Abstract
Impairments of endothelin receptor B (Ednrb/EDNRB) cause the development of Waardenburg-Shah syndrome with congenital hearing loss, hypopigmentation, and megacolon disease in mice and humans. Hearing loss in Waardenburg-Shah syndrome has been thought to be caused by an Ednrb-mediated congenital defect of melanocytes in the stria vascularis (SV) of inner ears. Here we show that Ednrb expressed in spiral ganglion neurons (SGNs) in inner ears is required for postnatal development of hearing in mice. Ednrb protein was expressed in SGNs from WT mice on postnatal day 19 (P19), whereas it was undetectable in SGNs from WT mice on P3. Correspondingly, Ednrb homozygously deleted mice (Ednrb−/− mice) with congenital hearing loss showed degeneration of SGNs on P19 but not on P3. The congenital hearing loss involving neurodegeneration of SGNs as well as megacolon disease in Ednrb−/− mice were markedly improved by introducing an Ednrb transgene under control of the dopamine β-hydroxylase promoter (Ednrb−/−;DBH-Ednrb mice) on P19. Neither defects of melanocytes nor hypopigmentation in the SV and skin in Ednrb−/− mice was rescued in the Ednrb−/−;DBH-Ednrb mice. Thus, the results of this study indicate a novel role of Ednrb expressed in SGNs distinct from that in melanocytes in the SV contributing partially to postnatal hearing development.
Keywords: G Protein-coupled Receptors(GPCR), Gene Knockout, Neurodegeneration, Neurons, Transgenic, Congenital Hearing Loss, Endothelin Receptor B, Spiral Ganglion Neuron, Waardenburg-Shah Syndrome
Introduction
Waardenburg syndrome (WS)3 involves hearing loss and hypopigmentation. The incidence of WS is 1/10,000–20,000 people (1). Waardenburg-Shah syndrome (WS type IV, WS-IV), caused by mutations of the transcription factor Sox10 (2), the cytokine endothelin (ET)-3 (3) and its receptor endothelin receptor B (Ednrb) (4), is characterized by hypopigmentation, megacolon disease and hearing loss.
Endothelin receptor B (Ednrb/EDNRB) belongs to the G protein-coupled receptor family, mediating pleiotropic actions of endothelins (5, 6). ET-1, ET-2, and ET-3 are ligands for Ednrb with equal affinity (6, 7). Impairments of Ednrb/EDNRB have been shown to cause embryonic defects of melanocytes and enteric ganglion neurons derived from the neural crest, resulting in hypopigmentation, megacolon disease, and congenital hearing loss. In rodent models, piebald-lethal rats in which Ednrb is spontaneously mutated (8) and Ednrb homozygous knock-out (Ednrb−/−) mice (9) have been shown to have typical WS-IV phenotypes. Thus, these previous studies indicate that Ednrb is one of the key regulatory molecules for embryonic development of melanocytes and peripheral neurons including neurons in the enteric nervous system.
The inner ears contain the organ of Corti and stria vascularis (SV). The organ of Corti, which contains two kinds of sensory cells (inner hair cells and outer hair cells), is responsible for mechanotransduction, by which sound impulses are converted into neural impulses. Auditory information from the sensory cells is transmitted to spiral ganglion neurons (SGNs) as the primary sensory carrier for the auditory system, followed by eventual transmission to the auditory cortex (10, 11). Impairments of SGNs have been shown to cause hearing loss (12). Our recent study has also shown that c-Ret-mediated degeneration of SGNs directly causes severe congenital hearing loss (13). The SV consists of marginal cells, melanocytes (also known as intermediate cells), and basal cells and has been shown to maintain high levels of potassium ion for endocochlear potential (EP) (14, 15). Melanocytes in the inner ear are specifically located in the SV, and these defects lead to impaired EP levels resulting in hearing loss (16). Thus, disturbance of these constituent cells in inner ears has been shown to cause hearing losses (10).
Dopamine β-hydroxylase (DBH) is an enzyme that converts the neurotransmitter dopamine to noradrenaline. DBH has been using as a specific marker of noradrenergic/adrenergic neurons because noradrenaline converted by DBH is secreted as a neurotransmitter from noradrenergic/adrenergic neurons. DBH promoter has been used as a valuable tool to allow a target gene to be expressed in peripheral neurons derived from the neural crest in vivo (17). A previous study showed that aganglionic megacolon disease in Ednrb homozygously deleted mice (Ednrb−/− mice) was recovered by the introduction of an Ednrb transgene driven by the human DBH promoter (Ednrb−/−;DBH-Ednrb mice) (18). However, there is no information about hearing levels in Ednrb−/−;DBH-Ednrb mice. Previous studies have shown that endogenous DBH is expressed in SGNs of inner ears (19), whereas neither endogenous DBH nor a transgene driven by the DBH promoter is expressed in melanocytes or their precursors (17, 20). Thus, these results of previous studies suggest that the DBH promoter enables Ednrb protein to be specifically expressed in SGNs.
Previous studies demonstrated that impairments of Ednrb/EDNRB cause hypopigmentation and megacolon disease due to defects of melanocytes and peripheral neurons such as enteric ganglion neurons, respectively (4–6, 8, 9). There has been only one report showing that Ednrb-mediated hearing loss involved a congenital defect of melanocytes in the SV (9). However, there was no information in that report about the role of Ednrb in SGNs, which serve as peripheral neurons in inner ears for the auditory system, although it was shown in the present study that Ednrb protein is expressed in SGNs. The results of the present study indicate a novel etiology for Ednrb-mediated hearing loss in mice that can involve postnatal degeneration of SGNs besides congenital defects of melanocytes in the SV.
