The Solomon-Bloembergen-Morgan theory predicts that the longitudinal relaxivity of a small Gd(III) complex, r1p, increases upon slowing down its rotational correlation time. This is usually achieved by grafting the complex onto a macromolecule. Previous efforts in designing such macromolecules have concentrated on the synthesis of dendrimeric analogues and covalent or reversible protein conjugates.2 These studies demonstrated the necessity not only of attaining fast water exchange but also of creating rigid systems in attaining high relaxivity agents.3
Surprisingly there has been little study of supramolecular self-assemblies. These complexes, however, present several advantages as their synthesis require fewer steps and they can be highly rigid. Although several mixed f block/d block self-assemblies have already been described,4 the macromolecular MRI contrast agents studied have been only Gd(III) chelates grafted onto tris-bidentate Fe(III) complexes.5 However, these do not constitute true supramolecular self-assemblies6 in that in each case the Gd(III) complexes were already completely synthesized prior to building the macromolecule.
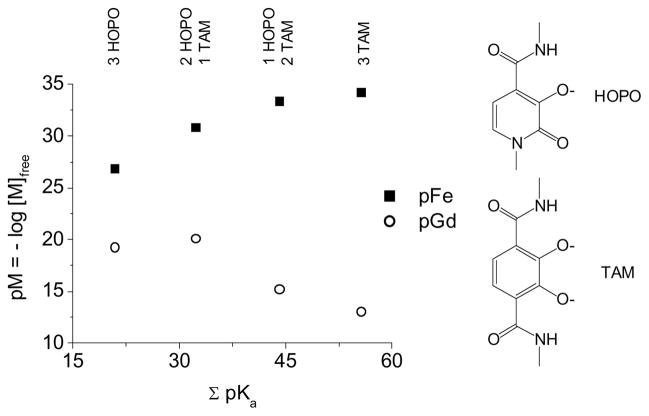
Herein we describe our approach to Gd(III)-containing supramolecular self-assemblies using Fe(III) as a template. Bis-bidentate ligands were designed comprising two different binding sites, one more selective for Gd(III) and the other for Fe(III). It has previously been shown (Figure 1) that Gd(III) is selective for 1-methyl-2,3-dihydroxypyridinone (HOPO) over 2,3-dihydroxyterephthalamide (TAM) (pGd TREN-trisHOPO = 19.2, pGd TREN-trisTAM < 13)7 whereas Fe(III) is selective for TAM over HOPO (pFe TREN-trisHOPO = 26.8, pFe TREN-trisTAM = 34.2).8,9 It was therefore predicted that three bis-bidentate ligands each comprising both one HOPO and one TAM moiety would coordinate both one Gd(III) and one Fe(III).
Figure 1.
Selectivity of 1-methyl-2,3-dihydroxypyridinone (HOPO) and 2,3-dihydroxyterephthalamide (TAM) for Fe(III) over Gd(III).7,8
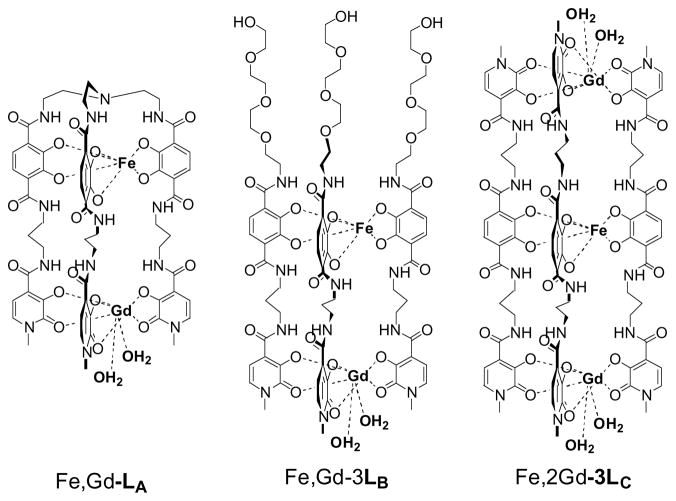
In the complexes studied (Figure 2), each ligand contains one TAM moiety (for Fe(III) coordination) linked to either one or two HOPO moieties (for Gd(III) coordination) through a three-carbon linker. This linker has been shown to favor the formation of triple-stranded helicates.10 In Fe,Gd-L1, the three strands are joined by a TREN backbone, while in the other two complexes Fe,Gd-3L2 and Fe,2Gd-3L3, they are left free to self-assemble (or not). All complexes are −3 charged. In Fe,Gd-3L2, a small PEG chain was grafted for increased water solubility.
