Abstract
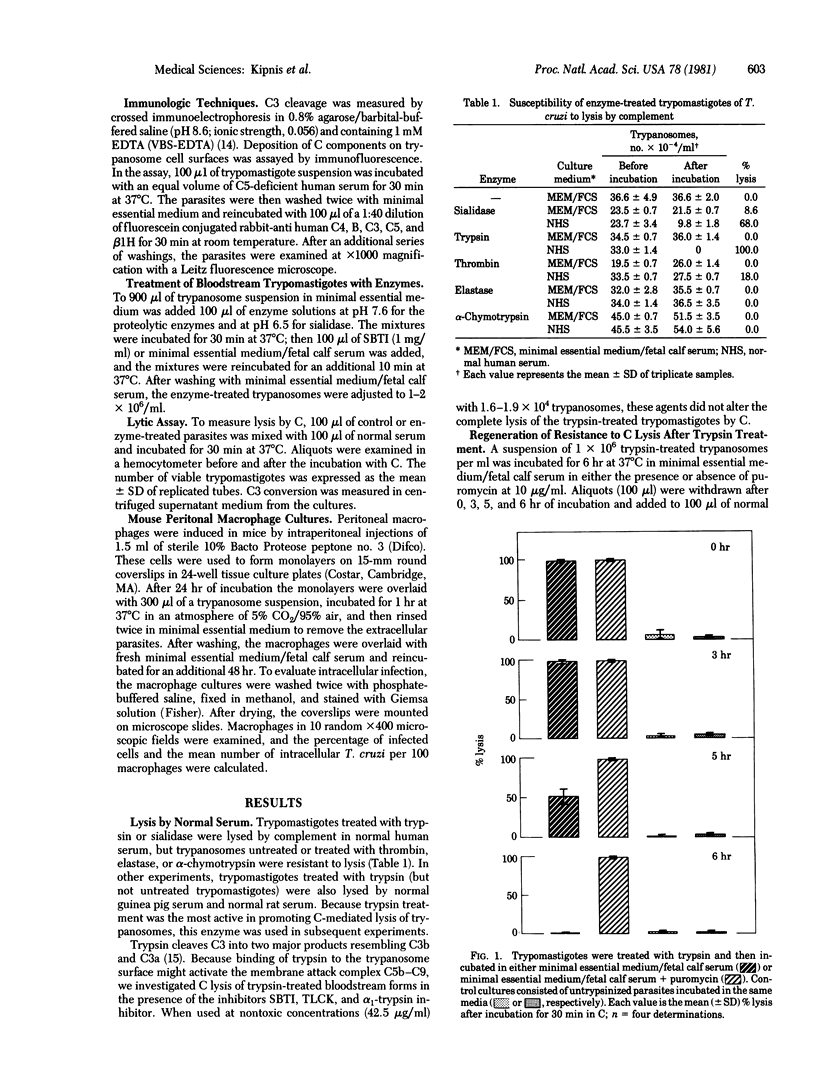
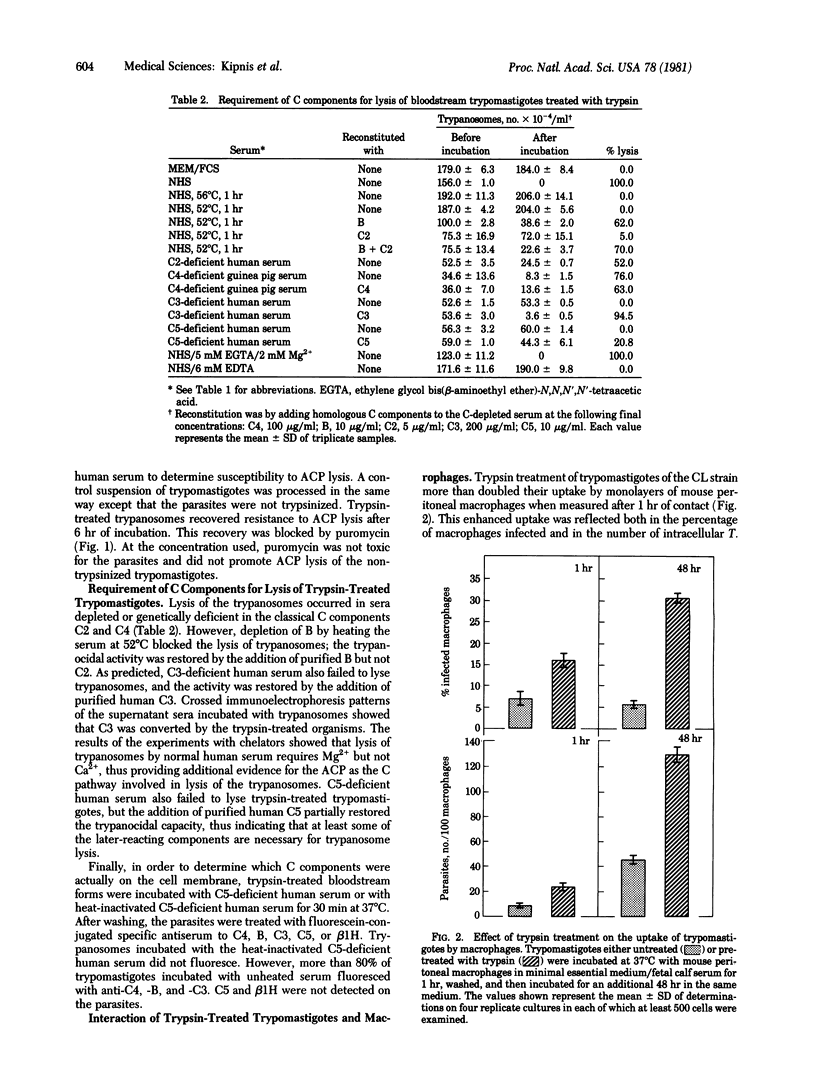
In the absence of bound antibody, trypomastigote bloodstream forms of Trypanosoma cruzi fail to activate the alternative complement pathway. We now demonstrate that treatment with trypsin and, to a lesser extent, with sialidase converts these protozoa into activators of the pathway, as judged by their lysis in normal sera or sera genetically deficient in fourth or second component of complement (C4 or C2) and their Mg2+-dependent consumption of C3 as measured by crossed immunoelectrophoresis. In addition, after pretreatment with enzyme and incubation in C5-deficient serum, trypomastigotes were shown to possess both C3 and properdin factor B (B) on their surface as judged by immunofluorescence. Requirement for the late components C5-C9 was suggested by the failure of C5-deficient sera to lyse trypsin-treated parasites. The inability to activate the alternative complement pathway was regained by these organisms after incubation in vitro. This restoration of insusceptibility was inhibited when puromycin was included in the culture medium. Treatment of the trypomastigotes with trypsin also potentiated their uptake by mouse peritoneal macrophages without apparent interference with their capacity to differentiate and multiply inside the cell. These findings suggest that untreated trypomastigotes normally escape recognition by the alternative pathway in vivo because of the presence on their surface of trypsin- and sialidase-sensitive regulatory molecules, the expression of which is dependent on protein synthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcantara A., Brener Z. The in vitro interaction of Trypanosoma cruzi bloodstream forms and mouse peritoneal macrophages. Acta Trop. 1978 Sep;35(3):209–219. [PubMed] [Google Scholar]
- Alves M. J., Colli W. Glycoproteins from trypanosoma cruzi: partial purification by gel chromatography. FEBS Lett. 1975 Apr 1;52(2):188–190. doi: 10.1016/0014-5793(75)80803-9. [DOI] [PubMed] [Google Scholar]
- Budzko D. B., Pizzimenti M. C., Kierszenbaum F. Effects of complement depletion in experimental chagas disease: immune lysis of virulent blood forms of Trypanosoma cruzi. Infect Immun. 1975 Jan;11(1):86–91. doi: 10.1128/iai.11.1.86-91.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N. R., Jensen F. C., Welsh R. M., Jr, Oldstone M. B. Lysis of RNA tumor viruses by human serum: direct antibody-independent triggering of the classical complement pathway. J Exp Med. 1976 Oct 1;144(4):970–984. doi: 10.1084/jem.144.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czop J. K., Fearon D. T., Austen K. F. Membrane sialic acid on target particles modulates their phagocytosis by a trypsin-sensitive mechanism on human monocytes. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3831–3835. doi: 10.1073/pnas.75.8.