EXPERIMENTAL PROCEDURES
Mice
Ednrb−/− mice (5) and Ednrb−/−;DBH-Ednrb mice (18) were reported previously. All experiments were authorized by the Institutional Animal Care and Use Committee in Chubu University (approval number 2110017) and the Institutional Recombinant DNA Experiment Committee in Chubu University (approval number 06-01) and followed the Japanese Government Regulations for Animal Experiments.
Measurement of Hearing
Tone burst-evoked auditory brainstem response (ABR) measurements (AD Instruments Pty. Ltd.) were performed as described previously (13, 21). Tone burst stimuli were measured 5 dB stepwise from 0 decibel sound pressure level (dB SPL) to 70 or 90 dB SPL. The threshold was obtained by identifying the lowest level of the I wave of ABR recognized. Data are presented as means ± S.E.
Morphological Analysis with a Light Microscope
After perfusion fixation by Bouin's solution, cochleae from postnatal day 19 (P19) mice were immersed in the same solution for 1 week. H&E staining and immunohistochemical analyses with anti-Ednrb (1:2000; Chemicon) and anti-Kir4.1 (1:500; Santa Cruz Biotechnology) antibodies were performed with paraffin sections. The VECTASTAIN ABC kit (Vector) and Envision kit/HRP (diaminobenzidine; DAKO) were used in the immunohistochemical analyses with a hematoxylin counterstain. In the case of anti-Kir4.1, the Vector VIP substrate kit for peroxidase (Vector) was used with counterstained methyl green. LacZ staining of dopachrome tautomerase (Dct)-LacZ melanocytes was performed as described previously (13). In brief, after fixation with PBS containing 0.25% glutaraldehyde, the inner ears were stained with 0.04% X-Gal by intracochlear perfusion. The samples were postfixed with 4% paraformaldehyde and decalcified with EDTA, and then paraffin sections were prepared. Estimation of cell density of SGNs with H&E staining basically followed the previous method (13, 22–24). In brief, the area of Rosenthal's canal in three sections from each mouse was measured with the software program WinROOF (version 6.2; Mitani Corp., Fukui, Japan) as reported previously (13, 24). Cell density of SGNs from three mice for each mouse strain was calculated by dividing the cell number of SGNs in the measured Rosenthal's canal by the area of the section examined. A total of 100–150 SGNs in three sections from each mouse were examined. Percentage of positive signals histochemically detected by antibodies or LacZ staining was estimated with WinROOF (version 6.2) as reported previously (13, 24). Briefly, the number of positive SGNs was divided by the total number of SGNs. A total of 100–150 SGNs in five sections from each mouse were examined. In the case of SV, positive signals in the measured SV were divided by the area of the section measured. To compare the positive levels among mouse strains, the normalized positive signals in Ednrb−/− mice and Ednrb−/−;DBH-Ednrb mice were divided by the normalized positive signals in WT mice. Rosenthal's canal or SV from three or four mice for each mouse strain was measured for each estimation.
Morphological Analysis by Transmission Electron Microscopy (TEM)
Preparation of tissues for TEM basically followed the previous method (13, 22). In brief, after perfusion fixation with a mixture of 2% paraformaldehyde and 2% glutaraldehyde in 0.3 m HEPES (pH 7.4), dissected murine cochleae were immersed in the same fixative solution overnight at 4 °C. The cochleae were then fixed with 2% osmium tetroxide in 0.3 m HEPES (pH 7.4) at 4 °C for 3 h. After rinsing off the fixative solution, the cochleae were dehydrated with a graded series of ethanol and embedded in epoxy resin (Quetol 651). Ultrathin sections (t = 70 nm) were observed under an electron microscope at 80 kV (JEOL JEM1200EX, Tokyo, Japan). Additional procedures are described in the supplemental Methods.
Statistics
Significant difference (*, p < 0.01; **, p < 0.05) from the control was analyzed by the Mann-Whitney U test.
RESULTS
Congenital Deafness in Ednrb−/− Mice and Tissue Distribution of Ednrb Protein in Inner Ears
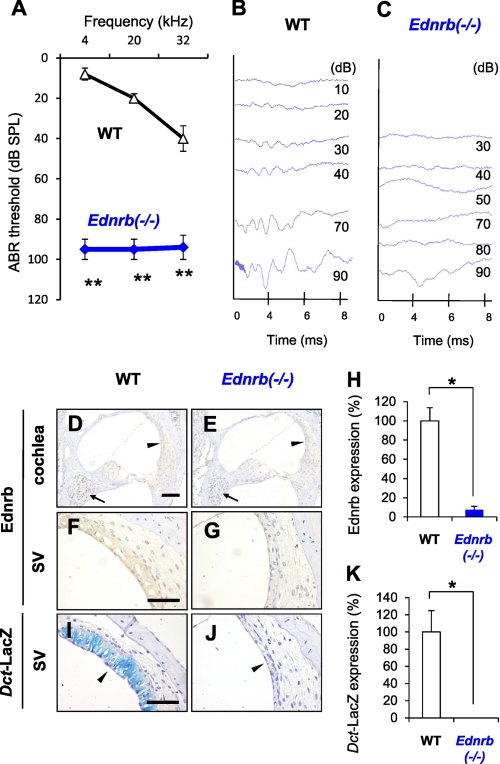
We first measured hearing levels of Ednrb−/− mice (5) and littermate WT mice on P19 (Fig. 1, A–C). ABR thresholds for 4–32-kHz sound in Ednrb−/− mice (90–95 dB SPL) were much higher than those in littermate WT mice (20–55 dB SPL) (Fig. 1A). This result indicates that the Ednrb −/− mice suffer from severe congenital hearing loss. Immunohistochemical analyses of inner ears showed expression of Ednrb protein in SGNs (arrow in Fig. 1D) and the SV (Fig. 1F) in WT mice but not in Ednrb−/− mice (Fig. 1, E, G, and H). Correspondingly, there were no melanocytes in the SV from Ednrb−/− mice (Fig. 1, I–K).
