Abstract
Aim
Although tobacco smoke is an established risk factor for adult cancer, studies of the association between parental smoking and childhood cancer have produced inconsistent results. To investigate the transgenerational relationship between pre-natal and post-natal tobacco smoke exposure from the grandmother’s pregnancies until after the post-natal period and childhood cancer.
Methods
Exposure to tobacco smoke was recorded for three generations. Data were collected through personal interviews using the paediatric environmental history, and were compared among 128 children with cancer and 128 matched controls. The contingency tables and a logistic multivariable regression model were used to control for possible confounding factors.
Results
Smoke exposure during oogenesis (maternal grandmother smokers) – odds ratio (OR) 2.2 (95% confidence interval (CI) 1.1–4.9) – and during the mother’ pregnancies – OR 1.8 (95% CI 1.1–3.3) – were significantly associated with an increased risk of childhood cancer.
Conclusions
Tobacco smoke exposure during the grandmother’s and mother’s pregnancies increase the risk of cancer in the descendants. The results suggest that the biological plausibility of the association between parental smoking and paediatric cancer can be explained by the large latency period of paediatric carcinogenesis.
Keywords: case-control studies, childhood cancer, tobacco smoke pollution
Introduction
Smoking and second-hand smoke are recognised as carcinogens for adult cancers, explaining about 30% of them. Despite this, studies about the association between parental smoking and childhood cancer have produced inconsistent results.1 Molecular and traditional epidemiology studies have indicated a possible relationship between in utero exposure to tobacco smoke and increased risk of childhood cancers.2 Studies have suggested that the foetal and germinal cells are susceptible to the genotoxic effects of tobacco smoke.3 Moreover, in rodents, exposure to chemical carcinogens during pregnancy does not only result in a high incidence of tumours in the progeny of the first generation, but also in an increased tumour incidence in subsequent generations.4 This topic is highly controversial but needs to be carefully assessed.
Medio Ambiente y Cáncer Pediátrico (Environment and Paediatric Cancer Group) is a project for the compilation of paediatric environmental history (PEH) in children with cancer in the United States, Argentina and Spain.5,6 As part of a larger and ongoing study about the determinants of paediatric cancer (PC) that uses the PEH, we analyse the association between PC and tobacco smoke along multigenerational exposures (from grandmother’s pregnancies to the post-natal period).
Methods
This is a case-control study conducted in Spain during 2004–2007. Cases were children born between 2001 and 2005 newly diagnosed with cancer between 1 January 2004 to 1 Janaury 2006 at one of six collaborating hospitals. Families were recruited from the hospital cancer registries. Centralised care in reference units of PC in Spain facilitated access to medical records in the hospitals of the network. Also, in each reference’s area, the hospital registry include over 98% of children younger than 15 years of age who are diagnosed with a malignant neoplasm.
Families were contacted by telephone to schedule interviews. Completion of the PEH questionnaire lasted 2–3 h. The interview was conducted in person, with one or both parents present. Informed consents were obtained from all parents. One paediatrician conducted the interviews at the collaborating hospitals and at the sites of local parent associations of children with cancer. The paediatrician has expertise in environmental health and oncology, and experience in interacting with PC patients and their families.
One control was sought for each case in the study. Random digit dialling was used to create probability sample of households. Controls were matched to the case by age of the child, year of mother’s birth (±2 years) and ZIP codes. Similar to the cases, matched controls were contacted by telephone to introduce the study and invite participation. Controls were asked to sign an informed consent form. Controls were excluded if they had been diagnosed with any childhood cancer. The study was approved by the hospital network ethics committees and the institutional review boards.
Data about smoking was collected – a smoker is someone who smokes any tobacco product, either daily or occasionally (smoked at least one cigarette every week along the any critical development period). Exposure and use of tobacco was registered for three generations, from the grandmother’s pregnancies until after the cancer diagnosis. We describe the consumption of tobacco during the critical development periods with the transgenerational and environmental tobacco exposure which includes:
The formation periods for the oogonies and spermatogonies during the maternal and paternal grandmother’s pregnancies. The consumption of tobacco in the grandparent’s house during the grandmother’s pregnancies.