Figure 2.
Heteronuclear Fe(III) templated Gd(III) self-assemblies based on a three carbon linker.
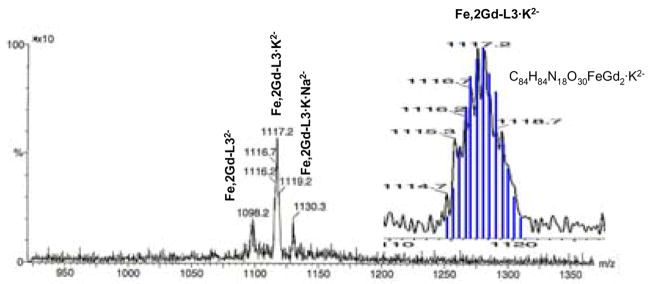
The mixed complexes were synthesized in six, four, and three steps, respectively, starting from previously described building blocks. Formation of the mixed self-assemblies, with the correct ratio of Gd(III) and Fe(III), was confirmed by high resolution electrospray mass spectrometry. The spectrum of Fe,2Gd-3LC (Figure 3), for instance, clearly shows only one product, containing one Fe(III) and two Gd(III).
Figure 3.
Electrospray mass spectrum of Fe,2Gd-3LC. The calculated (blue) and experimental (black) isotopic distribution of the molecular ion is shown in the expanded region.
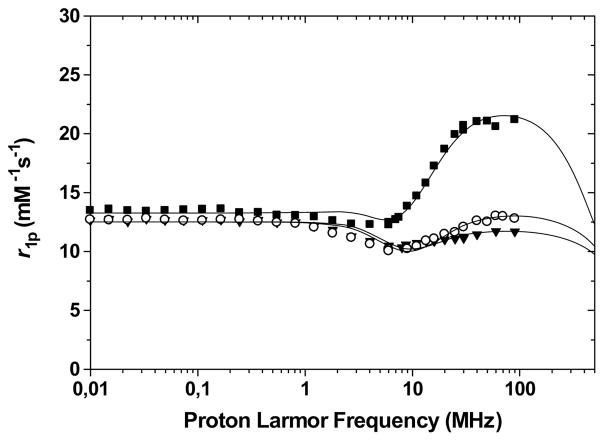
The Nuclear Magnetic Resonance Dispersion (NMRD) profiles of the heteronuclear self-assemblies are shown in Figure 4. Since not all complexes bear the same number of Gd(III) ions, the relaxivities given are per mM of Gd(III) for direct comparison. The complexes have high relaxivities, with a maximum centered around 60–100 MHz. The relaxivity of the trinuclear assembly, Fe,2Gd-3LC, is r1p = 21 mM−1s−1 per Gd(III) (or 42 mM−1s−1 per molecule) at 90 MHz; an unprecedented high value, especially given the relatively small molecular weight of the assembly. Unlike macromolecules based on commercial contrast agents, the relaxivity of the supramolecules increases significantly at the high Larmor frequencies that are relevant for medical applications. Such a peak at high field was previously observed for medium molecular weight systems such as the block dendrimer Gd-TREN-bisHOPO-TAM-Asp-Asp2-12OH11 and hydrophilic dendritic conjugates in which the Gd ion lies at the barycenter of the structure.12 The high relaxivity in the 2 Tesla field range is characteristic both of an optimally fast water exchange rate and a relatively long rotational correlation time arising from a rigid structure. The profiles thus strongly suggest that τM is still optimal for the heteronuclear complexes, and that the two-dimensional self-assemblies can efficiently slow down the molecular tumbling.
Figure 4.
