels with minimal impact on total tau or global proteostasis. One potential insight comes from the observation that there are more than 40 distinct J-proteins in humans [143]. These co-chaperones are thought to be especially important for client selection and recruitment into the early Hsp70 triage complex. Because mammals express over forty J-proteins, dozens of NEFs, hundreds of TPRs and other co-chaperones, combinatorial assembly will quickly permit a vast array of possible Hsp70/Hsp90 chaperone complexes. Thus, in this hypothetical and speculative model, groups of related substrates (e.g., hyperphosphorylated tau isoforms) might be expected to depend on a dedicated set of complexes. A major prediction of this model, then, is that targeting the ‘right’ chaperone complex with small molecules might allow selective degradation of abnormal tau without adverse effects on other targets. However, this model is likely too simple. In addition to the composition of the targeted chaperone complex, cellular outcomes are likely to be influenced by the relative substrate expression levels, age, cellular-folding capacity, current flux through the degradation pathways and cell type [144].
Control over protein–protein interactions in the Hsp70/Hsp90 complexes
Because substrate fate is thought to be dependent on a series of ‘hand-offs’ between dynamic, multiprotein chaperone complexes (as in Figure 2), another option for controlling the fate of tau might be to specifically target key protein–protein interactions. Consistent with this idea, data from our group and others have suggested that chemical modulators of co-chaperone interactions ‘tip’ the equilibrium and alter tau levels [72,73,117]. This general idea has been observed in other systems as well. For example, the Hsp90 inhibitor geldanamycin mimics ADP binding, but also inhibits recruitment of p23, which is a necessary step in client maturation [145]. Interestingly, p23 deletion causes hypersensitivity to geldanamycin and its overexpression exhibits a protective effect [146], suggesting that these chemical and co-chaperone factors are antagonistic. Together, these data suggest that blocking recruitment of specific co-chaperones or facilitating the assembly of others might directly impact client fate. In fact, this is believed to be what occurs during natural triage steps [147]. For example, BAG-2 inhibits client ubiquitination by CHIP by interfering with the interaction between CHIP and E2 ubiquitin-conjugating enzymes [148]. In another example, McClellan et al. showed that von Hippel–Lindau tumor-suppressor protein requires Hsp70 for its folding and degradation, whereas Hsp90 is only required for degradation [149]. HOP was also required for degradation, indicating that transfer of von Hippel–Lindau from the Hsp70 complex to Hsp90 is a necessary part of its degradation pathway. To the medicinal chemist or chemical biologist, these studies suggest numerous potential opportunities for achieving desired outcomes by manipulating protein contacts within the chaperone system.
Although there are significant challenges related to targeting protein–protein interactions, significant advances have been achieved in the past decade [150,151]. Emerging experimental methods, such as NMR-based fragment screening, are producing robust compounds [152] and comprehensive cheminformatics analyses have started to elucidate the features of good protein–protein interaction inhibitors [151]. As a result of these advances, protein–protein interaction inhibitors are being identified with increasing frequency, even in the Hsp70/Hsp90 systems [73,153]. For example, we found that dihydropyrimidine-based molecules can either force the association of a prokaryotic Hsp70 with its J-protein partner or, with a relatively modest change in chemical structure, related compounds could block this contact [73]. Using high-throughput screening against the J-protein-stimulated ATPase activity of an Hsp70, we also found polyphenols that selectively block J-stimulated activities by interfering with J-protein recruitment to the Hsp70 complex [72]. We termed this approach ‘gray box’ screening because it uses reconstituted, multichaperone complexes to uncover chemical inhibitors that target context-specific allosteric sites [65]. Together, these early studies suggest that the chaperone complexes might be amenable to chemical prodding, perhaps altering the fate of substrates, such as tau, that pass through this quality control system.
Induction of a stress response
Over the past decade, induction of Hsps has been shown to ameliorate proteotoxicity caused by misfolded proteins [154–159]. Accordingly, pharmacological activation of the heat shock response may be another viable strategy for the treatment of tauopathies. Towards that eventual goal, several compounds that activate HSF1 have been reported (reviewed in [160]). However, the mechanisms that link HSF1 induction to improved proteostasis are not yet clear. In the case of HSF1 inducers, it seems plausible that the resulting conditions (e.g., elevated levels of Hsp70/90) influence the average dwell time of misfolded substrates on chaperones, leading to improved capacity to degrade damaged proteins. In addition, high concentrations of chaperones, including Hsp27, might protect misfolded proteins from engaging in off-pathway, proteotoxic interactions. Because N-terminal Hsp90 inhibitors often induce a stress response, this mechanism may be particularly important in the response of abnormal tau to those compounds.
Future perspective
What does the future hold for molecular chaperones as therapeutic targets for tauopathies? As emphasized throughout these discussions, we expect that more detailed insights into the cellular and molecular mechanisms of protein quality control will allow us to safely promote the turnover of abnormal tau. Based on our current knowledge, it seems likely that these eventual therapies will include compounds targeting the ATP-binding clefts of Hsp70/90. These compounds might prolong the dwell time of abnormal tau on the chaperones, promoting their degradation. However, to achieve low toxicity, this list of possible drugs may also include molecules that target other regions on Hsp70 and Hsp90, especially co-chaperone binding surfaces that influence the selection of substrates. Finally, other chaperones, such as small Hsps, remain underexplored as drug targets because they lack obvious binding sites. Finding ways to target these factors, directly or via induction of a heat shock response, may expand our ability to accelerate tau turnover and ameliorate tauopathies.
Even with these expected advances in medicinal chemistry and compound discovery, a number of other challenges await their clinical deployment. For example, conducting trials in patients with aging-related neurodegenerative disorders is a daunting task. In the next decade, we expect that trials in this area will become more sophisticated, favoring more adaptive designs. Of equal importance, biomarkers that distinguish between different dementias, including tauopathies, will need to become better defined. Although these problems are not specific to tauopathies, they will need to be solved to encourage investment in this area.