FIGURE 1.
Congenital deafness in Ednrb−/− mice and expression of Ednrb in inner ears. A, hearing levels (means ± S.E. (error bars)) in WT mice (n = 9) and Ednrb−/− mice (n = 9) on P19 measured by ABR. B and C, ABR waveforms of littermate WT mice (WT, B) and Ednrb−/− mice (Ednrb−/−, C) on P19 at 10–90 dB SPL of 12 kHz sound. D–G, immunohistochemical analysis of Ednrb expression in the cochlea (D and E) and the SV (F and G) from Ednrb−/− mice (E and G) and littermate WT mice (D and F) on P19. Arrows and arrowheads in D and E indicate SGNs and the SV, respectively. H, percentage (means ± S.E.) of Ednrb expression in the SV from Ednrb−/− mice (Ednrb−/−, blue bar, n = 3) and littermate WT mice (WT, white bar, n = 3) to that in the SV from WT mice. I and J, LacZ staining of melanocytes in the SV. We employed Dct-LacZ mice, in which the Dct promoter is known as a specific marker of melanocytes (intermediate cells) (31), to establish Dct-LacZ;Ednrb−/− mice newly by crossing Ednrb−/− mice and Dct-LacZ mice (32). K, percentage (means ± S.E.) of LacZ-positive melanocytes in the SV from Ednrb−/− mice (Ednrb−/−, n = 3) and littermate WT mice (WT, white bar, n = 3) to that in the SV from WT mice. LacZ staining showed no positive cells in the SV from Dct-LacZ;Ednrb−/− mice (arrowhead in J and K), whereas Dct-LacZ mice with intact Ednrb showed LacZ-positive melanocytes in the SV (blue signals indicated by arrowhead in I and K). Significant difference (*, p < 0.05; **, p < 0.01) from the control was analyzed by the Mann-Whitney U test. Scale bars: 100 μm (D and E) and 50 μm (F–J).
Neurodegeneration of SGNs in Ednrb−/− Mice
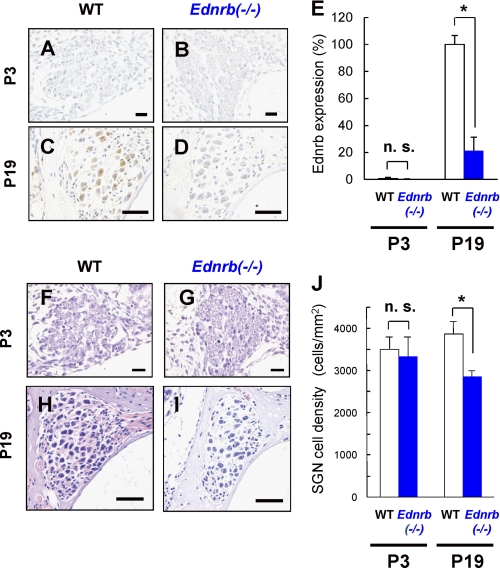
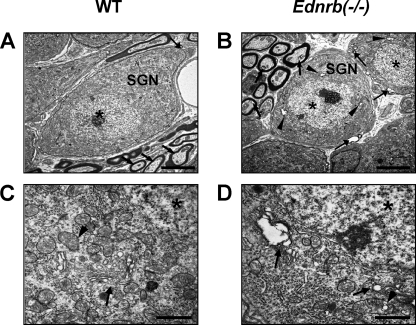
Immunohistochemical analysis of SGNs on P19 showed expression of Ednrb protein in WT mice (Fig. 2C) but not in Ednrb−/− mice (Fig. 2D), whereas it was undetectable in SGNs from WT mice on P3 (Fig. 2, A, B, and E). Ednrb protein was not detectable in hair cells from WT mice (supplemental Fig. S1). Moreover, cell density of SGNs in the basal turn from Ednrb−/− mice was 20–30% lower than that in the basal turn from littermate WT mice on P19 (Fig. 2, H–J) but not on P3 (Fig. 2, F, G, and J). Hair cells in the inner ear showed comparable morphology in WT and Ednrb−/− mice on P19 (supplemental Fig. S2). We further performed detailed morphological analyses of SGNs from Ednrb−/− mice and littermate WT mice on P16 by TEM analysis (Fig. 3). Gap areas (arrows in Fig. 3B) between SGNs (SGN in Fig. 3B) and Schwann cells were observed in Ednrb−/− mice but not in littermate WT mice on P16 (arrow in Fig. 3A). SGNs from Ednrb−/− mice showed vacuolar degeneration of the Golgi apparatus (arrows in Fig. 3D) and mitochondria (arrowhead in Fig. 3D), whereas WT mice showed intact morphology of the Golgi apparatus (arrow in Fig. 3C) and mitochondria (arrowhead in Fig. 3C). Because gaps between SGNs and Schwann cells and vacuolar degeneration have been shown to be neurodegeneration markers (13, 25), our results suggest that decreased cell density of SGNs in Ednrb−/− mice was caused by neurodegeneration.
