Abstract
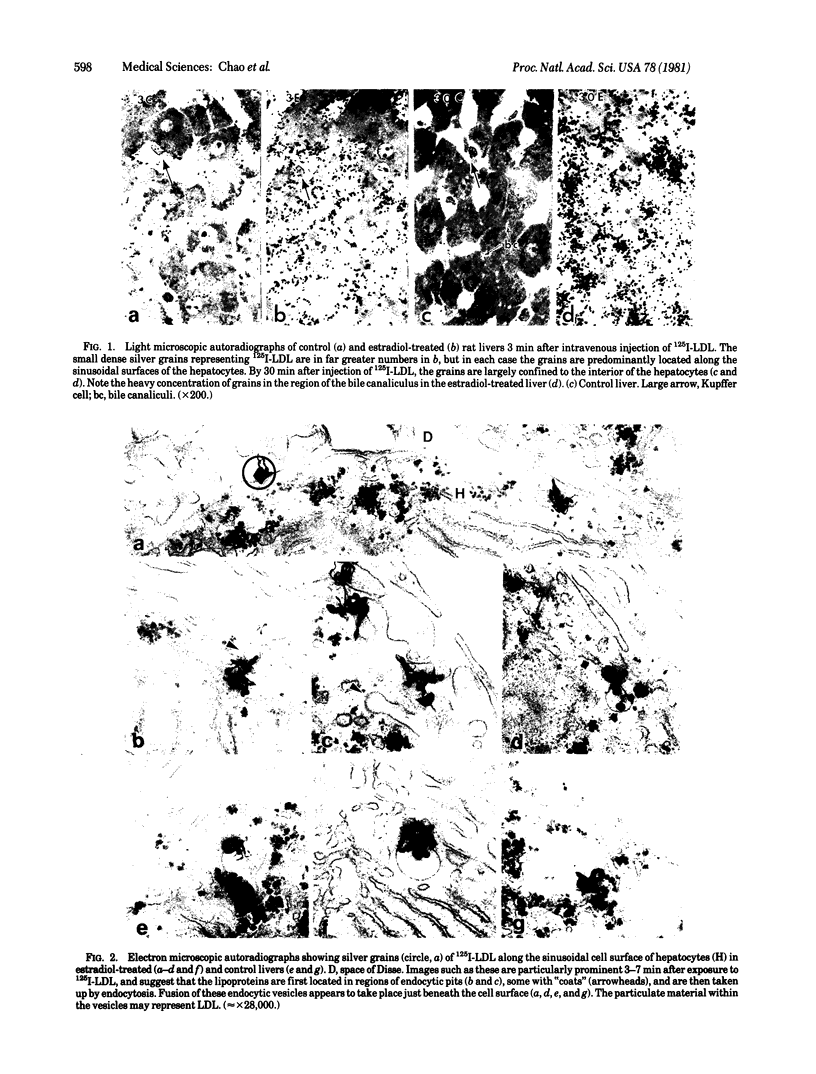
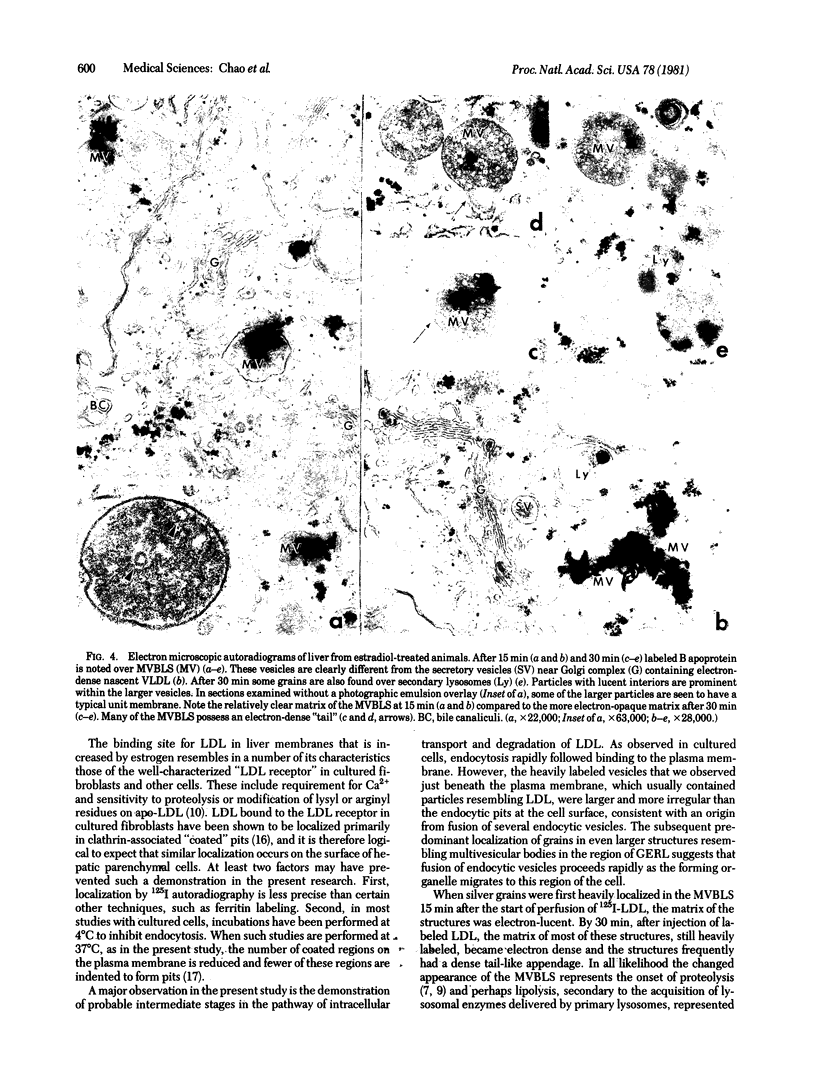
The hepatic uptake and catabolism of low density lipoproteins are stimulated severalfold in rats treated with large amounts of 17α-ethinylestradiol. To determine the sites within the liver at which these processes occur, 125I-labeled human low density lipoproteins were injected intravenously into intact control and estradiol-treated rats or added to perfusates of their isolated livers. The livers were fixed by perfusion and processed for light and electron microscopic autoradiography. Distribution of autoradiographic silver grains was estimated qualitatively in light micrographs and quantitatively in electron micrographs. Many more silver grains were seen in livers from estradiol-treated than from control rats, but the processing of labeled low density lipoprotein was indistinguishable. Three minutes after intravenous injection or perfusion of livers, the grains were concentrated over the microvillous surface of parenchymal cells bordering the space of Disse. Many of these grains were within two half-distances from endocytic pits. Only 5-15% of the grains were seen over endothelial and Kupffer cells. Silver grains were also observed over vesicles beneath the plasma membrane whose size and shape suggested that they were derived from fusion of endocytic vesicles. By 15 min, grains were predominantly located in structures like multivesicular bodies in the region of the GERL (Golgi complex-endoplasmic reticulum-lysosomes) near the bile canaliculi. These bodies were packed with small vesicle-like structures and a few larger vesicles, the latter possessing a unit membrane. Between 15 and 30 min, when proteolysis of low density lipoproteins is known to begin, the initially clear matrix of the multivesicular body-like structures became dark and the structures frequently had a dense tail-like appendage. At the same time, silver grains began to appear over secondary lysosomes. These and other results indicate that the hepatic uptake of low density lipoproteins that is stimulated in rats given large amounts of estradiol follows a pathway that closely resembles that of the well-defined “LDL receptor” in cultured cells. In the liver these lipoproteins appear to be transported in endocytic vesicles; the vesicles fuse to form multivesicular body-like structures that acquire lysosomal enzymes and are converted to secondary lysosomes as the lipoproteins are degraded.