Thus, in the next 10 years, we expect that new generations of chaperone inhibitors will emerge. These compounds will target many different aspects of chaperone biology and their discoveries will coincide with a deeper understanding of the mechanisms of protein quality control. Together with improved clinical trial design and better biomarkers, we expect that these advancements will significantly accelerate development of novel treatments for tauopathies.
Executive summary.
Tau is a microtubule-associated protein that, upon hyperphosphorylation, aggregates and forms neurofibrillary tangles, which is a characteristic feature seen in tauopathies such as Alzheimer’s disease.
Molecular chaperones including heat shock protein (Hsp)70, Hsp90 and their co-chaperones are involved in the quality control of tau and, thus, have gained attention as possible targets for tauopathies.
Hsp70 and Hsp90 inhibitors have been shown to reduce tau levels in models of tauopathy.
The molecular mechanisms linking Hsp70/Hsp90 inhibition to tau turnover are not yet clear, but they might involve differences in the ‘dwell time’ of the chaperone–tau interaction.
Emerging evidence suggests that tau levels might be reduced by targeting key protein–protein interactions and enzymatic activities that are involved in its quality control.
Acknowledgments
The authors thank Erik Zuiderweg, Bill Pratt and members of our laboratories for helpful conversations.
Key Terms
- Acetylcholinesterase inhibitors
These increase the level of acetylcholine, a neurotransmitter, by inhibiting the enzyme
- NMDA antagonists
These block the NMDA receptor and prevent binding of glutamate, which, at a high level, could lead to cell death
- Proteostasis
Proteostasis, or protein homeostasis, is maintained by numerous pathways that monitor protein synthesis, folding, assembly into complexes and turnover
- Heat shock protein
Family of molecular chaperones that facilitate protein folding and block protein aggregation
- Proteasome
Enzyme complex that degrades polypeptides
- Tetratricopeptide repeat
Structural motif that is involved in protein–protein interactions
- Ubiquitin E3 ligase
Protein that transfers ubiquitin molecules from an E2 ubiquitin-conjugating enzyme to target proteins
Footnotes
For reprint orders, please contact reprints@future-science.com
Financial & competing interests disclosure
The authors’ work on chaperones and tau is funded by grants from the NIH (NS095690) and NSF (MCB-0844512). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
- 1.Feany MB, Dickson DW. Neurodegenerative disorders with extensive tau pathology: a comparative study and review. Ann Neurol. 1996;40(2):139–148. doi: 10.1002/ana.410400204. [DOI] [PubMed] [Google Scholar]
- 2.Feany MB, Ksiezak-Reding H, Liu WK, et al. Epitope expression and hyperphosphorylation of tau protein in corticobasal degeneration: differentiation from progressive supranuclear palsy. Acta Neuropathol. 1995;90(1):37–43. doi: 10.1007/BF00294457. [DOI] [PubMed] [Google Scholar]
- 3.Goedert M, Spillantini MG, Crowther RA, et al. Tau gene mutation in familial progressive subcortical gliosis. Nat Med. 1999;5(4):454–457. doi: 10.1038/7454. [DOI] [PubMed] [Google Scholar]
- 4.Hutton M, Lendon CL, Rizzu P, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia ftdp-17. Nature. 1998;393(6686):702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 5.Mattila P, Togo T, Dickson DW. The subthalamic nucleus has neurofibrillary tangles in argyrophilic grain disease and advanced Alzheimer’s disease. Neurosci Lett. 2002;320(1–2):81–85. doi: 10.1016/s0304-3940(02)00006-x. [DOI] [PubMed] [Google Scholar]
- 6.Alonso AC, Grundke-Iqbal I, Iqbal K. Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med. 1996;2(7):783–787. doi: 10.1038/nm0796-783. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez F, Avila J. Tauopathies. Cell Mol Life Sci. 2007;64(17):2219–2233. doi: 10.1007/s00018-007-7220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grundke-Iqbal I, Iqbal K, Tung YC, et al. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramsden M, Kotilinek L, Forster C, et al. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (p301l) J Neurosci. 2005;25(46):10637–10647. doi: 10.1523/JNEUROSCI.3279-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santacruz K, Lewis J, Spires T, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309(5733):476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun W, Johnson GV. The role of tau phosphorylation and cleavage in neuronal cell death. Front Biosci. 2007;12:733–756. doi: 10.2741/2097. [DOI] [PubMed] [Google Scholar]
- 12.Andreadis A. Tau gene alternative splicing: expression patterns, regulation and modulation of function in normal brain and neurodegenerative diseases. Biochim Biophys Acta. 2005;1739(2–3):91–103. doi: 10.1016/j.bbadis.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Mandelkow E, von Bergen M, Biernat J, Mandelkow EM. Structural principles of tau and the paired helical filaments of Alzheimer’s disease. Brain Pathol. 2007;17(1):83–90. doi: 10.1111/j.1750-3639.2007.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gustke N, Trinczek B, Biernat J, Mandelkow EM, Mandelkow E. Domains of tau protein and interactions with microtubules. Biochemistry. 1994;33(32):9511–9522. doi: 10.1021/bi00198a017. [DOI] [PubMed] [Google Scholar]
