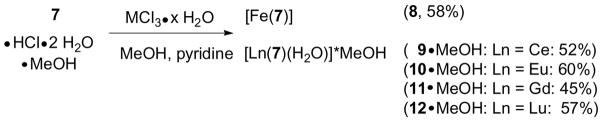Abstract
A cyclic, bidentate hydroxamic acid binding unit based on an isoquinoline scaffold has been utilized for the synthesis of a hexadentate tripodal ligand based on the TREN backbone. This prototype for a new class of multidentate chelators forms mononuclear iron(III) complexes and one-dimensional coordination polymers with lanthanide(III) cations. The latter has been determined by single crystal X-ray analysis of the cerium species. The solid state structure in the monoclinic space group P21/c (C36H34CeN7O11, a=12.341(2)Å, b=26.649(4)Å, c=10.621(2)Å, α=γ=90°, β=96.753(3)°, V=3468.6(9)Å3, Z=4) exhibits a trigonal-dodecahedral environment around the cerium cation. The proof of concept for the versatility of the new scaffold has been shown by the modification of the crucial precursor 3-carboxyisocoumarin through electrophilic aromatic substitutions to yield the corresponding chlorosulfonated and nitrated analogs.
The 1-hydroxy-2-pyridinone (1,2-HOPO) 3-carboxylic acid motif (1) (Figure 1) has been very successful for the design of multidentate ligands for a number of applications, such as actinide sequestering,1 magnetic resonance imaging,2 treatment of iron-overload,3 and lanthanide luminescence.4 Despite the very attractive properties of this class of cyclic hydroxamic acid ligands (facile synthetic access, high complex stability, etc.), the main drawback is the difficulty to introduce substituents at the heterocyclic pyridinone ring.
Figure 1.
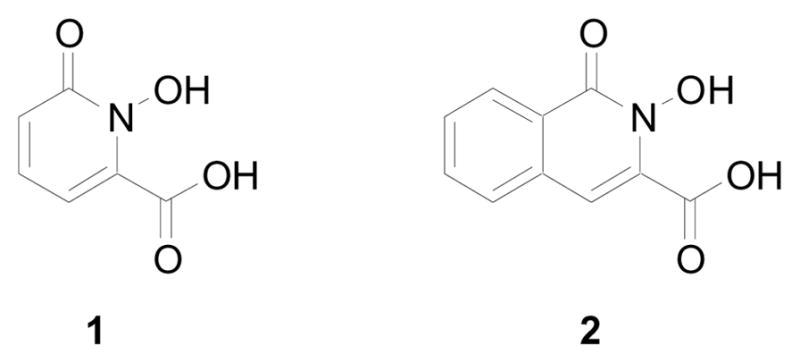
1,2-HOPO (left) and 1,2-HOIQO (right).
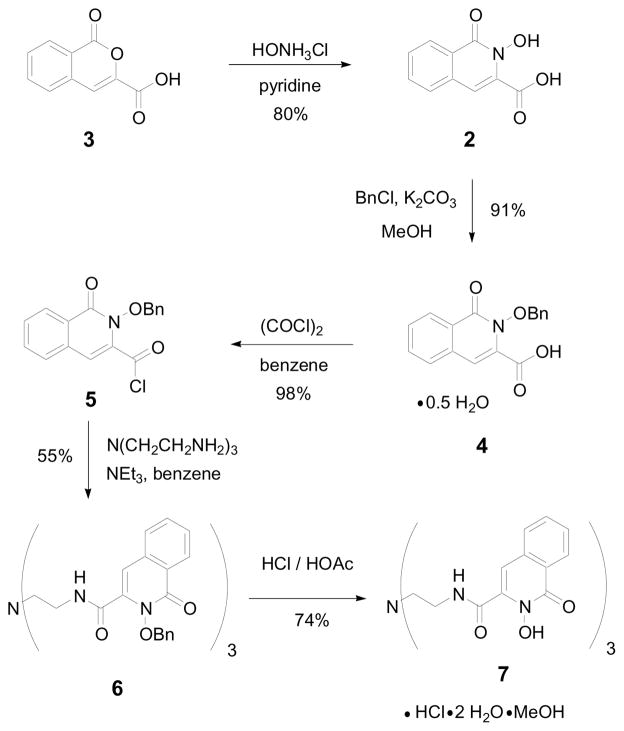
In an effort to overcome these limitations we decided to utilize the structurally related 2-hydroxy-2H-isoquinolin-1-one (1,2-HOIQO) 3-carboxylic acid scaffold (2) (Figure 1). Modification would allow for the tuning of a wide field of properties (e.g. solubility, electronics, photophysics, steric demand, functionality, etc.), which would greatly increase the value of the class of ligands based on cyclic hydroxamic acids. The key intermediate for its synthesis is 3-carboxyisocoumarin (3) prepared in a three-step sequence from homophthalic acid by a modification of literature precedence.5,6 Cyclic ester 3 was converted to the corresponding hydroxamic acid 2 according to a published procedure (Scheme 1).7 The remaining transformations were carried out following the analogous optimized route for the similar TREN-1,2-HOPO.8
Scheme 1.
