Abstract
Hydroxypyridinone-based Gd(III) complexes have previously been shown to exhibit high relaxivity, especially at the clinically relevant high magnetic fields relevant for present and future clinical use. This is due to more than one coordinated water molecules that exchange rapidly with bulk solvent.. These complexes, however, present poor water-solubility. Heteropodal complexes which include a terephthalamide moiety maintain the high relaxivity characteristics of the HOPO family and have been functionalized with solubilizing substituents of various charges. The charge of the substituent significantly affects the stability of the Gd(III) complex, with the most stable complex presenting a neutral charge. The solubilizing substituent also moderately affects the affinity of the complex for physiological anions, with the highest affinity observed for the positively charged complex. In any case, only two anions, phosphate and oxalate, measureably bind the Gd(III) complex with weak affinities for these two anions that are comparable to other q=1 complexes and much weaker than DO3A, q=2 based complexes. Furthermore, unlike poly(amino-carboxylate) based complexes, HOPO-based Gd(III) complexes do not show any noticeable interaction with carbonates. The nature of the substituent can also favorably stabilize the coordination of a third water molecule on the Gd(III) center and lead to a nine-coordinate ground state. Such complexes that attain q = 3 incorporate a substituent β to the terminal amide of the TAM podand which are hydrogen-bond acceptors, suggesting that the third water molecule is coordinated to the metal center through a hydrogen-bond network. These substituents include alcohols, primary amines, and acids. Moreover, the coordination of a third water molecule has been achieved without destabilizing the complex.
Keywords: Gadolinium, MRI, Contrast Agents, hydroxypyridinone
Introduction
In the last two decades, Magnetic Resonance Imaging (MRI) has evolved into one of the most powerful techniques in diagnostic medicine and biomedical research. This technique has been further strengthened by the use of contrast agents which catalytically shorten the nuclear magnetic relaxation time of nearby water protons, thereby highlighting their surrounding and improving the ability to distinguish different tissues. The Gd(III) ion, with seven unpaired electrons and a long electronic relaxation time, is ideal for use as such a relaxation agent. Its high toxicity, however, requires that it be complexed by a strong chelator for in vivo applications. Current commercial poly-(amino carboxylate) based chelates have only one coordinated water molecule which exchanges so slowly with the bulk solvent that it limits the image enhancing capability (relaxivity, r1p) of macromolecular derivatives.2 The Solomon-Bloembergen-Morgan equation indicates that the relaxivity can be significantly increased by increasing the number of inner sphere water molecules. Attempts to increase the relaxivity of poly(amino-carboxylate)-based ligands by increasing the number of water molecules coordinated to the Gd(III) and shortening their residence time have unfortunately led to substantially decreased stability of the complex.3–6
We have previously reported hydroxypyridinone-based Gd(III) complexes which display high relaxivity while maintaining high stability.7–9 This high relaxivity is due both to the presence of two coordinated water molecules and to their fast exchange rate. These two beneficial properties have been further exploited to design macromolecular derivatives of significantly high relaxivities at the high magnetic field which is clinically relevant.10–11 An important downside to the original hydroxypyridinone class of Gd(III) complexes is their poor water solubility. The concentration of a contrast agent after distribution throughout all of the extracellular space is typically 0.25 mmol contrast agent/kg body weight or ≈ 3 mM of blood.2 Contrast agents are therefore typically injected as 0.5 M solutions. Although high relaxivity agents could be injected at lower concentration, it remains imperative that it be highly soluble in water. While commercial agents easily reach such concentrations, this is not the case for the aromatic HOPO-based complexes. The solubility of Gd-TREN-1-Me-3,2-HOPO is less than 0.1 mM.12
The water solubility of HOPO-based complexes can readily be increased by grafting solubilizing substituents. However, in selecting the substituent it is important to consider the impact that it may have on the physical properties of the complex and its behavior in physiological conditions. It has been seen previously that functionalization of Gd-DOTA and Gd-DO3A with substituents of various charges can significantly impact their affinities for physiological anions.15–17 Previous studies have also indicated that for a given diastereomer of Eu-DOTA analogues the lifetime of the coordinated water molecule, τM, is directly related to the calculated solvent-accessible surface, which can in turn be affected by the position and size of the substituents.18 The change in ligand basicity that the substituents induce can also affect τM. Aime and coworkers have demonstrated that the rate of exchange (kex = 1/τM) of the two water molecules coordinated to a Gd(III) center in neutral complexes with heptadentate DO3A derivatives is modulated by the basicity of the macrocyclic nitrogen bearing the pendant group: a lower basicity results in a slower water-exchange rate.19 Even the charge of the Gd(III) complex can affect its water residence time. Aime and coworkers have recently been able to modulate the prototropic exchange rate of the coordinated water molecules of several Gd DTPA-BMA analogues by changing the overall charge of the complex.20 Similarly, Sherry and coworkers have shown that although the lanthanide coordination geometry of their Eu-DOTA analogues does play a role in determining τM, the identity of the ligand side chains, and particularly the charge and polarity they induce in the complex, can also have a determining effect.21.
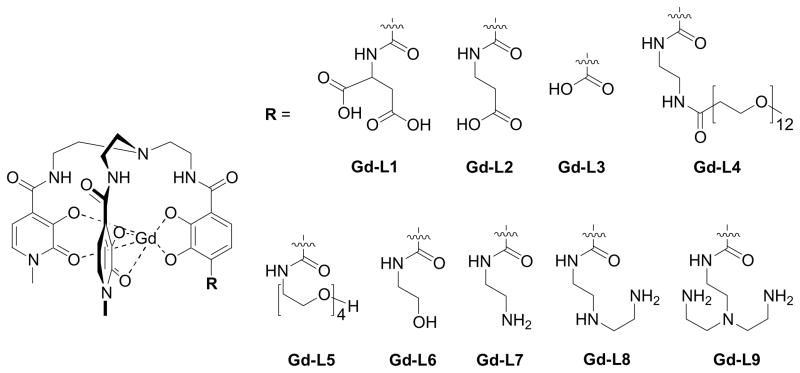
Initial studies have indicated that the most stable complex is achieved with a ligand of intermediate basicity, comprising two hydroxypyridinone and one terephthalamide moiety.9 A further advantage of this chelate is its ease of functionalization of the terminal acid of the TAM podand with solubilizing moiety and/or targeting groups. We have therefore investigated various Gd-TREN-bisHOPO-TAM complexes functionalized with differently charged water solubilizing substituents including amines, alcohols, PEG polymers, and carboxylates (Figure 1). All complexes maintain the same heteropodal chelate, thus enabling a direct study of the various effects of the substituents. Herein we present the thermodynamic and relaxivity evaluation of these derivatives and the effect of the solubilizing moieties on the stability of the complex and its selectivity versus physiological anions is discussed. The nature and position of the substituent can be used to advantage in further optimizing the number and exchange rate of coordinated water molecules.
Figure 1.
Functionalized Gd-TREN-bisHOPO-TAM complexes.
Results and Discussion
Stabilities of Gd(III) Complexes
The most important pharmacological requirement for a contrast agent is limited toxicity, which is in turn directly related to its stability.22–23 It has been previously determined that for hydroxypyridinone-based Gd(III) complexes, maximum stability is obtained with a heteropodal ligand of intermediate basicity comprising one terephthalamide moiety: TREN-bisHOPO-TAM.9 The effect of substituting the terephthalamide podand with solubilizing or targeting group had, however, not yet been determined.