- 15.Mukrasch MD, Bibow S, Korukottu J, et al. Structural polymorphism of 441-residue tau at single residue resolution. PLoS Biol. 2009;7(2):e34. doi: 10.1371/journal.pbio.1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6(3):197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 17.Narayanan RL, Durr UH, Bibow S, et al. Automatic assignment of the intrinsically disordered protein tau with 441-residues. J Am Chem Soc. 2010;132(34):11906–11907. doi: 10.1021/ja105657f. [DOI] [PubMed] [Google Scholar]
- 18.Uversky VN. Flexible nets of malleable guardians: intrinsically disordered chaperones in neurodegenerative diseases. Chem Rev. 2011;111(2):1134–1166. doi: 10.1021/cr100186d. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa M, Smith MJ, Goedert M. Tau proteins with ftdp-17 mutations have a reduced ability to promote microtubule assembly. FEBS Lett. 1998;437(3):207–210. doi: 10.1016/s0014-5793(98)01217-4. [DOI] [PubMed] [Google Scholar]
- 20.Jordan MA. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr Med Chem Anticancer Agents. 2002;2(1):1–17. doi: 10.2174/1568011023354290. [DOI] [PubMed] [Google Scholar]
- 21.Anderton BH, Betts J, Blackstock WP, et al. Sites of phosphorylation in tau and factors affecting their regulation. Biochem Soc Symp. 2001;67:73–80. doi: 10.1042/bss0670073. [DOI] [PubMed] [Google Scholar]
- 22.Hashiguchi M, Saito T, Hisanaga S, Hashiguchi T. Truncation of Cdk5 activator p35 induces intensive phosphorylation of ser202/thr205 of human tau. J Biol Chem. 2002;277(46):44525–44530. doi: 10.1074/jbc.M207426200. [DOI] [PubMed] [Google Scholar]
- 23.Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. Involvement of aberrant glycosylation in phosphorylation of tau by Cdk5 and GSK-3β. FEBS Lett. 2002;530(1–3):209–214. doi: 10.1016/s0014-5793(02)03487-7. [DOI] [PubMed] [Google Scholar]
- 24.Lund ET, McKenna R, Evans DB, Sharma SK, Mathews WR. Characterization of the in vitro phosphorylation of human tau by tau protein kinase ii (Cdk5/p20) using mass spectrometry. J Neurochem. 2001;76(4):1221–1232. doi: 10.1046/j.1471-4159.2001.00130.x. [DOI] [PubMed] [Google Scholar]
- 25.Engmann O, Giese KP. Crosstalk between Cdk5 and GSK3β: implications for Alzheimer’s disease. Front Mol Neurosci. 2009;2(2):1–5. doi: 10.3389/neuro.02.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hisanaga S, Endo R. Regulation and role of cyclin-dependent kinase activity in neuronal survival and death. J Neurochem. 2010;115(6):1309–1321. doi: 10.1111/j.1471-4159.2010.07050.x. [DOI] [PubMed] [Google Scholar]
- 27.Patrick GN, Zukerberg L, Nikolic M, et al. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402(6762):615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 28.Flaherty DB, Soria JP, Tomasiewicz HG, Wood JG. Phosphorylation of human tau protein by microtubule-associated kinases: GSK3β and Cdk5 are key participants. J Neurosci Res. 2000;62(3):463–472. doi: 10.1002/1097-4547(20001101)62:3<463::AID-JNR16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Kosuga S, Tashiro E, Kajioka T, et al. GSK-3β directly phosphorylates and activates MARK2/PAR-1. J Biol Chem. 2005;280(52):42715–42722. doi: 10.1074/jbc.M507941200. [DOI] [PubMed] [Google Scholar]
- 30.Mandelkow EM, Thies E, Trinczek B, Biernat J, Mandelkow E. Mark/par1 kinase is a regulator of microtubule-dependent transport in axons. J Cell Biol. 2004;167(1):99–110. doi: 10.1083/jcb.200401085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thies E, Mandelkow EM. Missorting of tau in neurons causes degeneration of synapses that can be rescued by the kinase MARK2/PAR-1. J Neurosci. 2007;27(11):2896–2907. doi: 10.1523/JNEUROSCI.4674-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drewes G, Ebneth A, Preuss U, Mandelkow EM, Mandelkow E. Mark, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997;89(2):297–308. doi: 10.1016/s0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- 33.Trinczek B, Ebneth A, Mandelkow EM, Mandelkow E. Tau regulates the attachment/detachment but not the speed of motors in microtubule-dependent transport of single vesicles and organelles. J Cell Sci. 1999;112(14):2355–2367. doi: 10.1242/jcs.112.14.2355. [DOI] [PubMed] [Google Scholar]
- 34.Gong CX, Iqbal K. Hyperphosphorylation of microtubule-associated protein tau: a promising therapeutic target for Alzheimer disease. Curr Med Chem. 2008;15(23):2321–2328. doi: 10.2174/092986708785909111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Calignon A, Fox LM, Pitstick R, et al. Caspase activation precedes and leads to tangles. Nature. 2010;464(7292):1201–1204. doi: 10.1038/nature08890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lleo A, Greenberg SM, Growdon JH. Current pharmacotherapy for Alzheimer’s disease. Ann Rev Med. 2006;57:513–533. doi: 10.1146/annurev.med.57.121304.131442. [DOI] [PubMed] [Google Scholar]
- 37.Lee VM, Trojanowski JQ. Progress from Alzheimer’s tangles to pathological tau points towards more effective therapies now. J Alzheimers Dis. 2006;9(Suppl 3):257–262. doi: 10.3233/jad-2006-9s328. [DOI] [PubMed] [Google Scholar]
- 38.Schneider A, Mandelkow E. Tau-based treatment strategies in neurodegenerative diseases. Neurotherapeutics. 2008;5(3):443–457. doi: 10.1016/j.nurt.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazanetz MP, Fischer PM. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat Rev Drug Discov. 2007;6(6):464–479. doi: 10.1038/nrd2111. [DOI] [PubMed] [Google Scholar]