The period of maturation of spermatogenesis (the 72 days before conception) to active and passive exposure.
Active and passive smoking (at work, and/or domestic exposure and/or in recreational activities) of the mother during the pregnancy. Exposure during the intrauterine period can be directly from the mother (2nd hand smoke) or due to environmental exposure from the father, the workplace, home, etc. (3rd hand exposure).
Passive post-natal exposure to the child until the cancer diagnosis and in age-matched controls.
Smoking data at the moment of the personal interview (from 4 to 10 months after of diagnosis).
SPSS version 13.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. Odds ratios (ORs) and 95% confidence intervals (CIs) were used to evaluate the association between tobacco exposure and PC. Analyses were done for all PCs. A logistic multivariable regression model was used to control for possible confounding factors, such as age, socio-economic status, mother’s and father’s educational level, history of the familial cancer syndrome, and transplacental ionising radiation. Effects were considered statistically significant with a P-value < 0.05 and ORs with a 95% CI that did not include 1.
Results
In total, 128 cases and 128 controls were analysed. Participation reached 100% of the cases originally identified in the study. One hundred and sixty-one families were contacted by phone calls (maximum nine calls) to recruit controls. Four families were non-responders, another four families did not want to participate because of lack of time, and 25 did not meet the age criteria.
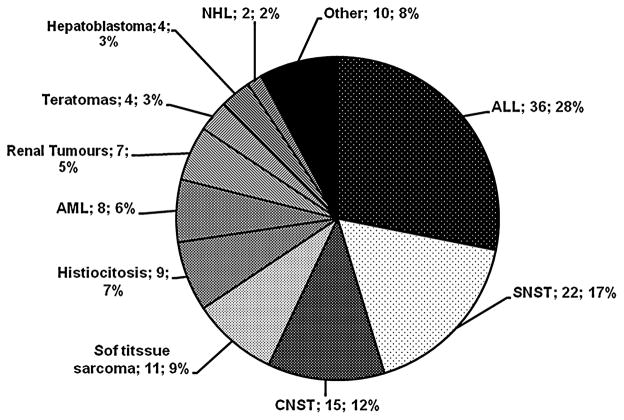
The distribution by tumour type appears in the Figure 1. The most frequent cancer subtypes were, in order, leukaemia, nervous system tumours and soft tissue sarcoma. Table 1 shows the characteristics of cases and controls. There were no significant differences between cases and controls in socio-demographic variables.
Fig. 1.
Cases grouped by cancer types. The proportion of ‘Other’ includes blastoma 1, hepatic hamartoma 1; neurofibromatosis 1, linfangioma 2, chronic myeloid leukaemia 1, thyroid 1, vascular tumours 3. ALL, acute lymphoblastic leukaemia; AML, acute myeloblastic leukaemia; CNST, central nervous system tumour; SNST, simpatic nervous system tumour; NHL, non-Hodgkin lymphoma.
Table 1.
Socio-demographic and confounding factors
| Cases | Controls | Cases (mean) | Controls (mean) | |
|---|---|---|---|---|
| Age | 1.7 | 1.6 | ||
| Children’s gender | ||||
| Male | 77 | 71 | ||
| Female | 51 | 57 | ||
| Mother’s age at pregnancy | 29.6 | 29.2 | ||
| Father’s age at pregnancy | 32.7 | 32.3 | ||
| Mother’s educational level | ||||
| None | 7 | 8 | ||
| Primary school | 31 | 30 | ||
| Incomplete secondary | 18 | 16 | ||
| Complete secondary | 10 | 15 | ||
| Incomplete college | 6 | 5 | ||
| Complete college | 28 | 26 | ||
| Father’s educational level | ||||
| None | 12 | 9 | ||
| Primary school | 39 | 42 | ||
| Incomplete secondary | 8 | 8 | ||
| Complete secondary | 14 | 15 | ||
| Incomplete college | 10 | 7 | ||
| Complete college | 17 | 19 | ||
| Net Income/month (€) | ||||
| <800 | 13 | 16 | ||
| 800–1500 | 34 | 30 | ||
| 1500–2500 | 43 | 46 | ||
| 2500–3500 | 15 | 18 | ||
| >3500 | 13 | 12 | ||
| Familial/hereditary cancer syndrome | 4 | 3 | ||
| Transplacental ionising radiation | 10 (7.8%) | 5 (3.9%) | ||
| Year of birth | ||||
| Mother | 1971 (58–85) Median: 1972 |
1972 (57–83) Median: 1973 |
||
| Father | 1968 (42–80) Median: 1969 |
1969 (45–78) Median: 1969 |
||
There were no significant differences in any of the variables.