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lederkremer R. M., Tanaka C. T., Alves M. J., Colli W. Lipopeptidophosphoglycan from Trypanosoma cruzi. Amide and ester-linked fatty acids. Eur J Biochem. 1977 Apr 1;74(2):263–267. doi: 10.1111/j.1432-1033.1977.tb11389.x. [DOI] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Activation of the alternative complement pathway due to resistance of zymosan-bound amplification convertase to endogenous regulatory mechanisms. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1683–1687. doi: 10.1073/pnas.74.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1971–1975. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. The C3-activator system: an alternate pathway of complement activation. J Exp Med. 1971 Sep 1;134(3 Pt 2):90s–108s. [PubMed] [Google Scholar]
- Kazatchkine M. D., Fearon D. T., Silbert J. E., Austen K. F. Surface-associated heparin inhibits zymosan-induced activation of the human alternative complement pathway by augmenting the regulatory action of the control proteins on particle-bound C3b. J Exp Med. 1979 Nov 1;150(5):1202–1215. doi: 10.1084/jem.150.5.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis T. L., Calich V. L., da Silva W. D. Active entry of bloodstream forms of Trypanosoma cruzi into macrophages. Parasitology. 1979 Feb;78(1):89–98. doi: 10.1017/s0031182000048617. [DOI] [PubMed] [Google Scholar]
- Krettli A. U., Weisz-Carrington P., Nussenzweig R. S. Membrane-bound antibodies to bloodstream Trypanosoma cruzi in mice: strain differences in susceptibility to complement-mediated lysis. Clin Exp Immunol. 1979 Sep;37(3):416–423. [PMC free article] [PubMed] [Google Scholar]
- LAURELL C. B. ANTIGEN-ANTIBODY CROSSED ELECTROPHORESIS. Anal Biochem. 1965 Feb;10:358–361. doi: 10.1016/0003-2697(65)90278-2. [DOI] [PubMed] [Google Scholar]
- Nilsson U. R., Mandle R. J., Jr, McConnell-Mapes J. A. Human C3 and C5: subunit structure and modifications by trypsin and C42-C423. J Immunol. 1975 Feb;114(2 Pt 2):815–822. [PubMed] [Google Scholar]
- Nogueira N., Bianco C., Cohn Z. Studies on the selective lysis and purification of Trypanosoma cruzi. J Exp Med. 1975 Jul 1;142(1):224–229. doi: 10.1084/jem.142.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. Complement C3 convertase: cell surface restriction of beta1H control and generation of restriction on neuraminidase-treated cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2416–2420. doi: 10.1073/pnas.75.5.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley M. J., Müller-Eberhard H. J. The second component of human complement: its isolation, fragmentation by C'1 esterase, and incorporation into C'3 convertase. J Exp Med. 1968 Sep 1;128(3):533–551. doi: 10.1084/jem.128.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack B. D., Prahl J. W. Third component of human complement: purification from plasma and physicochemical characterization. Biochemistry. 1976 Oct 5;15(20):4513–4521. doi: 10.1021/bi00665a028. [DOI] [PubMed] [Google Scholar]