- 40.Maccioni RB, Farias G, Morales I, Navarrete L. The revitalized tau hypothesis on Alzheimer’s disease. Arch Med Res. 2010;41(3):226–231. doi: 10.1016/j.arcmed.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Bulic B, Pickhardt M, Schmidt B, et al. Development of tau aggregation inhibitors for Alzheimer’s disease. Angew Chem Int Ed Engl. 2009;48(10):1740–1752. doi: 10.1002/anie.200802621. [DOI] [PubMed] [Google Scholar]
- 42.Cowan CM, Bossing T, Page A, Shepherd D, Mudher A. Soluble hyperphosphorylated tau causes microtubule breakdown and functionally compromises normal tau in vivo. Acta Neuropathol. 2010;120(5):593–604. doi: 10.1007/s00401-010-0716-8. [DOI] [PubMed] [Google Scholar]
- 43.Cowan CM, Chee F, Shepherd D, Mudher A. Disruption of neuronal function by soluble hyperphosphorylated tau in a drosophila model of tauopathy. Biochem Soc Trans. 2010;38(2):564–570. doi: 10.1042/BST0380564. [DOI] [PubMed] [Google Scholar]
- 44.O’Leary JC, 3rd, Li Q, Marinec P, et al. Phenothiazine-mediated rescue of cognition in tau transgenic mice requires neuroprotection and reduced soluble tau burden. Mol Neurodegener. 2010;5:45. doi: 10.1186/1750-1326-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dickey C, Kraft C, Jinwal U, et al. Aging analysis reveals slowed tau turnover and enhanced stress response in a mouse model of tauopathy. Am J Pathol. 2009;174(1):228–238. doi: 10.2353/ajpath.2009.080764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ittner LM, Gotz J. Amyloid-β and tau – a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci. 2011;12(2):65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- 47.Rapoport M, Dawson HN, Binder LI, Vitek MP, Ferreira A. Tau is essential to β-amyloid-induced neurotoxicity. Proc Natl Acad Sci USA. 2002;99(9):6364–6369. doi: 10.1073/pnas.092136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vossel KA, Zhang K, Brodbeck J, et al. Tau reduction prevents a β-induced defects in axonal transport. Science. 2010;330(6001):198. doi: 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125(3):443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 50.Tyedmers J, Mogk A, Bukau B. Cellular strategies for controlling protein aggregation. Nat Rev Mol Cell Biol. 2010;11(11):777–788. doi: 10.1038/nrm2993. [DOI] [PubMed] [Google Scholar]
- 51.Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11(8):545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patury S, Miyata Y, Gestwicki JE. Pharmacological targeting of the Hsp70 chaperone. Curr Top Med Chem. 2009;9(15):1337–1351. doi: 10.2174/156802609789895674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kettern N, Dreiseidler M, Tawo R, Hohfeld J. Chaperone-assisted degradation: multiple paths to destruction. Biol Chem. 2010;391(5):481–489. doi: 10.1515/BC.2010.058. [DOI] [PubMed] [Google Scholar]
- 54.Schubert U, Anton LC, Gibbs J, et al. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404(6779):770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 55.Bertelsen EB, Chang L, Gestwicki JE, Zuiderweg ER. Solution conformation of wild-type E. coli Hsp70 (DnaK) chaperone complexed with adp and substrate. Proc Natl Acad Sci USA. 2009;106(21):8471–8476. doi: 10.1073/pnas.0903503106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu X, Zhao X, Burkholder WF, et al. Structural analysis of substrate binding by the molecular chaperone DnaK. Science. 1996;272(5268):1606–1614. doi: 10.1126/science.272.5268.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flynn GC, Pohl J, Flocco MT, Rothman JE. Peptide-binding specificity of the molecular chaperone bip. Nature. 1991;353(6346):726–730. doi: 10.1038/353726a0. [DOI] [PubMed] [Google Scholar]
- 58.Rudiger S, Germeroth L, Schneider-Mergener J, Bukau B. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 1997;16(7):1501–1507. doi: 10.1093/emboj/16.7.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarkar M, Kuret J, Lee G. Two motifs within the tau microtubule-binding domain mediate its association with the hsc70 molecular chaperone. J Neurosci Res. 2008;86(12):2763–2773. doi: 10.1002/jnr.21721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang L, Bertelsen EB, Wisen S, et al. High-throughput screen for small molecules that modulate the ATPase activity of the molecular chaperone DnaK. Anal Biochem. 2008;372(2):167–176. doi: 10.1016/j.ab.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 61.Laufen T, Mayer MP, Beisel C, et al. Mechanism of regulation of Hsp70 chaperones by dnaj cochaperones. Proc Natl Acad Sci USA. 1999;96(10):5452–5457. doi: 10.1073/pnas.96.10.5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brodsky JL, Bracher A. Nucleotide exchange factors for Hsp70 molecular chaperones. In: Baltch GL, editor. Networking of Chaperones by Co-chaperones. Landes Bioscience; Austin, TX, USA: 2007. pp. 26–37. [Google Scholar]
- 63.Evans CG, Chang L, Gestwicki JE. Heat shock protein 70 (Hsp70) as an emerging drug target. J Med Chem. 2010;53(12):4585–4602. doi: 10.1021/jm100054f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang Y, Taldone T, Clement CC, et al. Design of a fluorescence polarization assay platform for the study of human Hsp70. Bioorg Med Chem Lett. 2008;18(13):3749–3751. doi: 10.1016/j.bmcl.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miyata Y, Chang L, Bainor A, et al. High-throughput screen for Escherichia coli heat shock protein 70 (Hsp70/DnaK): ATPase assay in low volume by exploiting energy transfer. J Biomol Screen. 2010;15(10):1211–1219. doi: 10.1177/1087057110380571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ricci L, Williams KP. Development of fluorescence polarization assays for the molecular chaperone Hsp70 family members: Hsp72 and DnaK. Curr Chem Genomics. 2008;2:90–95. doi: 10.2174/1875397300802010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wisen S, Gestwicki JE. Identification of small molecules that modify the protein folding activity of heat shock protein 70. Anal Biochem. 2008;374(2):371–377. doi: 10.1016/j.ab.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 68.Massey AJ. ATPases as drug targets: insights from heat shock proteins 70 and 90. J Med Chem. 2010;53(20):7280–7286. doi: 10.1021/jm100342z. [DOI] [PubMed] [Google Scholar]