FIGURE 2.
Decreased cell density of SGNs in Ednrb−/− mice. A–D, immunohistochemical analyses for serial sections of the levels of Ednrb expression in SGNs from Ednrb−/− mice (B and D) and littermate WT mice (A and C) on P3 (A and B) and P19 (C and D). E, percentage of positive-SGN number (means ± S.E. (error bars)) of Ednrb in Ednrb−/− mice (Ednrb−/−, blue bar, n = 3) and littermate WT mice (WT, white bars, n = 3) on P3 and P19 to that in WT mice on P19. F–I, H&E staining in SGNs at the basal turn from Ednrb−/− mice (G and I) and littermate WT mice (F and H) on P3 (F and G) and P19 (H and I). Scale bars: 20 μm (A, B, F, and G) and 50 μm (C, D, H, and I). J, cell density (means ± S.E.) of SGNs from littermate WT mice (white bars) and Ednrb−/− mice (blue bars) on P3 and P19. Significant difference (*, p < 0.05) from the control was analyzed by the Mann-Whitney U test. n.s., not significant.
FIGURE 3.
Neurodegeneration of SGNs in Ednrb−/− mice. TEM of SGNs from Ednrb−/− mice (B and D) and littermate WT mice (A and C) on P16. A and B, gap areas (arrows in B) between SGNs (SGN in B) and Schwann cells were observed in Ednrb−/− mice but not in littermate WT mice (arrow in A). C and D, vacuolar degeneration of the Golgi apparatus (arrows in D) and mitochondria (arrowhead in D) in SGNs from Ednrb−/− mice and intact morphology of the Golgi apparatus (arrow in C) and mitochondria (arrowhead in C) from WT mice. Asterisks indicate nuclei. Scale bars: 5 μm (A and B) and 1 μm (C and D).
Improved Hearing Levels of Ednrb−/−;DBH-Ednrb Mice
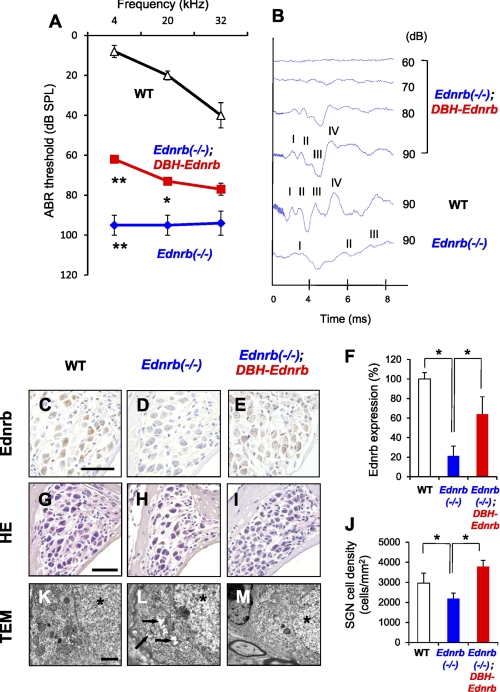
Our results showed not only a defect in melanocytes of the SV (Fig. 1) but also neurodegeneration of SGNs (Fig. 2) in Ednrb−/− mice. Previous studies showed that megacolon disease in Ednrb−/− mice was recovered in Ednrb−/−;DBH-Ednrb mice, suggesting that Ednrb transgene driven by the human DBH promoter recovers development of enteric ganglion neurons (18, 26). To clarify the role of Ednrb expressed in SGNs in neurodegeneration of SGNs, we next crossed Ednrb−/− mice with DBH-Ednrb transgenic mice (18, 26) to develop Ednrb−/−;DBH-Ednrb mice. Comparative analysis of ABR thresholds between Ednrb−/− mice and littermate Ednrb−/−;DBH-Ednrb mice on P19 revealed marked improvement (more than 30 dB SPL at 4 kHz) of hearing levels in Ednrb−/−;DBH-Ednrb mice (Fig. 4A). Latencies of all four ABR waves in Ednrb−/− mice were also recovered in Ednrb−/−;DBH-Ednrb mice (Fig. 4B). We next determined the rescue effect of Ednrb transgene driven by the DBH promoter on SGNs in Ednrb−/− mice. Comparable expression levels of Ednrb in SGNs were observed in Ednrb−/−;DBH-Ednrb mice and littermate WT mice on P19 (Fig. 4, C–F). Cell density of SGNs from Ednrb−/−;DBH-Ednrb mice was also comparable with that of SGNs from littermate WT mice and was significantly higher than that of SGNs from Ednrb−/− mice (Fig. 4, G–J). Gaps between SGNs and Schwann cells and vacuolar degeneration of the Golgi apparatus and mitochondria were nearly undetectable by TEM in Ednrb−/−;DBH-Ednrb mice as well as WT mice (Fig. 4, K–M). These results suggest that not only enteric ganglion neurons but also SGNs in Ednrb−/−;DBH-Ednrb mice were recovered by Ednrb transgene driven by the DBH promoter.