1/T1 NMRD profile at 298 K and pH 7.4 of Fe,Gd-LA (filled triangles), Fe,Gd-3LB (open circle) and Fe,2Gd-3LC (filled squares). Longitudinal relaxivities are given per Gd(III) ion.
Details of the refinements of the NMRD profiles of the self-assemblies (Table 1), as compared to the parent mono-nuclear Gd-TREN-bisHOPO-TAM13, indicate that most of the increase in molecular weight is translated into an increase in the rotational correlation time τR. The larger tri-nuclear Fe,2Gd-3LC (MW = 2306 g/mol), for instance is 2.7 times larger than Gd-TREN-bisHOPO-TAM (MW = 831 g/mol), and its rotational correlation time 2.5 times that of the parent complex (τR = 312 ps vs. 125 ps). This linear relationship between τR and the molecular weight is indicative of a highly rigid structure with little internal motion. It has also been observed in highly rigid dendrimers for which the effective increase in τR of the Gd(III) complex is higher than that of derivatives of the commercial PAMAM.11,14 Each Gd(III) centers of each assembly still coordinates two water molecules, which continue rapid exchange with bulk solvent. The electronic relaxation times of the supramolecules are also comparable to the parent mononuclear complex. A comparison of the cylindrical architecture of the self-assemblies with the spherical dendrimers or metallostars indicate that the rod-like architectures of former are still well suited to the attainment of highly rigid, high relaxivity MRI contrast agents. Furthermore, unlike the metallostars whose relaxivity were only measured under acidic conditions,5d the HOPO/TAM assemblies maintain the high relaxivity at physiological pH and when dissolved in human serum.
Table 1.
Refinement parameters of the 1/T1 NMRD profiles of Fe,Gd-LA, Fe,Gd-3LB and Fe,2Gd-3LC.
| Fe,Gd-LA | Fe,Gd-3LB | Fe,2Gd-3LC | |
|---|---|---|---|
| r1p at 20 MHz (mM−1 s−1) | 11.0 | 11.5 | 18.7 |
| q | 2 | 2 | 2 |
| τR (ns) | 150 | 172 | 312 |
| Δ2 (s−2 × 1019) | 5.4 | 6.5 | 4.9 |
| τv (ps) | 25.9 | 22.5 | 31.7 |
| τM (ns)* | 10 | 10 | 10 |
| r (Ǻ) | 3.06 | 3.06 | 3.06 |
| a (Ǻ)* | 4.0 | 4.0 | 4.0 |
| D (cm2 s−1 × 105)* | 2.24 | 2.24 | 2.24 |
fixed
In summary, the templated self-assembly of Gd(III) hydroxypyridinone and Fe(III) terephthalamide components of a supramolecular structure presents a new class of MRI contrast agents. These compounds utilize the rapid assembly of rigid macromolecules in few synthetic steps. Relaxivity studies indicate that the high molecular weight clusters effectively slow down the molecular tumbling. This and the fast water exchange produce high relaxivity at the high magnetic fields relevant to future medical applications.
Supplementary Material
Acknowledgments
This research (UCB) was supported by NIH grant HL69832, NATO Travel Grant PST.CLG.980380, and an unrestricted research gift from Schering AG.
Footnotes
Supporting Information Available: Detailed experimental procedures and characterization data for the synthesis of the complexes Fe,Gd-LA, Fe,Gd-3LB and Fe,2Gd-3LC; pH-dependence of the selectivity of Fe(III) for TAM over HOPO ligands; inner- and outer-sphere contributions to the NMRD profiles of the assemblies; MM3 energy minimization of the assemblies. This information is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Paper no. 17 in the series “High Relaxivity MRI Agents”; for the previous paper in the series see Pierre VC, Botta M, Aime S, Raymond KN. J Am Chem Soc. 2006;128:5344–5345. doi: 10.1021/ja057805x.