- 69.Massey AJ, Williamson DS, Browne H, et al. A novel, small molecule inhibitor of Hsc70/Hsp70 potentiates Hsp90 inhibitor induced apoptosis in Hct116 colon carcinoma cells. Cancer Chemother Pharmacol. 2010;66(3):535–545. doi: 10.1007/s00280-009-1194-3. [DOI] [PubMed] [Google Scholar]
- 70.Williamson DS, Borgognoni J, Clay A, et al. Novel adenosine-derived inhibitors of 70 kDa heat shock protein, discovered through structure-based design. J Med Chem. 2009;52(6):1510–1513. doi: 10.1021/jm801627a. [DOI] [PubMed] [Google Scholar]
- 71.Chang L, Thompson AD, Ung P, Carlson HA, Gestwicki JE. Mutagenesis reveals the complex relationships between ATPase rate and the chaperone activities of escherichia coli heat shock protein 70 (Hsp70/DnaK) J Biol Chem. 2010;285(28):21282–21291. doi: 10.1074/jbc.M110.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang L, Miyata Y, Ung PM, et al. Chemical screens against a reconstituted multiprotein complex: myricetin blocks dnaj regulation of DnaK through an allosteric mechanism. Chem Biol. 2011;18(2):210–221. doi: 10.1016/j.chembiol.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wisen S, Bertelsen EB, Thompson AD, et al. Binding of a small molecule at a protein–protein interface regulates the chaperone activity of Hsp70-Hsp40. ACS Chem Biol. 2010;5(6):611–622. doi: 10.1021/cb1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bischofberger P, Han W, Feifel B, Schonfeld HJ, Christen P. D-peptides as inhibitors of the DnaK/dnaj/grpe chaperone system. J Biol Chem. 2003;278(21):19044–19047. doi: 10.1074/jbc.M300922200. [DOI] [PubMed] [Google Scholar]
- 75.Haney CM, Schneider C, Beck B, Brodsky JL, Domling A. Identification of Hsp70 modulators through modeling of the substrate binding domain. Bioorg Med Chem Lett. 2009;19(14):3828–3831. doi: 10.1016/j.bmcl.2009.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ali MM, Roe SM, Vaughan CK, et al. Crystal structure of an Hsp90-nucleotide-p23/sba1 closed chaperone complex. Nature. 2006;440(7087):1013–1017. doi: 10.1038/nature04716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krukenberg KA, Bottcher UM, Southworth DR, Agard DA. Grp94, the endoplasmic reticulum Hsp90, has a similar solution conformation to cytosolic Hsp90 in the absence of nucleotide. Protein Sci. 2009;18(9):1815–1827. doi: 10.1002/pro.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krukenberg KA, Forster F, Rice LM, Sali A, Agard DA. Multiple conformations of E. coli Hsp90 in solution: insights into the conformational dynamics of Hsp90. Structure. 2008;16(5):755–765. doi: 10.1016/j.str.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bron P, Giudice E, Rolland JP, et al. Apo-Hsp90 coexists in two open conformational states in solution. Biol Cell. 2008;100(7):413–425. doi: 10.1042/BC20070149. [DOI] [PubMed] [Google Scholar]
- 80.Southworth DR, Agard DA. Species-dependent ensembles of conserved conformational states define the Hsp90 chaperone ATPase cycle. Mol Cell. 2008;32(5):631–640. doi: 10.1016/j.molcel.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ratzke C, Mickler M, Hellenkamp B, Buchner J, Hugel T. Dynamics of heat shock protein 90 c-terminal dimerization is an important part of its conformational cycle. Proc Natl Acad Sci USA. 2010;107(37):16101–16106. doi: 10.1073/pnas.1000916107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- 83.Shiau AK, Harris SF, Southworth DR, Agard DA. Structural analysis of E. coli Hsp90 reveals dramatic nucleotide-dependent conformational rearrangements. Cell. 2006;127(2):329–340. doi: 10.1016/j.cell.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 84.Taipale M, Jarosz DF, Lindquist S. Hsp90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11(7):515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 85.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic Hsp90 complex in cancer. Nat Rev Cancer. 2010;10(8):537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Workman P, Burrows F, Neckers L, Rosen N. Drugging the cancer chaperone Hsp90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann NY Acad Sci. 2007;1113:202–216. doi: 10.1196/annals.1391.012. [DOI] [PubMed] [Google Scholar]
- 87.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein Hsp90–pp60v–src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA. 1994;91(18):8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Taldone T, Sun W, Chiosis G. Discovery and development of heat shock protein 90 inhibitors. Bioorg Med Chem. 2009;17(6):2225–2235. doi: 10.1016/j.bmc.2008.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hadden MK, Lubbers DJ, Blagg BS. Geldanamycin, radicicol, and chimeric inhibitors of the Hsp90 n-terminal ATP binding site. Curr Top Med Chem. 2006;6(11):1173–1182. doi: 10.2174/156802606777812031. [DOI] [PubMed] [Google Scholar]