FIGURE 4.
Improvements of hearing levels in Ednrb−/− mice by DBH-Ednrb transgene. A, hearing levels (means ± S.E. (error bars)) in WT (n = 9), Ednrb−/− (n = 9), and Ednrb−/−;DBH-Ednrb mice (n = 12) on P19 measured by ABR. B, ABR waveforms of littermate WT, Ednrb−/−, and Ednrb−/−;DBH-Ednrb mice on P19 at 12-kHz sound. ABR wave peaks correspond to cochlear nerve activity (wave I) and downstream neural activities (waves II-–IV) (33, 34). C–E, immunohistochemical analysis of Ednrb expression in SGNs from WT (C), Ednrb−/− (D), and Ednrb−/−;DBH-Ednrb mice (E) on P19. F, percentage of positive SGN number (means ± S.E.) of Ednrb in Ednrb−/− mice (Ednrb−/−, blue bar, n = 3), Ednrb−/−;DBH-Ednrb mice (Ednrb−/−;DBH-Ednrb, red bar, n = 3) and littermate WT mice (WT, white bar, n = 3) to that in WT mice. G–I, H&E staining in SGNs at the basal turn from WT (G), Ednrb−/− (H), and Ednrb−/−;DBH-Ednrb mice (I) on P19. J, cell density (means ± S.E.) of SGNs from WT, Ednrb−/−, and Ednrb−/−;DBH-Ednrb mice on P19. K–M, TEM of SGNs from WT (K), Ednrb−/− (L), and Ednrb−/−;DBH-Ednrb mice (M) on P16. Vacuolar degeneration in SGNs from Ednrb−/− mice (arrows in L) was not observed in Ednrb−/−;DBH-Ednrb mice (M). Asterisks indicate nuclei (K–M). Scale bars: 50 μm (C–E, G–I), 1 μm (K–M). Significant difference (*, p < 0.05; **, p < 0.01) from the control was analyzed by the Mann-Whitney U test.
Defects of Melanocytes in the SV from Ednrb−/−;DBH-Ednrb Mice
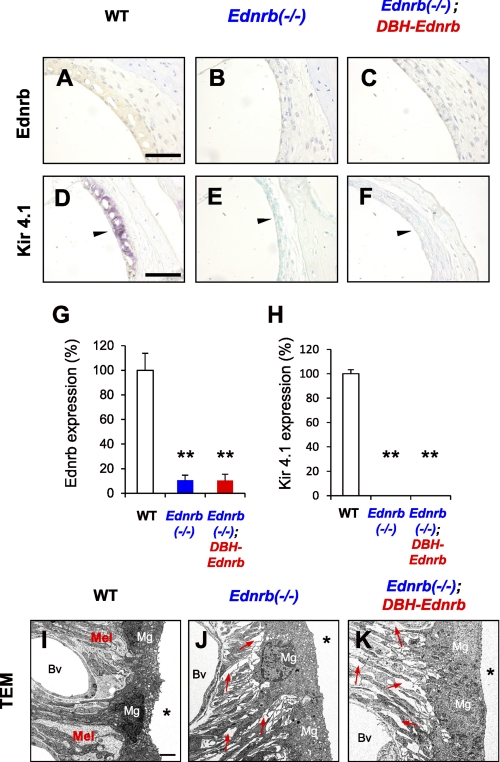
We finally examined whether Ednrb transgene driven by the DBH promoter affects defects of melanocytes in the SV from Ednrb−/− mice. Neither Ednrb-positive cells (Fig. 5, A–C, G) nor Kir4.1-positive cells (Fig. 5, D–F, H), a specific marker of melanocytes (27), were detectable in the SV from Ednrb−/− mice (Fig. 5, B, E, G, and H) and Ednrb−/−;DBH-Ednrb mice (Fig. 5, C, F, G, and H). TEM analysis further revealed that Ednrb−/− mice and Ednrb−/−;DBH-Ednrb mice similarly exhibited no melanocytes and many gap areas (red arrows in Fig. 5, J and K) among marginal cells and blood vessels, whereas WT mice showed melanocytes among marginal cells and blood vessels (Fig. 5I). In addition, EP of Ednrb−/−;DBH-Ednrb mice (79 ± 6 mV) was significantly (p < 0.01) lower than that of WT mice (109 ± 4 mV) (supplemental Fig. S4). These results suggest that defects of melanocytes in the SV from Ednrb−/− mice were not recovered by Ednrb transgene driven by the DBH promoter. Correspondingly, there was no difference in coat color or defects of melanocytes between Ednrb−/− mice and Ednrb−/−;DBH-Ednrb mice (supplemental Fig. S5). These results suggest that Ednrb transgene driven by the DBH promoter did not affect development of melanocytes in the skin and SV.
FIGURE 5.