Synthesis of TREN-1,2-HOIQO (7).
With the new ligand TREN-1,2-HOIQO (7) in hand, complexes with a variety of metal ions were prepared. Due to the relevance to the applications mentioned in the introduction, iron(III) and lanthanide(III) (Ln = Ce, Eu, Gd, Lu) were chosen. The synthesis involved heating equimolar amounts of the ligand and the metal(III) chloride precursors in methanol with pyridine as a base. The products were isolated in analytically pure form by simply filtering off the precipitates, followed by washing with methanol and drying in vacuo (Scheme 2).
Scheme 2.
Synthesis of metal complexes with TREN-1,2-HOIQO.
In the case of the lanthanide complexes one molecule of water was assigned to the inner coordination sphere based on the findings by X-ray analysis (vide infra). The properties of the iron species 8 (soluble in nonpolar solvents such as CHCl3, with [Fe(7)H]+ as the prevalent peak in the mass spectrum) are consistent with a neutral, mononuclear complex. In contrast to this, the lanthanide compounds are only soluble in highly polar solvents like DMF or DMSO. In DMF solution the predominant species also seems to be mononuclear in nature as indicated by mass spectrometry of the europium complex 10·MeOH (detection of [Eu(7)H]+). The 1H-NMR spectrum of the lutetium complex 12·MeOH in DMSO-d6 shows the presence of one single species, with all three arms of the tripodal ligand 7 equivalent, indicating a fast equilibration of different coordination isomers. The low solubility, suggests that the solid species is polymeric, which upon dissolution is broken up by coordination of DMF or DMSO to the metal center. This hypothesis was corroborated by growing single crystals of the cerium complex by vapor diffusion of water into a DMF solution. The crystal structure shows a complex with the composition [Ce(7)(H2O)]·H2O (9·H2O) with one water molecule bound to the metal and another one isolated in the lattice (Figure 2).9
Figure 2.
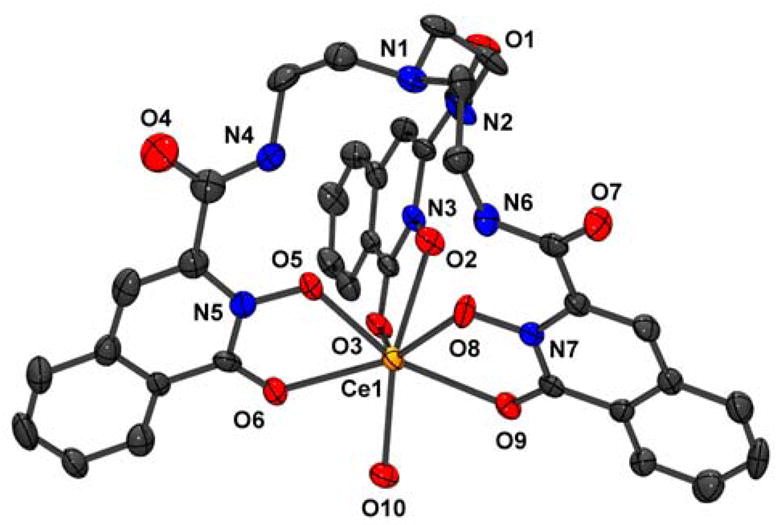
Asymmetric unit of [Ce(7)(H2O)]·H2O (9·H2O). Thermal ellipsoid plot (Ortep 3 for Windows,10 50% probability level) with atom numbering scheme. Hydrogens and the isolated water molecule are omitted for clarity.11
The coordination sphere of the cerium atom consists of the three bidentate 1,2-HOIQO moieties, one water molecule and oxygen O7′ from a neighboring complex, resulting in one-dimensional polymeric coordination polymers (Figure 3). This design principle is similar to the dimeric nature of the previously reported gadolinium complex with TREN-1,2-HOPO.2 The geometry around the cerium is best described as trigonal-dodecahedral with a mean angular deviation (shape measure)12 of the dihedral angles between adjacent faces compared to the ideal polyhedron of S = 10.8°.