- 90.Donnelly A, Blagg BS. Novobiocin and additional inhibitors of the Hsp90 c-terminal nucleotide-binding pocket. Curr Med Chem. 2008;15(26):2702–2717. doi: 10.2174/092986708786242895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marcu MG, Neckers LM. The c-terminal half of heat shock protein 90 represents a second site for pharmacologic intervention in chaperone function. Curr Cancer Drug Targets. 2003;3(5):343–347. doi: 10.2174/1568009033481804. [DOI] [PubMed] [Google Scholar]
- 92.Biamonte MA, Van de Water R, Arndt JW, et al. Heat shock protein 90: inhibitors in clinical trials. J Med Chem. 2010;53(1):3–17. doi: 10.1021/jm9004708. [DOI] [PubMed] [Google Scholar]
- 93.Allan RK, Mok D, Ward BK, Ratajczak T. Modulation of chaperone function and cochaperone interaction by novobiocin in the c-terminal domain of Hsp90: evidence that coumarin antibiotics disrupt Hsp90 dimerization. J Biol Chem. 2006;281(11):7161–7171. doi: 10.1074/jbc.M512406200. [DOI] [PubMed] [Google Scholar]
- 94.Li Y, Zhang T, Jiang Y, et al. (−)-epigallocatechin-3-gallate inhibits Hsp90 function by impairing Hsp90 association with cochaperones in pancreatic cancer cell line mia paca-2. Mol Pharm. 2009;6(4):1152–1159. doi: 10.1021/mp900037p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang T, Hamza A, Cao X, et al. A novel Hsp90 inhibitor to disrupt Hsp90/cdc37 complex against pancreatic cancer cells. Mol Cancer Ther. 2008;7(1):162–170. doi: 10.1158/1535-7163.MCT-07-0484. [DOI] [PubMed] [Google Scholar]
- 96.Zhang T, Li Y, Yu Y, et al. Characterization of celastrol to inhibit Hsp90 and Cdc37 interaction. J Biol Chem. 2009;284(51):35381–35389. doi: 10.1074/jbc.M109.051532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol. 2005;12(10):842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- 98.Sun Y, MacRae TH. Small heat shock proteins: molecular structure and chaperone function. Cell Mol Life Sci. 2005;62(21):2460–2476. doi: 10.1007/s00018-005-5190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haslbeck M, Kastenmuller A, Buchner J, Weinkauf S, Braun N. Structural dynamics of archaeal small heat shock proteins. J Mol Biol. 2008;378(2):362–374. doi: 10.1016/j.jmb.2008.01.095. [DOI] [PubMed] [Google Scholar]
- 100.Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18(3):306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 101.Smith DF, Toft DO. Minireview: the intersection of steroid receptors with molecular chaperones: observations and questions. Mol Endocrinol. 2008;22(10):2229–2240. doi: 10.1210/me.2008-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carrigan PE, Nelson GM, Roberts PJ, et al. Multiple domains of the co-chaperone HOP are important for Hsp70 binding. J Biol Chem. 2004;279(16):16185–16193. doi: 10.1074/jbc.M314130200. [DOI] [PubMed] [Google Scholar]
- 103.Hernandez MP, Sullivan WP, Toft DO. The assembly and intermolecular properties of the Hsp70-HOP-Hsp90 molecular chaperone complex. J Biol Chem. 2002;277(41):38294–38304. doi: 10.1074/jbc.M206566200. [DOI] [PubMed] [Google Scholar]
- 104.Connell P, Ballinger CA, Jiang J, et al. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat Cell Biol. 2001;3(1):93–96. doi: 10.1038/35050618. [DOI] [PubMed] [Google Scholar]
- 105.Pearl LH. Hsp90 and cdc37 – a chaperone cancer conspiracy. Curr Opin Genet Dev. 2005;15(1):55–61. doi: 10.1016/j.gde.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 106.Panaretou B, Siligardi G, Meyer P, et al. Activation of the ATPase activity of Hsp90 by the stress-regulated cochaperone aha1. Mol Cell. 2002;10(6):1307–1318. doi: 10.1016/s1097-2765(02)00785-2. [DOI] [PubMed] [Google Scholar]
- 107.Kundrat L, Regan L. Balance between folding and degradation for Hsp90-dependent client proteins: a key role for CHIP. Biochemistry. 2010;49(35):7428–7438. doi: 10.1021/bi100386w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morishima Y, Wang AM, Yu Z, et al. CHIP deletion reveals functional redundancy of e3 ligases in promoting degradation of both signaling proteins and expanded glutamine proteins. Hum Mol Genet. 2008;17(24):3942–3952. doi: 10.1093/hmg/ddn296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stankiewicz M, Nikolay R, Rybin V, Mayer MP. CHIP participates in protein triage decisions by preferentially ubiquitinating Hsp70-bound substrates. FEBS J. 2010;277(16):3353–3367. doi: 10.1111/j.1742-4658.2010.07737.x. [DOI] [PubMed] [Google Scholar]
- 110.Jinwal UK, Koren J, O’Leary JC, et al. Hsp70 ATPase modulators as therapeutics for Alzheimer’s and other neurodegenerative diseases. Mol Cell Pharmacol. 2010;2(2):43–46. [PMC free article] [PubMed] [Google Scholar]
- 111.Koren J, 3rd, Jinwal UK, Lee DC, et al. Chaperone signalling complexes in Alzheimer’s disease. J Cell Mol Med. 2009;13(4):619–630. doi: 10.1111/j.1582-4934.2008.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jinwal UK, O’Leary JC, 3rd, Borysov SI, et al. Hsc70 rapidly engages tau after microtubule destabilization. J Biol Chem. 2010;285(22):16798–16805. doi: 10.1074/jbc.M110.113753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weaver CL, Espinoza M, Kress Y, Davies P. Conformational change as one of the earliest alterations of tau in Alzheimer’s disease. Neurobiol Aging. 2000;21(5):719–727. doi: 10.1016/s0197-4580(00)00157-3. [DOI] [PubMed] [Google Scholar]