Melanocyte defect in the SV from Ednrb−/− mice was not rescued in Ednrb−/−;DBH-Ednrb mice. A–C, immunohistochemical analysis of Ednrb expression in the SV from WT mice (A), Ednrb−/− mice (B), and Ednrb−/−;DBH-Ednrb mice (C) on P19. D–F, immunohistochemical analysis of Kir4.1 expression, which is known as one of the melanocyte markers in the SV (27). Kir4.1-expressing cells were found in WT mice (purple signals indicated by arrowhead in D) but were not found in Ednrb−/− mice and Ednrb−/−;DBH-Ednrb mice (E and F). The methods used for staining are described in detail under “Experimental Procedures.” G and H, percentage (means ± S.E. (error bars)) of Ednrb (G) and Kir4.1 (H) expression levels in the SV from Ednrb−/− mice (Ednrb−/−, blue bar, n = 3), Ednrb−/−;DBH-Ednrb mice (Ednrb−/−;DBH-Ednrb, red bar, n = 3) and littermate WT mice (WT, white bar, n = 3) to that in the SV from WT mice. Significant difference (*, p < 0.05; **, p < 0.01) from the control was analyzed by the Mann-Whitney U test. I–K, TEM of the SV from Ednrb−/− mice (J), Ednrb−/−;DBH-Ednrb mice (K), and littermate WT mice (I) on P19. WT mice exhibited melanocytes (Mel in I) among marginal cells (Mg in I) and blood vessels (Bv in I), whereas Ednrb−/− mice and Ednrb−/−;DBH-Ednrb mice exhibited no melanocytes and many gaps (indicated by red arrows in J and K) among marginal cells (Mg in J and K) and blood vessels (Bv in J and K). Asterisk indicates endolymphatic space (I–K). Scale bars: 50 μm (A–F), 2 μm (I–K).
DISCUSSION
This study demonstrated that Ednrb−/− mice had severe congenital deafness (ABR threshold > 90 dB SPL) with not only a defect of melanocytes in the SV (Figs. 1 and 5) but also neurodegeneration of SGNs (Figs. 2 and 3). These results indicate a novel etiology for Ednrb-mediated hearing loss in Ednrb−/− mice that involves degeneration of SGNs, which serve as peripheral neurons in inner ears, besides defects of melanocytes in the SV.
This study showed neurodegeneration of SGNs resulting in decreased numbers of SGNs in Ednrb−/− mice on P19 (Figs. 2 and 3), whereas cell density and morphology of SGNs were comparable in Ednrb−/− mice and WT mice on P3 (Fig. 2). These results suggest that SGNs in Ednrb−/− mice developed normally at least until P3, when the level of Ednrb expression in SGNs from WT mice was undetectable (Fig. 2A). However, SGNs from Ednrb−/− mice no longer survived on P19 (Fig. 2, I and J), when the level of Ednrb expression in SGNs from WT mice was clearly detectable (Fig. 2C). We therefore conclude that a defect of Ednrb expression affects survival of SGNs during hearing development after birth in mice.
Degeneration of SGNs but not the defect of melanocytes in the SV from Ednrb−/− mice was recovered in Ednrb−/−;DBH-Ednrb mice (Figs. 4 and 5). The defect of Ednrb protein in SGNs, but not in melanocytes in the SV (Fig. 5) or in inner and outer hair cells (supplemental Fig. S1), from Ednrb−/− mice was correspondingly rescued by Ednrb transgene driven by the DBH promoter. In addition, the suprathreshold ABR, which has been shown to reflect auditory nerve activity (28), showed similar growth rates in Ednrb−/−;DBH-Ednrb mice and littermate WT mice (supplemental Fig. S6), suggesting that development of SGNs was similar in Ednrb−/−;DBH-Ednrb mice and WT mice. On the other hand, Ednrb−/−;DBH-Ednrb mice showed a significantly lower level of EP than that in WT mice (supplemental Fig. S4), although a previous study has shown that there is a link between EP levels and auditory nerve activities (29). TEM analysis also showed no melanocytes with many gap areas in the SV from Ednrb−/−;DBH-Ednrb mice as well as Ednrb−/− mice (Fig. 5, I–K), suggesting impairments of the SV in Ednrb−/−;DBH-Ednrb mice. Thus, these results suggest that degeneration of SGNs in Ednrb−/− mice was specifically recovered in inner ears of Ednrb−/−;DBH-Ednrb mice. We further showed that hearing levels in Ednrb−/−;DBH-Ednrb mice were partially (20–30 dB SPL) recovered compared with those in Ednrb−/− mice (Fig. 4). Thus, these results suggest that Ednrb expressed in SGNs is partially required for postnatal development of hearing.
Ednrb has been reported to mediate embryonic development of melanocytes (30) and the enteric nervous system (18, 26) derived from the neural crest. Our results indicate a novel possibility that Ednrb is essential for postnatal development of SGNs, although the development process of SGNs (e.g. differentiation or migration of precursors) during prenatal and postnatal hearing development has not been completely elucidated (12). Our previous study also showed that impairment of c-Ret causes severe congenital hearing loss with degeneration of SGNs and with intact morphology of hair cells and the SV (13). Because both EDNRB and c-RET cause megacolon disease with congenital intestinal aganglionosis in mice and humans, further study is needed to determine whether megacolon-related molecules such as SOX10 and PAX3 are involved in congenital hearing loss caused by degeneration of SGNs.