Table 2 describes presence of exposure to tobacco smoke during critical periods of development.
Table 2.
The transgenerational tobacco smoke pollution exposure (%)
| Period | Tobacco smoke exposure | Cases | Controls | OR cases/controls |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Germinal Cells. Grandmother’s pregnancies | Any smoker in the house (maternal grandparents) | 88 (73.3) | 78 (67.2) | 1.0 (0.9–1.2) |
| Maternal grandmother smoker | 22 (19) | 12 (9.6) | 1.9 (1.1–3.8) | |
| Any smoker in the house (paternal grandparents) | 99 (82.5) | 85 (72.6) | 1.1 (0.9–1.3) | |
| Paternal grandmother smoker | 12 (10.2) | 11 (8.7) | 1.1(0.5–2.5) | |
| Germinal cells. 6 months before conception | Mother smoker | 56 (45.5) | 50 (39.1) | 1.1 (0.8–1.5) |
| Father smoker | 60 (49.6) | 55 (43) | 1.1 (0.8–1.5) | |
| Any smoker | 79 (64.8) | 74 (57.8) | 1.1 (0.9–1.3) | |
| Pregnancy | Mother smoker | 53 (43.1) | 49 (38.3) | 1.1 (0.8–1.5) |
| Father smoker | 59 (49.2) | 54 (42.2) | 1.1 (0.8–1.5) | |
| Intrauterine smoker† | 87 (71.3) | 72 (56.3) | 1.2 (1.1–1.5) | |
| Post-natal (at diagnosis) | Mother | 46 (37.4) | 45 (35.2) | 1.0 (0.7–1.4) |
| Father | 58 (48.3) | 53 (41.4) | 1.1 (0.8–1.5) | |
| Any smoker at home | 74 (60.7) | 71 (55.5) | 1.0 (0.8–1.3) | |
| Smokers at the time of interview | Mother | 42 (34.1) | 49 (38.3) | 1.0 (0.8–1.3) |
| Father | 50 (41.7) | 52 (40.6) | 1.2 (0.9–1.5) | |
| Any smoker at home | 63 (51.6) | 70 (54.7) | 1.1 (0.9–1.3) |
Exposure during pregnancy includes maternal smoking and second-hand smoking exposure.
The age of tobacco exposure initiation of the mothers and fathers of the children with cancer was 16.5 and 15.7 years, respectively. The most frequent time of tobacco smoke exposure for the children with cancer coincides with the foetal periods (71.3% were exposed in some form during this period).
Comparing the differences among the tumour types, acute linfoblastic leukaemia (ALL) stands out because it had the highest frequency of active smoking consumption of the maternal grandmother during pregnancy (26.7% smoked, P < 0.05). The mothers of children with neuroblastoma (61.9% smoked) were significantly more likely to have been smokers during their pregnancy than the mothers in the rest of the tumour types (P < 0.05).
The OR of exposure during the oogenesis and the intrauterine period was 1.9 (1.1–3.8) and 1.2 (1.1–1.5), respectively. After logistic regression, factors remaining significant were exposure during oogenesis (maternal grandmother) with an OR of 2.2 (95% CI 1.1–4.9) and intrauterine exposure (foetal smoker or second- or third-hand exposure) with an OR of 1.8 (95% CI 1.1–3.3). Although the overall number of cases is small, the exploratory study of the ALL, in the stepwise logistic regression analysis, showed that maternal grandmother smoking during pregnancy and the transplacental ionising radiation remained in the model, with an OR 3.0 (95% CI 1.1–8.8) and 5.1 (95%CI 1.3–19.6), respectively.