- 114.Dou F, Netzer WJ, Tanemura K, et al. Chaperones increase association of tau protein with microtubules. Proc Natl Acad Sci USA. 2003;100(2):721–726. doi: 10.1073/pnas.242720499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Petrucelli L, Dickson D, Kehoe K, et al. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum Mol Genet. 2004;13(7):703–714. doi: 10.1093/hmg/ddh083. [DOI] [PubMed] [Google Scholar]
- 116.Shimura H, Schwartz D, Gygi SP, Kosik KS. CHIP-Hsc70 complex ubiquitinates phosphorylated tau and enhances cell survival. J Biol Chem. 2004;279(6):4869–4876. doi: 10.1074/jbc.M305838200. [DOI] [PubMed] [Google Scholar]
- 117.Jinwal UK, Miyata Y, Koren J, 3rd, et al. Chemical manipulation of Hsp70 ATPase activity regulates tau stability. J Neurosci. 2009;29(39):12079–12088. doi: 10.1523/JNEUROSCI.3345-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tortosa E, Santa-Maria I, Moreno F, et al. Binding of Hsp90 to tau promotes a conformational change and aggregation of tau protein. J Alzheimers Dis. 2009;17(2):319–325. doi: 10.3233/JAD-2009-1049. [DOI] [PubMed] [Google Scholar]
- 119.Dickey CA, Dunmore J, Lu B, et al. Hsp induction mediates selective clearance of tau phosphorylated at proline-directed ser/thr sites but not KXGS (MARK) sites. FASEB J. 2006;20(6):753–755. doi: 10.1096/fj.05-5343fje. [DOI] [PubMed] [Google Scholar]
- 120.Dickey CA, Eriksen J, Kamal A, et al. Development of a high throughput drug screening assay for the detection of changes in tau levels – proof of concept with Hsp90 inhibitors. Curr Alzheimer Res. 2005;2(2):231–238. doi: 10.2174/1567205053585927. [DOI] [PubMed] [Google Scholar]
- 121.Dickey CA, Kamal A, Lundgren K, et al. The high-affinity Hsp90–CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest. 2007;117(3):648–658. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Luo W, Dou F, Rodina A, et al. Roles of heat-shock protein 90 in maintaining and facilitating the neurodegenerative phenotype in tauopathies. Proc Natl Acad Sci USA. 2007;104(22):9511–9516. doi: 10.1073/pnas.0701055104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dickey CA, Koren J, Zhang YJ, et al. Akt and CHIP coregulate tau degradation through coordinated interactions. Proc Natl Acad Sci USA. 2008;105(9):3622–3627. doi: 10.1073/pnas.0709180105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lindquist S. Protein folding sculpting evolutionary change. Cold Spring Harb Symp Quant Biol. 2009;74:103–108. doi: 10.1101/sqb.2009.74.043. [DOI] [PubMed] [Google Scholar]
- 125.Kosik KS, Shimura H. Phosphorylated tau and the neurodegenerative foldopathies. Biochim Biophys Acta. 2005;1739(2–3):298–310. doi: 10.1016/j.bbadis.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 126.Shimura H, Miura-Shimura Y, Kosik KS. Binding of tau to heat shock protein 27 leads to decreased concentration of hyperphosphorylated tau and enhanced cell survival. J Biol Chem. 2004;279(17):17957–17962. doi: 10.1074/jbc.M400351200. [DOI] [PubMed] [Google Scholar]
- 127.Bjorkdahl C, Sjogren MJ, Zhou X, et al. Small heat shock proteins Hsp27 or αb-crystallin and the protein components of neurofibrillary tangles: tau and neurofilaments. J Neurosci Res. 2008;86(6):1343–1352. doi: 10.1002/jnr.21589. [DOI] [PubMed] [Google Scholar]
- 128.Schwarz L, Vollmer G, Richter-Landsberg C. The small heat shock protein Hsp25/27 (Hspb1) is abundant in cultured astrocytes and associated with astrocytic pathology in progressive supranuclear palsy and corticobasal degeneration. Int J Cell Biol. 2010:717520. doi: 10.1155/2010/717520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Abisambra JF, Blair LJ, Hill SE, et al. Phosphorylation dynamics regulate Hsp27-mediated rescue of neuronal plasticity deficits in tau transgenic mice. J Neurosci. 2010;30(46):15374–15382. doi: 10.1523/JNEUROSCI.3155-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Elliott E, Laufer O, Ginzburg I. Bag-1m is up-regulated in hippocampus of Alzheimer’s disease patients and associates with tau and app proteins. J Neurochem. 2009;109(4):1168–1178. doi: 10.1111/j.1471-4159.2009.06047.x. [DOI] [PubMed] [Google Scholar]
- 131.Elliott E, Tsvetkov P, Ginzburg I. Bag-1 associates with hsc70. Tau complex and regulates the proteasomal degradation of tau protein. J Biol Chem. 2007;282(51):37276–37284. doi: 10.1074/jbc.M706379200. [DOI] [PubMed] [Google Scholar]
- 132.Carrettiero DC, Hernandez I, Neveu P, Papagiannakopoulos T, Kosik KS. The cochaperone bag2 sweeps paired helical filament-insoluble tau from the microtubule. J Neurosci. 2009;29(7):2151–2161. doi: 10.1523/JNEUROSCI.4660-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Andreasson C, Fiaux J, Rampelt H, Mayer MP, Bukau B. Hsp110 is a nucleotide-activated exchange factor for Hsp70. J Biol Chem. 2008;283(14):8877–8884. doi: 10.1074/jbc.M710063200. [DOI] [PubMed] [Google Scholar]
- 134.Dragovic Z, Broadley SA, Shomura Y, Bracher A, Hartl FU. Molecular chaperones of the Hsp110 family act as nucleotide exchange factors of Hsp70s. EMBO J. 2006;25(11):2519–2528. doi: 10.1038/sj.emboj.7601138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eroglu B, Moskophidis D, Mivechi NF. Loss of Hsp110 leads to age-dependent tau hyperphosphorylation and early accumulation of insoluble amyloid β. Mol Cell Biol. 2010;30(19):4626–4643. doi: 10.1128/MCB.01493-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jinwal UK, Koren J, 3rd, Borysov SI, et al. The Hsp90 cochaperone, fkbp51, increases tau stability and polymerizes microtubules. J Neurosci. 2010;30(2):591–599. doi: 10.1523/JNEUROSCI.4815-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Salminen A, Ojala J, Kaarniranta K, Hiltunen M, Soininen H. Hsp90 regulates tau pathology through co-chaperone complexes in Alzheimer’s disease. Prog Neurobiol. 2011;93(1):99–110. doi: 10.1016/j.pneurobio.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 138.Van Duyne GD, Standaert RF, Karplus PA, Schreiber SL, Clardy J. Atomic structure of fkbp-fk506, an immunophilin–immunosuppressant complex. Science. 1991;252(5007):839–842. doi: 10.1126/science.1709302. [DOI] [PubMed] [Google Scholar]