The degeneration of SGNs from Ednrb−/− mice did not involve the hallmark of apoptotic signals (supplemental Fig. S3). The results of a previous study also showed that neurodegeneration of enteric neurons did not involve apoptotic signals during the developmental stage in mice with deletion of Ednrb (30). On the other hand, our results showed that hair bundles of inner and outer hair cells in Ednrb−/− mice, which have already developed congenital hearing loss, were comparable with those in littermate WT mice (supplemental Fig. S2). Immunohistochemical analysis correspondingly showed that expression of Ednrb protein was nearly undetectable in hair cells from WT mice (supplemental Fig. S1). These results suggest that the congenital hearing loss in Ednrb−/− mice involves postnatal degeneration of SGNs as well as defects of melanocytes in the SV rather than disturbance of hair cells.
Several mouse models for Ednrb-mediated WS-IV have been reported (summarized in supplemental Fig. S7). sl mice, in which exon 1 and intron 1 are spontaneously deleted, and WS-IV mice, in which exons 2 and 3 are spontaneously deleted, have been shown to develop megacolon disease and hearing loss. On the other hand, the hearing level of Ednrb−/− mice with deletion of exon 3, which we analyzed in this study, has not been reported. In humans, although impairments of EDNRB caused by nonsense mutations of exon 3 have been reported also to result in the development of WS with hearing loss, the etiology has not been clarified. Thus, this study for the first time provides an insight into the pathogenesis of congenital hearing loss caused by impairment of Ednrb−/− by deletion of exon 3 in mice.
Our results suggest that 60–70 dB SPL of hearing levels could be maintained even if there are no melanocytes in the SV in inner ears of Ednrb−/−;DBH-Ednrb mice. Because a previous study has shown that a transgene driven by the Dct promoter is expressed in melanocytes (31), further study is needed to determine the concurrent rescue effect of Ednrb transgene driven by the Dct promoter and the DBH promoter on congenital deafness in Ednrb−/− mice.
In summary, this study demonstrates a novel role of Ednrb expression in SGNs distinct from that in melanocytes in the SV contributing partially to postnatal hearing development via survival of SGNs. A therapeutic strategy for congenital hearing loss in WS-IV patients has not been established. Enhancement of EDNRB expression in SGNs could be a novel potential therapeutic strategy for congenital hearing loss in WS-IV patients.
Supplementary Material
Acknowledgments
We thank Drs. Ian J. Jackson (Western General Hospital, Edinburgh, UK), Takahiro Kunisada (Gifu University, Japan), and Masatake Osawa (Harvard Medical School, Boston, MA) for supplying the Dct-LacZ mice; Haruka Tamura, Harumi Ohno, Kyoko Ohgami, and Yoko Kato for technical assistance; and laboratory members for helpful discussions.
This work was supported in part by Grants-in-aid for Scientific Research (B) 19390168 and 20406003 (to M. K.), Grants-in-aid for Young Scientists (B) 18790738 (to N. O.) and 20791232 (to M. I.-E.) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Center of Excellence Project (Health Science Hills) for Private Universities from MEXT and Chubu University Grant S0801055 (to N. O. and M. K.), Lydia O'Leary Memorial Foundation (to M. K.), Asia and Africa Science Platform Program from the Japan Society for the Promotion of Science (to M. K.), a research grant from the Tokyo Biochemical Research Foundation (to M. K.), Adaptable and Seamless Technology Transfer Program through Target-driven R&D, Japan Science and Technology Agency (to N. O.), Research Foundation from the Institute of Science and Technology Research in Chubu University (to M. K.), and Chubu University Grants A, B, and C (to N. O. and M. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Methods, additional references, and Figs. S1–S7.
- WS
- Waardenburg syndrome
- ABR
- auditory brainstem response
- dB SPL
- decibel sound pressure level
- DBH
- dopamine β-hydroxylase
- Dct
- dopachrome tautomerase
- EDNRB
- endothelin receptor B
- EP
- endocochlear potential
- ET
- endothelin
- Kir4.1
- inward rectifier potassium channel
- P3
- P16, and P19, postnatal days 3, 16, and 19, respectively
- SGN
- spiral ganglion neuron
- SV
- stria vascularis
- TEM
- transmission electron microscopy.