Discussion
Our results suggest that passive exposures in the preconceptional intrauterine period of oogenesis (maternal grandmothers) and intrauterine (mothers) period increase the risk of cancer in descendants. It is possible that effects of tobacco translate to the second generation descendants by its actions on the germ cells.
The data support the transgeneration hypothesis of oncogenesis, lengthening the latency period of paediatric cancers to various generations.
The greatest risk of germline mutations occurs during the fertile period for both sexes (especially the male) and in the women, in addition, around the fourth to the seventh month of foetal life.7 Carcinogenic substances of tobacco affect all the cells of the organism, including the germ cells.8 Three mechanisms of transplacental action of tobacco carcinogens exist that explain our results9–11: (i) the direct lesion to the DNA in the foetal cells, activation oncogenes or inactivation of tumour suppressor genes; in both cases the cancer develops in the first months to years of life; (ii) a lesion to the foetal tissue structures, making one or more tissues vulnerable to cancer later in life; and (iii) by deleting only one foetal tumour suppressor gen, converting a cell line and the tissues derived more susceptible to carcinogenic agents. In the later two, the cancers develop both during the paediatric and adult period. It has also been demonstrated that foetal hemoglobin presents greater susceptibility to the carcinogenic substances in tobacco than adult hemoglobin, increasing the foetal concentrations of carcinogenic substances. In the same way it has been described that benzene (a) induces mutations in foetal DNA with loss of function of the p53 tumour suppressor gene.12
It is interesting that there was a small association with maternal grandomother but not with gestational (mother) smoking. Maybe, it explains by the difference susceptibility between the somatic and germ cells and the models of exposure to tobacco carcinogens.
We believe that the intrauterine actions on foetal cells (somatic and germ) by tobacco smoke have increased because of the gradual incorporation of women to smoking in Spain and the world. All of the previous can points can in part explain the global increase in childhood cancers. The contribution of tobacco to PC is due to the combined action during the critical transplacental development periods during the 1st or 2nd generations at the minimum. In effect, the latent period is greater than for adult cancer, increasing the opportunities for intervention and prevention of paediatric cancers through the action of the previous generations.
Considering the plausibility of passive tobacco exposure on the total of paediatric cancers, the tobacco can be 2nd hand when the mother is a smoker or 3rd hand when the mother is a passive smoker. A meta-analysis based on 11 studies (4 cohort and 7 case control) showed a RR of 1.1 (95% CI 1.0–1.2), between maternal tobacco use during gestation and the risk of pedatric cancer in her children.13 Another study about the association of PC and paternal tobacco use showed an OR of 1.3 (95% CI 1.0–1.7).14 There is a growing body of literature suggesting that paternal smoking has the potential for initiating a carcinogenic process in offspring thorough its genotoxic effect on sperm. However, other studies have suggested that in utero exposure to tobacco smoke can have genotoxic effects on the foetus. The current hypothesis is that the development of childhood leukaemia requires at least a pre-natal initiating event and a post-natal promoting event; therefore it is important to study the joint influence between pre-natal and post-natal smoking exposures.15
In our study, the maternal grandmothers of our patients with ALL smoked more that the rest during oogenesis and the mothers of children with neuroblastomas smoked more during their pregnancy than the rest. The National Cancer Institute identifies as a factor with limited or inconsistent evidence exposure to tobacco for ALL and neuroblastomas.16 Also, in agreement with the previous data, the transplacental ionising radiation is a risk factor recognised for ALL.17
We must carefully consider the limitations of the study. In our study, all PCs were analysed concomitantly because it is plausible that similar carcinogenic mechanisms may apply for all childhood tumours, and the sample size limited. But, childhood cancer is a heterogeneous disease. In addition to categorisation by histology, childhood cancer can be further categorised by molecular techniques into more homogeneous subgroups, which will increase the power to establish the associations between specific environmental exposures and specific molecular changes. Also, for a more complete picture of a child’s in utero exposure to carcinogens, it is important to consider the mother’s, grandmother’s and the child’s genetic information. This lack of genetic information is important in determining an individual’s susceptibility to carcinogens.