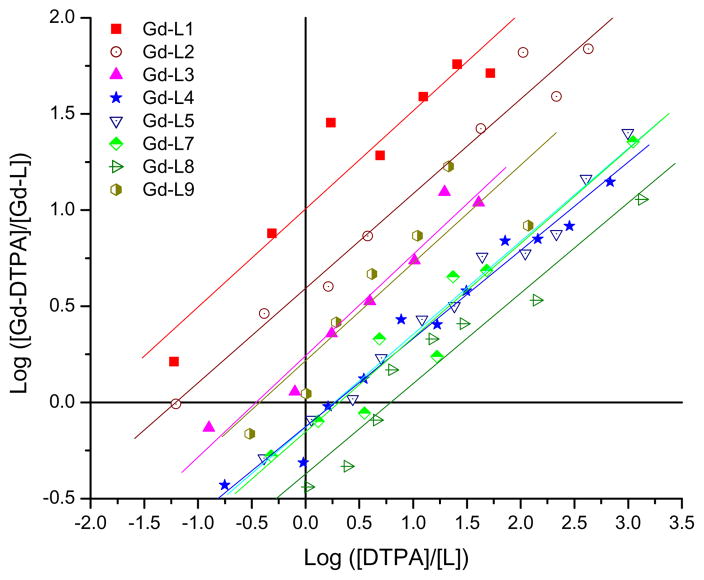
The affinities of the various functionalized TREN-bisHOPO-TAM ligands for Gd(III) were measured via competition titration against the commercial agent DTPA. Throughout each titration, the pH as well as the total concentration of the heteropodal ligand and Gd(III) were kept constant, whereas the concentration of the competing ligand was progressively increased. The concentrations of free and complexed TREN-bisHOPO-TAM (L) were then determined by UV-visible spectroscopy by using solutions of L and Gd-L at identical conditions (concentrations, pH and electrolyte and buffer concentration) as references. The proportion of transmetallation resulting from the titration of each TREN-bisHOPO-TAM ligand comprising either an amine, acid or PEG substituent against DTPA is shown in Figure 2. Since the stability of Gd-DTPA is known,22,24 the concentration of competing ligand necessary to generate an equal partition of Gd(III) between the two ligands (log([DTPA]/[L]) when log([Gd-DTPA]/[Gd-L]) = 0) directly gives the pM of the heteropodal ligand at physiological pH. The refined relative stabilities (ΔpM = pGdL - pGdC.L.) of each Gd-TREN-bisHOPO-TAM complex versus DTPA and the resulting pGd are given in Table 1.
Figure 2.
Competition titrations of Gd-TREN-bisHOPO-TAM complexes against DTPA. The x-intercepts indicate the difference in pGd between each heteropodal ligand and the competing poly(amino-carboxylate). Experimental conditions: 0.1 M KCl, pH 7.4, 25 °C.
Table 1.
Relative stabilities of Gd-TREN-bisHOPO-TAM complexes versus DTPA.*
| complex | charge | pGdL-pGdDTPA | pGd |
|---|---|---|---|
| Gd-L1 | −3 | −1.97 | 17.1 |
| Gd-L2 | −2 | − 1.21 | 17.9 |
| Gd-L3 | −2 | − 0.46 | 18.6 |
| Gd-L4 | −1 | + 0.27 | 19.4 |
| Gd-L5 | −1 | + 0.28 | 19.4 |
| Gd-L7 | −1 | + 0.32 | 19.4 |
| Gd-L8 | 0 | + 0.79 | 19.9 |
| Gd-L9 | +1 | − 0.42 | 18.7 |
Experimental conditions: 0.1 M KCl, pH 7.4, 25 °C.
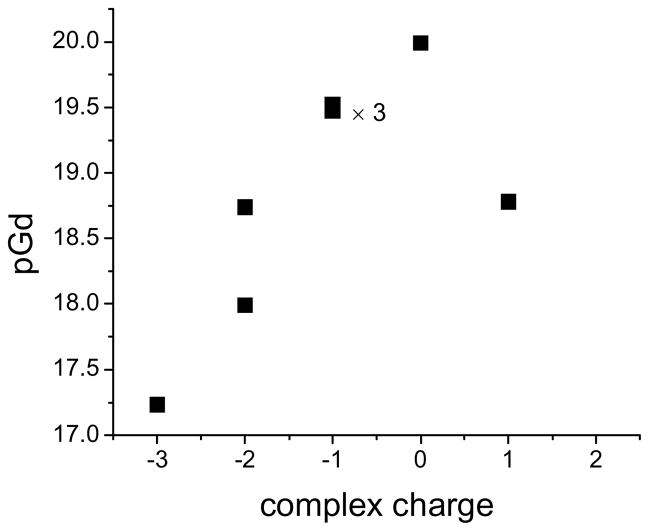
The solubilizing substituent of the TREN-bisHOPO-TAM ligand significantly affects the stability of its Gd(III) complex. The stability of the various heteropodal complexes spans over three orders of magnitude, with the neutral amine terminated Gd-L8 being the most stable, and the negatively charged acid terminated Gd-L1 being the least stable. Figure 3 indicates the effect of the charge of the Gd-TREN-bisHOPO-TAM derivatives on their stability. It is apparent from this graph that acidic or negatively charged substituents drastically decrease the stability of the complex and that this decrease is nearly proportional to the number of negative charges. The addition of positive charges, as in the +1 charged Gd-L9 also significantly decreases the pGd by one order of magnitude. It appears that in order to achieve maximum stability, not only should the ligand basicity be optimized,9 but the overall charge of the complex should be maintained as close to zero as possible. One should note that Gd(III) complexes whose ligands have similar basicity and who bear similar overall charge, such as the −1 charged Gd-L7, Gd-L4, and Gd-L5, have equivalent stability. Furthermore, the position of the negative acid moiety on the ligand appears to influence its impact on stability. In Gd-L3, the carboxylate moiety is kept far away from the Gd(III) center. The complex is more stable than Gd-L2, whose terminal acid is hydrogen-bonded close to the metal.
Figure 3.
Effect of the charge of the Gd(III) complex on its stability.
Affinities for Physiological Cations
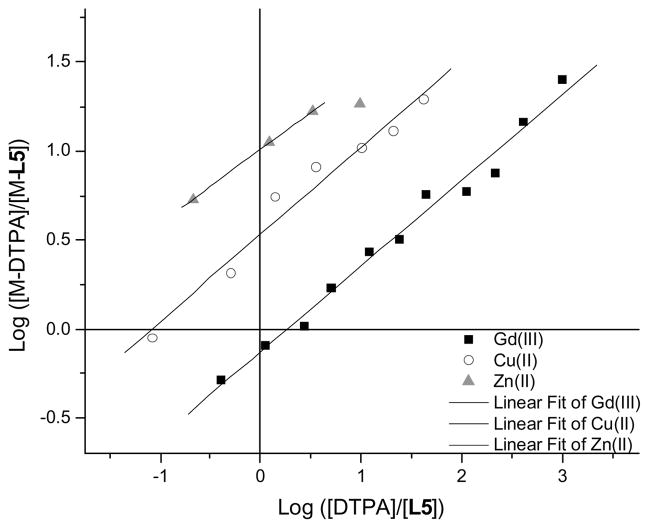
The tripodal hydroxypyridinone-based gadolinium complexes, like their DTPA-based counterparts, are highly labile; their transmetallation readily occurs within hours. Their physiological toxicity is therefore not only controlled by their stability, but also by their selectivity versus endogenous metals, especially those present in non-negligible concentrations in the uncomplexed form in the serum. Of concern are Ca2+ (concentration of the free ion in serum = 2.5 mM), Zn2+ (50 μM), and Cu2+ (1 μM).22 Selectivity is a measure of the relative stabilities of the different metal complexes formed by the same ligand. Previous studies with L6 have determined that the ligand does not noticeably form any complexes with Ca(II) under physiological conditions and that its Zn(II) complex was significantly less stable than Zn(DTPA).8 No studies had been performed on Cu(II) hydroxypyridinone based complexes. In order to determine the selectivity of the heteropodal ligand L5 for Gd3+ over Zn2+ and Cu2+, the stability of the two latter complexes first had to be established. The stability, in terms of pM, of Zn-L5, and Cu-L5 was determined following the same protocol used for the pGd. The titrations (Figure 4 and Table 2) indicate that the −1 charged hydroxypyridinone-based ligand is significantly more selective for Gd(III) over Cu(II) (pGd – pCu = 2.5) and Zn(II) (pGd – pZn = 7.0) than the commercial DTPA (pGd – pCu = 1.1; pGd – pZn = 3.7).22 Since L5 is of comparable stability and of higher selectivity against endogenous cations than the commercial agents, it is predicted that it would be of low toxicity.
Figure 4.