Figure 3.
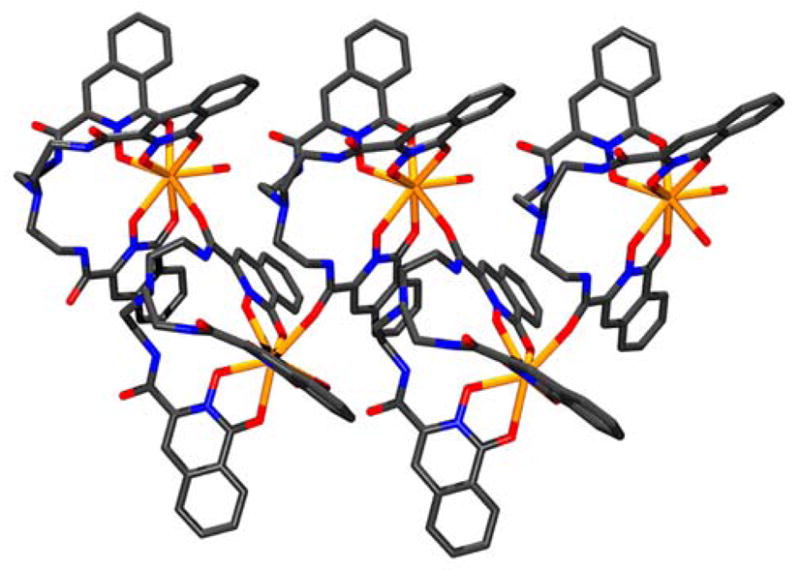
Part of the extended one-dimensional chain for the complex [Ce(7)(H2O)]·H2O (9·H2O). Hydrogens and the isolated molecule of water in the crystal omitted for clarity (Mercury 1.413).
The rigidity of the structure is enhanced by intermolecular π-π-interactions of the aromatic 1,2-HOIQO moieties of different one-dimensional polymeric strands (Figure 4). The distance of 3.4 Å between the almost parallel ring planes is consistent with interactions of that kind.14 Since the composition, as well as the properties of the related lanthanide complexes with europium(III), gadolinium(III) and lutetium(III) are exactly the same, it is highly likely that these species have the same polymeric structure as the cerium(III) complex.
Figure 4.