Recall bias is crucial in studies of childhood cancer. Mothers of children with cancer might be more likely to remember possible noxious events during pregnancy than mothers of healthy children. This worry is real and exists in this type of study. We have tried to control it by: validating information about the mother’s smoking status by verifying the information with the information in her medical charts or/and contacting her health centre. With regards to the grandparent’s exposure (in the cases and controls) we tried to reconfirm with a second call and/or personal interview when it was necessary. Also, the mean ages of cases and controls were not different in our study. Therefore, on average, recall periods were similar between the two groups. Tobacco is tolerated in Spain more than in any other countries of Europe, and the association between smoking and childhood cancer is not well known by parents as it is not even known by most clinicians in Spain.
On the other hand, the interpretation of our study is hampered by the crude exposure assessment based on a dichotomous indicator of smoking by the parents, without considering quantitative exposure variables. Most of the studies reporting results for different exposure levels did not provide evidence of a dose–response relationship, which also detracts from a causal interpretation of the summary risk estimates.
We have incorporated during the personal interview some confounding factors (socio-economic status, transplacental exposure to ionising radiation) but we can still not eliminate unknown confounders. Thus, confounding remains a plausible explanation for the observed increased risk. Also, interactions may be important and should be fully explored. Our work is part of an ongoing research effort and we expect to be able to address these issues in the future.
Sixty-one percent of the children with cancer lived in a house where someone smoked at diagnosis compared with 55% of the children without cancer. In Spain, less than 5% of cancer survivorship programmes offer smoking prevention programme and only one offer smoking cessation services. Despite this, once the child was diagnosed, we observed a reduction in the consumption in the parents. Our data indicate that we should continue looking for aetiology of paediatric cancers, but there is significant evidence that the parents and family of children with cancer should urgently consider smoking cessation.18 Without a doubt, the quality of life and the environment will improve, as may the long survival of the oncologic patient during adult age.19 Independently of preventative actions, paediatricians should know and be familiar with the diverse therapies for smoking cessations for two major reasons. First, to inform, help, stimulate, and obtain that the parents and family of the child with cancer abandon their smoking. Second, to directly treat the adolescent smokers, given the greater effect of the cessation therapy during the initial phases of the adolescent smoking. It is need stop smoking before it starts among survivors of childhood cancer.
What is already known on this topic
Tobacco smoke contains at least 60 known human or animal carcinogens.
The germ and foetal cells are especially susceptible to initiating the oncogenic processes generated by carcinogenic substances in tobacco smoke.
The studies of association between parental smoking and childhood cancer have produced inconsistent results.
What this study adds
The exposure to tobacco smoke during the mother’s and grandmother’s pregnancies increase the risk of cancer in the decedents.
This study supports the human transgenerational hypothesis of oncogenesis, lengthening the latency period of paediatric cancers to various generations.
It needs to offer smoking cessation and prevention services as a priority for teenage survivors, their parents and other family members.
Acknowledgments
The authors express their gratitude for the support and funding granted by the Scientific Foundation of the AECC (Asociación Española Contra el Cáncer). Additionally, we thank the International Exchange Program for Minority Students funded by the National Institutes of Health (T37 MD001452) and the International Training and Research Program in Environmental and Occupational Health at the Mount Sinai Medical Center (New York), funded by the Fogarty International Center (Fogarty International Center TW00640).