Competition titrations of Gd(III) (filled squares), Cu(II) (open circles) and Zn(II) (grey triangles) of L5 complexes against DTPA. The x-intercepts indicate the difference in pM between each heteropodal ligand and the competing poly(amino-carboxylate). Experimental conditions: 0.1 M KCl, pH 7.4, 25 °C.
Table 2.
Relative stabilities of Gd- Cu- and Zn-L5 complexes versus DTPA.*
| complex | pML5-pMDTPA | pM |
|---|---|---|
| Gd-L5 | + 0.28 | 19.4 |
| Cu-L5 | − 1.07 | 16.9 |
| Zn-L5 | − 2.86 | 12.4 |
Experimental conditions: 0.1 M KCl, pH 7.4, 25 °C.
Affinities for Physiological Anions
An important parameter to consider in designing MRI contrast agents is their in vivo behavior, in particular, their relaxivity and stability in the presence of physiological cations and anions. Like their metal counterparts, physiological anions may be of a toxicity concern: if the complex is not stable enough, phosphates can compete with the ligand for GdIII thus resulting in insoluble adducts that would precipitate in the blood stream.22 Endogenous anions can also decrease the contrast enhancing effectiveness of stable complexes as they compete directly with bulk water molecules for coordination to Gd3+. If their affinities are sufficient to replace the coordinated inner sphere water molecules, the Gd3+ center can no longer effectively relax bulk water and its relaxivity sharply decreases. For instance, previous work on the DO3A family of complexes has demonstrated that both inner sphere water molecules were easily replaced by citrate, malonate or lactate and that at least one water molecule could be replaced by carbonate or phosphate.15–17 Minor structural modifications of the ligand, such as N-methylation of the coordinating carboxylates, were found to significantly influence the affinities of oxy-anions for the complex.17,25 More importantly, the affinity of the anions for a complex decreased as a function of its overall negative charge.17
The affinity of physiological anions for hydroxypyridinone-based Gd3+ complexes was investigated on two differently charged complexes: Gd-L5 which bears an overall −1 charge at physiological pH, and Gd-L9 which bears an overall +1 charge. The physiologically available anions studied are those found in sufficient concentrations in the plasma to be potentially problematic. These include HCO3− (24.5 mM), HPO42− (0.38 mM) and citrate (0.11 mM) whose concentrations in blood plasma are comparable to that of a contrast agent after distribution throughout all of the extracellular space.2 Other biologically relevant anions include acetate, lactate, oxalate, malonate and the amino acids glycinate, glutamate, and aspartate. Fluoride, with the ability to replace a water molecule coordinated to a lanthanide and remain tightly,25 was also included in the study.
The interactions between the Gd(III) complexes and the anions were followed by relaxometry. In this protocol, solutions of the Gd(III) complex in the presence of a 200-fold molar excess of the respective anion were neutralized to pH 7.4 and equilibrated for two weeks. The relaxivities of the complex in the presence of each anion were then compared to that measured in pure water at identical pH and temperature. Anions that have high affinities for the lanthanide displace one or more coordinated water molecules, thereby reducing the relaxivity of the complex. Table 3 indicates the effect of each physiological anion on the relaxivity of either Gd(III) complex.
Table 3.
Corrected longitudinal relaxivity, r1, and difference in relaxivity, Δr1 = r1GdL+anion − r1GdL, for Gd-L5 and Gd-L9 in the presence of 200 equivalents of each physiological anion.*
| Anion | Gd-L5 | Gd-L9 | ||
|---|---|---|---|---|
| r1 (mM−1s−1) | Δr1 (mM−1s−1) | r1 (mM−1s−1) | Δr1 (mM−1s−1) | |
| - | 9.94 (2) | - | 8.96 (2) | - |
| Fluoride | 9.26 (3) | − 0.68 (5) | 6.45 (1) | − 2.51 (3) |
| biphosphate | 7.11 (2) | − 2.83 (4) | 7.21 (2) | − 1.75 (4) |
| bicarbonate | 9.78 (3) | − 0.16 (5) | 8.84 (2) | − 0.12 (4) |
| Lactate | 9.90 (1) | − 0.04 (3) | 9.82 (1) | + 0.86 (3) |
| Oxalate | 5.79 (1) | − 4.15 (3) | 6.31 (2) | − 2.65 (4) |
| Acetate | 10.00 (3) | + 0.06 (5) | 9.88 (3) | + 0.92 (5) |
| Malonate | 9.40 (2) | − 0.54 (4) | 9.40 (2) | + 0.44 (4) |
| Citrate | 10.00 (2) | + 0.06 (4) | 9.24 (1) | + 0.28 (3) |
| Glycinate | 9.48 (2) | − 0.46 (4) | 9.40 (1) | + 0.44 (3) |
| Aspartate | 10.00 (2) | + 0.06 (4) | 10.46 (2) | + 1.50 (4) |
| Glutamate | 9.72 (3) | − 0.22 (5) | 10.68 (2) | + 1.72 (4) |
Experimental conditions: 20 MHz, 25 °C, pH = 7.40, [Gd complex] = 0.501 mM, [physiological anions] = 0.100 M. Values in parentheses represent one standard deviation.
Only two physiological anions, hydrogen phosphate and oxalate, have a significant effect on the relaxivity of both HOPO-based complexes. As a comparison, bicarbonate interacts significantly not only with the commercial Gd-DO3A (q = 2, log Ka = 4.8) but also with Gd-DOTA (q = 1, log Ka = 1.9) and Gd-DTPA (q = 1, log Ka = 1.4).2 It does not, however, have any noticeable interaction with either of the Gd-TREN-bisHOPO-TAM complexes, and this regardless of the charge or substituent of the complex. In the case of the negatively charged Gd-L5, malonate and glycine also interact mildly, suggesting a very low association constant. No other anions were found to interact noticeably with the Gd(III) complex. More interestingly, in the case of the positively charged Gd-L9, several anions such as glutamate, aspartate, acetate and lactate, increase the relaxivity of the Gd(III) complex. This suggests that theses anions do not replace coordinated water molecules, but rather form a strong ionic pair with the pendant amines, resulting in a ternary complex with a longer rotational correlation time, and hence higher relaxivity. The increase in relaxivity is indeed proportional to the size of the anions: a greater increase is observed for aspartate than for glycine, and an even greater one is observed for glutamate. On the other hand, a contribution to the relaxivity from second coordination sphere water molecules following the formation of the ion pairing interaction cannot be excluded.
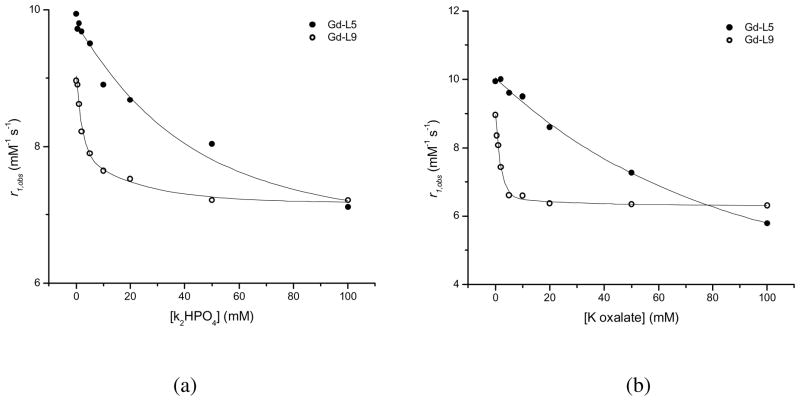
Only two physiological anions, oxalate and phosphate, interact significantly with either of the Gd-TREN-bisHOPO-TAM complexes. The association constants, Ka, of these anions for each Gd(III) complex were determined by titrating the solution of the Gd(III) complex containing a 200-fold molar excess of the anion considered with a stock solution of the Gd(III) complex at the same concentration and pH (Figure 5). The Ka for each ternary complex was analyzed according to the Proton Relaxation Enhancement (PRE) method.2 They are given in Table 4 together with the refinement parameters for the four titrations. Both anions have higher affinity for the positively charged Gd-L9 than for the negatively charged Gd-L5. Botta and coworkers have observed a similar trend for Gd(III) complexes of the DO3A family in which the affinity of anions for the lanthanide decreased with its overall negative charge.25 Interestingly, hydrogen phosphate forms stronger complexes than oxalate with the negatively charged Gd-L5, but weaker ones with the positively charged Gd-L9. In both cases, hydrogen phosphate only replaces one of the two bound water molecules such that the relaxivity of the ternary adduct is only partially diminished. Oxalate, on the other hand, displaces both inner-sphere water molecules of the negatively charged Gd-L5, but apparently only one of the positively charged Gd-L9. The association constants with hydrogen phosphate for the two hydroxypyridinone q = 2 complexes are comparable to that of commercial q = 1 complexes (Gd-DTPA - log Ka = 2.0, Gd-DTPA-BMA - log Ka = 2.0 and Gd-DOTA - log Ka = 2.2) and are significantly lower than that of another q = 2 complex, Gd-DO3A (log Ka = 4.8).2 This difference stresses the importance in achieving appropriate coordination geometry for complexes with several inner-sphere water molecules. A higher number of inner sphere water molecules does not necessarily correlate with their ease of displacement by physiological anions as long as they are positioned anti from one another.