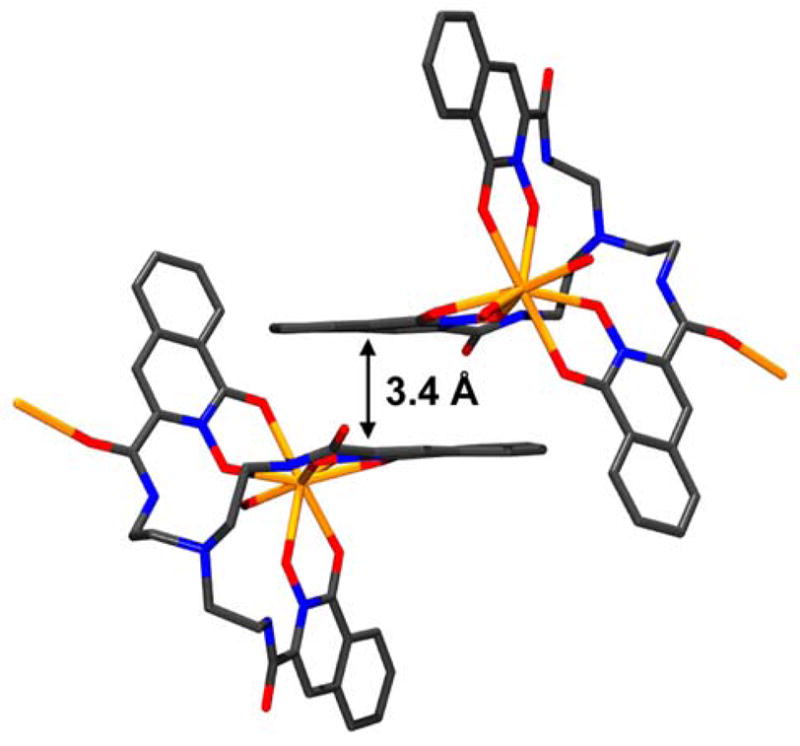
π- π-Stacking of the 1,2-HOIQO rings in [Ce(TREN-1,2-HOIQO)(H2O)]*H2O(9*H2O).11 Hydrogens and the isolated molecule of water in the crystal omitted for clarity (Mercury 1.413).
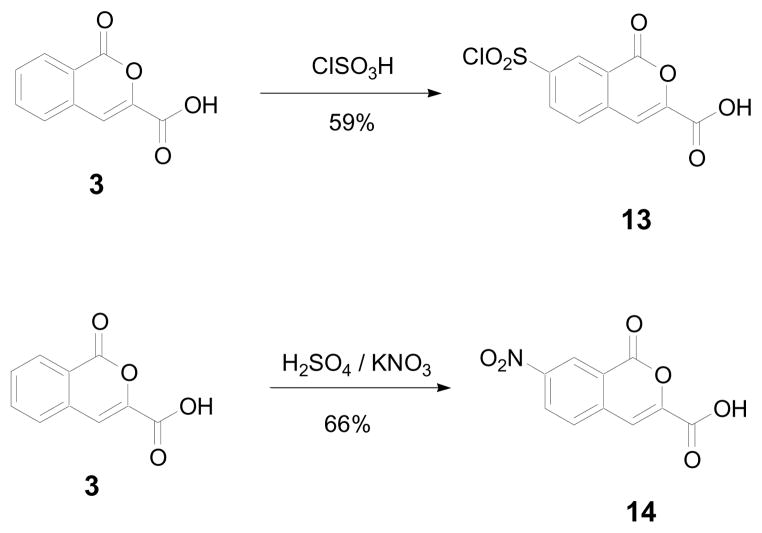
To test the hypothesis that the extended aromatic system could be modified, two prototypical electrophilic aromatic substitutions were carried out on the precursor 3-carboxyisocoumarin (3). The first was a chlorosulfonation with chlorosulfonic acid, the second a nitration under non-aqueous conditions with concentrated sulfuric acid/potassium nitrate (Scheme 3).
Scheme 3.
Electrophilic aromatic substitution of isocoumarin 3.
The selectivity of the substitution reactions for the 7-position is established by 2D NMR techniques. These two building blocks should be very valuable for the synthesis of functionalized 1,2-HOIQO analogs. Investigations in that direction are currently underway.
In conclusion, we have synthesized the first example of ligands with the new class of cyclic hydroxamic acid binding units, 1,2-HOIQO; TREN-1,2-HOIQO forms mononuclear complexes with iron(III) and one-dimensional coordination polymers with lanthanide(III) cations (Ln = Ce, Eu, Gd, Lu). The binding properties seem to be comparable to the characteristics of the analogous 1,2-HOPO complexes. As a proof of concept for the versatility for structural modifications, two precursors for the synthesis of functionalized 1,2-HOIQO building blocks were prepared by electrophilic aromatic substitution reactions, promising a rich chemistry based around the new 1,2-HOIQO metal coordinating group.