References
- 1.US Department of Health and Human Services. The Health Consequences of Tobacco Use: A Report of the Surgeon General. Atlanta, US: Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- 2.Sasco AJ, Vainio H. From in utero and childhood exposure to parental smoking to childhood cancer: a possible link and the need for action. Hum Exp Toxicol. 1999;18:192–201. doi: 10.1191/096032799678839905. [DOI] [PubMed] [Google Scholar]
- 3.Zenzes MT, Puy LA, Bielecki R, et al. Detection of benzo(a)pyrene diol epoxide-DNA adducts in embryos from smoking couples: evidence for transmission by spermatozoa. Mol Hum Reprod. 1999;5:125–31. doi: 10.1093/molehr/5.2.125. [DOI] [PubMed] [Google Scholar]
- 4.Tomatis L, Ponomarkov V, Turusov V. The effect of ethylnitrosourea administration during pregnancy on three subsequent generations. Int J Cancer. 1977;19:240–8. doi: 10.1002/ijc.2910190214. [DOI] [PubMed] [Google Scholar]
- 5.Ferrís Tortajada J, Ortega García JA, Marco Macián A, et al. Environment and pediatric cancer. An Pediatr (Barc) 2004;61:42–50. doi: 10.1016/s1695-4033(04)78352-6. [DOI] [PubMed] [Google Scholar]
- 6.Ortega-García JA, Ferrís-Tortajada J, Torres-Cantero AM, et al. Full breastfeeding and paediatric cancer. J Paediatr Child Health. 2008;44:10–3. doi: 10.1111/j.1440-1754.2007.01252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadler TW. Langman Embriología Médica. 9. Panamericana; Buenos Aires: 2004. [Google Scholar]
- 8.Cooper AR, Moley KH. Maternal tobacco use and its preimplantation effects on fertility: more reasons to stop smoking. Semin Reprod Med. 2008;26:204–12. doi: 10.1055/s-2008-1042959. [DOI] [PubMed] [Google Scholar]
- 9.U.S. EPA. Supplemental Guidance for Assessing Susceptibility from Early-Life Exposure to Carcinogens. Washington, DC: U.S. Environmental Protection Agency; 2005. EPA/630/R-03/003F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Environmental Protection Agency. Critical Periods in Development. Paper 2003-2. Washington, DC: 2003. OCHP Paper Series on Children’s Health and the Environment. [Google Scholar]
- 11.Ferrís i Tortajada J, Ortega García JA, López Andreu JA, et al. Tabaquismo parental y cancer pediátrico. Rev Esp Pediatr. 2004;60:225–36. [Google Scholar]
- 12.Zenzes MT, Bielecki R, Reed TE. Detection of benzo (a) pyrene diol epoxide-DNA adducts in sperm of men exposed to cigarette smoke. Fertil Steril. 1999;72:330–5. doi: 10.1016/s0015-0282(99)00230-7. [DOI] [PubMed] [Google Scholar]
- 13.Boffetta P, Tredaniel J, Greco A. International Agency for Research on Cancer and World Health Organization. Lyon: IARC Press; 1999. Parental Tobacco Smoke and Childhood Cancer. [Google Scholar]
- 14.Ji BT, Shu XO, Linet MS, et al. Paternal cigarette smoking and the risk of childhood cancer among offspring of non-smoking mothers. J Natl Cancer Inst. 1997;89:238–44. doi: 10.1093/jnci/89.3.238. [DOI] [PubMed] [Google Scholar]
- 15.Chang JS. Parental smoking and childhood leukemia. Methods Mol Biol. 2009;472:103–37. doi: 10.1007/978-1-60327-492-0_5. [DOI] [PubMed] [Google Scholar]
- 16.Ries LAG, Smith MA, Gurney JG, et al., editors. SEER Program NIG Pub No. 99-4649. Bethesda, MD: National Cancer Institute; 1999. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. [Google Scholar]
- 17.Wakeford R. The cancer epidemiology of radiation. Oncogene. 2004;23:6404–28. doi: 10.1038/sj.onc.1207896. [DOI] [PubMed] [Google Scholar]
- 18.Frobisher C, Winter DL, Lancashire ER, et al. Extent of smoking and age at initiation of smoking among adult survivors of childhood cancer in Britain. J Natl Cancer Inst. 2008;100:1068–81. doi: 10.1093/jnci/djn210. [DOI] [PubMed] [Google Scholar]
- 19.Tyc VL, Throckmorton-Belzer L, Klosky JL, et al. Smoking among parents of pediatric cancer patients and children’s exposure to environmental tobacco smoke. J Child Health Care. 2004;8:288–300. doi: 10.1177/1367493504047319. [DOI] [PubMed] [Google Scholar]