Figure 5.
Titrations of Gd-L5 (filled circles) and Gd-L9 (open circles) with (a) K2HPO4 and (b) K oxalate. Experimental conditions: 25°C, pH = 7.40, [Gd complex] = 0.501 mM.
Table 4.
Refinement parameters for the binding of biphosphate and oxalate to Gd-L5 and Gd-L9.*
| Gd-L5 | Gd-L9 | |||
|---|---|---|---|---|
| biphosphate | oxalate | biphosphate | oxalate | |
| r1 free (mM−1s−1) | 9.94 | 9.94 | 8.96 | 8.96 |
| r1 bound (mM−1s−1) | 6.32 | 2.40 | 7.15 | 6.22 |
| q bound | 1 | 0 | 1 | 1 |
| log Ka | 1.4 | 1.0 | 2.4 | 2.9 |
| χ2 | 0.0061 | 0.0086 | 0.0018 | 0.0031 |
Experimental conditions: 25 °C, pH = 7.40, [Gd complex] = 0.501 mM.
The bidentate oxalate anion appears to replace both inner-sphere water molecules of the negatively charged Gd-L5 (r1bound = 2.40 mM−1s−1), but only one of the positively charged Gd-L9 (r1bound = 6.22 mM−1s−1). This apparent discrepancy in the binding mode of the oxalate for the amine-terminated Gd(III) complex could be explained by two different mechanisms. Either the oxalate binds the Gd(III) in an unusual monodentate manner while its second carboxylate forms a strong ionic bond with the pendant tertiary nitrogen of the ligand (in this case Gd(III) would maintain a coordination number of eight); or the oxalate binds the Gd(III) in a bidentate manner but constrains the metal center to a nine coordinate state that still includes one water molecule.
Some useful indications to discriminate between the two different binding modes for the oxalate ion can be obtained by analyzing the NMRD profile at low magnetic fields of Gd-L9 with and without 200 equivalents of oxalate (Figure 6). The parameters for the refinements of these two profiles are given in Table 5 and in the caption of Figure 6. In the presence of a large excess of oxalate, the complex indeed seems to have only one coordinated water molecule. However, its NMRD profile is very different from the simulated curve of the free complex with q = 1. The relaxivity of the ternary adducts is much lower than that of the free complex at high magnetic field (>10 MHz), but identical at lower magnetic field (< 1 MHz). This variation results from a large difference in the electronic relaxation parameters upon complexation of the oxalate: the ternary complex has a significantly longer T1,2e and thus higher relaxivity at low magnetic field. The electronic relaxation times are sensitive to the coordination geometry of the Gd(III) center. A more symmetric coordination cage results in a smaller mean-square zero-field-splitting energy, Δ2, and hence longer electronic relaxation times, T1,2e.24 In the presence of oxalate, the value of Δ2 of Gd-L9 decreases by ca. 50%. The resulting longer electronic relaxation times suggest a more symmetric environment around the Gd(III) center. Our interpretation is that upon binding of oxalate, Gd(III) shifts from an 8-coordinate bi-capped trigonal prismatic geometry to the more symmetric 9-coordinate tri-capped trigonal prism (Figure 7). In this proposed mechanism, oxalate still acts as a bidentate ligand forming a ternary complex that still coordinates one water molecule. One should note that the ternary adduct of the Gd(III) complex with oxalate also has a slightly higher molecular weight than the free complex, and therefore a slightly longer rotational correlation time, τR.
Figure 6.
Nuclear Magnetic Resonance Dispersion profile of Gd-L9 in water (filled circles) and in the presence of 200 equivalents of oxalate (open circles). The solid curve through the data of the ternary complex were fitted with the following parameters: r=3.0 Å, q=1, τR=145 ps, τV=17 ns, Δ2=6.3×1019 s−1, a = 4.0 Å, D = 2.24×10−5 cm2s−1. The dotted curve is a simulation of the NMRD profile of the complex in water with a single water molecule. Experimental conditions: 25 °C, pH 7.40.
Figure 7.
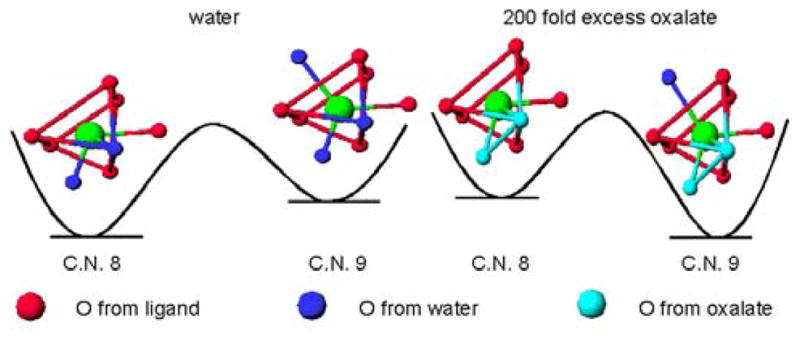
Proposed water-exchange mechanisms for Gd-L9 and its ternary complex with oxalate. The 8-and 9-coordinate geometries are from the single crystal X-ray structure of La-TREN-1-Me-3,2-HOPO.14
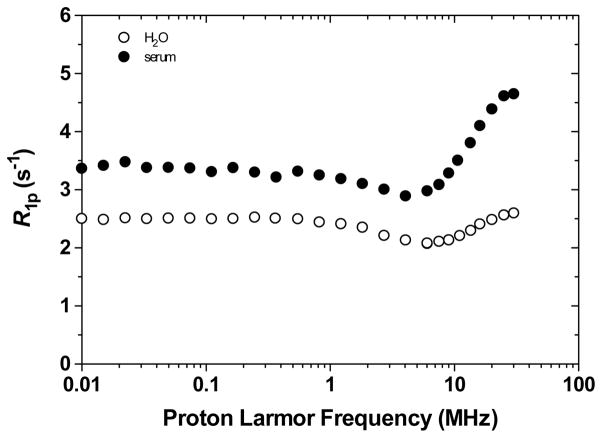
The overall weak interactions between Gd-L5 and physiological anions, and the high selectivity for the ligand over physiological metals suggest that the Gd(III) complex and its relaxivity should be unaffected in human serum. The NMRD (Nuclear Magnetic Relaxation Dispersion) profiles of 0.25 mM Gd-L5 in human serum at 25 °C, and for comparison, in water at 25 °C are shown in Figure 8. The proton relaxation enhancement, ε*,2 of Gd-L5 in human serum is 1.76 at 20 MHz. Since there is no significant interactions between the Gd(III) complex and physiological cations and anions there is no decrease in relaxivity. The observed increase in relaxivity is consistent with previous reports of weak interactions between the small PEG chain and plasma proteins.26–28 The adducts of the Gd(III) complex with the proteins behave as macromolecules which tumble significantly slower; the resulting longer τR thus increases the relaxivity of the complex.
Figure 8.
1H Nuclear Magnetic Relaxation Dispersion profiles of 0.25 mM Gd-L5 in water and human serum at 25 °C. The data are corrected for the diamagnetic contributions of water and serum, respectively..