Supplementary Material
Acknowledgments
M.S. thanks the German Research Foundation (DFG) for a research fellowship. M.D.P. thanks the NSF for a predoctoral fellowship. This work was supported in part by NIH grant R01-HL69832 and by the Director, Office of Science, Office of Advanced Scientific Computing Research, Office of Basic Energy Sciences (U.S. Department of Energy) under contract DE-AC02-05CH11231.
Footnotes
Supporting Information Available: Experimental procedures and analytical details for the ligand synthesis and the preparation of the metal complexes. Crystallographic data and cif-file for complex 9·H2O.
References
- 1.Gorden AEV, Xu J, Raymond KN, Durbin PW. Chem Rev. 2003;103:4207. doi: 10.1021/cr990114x. and refs cited therein. [DOI] [PubMed] [Google Scholar]
- 2.Xu J, Churchill DG, Botta M, Raymond Kenneth N. Inorg Chem. 2004;43:5492–5494. doi: 10.1021/ic049028s. [DOI] [PubMed] [Google Scholar]
- 3.a) Raymond KN, Scarrow RC, White DL. 796815. patent US. 1987; b) Turcot I, Stintzi A, Xu J, Raymond KN. J Biol Inorg Chem. 2000;5:634. doi: 10.1007/s007750000149. [DOI] [PubMed] [Google Scholar]
- 4.Moore EG, Xu J, Jocher CJ, Werner EJ, Raymond KN. J Am Chem Soc. 2006;128:10648–10649. doi: 10.1021/ja062597+. [DOI] [PubMed] [Google Scholar]
- 5.Johnston HW, Kaslow CE, Langsjoen A, Shriner RL. J Org Chem. 1948;13:477–483. doi: 10.1021/jo01162a003. [DOI] [PubMed] [Google Scholar]
- 6.For the modifiction in the last step of this sequence see the Supporting Information.
- 7.Chatterjea JN, Mukherjee SK, Bhakta C. J Ind Chem Soc. 1982;59:707–709. [Google Scholar]
- 8.Xu J, Durbin PW, Kullgren B, Ebbe SN, Uhlir LC, Raymond KN. J Med Chem. 2002;45:3963–3971. doi: 10.1021/jm010564t. [DOI] [PubMed] [Google Scholar]
- 9.Crystal data for 9·H2O: C36H34CeN7O11 (880.81 g/mol), monoclinic, a=12.341(2)Å, b=26.649(4)Å, c=10.621(2)Å, α=γ=90°, β=96.753(3)°, V=3468.6(9)Å3, space group P21/c (No. 14), Z=4, T=189(2)K,λ =0.71073Å, ρdiff=1.69g/cm3, μ=1.39mm−1, R(F0)=0.0505, wR(F02)=0.0983, GOF=0.887.
- 10.Farrugia LJ. J Appl Cryst. 1997;30:565. [Google Scholar]
- 11.Selected bond lengths and angles [Å,°] in 9·H2O: Ce1-O2=2.468(4), Ce1-O3=2.432(4), Ce1-O5=2.426(4), Ce1-O6=2.445(4), Ce1-O7′=2.478(4), Ce1-O8=2.445(4), Ce1-O9=2.450(4), Ce1-O10=2.573(4); O2-Ce1-O3=63.05(12), O5-Ce1-O6=63.27(13), O8-Ce1-O9=63.44(12).
- 12.Xu J, Radkov E, Ziegler M, Raymond KN. Inorg Chem. 2000;39:4156–4164. doi: 10.1021/ic000063i. [DOI] [PubMed] [Google Scholar]
- 13.Bruno IJ, Cole JC, Edgington PR, Kessler MK, Macrae CF, McCabe P, Pearson J, Taylor R. Acta Crystallogr. 2002;B58:389–397. doi: 10.1107/s0108768102003324. [DOI] [PubMed] [Google Scholar]
- 14.a) Dahl T. Acta Chem Scand. 1994;48:96–106. [Google Scholar]; b) Janiak C. J Chem Soc, Dalton Trans. 2000:3885–3896. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.