REFERENCES
- 1. Pardono E., van Bever Y., van den Ende J., Havrenne P. C., Iughetti P., Maestrelli S. R., Costa F. O., Richieri-Costa A., Frota-Pessoa O., Otto P. A. (2003) Am. J. Med. Genet. A 117A, 223–235 [DOI] [PubMed] [Google Scholar]
- 2. Pingault V., Bondurand N., Kuhlbrodt K., Goerich D. E., Préhu M. O., Puliti A., Herbarth B., Hermans-Borgmeyer I., Legius E., Matthijs G., Amiel J., Lyonnet S., Ceccherini I., Romeo G., Smith J. C., Read A. P., Wegner M., Goossens M. (1998) Nat. Genet. 18, 171–173 [DOI] [PubMed] [Google Scholar]
- 3. Edery P., Attié T., Amiel J., Pelet A., Eng C., Hofstra R. M., Martelli H., Bidaud C., Munnich A., Lyonnet S. (1996) Nat. Genet. 12, 442–444 [DOI] [PubMed] [Google Scholar]
- 4. Puffenberger E. G., Hosoda K., Washington S. S., Nakao K., deWit D., Yanagisawa M., Chakravart A. (1994) Cell 79, 1257–1266 [DOI] [PubMed] [Google Scholar]
- 5. Hosoda K., Hammer R. E., Richardson J. A., Baynash A. G., Cheung J. C., Giaid A., Yanagisawa M. (1994) Cell 79, 1267–1276 [DOI] [PubMed] [Google Scholar]
- 6. Bagnato A., Spinella F., Rosanò L. (2005) Endocr. Relat. Cancer 12, 761–772 [DOI] [PubMed] [Google Scholar]
- 7. Sakurai T., Yanagisawa M., Takuwa Y., Miyazaki H., Kimura S., Goto K., Masaki T. (1990) Nature 348, 732–735 [DOI] [PubMed] [Google Scholar]
- 8. Gariepy C. E., Cass D. T., Yanagisawa M. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 867–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsushima Y., Shinkai Y., Kobayashi Y., Sakamoto M., Kunieda T., Tachibana M. (2002) Mamm. Genome 13, 30–35 [DOI] [PubMed] [Google Scholar]
- 10. Gürtler N., Lalwani A. K. (2002) Otolaryngol. Clin. North Am. 35, 891–908 [DOI] [PubMed] [Google Scholar]
- 11. Brown S. D., Hardisty-Hughes R. E., Mburu P. (2008) Nat. Rev. Genet. 9, 277–290 [DOI] [PubMed] [Google Scholar]
- 12. Rubel E. W., Fritzsch B. (2002) Annu. Rev. Neurosci. 25, 51–101 [DOI] [PubMed] [Google Scholar]
- 13. Ohgami N., Ida-Eto M., Shimotake T., Sakashita N., Sone M., Nakashima T., Tabuchi K., Hoshino T., Shimada A., Tsuzuki T., Yamamoto M., Sobue G., Jijiwa M., Asai N., Hara A., Takahashi M., Kato M. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 13051–13056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salt A. N., Melichar I., Thalmann R. (1987) Laryngoscope 97, 984–991 [PubMed] [Google Scholar]
- 15. Steel K. P., Barkway C. (1989) Development 107, 453–463 [DOI] [PubMed] [Google Scholar]
- 16. Nin F., Hibino H., Doi K., Suzuki T., Hisa Y., Kurachi Y. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 1751–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kapur R. P., Yost C., Palmiter R. D. (1992) Development 116, 167–175 [DOI] [PubMed] [Google Scholar]
- 18. Laghmani K., Preisig P. A., Moe O. W., Yanagisawa M., Alpern R. J. (2001) J. Clin. Invest. 107, 1563–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Trigueiros-Cunha N., Renard N., Humbert G., Tavares M. A., Eybalin M. (2003) Eur. J. Neurosci. 18, 2653–2662 [DOI] [PubMed] [Google Scholar]
- 20. Kapur R. P., Hoyle G. W., Mercer E. H., Brinster R. L., Palmiter R. D. (1991) Neuron 7, 717–727 [DOI] [PubMed] [Google Scholar]
- 21. Zheng Q. Y., Johnson K. R., Erway L. C. (1999) Hear. Res. 130, 94–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lang H., Schulte B. A., Zhou D., Smythe N., Spicer S. S., Schmiedt R. A. (2006) J. Neurosci. 26, 3541–3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shimada A., Ohta A., Akiguchi I., Takeda T. (1992) J. Neuropathol. Exp. Neurol. 51, 440–450 [DOI] [PubMed] [Google Scholar]
- 24. Ohgami N., Ida-Eto M., Sakashita N., Sone M., Nakashima T., Tabuchi K., Hoshino T., Shimada A., Tsuzuki T., Yamamoto M., Sobue G., Jijiwa M., Asai N., Hara A., Takahashi M., Kato M. (2011) Neurobiol. Aging, in press [DOI] [PubMed] [Google Scholar]
- 25. Hubbs A. F., Benkovic S. A., Miller D. B., O'Callaghan J. P., Battelli L., Schwegler-Berry D., Ma Q. (2007) Am. J. Pathol. 170, 2068–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gariepy C. E., Williams S. C., Richardson J. A., Hammer R. E., Yanagisawa M. (1998) J. Clin. Invest. 102, 1092–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Knipper M., Claussen C., Rüttiger L., Zimmermann U., Lüllmann-Rauch R., Eskelinen E. L., Schröder J., Schwake M., Saftig P. (2006) J. Physiol. 576, 73–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Graham C. E., Vetter D. E. (2011) J. Neurosci. 31, 1267–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sewell W. F. (1984) J. Physiol. 347, 685–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stanchina L., Baral V., Robert F., Pingault V., Lemort N., Pachnis V., Goossens M., Bondurand N. (2006) Dev. Biol. 295, 232–249 [DOI] [PubMed] [Google Scholar]
- 31. Steel K. P., Davidson D. R., Jackson I. J. (1992) Development 115, 1111–1119 [DOI] [PubMed] [Google Scholar]
- 32. Mackenzie M. A., Jordan S. A., Budd P. S., Jackson I. J. (1997) Dev. Biol. 192, 99–107 [DOI] [PubMed] [Google Scholar]
- 33. Møller A. R., Jannetta P. J. (1985) in The Auditory Brainstem Response (Jacobson J. T. ed) pp. 13–31, Taylor & Francis, London [Google Scholar]
- 34. Stockard J. J., Rossiter V. S. (1977) Neurology 27, 316–325 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.