Relaxivity of Gd(III) complexes
Hydroxypyridinone/terephthalamide Gd(III) complexes exchange water rapidly (kex ~ 108 s−1) through an associative interchange mechanism, suggesting that the eight and nine coordination states are close in energy.29 This model is supported by the structure of the LaIII analogue of TREN-1-Me-3,2-HOPO which crystallized as a dimer, with one La being eight-coordinate and the other nine.14 The crystal structure of Gd-TREN-1-Me-3,2-HOPO indicates that filling the open coordination site of Gd(III) with a third water molecule would result in minimal distortion of the complex.13 This indicates that it would be possible to stabilize the nine coordination state to achieve q=3 complexes which would still maintain high stability. Indeed, we have shown that grafting a primary amine on the terminal acid of the terephthalamide moiety facilitates the coordination of a third water molecule on the Gd(III) center.30 This favorable stabilization of the nine coordination state of the Gd(III) center is probably due to a hydrogen-bonding network that can, in theory, be achieved by any hydrogen-bond acceptor such as alcohols and carboxylates.
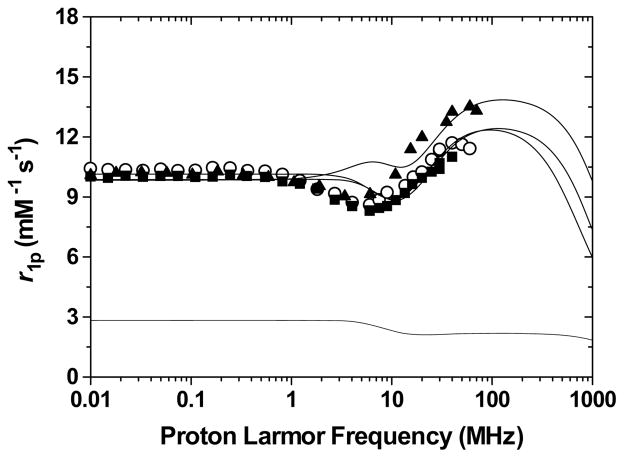
The Nuclear Magnetic Relaxation Dispersion (NMRD) profiles of the −1 charged poly(ethylene-glycol) and alcohol terminated heteropodal complexes, Gd-L4, Gd-L5, and Gd-L6 are shown in Figure 9. The parameters obtained from the refinement of these profiles are given in Table 5. Although it has a shorter rotational correlation time, the smaller alcohol-terminated complex displays higher relaxivity than either PEG derivatives, indicating that it has a higher number of inner sphere water molecules. This increase in coordination number for the alcohol-terminated complex is probably due to a hydrogen-bonding network similar to that of Gd-L7.30 As shown in Figure 10, the ethylene bridge to the alcohol enables its oxygen to intramolecularly hydrogen-bond to the amide proton via a stable five-membered ring. This amide proton is already hydrogen-bonded via a six-membered ring to the catechol oxygen such that intermolecular hydrogen-bonding of the alcohol to a water molecule brings the water very close to the Gd(III) center and facilitates its coordination. Intermolecular hydrogen-bonding to water should be much weaker for the PEG derivatives, which therefore maintain a q = 2, eight-coordinate ground state.
Figure 9.
Nuclear Magnetic Relaxation Dispersion profiles of Gd-L4 (open circles; dotted line), Gd-L5 (filled squares), and Gd-L6 (filled triangles). The best fitting curve for Gd-L4 was calculated with a model that considers a contribution of second hydration sphere water molecules (lower dotted line): q′=4, r′=3.9 Å, τR′=70 ps. Experimental conditions: 25 °C and pH = 7.4.
Table 5.
| Gd complex | L1 | L2 | L3 | L4 | L5 | L6 | L7 | L8 | L9 |
|---|---|---|---|---|---|---|---|---|---|
| charge | −3 | −2 | −2 | −1 | −1 | −1 | −1 | 0 | +1 |
| MW (g/mol) | 896 | 852 | 781 | 1323 | 957 | 824 | 823 | 866 | 910 |
| r1p (20 MHz) | 13.20 | 13.45 | 7.30 | 10.24 | 9.94 | 12.28 | 11.10 | 9.70 | 8.96 |
| Δ2 (1020 s−1) | 1.5 (2) | 1.1 (2) | 1.5 (4) | 0.9 (1) | 1.2 (1) | 1.6 (2) | 1.3 (2) | 1.4 (1) | 1.3 (1) |
| τV (ps) | 18 (3) | 20 (2) | 19 (3) | 21 (2) | 19 (2) | 20 (1) | 19.0 (9) | 19.0 (4) | 20.0 (9) |
| τm (ns) | 6.0 (8)b | 4.5 (6)b | 10c | 20c | 20c | 10c | 2.6 (9)b | 3.0c | 2.1 (8)b |
| τR (ps) | 135 (4) | 124 (2) | 94 (3) | 234 (7) | 145 (4) | 109 (4) | 110 (5) | 114 (3) | 127 (3) |
| q | 3 | 3 | 2 | 1 | 2 | 3 | 3 | 2.4d | 2 |
| Δ HM# (kJ/mol) | 19 (1) | 17 (1) | / | / | / | / | 14 (1) | d | 17 (1) |
| ΔHv# (kJ/mol) | 5 (2) | 3 (1) | / | / | / | / | 1 | 1 | 3 |
| A/ħ (106 rad/s) | −3.6 (1) | −3.5 (2) | / | / | / | / | −3.6 (1) | d | −3.6 (1) |
Experimental conditions: 25 °C, pH = 7.40;
for the parameters r (distance between the gadolinium ion and the protons of the bound water molecules), D (relative diffusion coefficent) and a (distance of closest approach of outer sphere water molecules to the paramagnetic metal ion) the standard values of 3.0 Å, 2.24×10−5 cm2 s−1 and 4.0 Å were used, respectively;
measured by 17O VT VMR;
fixed;
see ref. 28.
Figure 10.
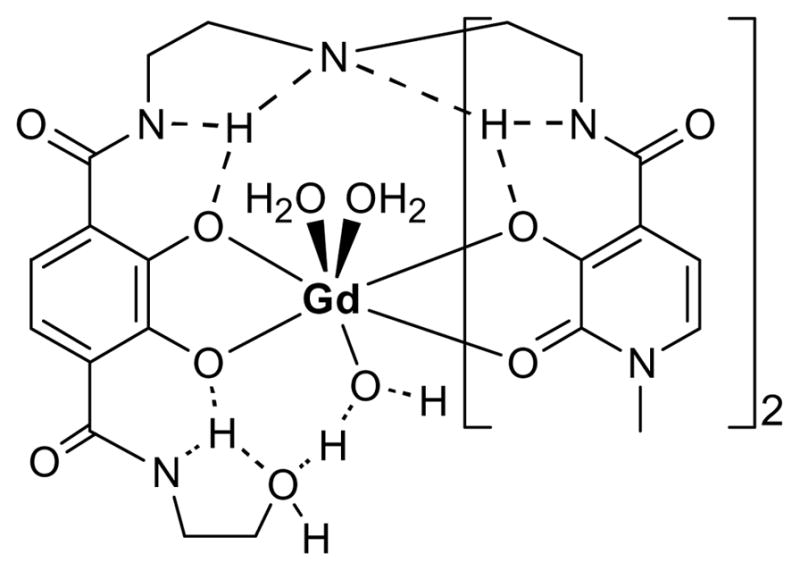
Proposed hydrogen-bonding network to a third water molecule resulting in the stabilization of the 9-coordinate geometry at for Gd-L6.