Keywords: hepatic receptors, endocytosis, multivesicular body-like structures, lysosomes
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Brown M. S., Goldstein J. L. Role of the coated endocytic vesicle in the uptake of receptor-bound low density lipoprotein in human fibroblasts. Cell. 1977 Mar;10(3):351–364. doi: 10.1016/0092-8674(77)90022-8. [DOI] [PubMed] [Google Scholar]
- Chao Y. S., Windler E. E., Chen G. C., Havel R. J. Hepatic catabolism of rat and human lipoproteins in rats treated with 17 alpha-ethinyl estradiol. J Biol Chem. 1979 Nov 25;254(22):11360–11366. [PubMed] [Google Scholar]
- Cooper A. D., Yu P. Y. Rates of removal and degradation of chylomicron remnants by isolated perfused rat liver. J Lipid Res. 1978 Jul;19(5):635–643. [PubMed] [Google Scholar]
- Faergeman O., Havel R. J. Metabolism of cholesteryl esters of rat very low density lipoproteins. J Clin Invest. 1975 Jun;55(6):1210–1218. doi: 10.1172/JCI108039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faergeman O., Sata T., Kane J. P., Havel R. J. Metabolism of apoprotein B of plasma very low density lipoproteins in the rat. J Clin Invest. 1975 Dec;56(6):1396–1403. doi: 10.1172/JCI108220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Jones A. L., Schmucker D. L., Mooney J. S., Adler R. D., Ockner R. K. Morphometric analysis of rat hepatocytes after total billary obstruction. Gastroenterology. 1976 Dec;71(6):1050–1060. [PubMed] [Google Scholar]
- Kovanen P. T., Brown M. S., Goldstein J. L. Increased binding of low density lipoprotein to liver membranes from rats treated with 17 alpha-ethinyl estradiol. J Biol Chem. 1979 Nov 25;254(22):11367–11373. [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Nilsson A., Zilversmit D. B. Distribution of chylomicron cholesteryl ester between parenchymal and Kupffer cells of rat liver. Biochim Biophys Acta. 1971 Oct 5;248(1):137–142. doi: 10.1016/0005-2760(71)90085-3. [DOI] [PubMed] [Google Scholar]
- Novikoff P. M., Yam A. The cytochemical demonstration of GERL in rat hepatocytes during lipoprotein mobilization. J Histochem Cytochem. 1978 Jan;26(1):1–13. doi: 10.1177/26.1.563889. [DOI] [PubMed] [Google Scholar]
- Renston R. H., Maloney D. G., Jones A. L., Hradek G. T., Wong K. Y., Goldfine I. D. Bile secretory apparatus: evidence for a vesicular transport mechanism for proteins in the rat, using horseradish peroxidase and [125I]insulin. Gastroenterology. 1980 Jun;78(6):1373–1388. [PubMed] [Google Scholar]
- Sherrill B. C., Dietschy J. M. Characterization of the sinusoidal transport process responsible for uptake of chylomicrons by the liver. J Biol Chem. 1978 Mar 25;253(6):1859–1867. [PubMed] [Google Scholar]
- Sigurdsson G., Noel S. P., Havel R. J. Catabolism of the apoprotein of low density lipoproteins by the isolated perfused rat liver. J Lipid Res. 1978 Jul;19(5):628–634. [PubMed] [Google Scholar]
- Sigurdsson G., Noel S. P., Havel R. J. Quantification of the hepatic contribution to the catabolism of high density lipoproteins in rats. J Lipid Res. 1979 Mar;20(3):316–324. [PubMed] [Google Scholar]
- Stein O., Rachmilewitz D., Sanger L., Eisenberg S., Stein Y. Metabolism of iodinated very low density lipoprotein in the rat. Autoradiographic localization in the liver. Biochim Biophys Acta. 1974 Aug 22;360(2):205–216. doi: 10.1016/0005-2760(74)90170-2. [DOI] [PubMed] [Google Scholar]
- Stein O., Stein Y., Goodman D. S., Fidge N. H. The metabolism of chylomicron cholesteryl ester in rat liver. A combined radioautographic-electron microscopic and biochemical study. J Cell Biol. 1969 Dec;43(3):410–431. doi: 10.1083/jcb.43.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Berkel T. J., Van Tol A. Role of parenchymal and non-parenchymal rat liver cells in the uptake of cholesterolester-labeled serum lipoproteins. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1097–1101. doi: 10.1016/0006-291x(79)92120-x. [DOI] [PubMed] [Google Scholar]
- Windler E. E., Kovanen P. T., Chao Y. S., Brown M. S., Havel R. J., Goldstein J. L. The estradiol-stimulated lipoprotein receptor of rat liver. A binding site that membrane mediates the uptake of rat lipoproteins containing apoproteins B and E. J Biol Chem. 1980 Nov 10;255(21):10464–10471. [PubMed] [Google Scholar]