- 2.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chem Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 3.Nicolle GM, Toth E, Schmitt-Willich H, Raduchel B, Merbach AE. Chem-Eur J. 2002;8:1040–1048. doi: 10.1002/1521-3765(20020301)8:5<1040::aid-chem1040>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 4.a) Bunzli JCG, Piguet C. Chem Rev. 2002;102:1897–1928. doi: 10.1021/cr010299j. and references therein. [DOI] [PubMed] [Google Scholar]; b) Piguet C, Hopfgartner G, Williams AF, Bunzli JC. Chem Commun. 1995:491–493. [Google Scholar]; c) Piguet C, Edder C, Rigault S, Bernardinelli G, Bunzli JCG, Hopfgartner G. J Chem Soc, Dalton Trans. 2000:3999–4006. [Google Scholar]; d) Stemmler AJ, Kampf JW, Kirk ML, Atasi BH, Pecoraro VL. Inorg Chem. 1999;38:2807–2817. doi: 10.1021/ic9800233. [DOI] [PubMed] [Google Scholar]; e) Pecoraro VL, Stemmler AJ, Gibney BR, Bodwin JJ, Wang H, Kampf JW, Barwinski A. Progress Inorg Chem. 1997;45:83–177. [Google Scholar]
- 5.a) Aime S, Botta M, Fasano M, Terreno E. Spectrochim Acta, Part A. 1993;49:1315–1322. [Google Scholar]; b) Comblin V, Gilsoul D, Hermann M, Humblet V, Jacques V, Mesbahi M, Sauvage C, Desreux JF. Coord Chem Rev. 1999;186:451–470. [Google Scholar]; c) Parac-Vogt TN, Kimpe K, Binnemans K. J Alloys Compd. 2004;374:325–329. [Google Scholar]; d) Livramento JB, Toth E, Sour A, Borel A, Merbach AE, Ruloff R. Angew Chem, Int Ed Engl. 2005;44:1480–1484. doi: 10.1002/anie.200461875. [DOI] [PubMed] [Google Scholar]; e) Livramento JB, Sour A, Borel A, Merbach AE, Toth E. Chem Eur J. 2006;12:989–1003. doi: 10.1002/chem.200500969. [DOI] [PubMed] [Google Scholar]
- 6.Lehn J-M. Supramolecular Chemistry: Concepts and Perspectives. VCH, Weinheim; Germany: 1995. [Google Scholar]
- 7.Doble DMJ, Melchior M, O'Sullivan B, Siering C, Xu JD, Pierre VC, Raymond KN. Inorg Chem. 2003;42:4930–4937. doi: 10.1021/ic026240s. [DOI] [PubMed] [Google Scholar]
- 8.Jurchen KMC. PhD dissertation. University of California; Berkeley: 2003. [Google Scholar]
- 9.This selectivity is pH dependent and reverses below pH 5.5 – see supporting information for details
- 10.Scarrow RC, Riley PE, Abudari K, White DL, Raymond KN. Inorg Chem. 1985;24:954–967. [Google Scholar]
- 11.Pierre VC, Botta M, Raymond KN. J Am Chem Soc. 2005;127:504–505. doi: 10.1021/ja045263y. [DOI] [PubMed] [Google Scholar]
- 12.Fulton DA, O’Halloran M, Parker D, Senanayake K, Botta M, Aime S. Chem Commun. 2005:474–476. doi: 10.1039/b413536a. [DOI] [PubMed] [Google Scholar]
- 13.a) Cohen SM, Xu J, Radkov E, Raymond KN, Botta M, Barge A, Aime S. Inorg Chem. 2000;39:5747–5756. doi: 10.1021/ic000563b. [DOI] [PubMed] [Google Scholar]; b) Raymond KN, Pierre VC. Bioconjugate Chem. 2005;16:3–8. doi: 10.1021/bc049817y. [DOI] [PubMed] [Google Scholar]
- 14.Rudovsky J, Hermann P, Botta M, Aime S, Lukes I. Chem Commun. 2005:2390–2392. doi: 10.1039/b418712a. [DOI] [PubMed] [Google Scholar]
- 15.The relaxivity of Fe,2Gd-3LC in distilled water (pH=7.1) and in human serum (Seronorm Human, SERO AS ASKER, Norway) are 18.3 mM−1 s−1 and 20.5 mM−1s−1, respectively (20 MHz and 25°C). No observable change in r1 was observed over 8 hours.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.