The relaxivity of the 12 ether-long PEG derivative Gd-L4 is comparable to that of the 4 ether-long one Gd-L5, and this is despite a longer rotational correlation time resulting from its 1.4 times bigger molecular weight. Its lower than expected relaxivity is, therefore, a consequence of its lower number of inner sphere water molecule (q = 1). This deduction is in agreement with previous studies26–28 that have demonstrated that long poly(ethylene-glycol) chains almost always displace one water molecule. Moreover, a significant contribution to the total relaxivity results from H-bonded water molecules in the proximity of the paramagnetic center. This contribution corresponds approximately to that of four second-sphere water molecules (q′) located at a distance of ca. 3.9 Å from the Gd(III) center. This effect is probably due to the hydrophilic polymer, which organizes the water molecules by forming a network of hydrogen-bonded solvent molecules around it.28
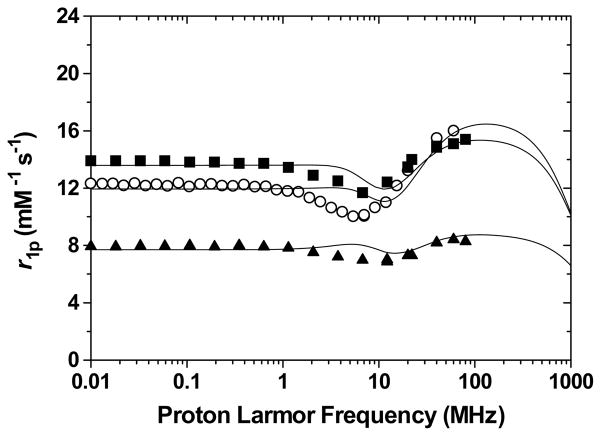
The NMRD profiles of the −2 and −3 charged acid-terminated heteropodal complexes Gd-L1, Gd-L2 and Gd-L3, are shown in Figure 11. The parameters obtained from the refinement of these profiles are given in Table 5. As it is apparent from the NMRD profile, the amino acid derivatives demonstrate much higher relaxivity, corresponding to q = 3, than the non-functionalized q = 2 Gd-L3. Again, the higher coordination number of the alanine and aspartic derivatives can be explained by an intra- and intermolecular hydrogen-bonding network which stabilizes the coordination of a third water molecule on the Gd(III) center (Figure 12). Although the carboxylate of Gd-L3 is capable of hydrogen-bonding a solvent molecule, it is sterically not close enough to the metal center to be able to stabilize the coordination of a third water molecule.
Figure 11.
Nuclear Magnetic Relaxation Dispersion profiles of Gd-L1 (open circles), Gd-L2 (filled squares), and Gd-L3 (filled triangles). Experimental conditions: 25 °C and pH = 7.4.
Figure 12.
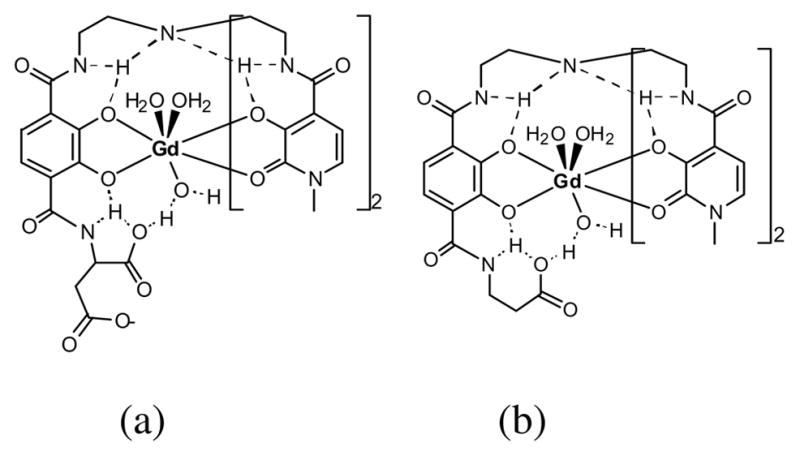
Proposed hydrogen-bonding network to a third water molecule resulting in the stabilization of the 9-coordinate geometry at for (a) Gd-L1 and (b) Gd-L2.
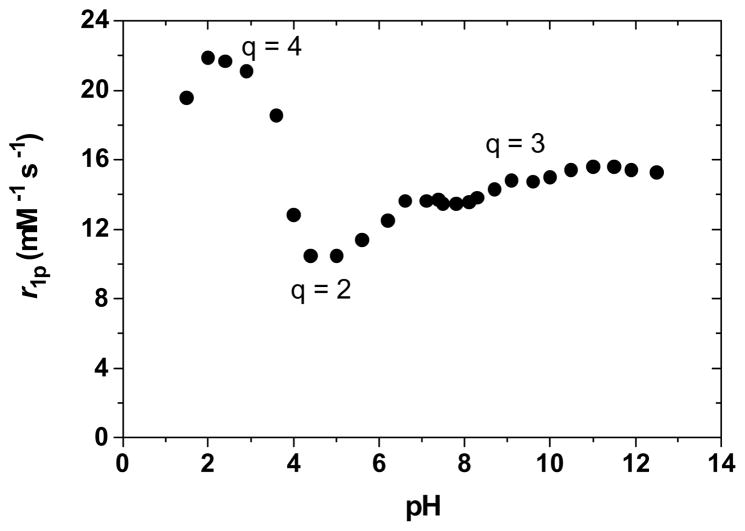
The pH dependence of the relaxivity of the β-Alanine derivative (Gd-L2), shown in Figure 13, is very similar to that of the mono-amine substituted complex;30 this further supports the theory of the hydrogen-bonding network. The relaxivity remains constant between pH 13 and 7, corresponding to the nine-coordinate, q = 3 form. It then decreases by about a third, to presumably a q = 2 complex at pH 4, before increasing again at pH 3 to the partially dissociated q = 4 form. This pH of 4 corresponds to the typical pKa of carboxylic acids such as the one of the terminal substituent. The protonated form of the acid, however, does not have the same hydrogen-bonding network as the deprotonated one. It may therefore no longer hydrogen-bond and stabilize the coordination of a third water molecule on the Gd(III) center. The resulting neutral complex is thus only a eight-coordinate (q = 2) species with lower relaxivity. As the pH decreases to 3, the TAM podand is protonated; as it dissociates from the Gd(III), it is replaced by two water molecules, resulting in a two-fold increase in relaxivity. The relaxivity then decreases toward pH 1 as the complex completely dissociates.
Figure 13.
Effect of pH on the longitudinal relaxivity, r1p, of Gd-L2. Experimental conditions: 25 °C and 20 MHz.
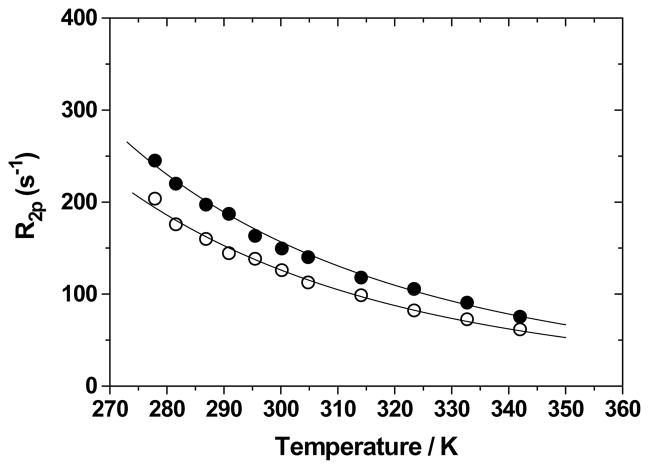
The temperature dependences of the paramagnetic contribution to the water 17O NMR transverse relaxation rate (R2p) for the two acid derivatives are shown in Figure 14. The curves of both complexes are characterized only by the fast exchange region where R2p decreases with temperature, a behavior associated with short τM values. Furthermore, since these complexes have a nine-coordinate ground state, the mechanism of exchange is probably dissociative. The refinement parameters for these dependences, given in Table 5, indicate that the water residence mean lifetimes of the −2 charged alanine derivative Gd-L2 and of the −3 charged aspartic derivative Gd-L1 are of the order of few ns at 298 K and rather similar to those of the −1 and +1 charged amine derivatives Gd-L7 and Gd-L9, respectively. These results might be an indication that the three water molecules in the nine-coordinate ground state are more sterically destabilized and their mean lifetimes remain very short. Then, all these complexes possess a very similar rate of water exchange, regardless of their charge and type of coordination ground state, confirming that in the HOPO family of complexes the eight- and nine-coordinate states are very close in energy and small perturbation in the ligand structure may change the nature of the ground state. Recently, an even shorter τM value was reported for a −3 charged Gd(III) complex were the exchange occurs via a dissociative mechanism and this was also explained on the basis of a very small energy gap between the nine-coordinate ground state and the eight-coordinate transition state.31
Figure 14.