- 139.Frydman J. Folding of newly translated proteins in vivo: the role of molecular chaperones. Ann Rev Biochem. 2001;70:603–647. doi: 10.1146/annurev.biochem.70.1.603. [DOI] [PubMed] [Google Scholar]
- 140.Arndt V, Rogon C, Hohfeld J. To be, or not to be – molecular chaperones in protein degradation. Cell Mol Life Sci. 2007;64(19–20):2525–2541. doi: 10.1007/s00018-007-7188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Park HJ, Mylvaganum M, McPherson A, et al. A soluble sulfogalactosyl ceramide mimic promotes δ f508 cftr escape from endoplasmic reticulum associated degradation. Chem Biol. 2009;16(4):461–470. doi: 10.1016/j.chembiol.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schneider C, Sepp-Lorenzino L, Nimmesgern E, et al. Pharmacologic shifting of a balance between protein refolding and degradation mediated by Hsp90. Proc Natl Acad Sci USA. 1996;93(25):14536–14541. doi: 10.1073/pnas.93.25.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kampinga HH, Hageman J, Vos MJ, et al. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14(1):105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Ann Rev Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 145.Smith DF, Whitesell L, Nair SC, et al. Progesterone receptor structure and function altered by geldanamycin, an Hsp90-binding agent. Mol Cell Biol. 1995;15(12):6804–6812. doi: 10.1128/mcb.15.12.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Forafonov F, Toogun OA, Grad I, et al. P23/sba1p protects against Hsp90 inhibitors independently of its intrinsic chaperone activity. Mol Cell Biol. 2008;28(10):3446–3456. doi: 10.1128/MCB.02246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hohfeld J, Cyr DM, Patterson C. From the cradle to the grave: molecular chaperones that may choose between folding and degradation. EMBO Rep. 2001;2(10):885–890. doi: 10.1093/embo-reports/kve206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Arndt V, Daniel C, Nastainczyk W, Alberti S, Hohfeld J. Bag-2 acts as an inhibitor of the chaperone-associated ubiquitin ligase CHIP. Mol Biol Cell. 2005;16(12):5891–5900. doi: 10.1091/mbc.E05-07-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.McClellan AJ, Scott MD, Frydman J. Folding and quality control of the vhl tumor suppressor proceed through distinct chaperone pathways. Cell. 2005;121(5):739–748. doi: 10.1016/j.cell.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 150.Gestwicki JE, Marinec PS. Chemical control over protein–protein interactions: beyond inhibitors. Comb Chem High Throughput Screen. 2007;10(8):667–675. doi: 10.2174/138620707782507296. [DOI] [PubMed] [Google Scholar]
- 151.Wells JA, McClendon CL. Reaching for high-hanging fruit in drug discovery at protein–protein interfaces. Nature. 2007;450(7172):1001–1009. doi: 10.1038/nature06526. [DOI] [PubMed] [Google Scholar]
- 152.Hajduk PJ, Meadows RP, Fesik SW. Nmr-based screening in drug discovery. Q Rev Biophys. 1999;32(3):211–240. doi: 10.1017/s0033583500003528. [DOI] [PubMed] [Google Scholar]
- 153.Li Y, Zhang T, Schwartz SJ, Sun D. New developments in Hsp90 inhibitors as anti-cancer therapeutics: mechanisms, clinical perspective and more potential. Drug Resist Updat. 2009;12(1–2):17–27. doi: 10.1016/j.drup.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of α-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science. 2002;295(5556):865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- 155.Bonini NM. Chaperoning brain degeneration. Proc Natl Acad Sci USA. 2002;99(Suppl 4):16407–16411. doi: 10.1073/pnas.152330499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fujimoto M, Takaki E, Hayashi T, et al. Active hsf1 significantly suppresses polyglutamine aggregate formation in cellular and mouse models. J Biol Chem. 2005;280(41):34908–34916. doi: 10.1074/jbc.M506288200. [DOI] [PubMed] [Google Scholar]
- 157.Muchowski PJ, Schaffar G, Sittler A, et al. Hsp70 and Hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc Natl Acad Sci USA. 2000;97(14):7841–7846. doi: 10.1073/pnas.140202897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wacker JL, Zareie MH, Fong H, Sarikaya M, Muchowski PJ. Hsp70 and Hsp40 attenuate formation of spherical and annular polyglutamine oligomers by partitioning monomer. Nat Struct Mol Biol. 2004;11(12):1215–1222. doi: 10.1038/nsmb860. [DOI] [PubMed] [Google Scholar]
- 159.Wyttenbach A, Sauvageot O, Carmichael J, et al. Heat shock protein 27 prevents cellular polyglutamine toxicity and suppresses the increase of reactive oxygen species caused by huntingtin. Hum Mol Genet. 2002;11(9):1137–1151. doi: 10.1093/hmg/11.9.1137. [DOI] [PubMed] [Google Scholar]
- 160.Nagai Y, Fujikake N, Popiel HA, Wada K. Induction of molecular chaperones as a therapeutic strategy for the polyglutamine diseases. Curr Pharm Biotechnol. 2010;11(2):188–197. doi: 10.2174/138920110790909650. [DOI] [PubMed] [Google Scholar]