Temperature dependence of the paramagnetic contribution to the water 17O NMR transverse relaxation rate (R2p) for Gd-L1 (17 mM; filled circles) and Gd-L2 (14 mM; open circles). Experimental conditions: pH 7.03, 2.1 T.
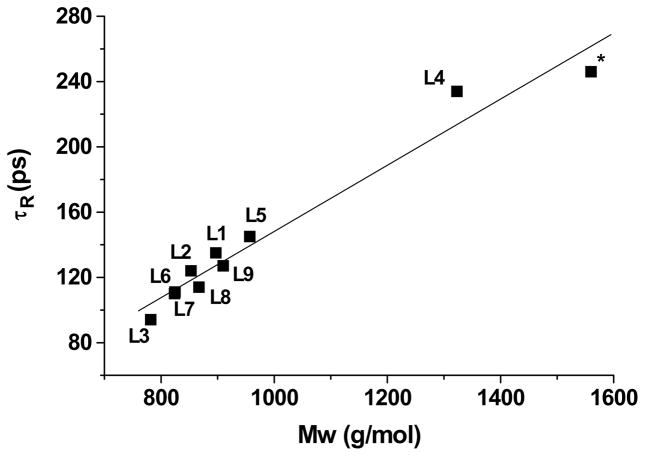
One method to ensure that the number of coordinated water molecules, q, has been assigned correctly it to graph the rotational correlation time, τR, versus the molecular weight (Figure 15). This linear relationship, described by the Debye-Stokes equation (Equation 1) is independent of the number of inner-sphere water molecules.
Figure 15.
Rotational correlation time, τR, versus molecular weight for Gd-TREN-bisHOPO-TAM derivatives (R=0.981 for the straight line). The data labelled with an asterisk refers to a dendritic complex reported in ref. 9. Note that the fact that L4 is above the line supports our view that a significant number of water molecules are H-bonded to the PEG chain and contribute to the relaxivity but also result in a higher effective Mw.
| Equation 1 |
In this correlation, kB is Boltzmann’s constant, T is the temperature, reff is the radius of the molecule in the medium considered, and η is the microviscosity which can be considerably different from the macroviscosity of the solution. At first approximation, which is for rigid spherical molecules with negligible internal motion such as small complexes, the volume r3eff is linearly proportional to the molecular weight of the compound, and hence so is τR.
In the refinement of a NMRD profile, the effect of q is inversely proportional to that of τR. If the value of q is set too low, then in order to compensate for it, τR would be refined to a value too high by the same ratio (and vice versa). For instance, initial refinement of the NMRD profile of Gd-L5 suggested that the complex comprised one inner sphere water molecules. However, this refinement led to a rotational correlation time of 240 ps. Since the complex has a MW = 957 g/mol, this was clearly an outlier in the τR vs. Mw graph, indicating that the parameters were not refined correctly. Refining the profile with q = 2 resulted in a τR = 145 ps which is in agreement with the Debye-Stokes relationship indicating that the Gd(III) center most probably coordinates two water molecules. As can be seen in Figure 15, since all complexes follow this linear relationship, the numbers of inner sphere water molecules are in all probability assigned correctly.
Conclusion
The solution thermodynamics and relaxometric properties of nine heteropodal complexes bearing differently charged water solubilizing substituents have been presented. The complexes maintain the same TREN-bisHOPO-TAM chelating scaffold, which enabled the direct study of the effects of the charged substituents. The stability of each complex was determined by competition titration against the commercial agent DTPA. The charge of the substituent has a significant impact on the stability of the Gd(III) complex: the closer it is to neutral, the more stable it is. In particular, the addition of negative charges through functionalization of the TAM podand with acid moieties significantly decreases the stability of the complex by several orders of magnitude.
The affinities of physiologically relevant anions for both a negatively and a positively charged heteropodal complex have been presented. Only two anions: phosphate and oxalate, coordinate the Gd(III) and partially replace the inner-sphere water molecules. The affinities for these anions are, however, very weak, with the negatively charged complex displaying lower affinity than the positively charged one. The association constants of the ternary adducts are comparable to that of other q = 1 commercial contrast agent and are significantly lower than those of other q = 2 complexes. Consequently the relaxivity, and thus, the contrast ability, of the heteropodal complexes are not affected by human serum.
The relaxivity of the heteropodal complexes, including their NMRD profile, pH dependence and water exchange rates have also been presented. The nature of the substituent can favorably stabilize the coordination of a third water molecule on the Gd(III) center and lead to a nine-coordinate ground state. Such complexes that attain q = 3 incorporate a substituent β to the terminal amide of the TAM podand which are hydrogen-bond acceptors, suggesting that the third water molecule is coordinated to the metal center through a hydrogen-bonding network. These substituents include alcohols, primary amines, and acids. Since this q = 3 can be achieved without destabilizing the complex, the significantly higher relaxivity of these small complexes makes them promising candidates for the development of second-generation contrast agents.
Experimental Section
Solution Thermodynamics
Relative Stability by Competition Titration
Varying volumes of DTPA stock solution were added to solutions of identical concentrations of ligand (L), standardized metal (Gd(III), Cu(II) or Zn(II)), electrolyte (KCl), HEPES buffer (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pKa = 7.5), and MES buffer (4-morpholineethanesulfonic acid, pKa = 7.5). All solutions were brought to pH 7.40 ± 0.01 by the addition of standardized 0.1 M KOH and diluted to identical volumes. A molar ratio of 1:1 for Gd(III):ligand, and molar ratios of 1:0.5 up to 1:2000 for ligand:C.L. were used. Typical final concentrations were 50 μM of ligand and metal, 0.1 M KCl, 0.5 mM MES, and 0.7 mM HEPES. All samples were equilibrated in a thermostatic shaker at 25 °C for at least 48 h. The UV-visible spectrum of each solution was measured on a Cary 300 (Varian) using a 1-cm quartz cell. A solution of DTPA at identical concentration and pH was used as a background. The concentrations of free and complexed ligand in each solution were averaged over 100 wavelengths between 325 nm and 375 nm by using solutions of L and GdL at identical conditions (concentrations, pH, electrolyte and buffer concentration) as references.
Affinities for Physiological Anions
Stock solutions (0.2000 M) of each anion (glycine, monopotassium L-glutamate, monopotassium L-aspartate, potassium oxalate, potassium citrate tribasic, L-malic acid, L-lactic acid, potassium acetate, potassium bicarbonate, potassium phosphate dibasic, potassium fluoride, all from Aldrich 99.9%) were prepared by dissolving a precisely weighed mass of the respective acid or potassium salt in degassed Millipore water and neutralizing it to pH 7.40 with standardized 0.1000 M KOH or standardized 0.1000 M HCl. Stock solutions (1.00 mM GdL) of each GdL complex were prepared by dissolving a precisely weighed mass of the respective ligand (L5 and L9) with a precise volume of standardized Gd(III) stock solution in degassed Millipore water and neutralizing it to pH 7.40 with standardized 0.1000 M KOH. A molar ratio of ligand to Gd(III) of 1.05:1 was used to ensure complete complexation of the Gd(III). Equal volumes of the GdL and the anion stock solutions were mixed and let to equilibrate for one week (typical final concentrations were 0.500 mM GdL and 0.100 M anion). The relaxivity at 20 MHz (vide infra) of the complex in the presence of each anion was compared to that in pure water at identical concentration and pH. If a significant interaction was observed, the GdL/anion solution was titrated with incremental addition of the pure GdL complex at identical concentration and pH, and the relaxivity at 20 MHz measured after each addition.
Relaxivity Measurements
1H NMR
The longitudinal water proton relaxation rate at 20 MHz was measured by using a Spinmaster spectrometer (Stelar, Mede, Italy) operating at 0.47 T; the standard inversion-recovery technique was employed (16 experiments, 4 scans). A typical 90° pulse width was 3.5 ms, and the reproducibility of the T1 data was ± 0.5%. The temperature was controlled with a Stelar VTC-91 air-flow heater equipped with a copper-constantan thermocouple (uncertainty of ± 0.1 °C). The proton 1/T1 NMRD profiles were measured on a Stelar fast field-cycling FFC-2000 (Mede, Pv, Italy) relaxometer on about 0.25–1.0 mmol gadolinium solutions in non-deuterated water. The relaxometer operates under computer control with an absolute uncertainty in 1/T1 of ± 1%. The NMRD profiles were measured in the range of magnetic fields from 0.00024 to 1.6 T (corresponding to 0.01–70 MHz proton Larmor frequencies). Details of the instrument and of the data acquisition procedure are given elsewhere.14 Samples in human serum were prepared by dissolving lyophilized human serum (Sigma-Aldrich) in 5 mL of a Gd(III) complex of known concentration.
17O NMR
Variable-temperature 17O NMR measurements were recorded on JEOL EX-90 (2.1 T) spectrometer equipped with a 5 mm probe. A D2O external lock and solutions containing 2.0% of the 17O isotope (Cambridge Isotope) were used. The observed transverse relaxation rates were calculated from the signal width at half-height. Other details of the instrumentation, experimental methods, and data analysis have been previously reported.14
Supplementary Material
Acknowledgments
The research (UCB) was supported by the NIH (grant HL69832), NATO Travel Grant (PST.CLG.980380), and an unrestricted research gift from Schering AG.
Footnotes
Supporting Information Available. Detailed experimental procedures and characterization data for the synthesis of the ligands and complexes, detailed method for the determination of pM values, and NMRD profile showing second sphere contributions of Gd-L4. This information is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Paper no. 19 in the series “High Relaxivity Gadolinium MRI Agents”; for the previous paper in the series see Puerta DT, Botta M, Jocher CJ, Werner EJ, Avedano S, Raymond KN, Cohen SM. J Am Chem Soc. 2006;128:2222–2223. doi: 10.1021/ja057954f.
- 2.Merbach AE, Tóth E. Wiley. The Chemistry of Contrast Agents. Chichester: 2001. [Google Scholar]
- 3.Laus S, Ruloff R, Toth E, Merbach AE. Chem-Eur J. 2003;9:3555–3566. doi: 10.1002/chem.200204612. [DOI] [PubMed] [Google Scholar]
- 4.Ruloff R, Toth E, Scopelliti R, Tripier R, Handel H, Merbach AE. Chem Comm. 2002:2630–2631. doi: 10.1039/b207713b. [DOI] [PubMed] [Google Scholar]
- 5.Wang YM, Lee C, Liu Hl, Sheu GCRS. Dalton Trans. 1998;24:4113–4118. [Google Scholar]
- 6.Cheng TH, Wang YM, Lin KT, Liu GC. Dalton Trans. 2001;22:3357–3366. [Google Scholar]
- 7.Raymond KN, Pierre VC. Bioconjugate Chem. 2005;16:3–8. doi: 10.1021/bc049817y. [DOI] [PubMed] [Google Scholar]
- 8.Pierre VC, Melchior M, Doble DMJ, Raymond KN. Inorg Chem. 2004;43:8520–8525. doi: 10.1021/ic0493447. [DOI] [PubMed] [Google Scholar]
- 9.Doble DMJ, Melchior M, O’Sullivan B, Siering C, Xu J, Pierre VC, Raymond KN. Inorg Chem. 2003;42:4930–4937. doi: 10.1021/ic026240s. [DOI] [PubMed] [Google Scholar]
- 10.Pierre VC, Botta M, Raymond KN. J Am Chem Soc. 2005;127:504–505. doi: 10.1021/ja045263y. [DOI] [PubMed] [Google Scholar]
- 11.Pierre VC, Botta M, Aime S, Raymond KN. Fe(III) templated Gd(III) self-assemblies. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajela S, Botta M, Giraudo S, Xu J, Raymond KN, Aime S. J Am Chem Soc. 2000;122:11228–11229. [Google Scholar]
- 13.Xu J, Franklin SJ, Whisenhunt DW, Raymond KN. J Am Chem Soc. 1995;117:7245–7246. [Google Scholar]
- 14.Cohen SM, Xu J, Radkov E, Raymond KN, Botta M, Barge A, Aime S. Inorg Chem. 2000;39:5747–5756. doi: 10.1021/ic000563b. [DOI] [PubMed] [Google Scholar]
- 15.Dickins RS, Aime S, Batsanov AS, Beeby A, Botta M, Bruce J, Howard JAK, Love CS, Parker D, Peacock RD, Puschmann H. J Am Chem Soc. 2002;124:12697–12705. doi: 10.1021/ja020836x. [DOI] [PubMed] [Google Scholar]
- 16.Aime S, Botta M, Bruce JI, Mainero V, Parker D, Terreno E. Chem Commun. 2001:115–116. [Google Scholar]
- 17.Bruce JI, Dickins RS, Govenlock LJ, Gunnlaugsson T, Lopinski S, Lowe MP, Parker D, Peacock RD, Perry JJB, Aime S, Botta M. J Am Chem Soc. 2000;122:9674–9684. [Google Scholar]
- 18.Aime S, Barge A, Batsanov AS, Botta M, Castelli DD, Fedeli F, Mortillaro A, Parker D, Puschmann H. Chem Commun. 2002:1120–1121. doi: 10.1039/b202862j. [DOI] [PubMed] [Google Scholar]
- 19.Terreno E, Boniforte P, Botta M, Fedeli F, Milone L, Mortillaro A, Aime S. Eur J Inorg Chem. 2003:3530–3533. doi: 10.1021/ic034321y. [DOI] [PubMed] [Google Scholar]
- 20.Aime S, Gianolio E, Barge A, Kostakis D, Plakatouras IC, Hadjiliadis N. Eur J Inorg Chem. 2003:2045–2048. [Google Scholar]
- 21.Zhang SR, Merritt M, Woessner DE, Lenkinski RE, Sherry AD. Acc Chem Res. 2003;36:783–790. doi: 10.1021/ar020228m. [DOI] [PubMed] [Google Scholar]
- 22.Cacheris WP, Quay SC, Rocklage SM. Magn Reson Imaging. 1990;8:467–481. doi: 10.1016/0730-725x(90)90055-7. [DOI] [PubMed] [Google Scholar]
- 23.Wedeking P, Kumar K, Tweedle MF. Magn Reson Imaging. 1992;10:641–648. doi: 10.1016/0730-725x(92)90016-s. [DOI] [PubMed] [Google Scholar]
- 24.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chem Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 25.Botta M, Aime S, Barge A, Bobba G, Dickins RS, Parker D, Terreno E. Chem-Eur J. 2003;9:2102–2109. doi: 10.1002/chem.200204475. [DOI] [PubMed] [Google Scholar]
- 26.Doble DMJ, Botta M, Wang J, Aime S, Barge A, Raymond KN. J Am Chem Soc. 2001;123:10758–10759. doi: 10.1021/ja011085m. [DOI] [PubMed] [Google Scholar]
- 27.Thompson MK, Doble DMJ, Tso LS, Barra S, Botta M, Aime S, Raymond KN. Inorg Chem. 2004;43:8577–8586. doi: 10.1021/ic048607u. [DOI] [PubMed] [Google Scholar]
- 28.Botta M, Quici S, Pozzi G, Marzanni G, Pagliarin R, Barra S, Crich SG. Org Biomol Chem. 2004;2:570–577. doi: 10.1039/b313677a. [DOI] [PubMed] [Google Scholar]
- 29.Thompson MK, Botta M, Nicolle G, Helm L, Aime S, Merbach AE, Raymond KN. J Am Chem Soc. 2003;125:14274–14275. doi: 10.1021/ja037441d. [DOI] [PubMed] [Google Scholar]
- 30.Pierre VC, Botta M, Aime S, Raymond KN. J Am Chem Soc. 2006;128:5344–5345. doi: 10.1021/ja057805x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mato-Iglesias M, Platas-Iglesias C, Djanashvili K, Peters JA, Tóth E, Balogh E, Muller RN, Vander Elst L, de Blas A, Rodríguez-Blas T. Chem Commun. 2005:4729–4731. doi: 10.1039/b508180g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.