Abstract
Withdrawal stress is a common occurrence in opioid users, yet very few studies have examined the effects of morphine withdrawal (MW) on immune functioning or the role of glucocorticoids in MW-induced immunomodulation. This study investigated for the first time the role of glucocorticoids in MW modulation of LPS-induced IL-12p40, a key cytokine playing a pivotal role in immunoprotection. Using WT and μ-opioid receptor knock-out mice, we show that MW in vivo significantly attenuated LPS-induced IL-12p40 mRNA and protein expression. The role of glucocorticoids in MW modulation of IL-12p40 was investigated using a murine macrophage cell line, CRL2019, in an in vitro MW model. Interestingly, MW alone in the absence of glucocorticoids resulted in a significant reduction in IL-12p40 promoter activity and mRNA and protein expression. EMSA revealed a concurrent decrease in consensus binding to transcription factors NFκB, Activator Protein-1, and CCAAT/enhancer-binding protein and Western blot analysis demonstrated a significant activation of LPS-induced ERK1/2 phosphorylation. Interestingly, although glucocorticoid treatment alone also modulated these transcription factors and ERK1/2 activation, the addition of glucocorticoids to MW samples resulted in a greater than additive reduction in the transcription factors and significant hyperactivation of LPS-induced ERK1/2 phosphorylation. ERK inhibitors reversed MW and MW plus corticosterone inhibition of LPS-induced IL-12p40. The potentiating effects of glucocorticoids were non-genomic because nuclear translocation of glucocorticoid receptor was not significantly different between MW and corticosterone treatment. This study demonstrates for the first time that MW and glucocorticoids independently modulate IL-12p40 production through a mechanism involving ERK1/2 hyperactivation and that glucocorticoids can significantly augment MW-induced inhibition of IL-12p40.
Keywords: Glucocorticoid Receptor, Immunosuppression, Lipopolysaccharide (LPS), Macrophages, MAP Kinases (MAPKs), ERK1/2, IL-12p40, Morphine Withdrawal, Mu-Opioid Receptor, Transcription Factors
Introduction
Drugs of abuse have long been known to contribute to immunosuppression (1) affecting both innate and adaptive immunity in experimental animals as well as in humans (2). Although a wealth of data exists on the effects of morphine treatment on immune function, very few studies have investigated the effects of morphine withdrawal on immune parameters, and of these even fewer describe the underlying mechanisms that modulate morphine withdrawal-induced immunosuppression. Morphine withdrawal can be induced either by simple cessation of morphine (abrupt withdrawal) (3) or by cessation of morphine with administration of morphine antagonists (precipitated withdrawal) (4). Elegant studies from the Eisenstein laboratory (3, 5–10) were the first to demonstrate that both abrupt and precipitated morphine withdrawal result in significant immunosuppression with significant changes in several key cytokines, including IL-12p40.
Stress has been shown to play a major role in several aspects of morphine use and abuse. These roles include precipitating abuse, enhancing drug seeking (11), potentiating responses to other stressors, and facilitating drug relapse. Physiological alterations are elicited as a coping mechanism in response to a wide variety of stressors, including physical, psychological, infectious-immunological, and pharmacological insults. These include activation of the Hypothalamic-pituitary-adrenal (HPA)3 axis, resulting in the release of glucocorticoids, stimulation of the sympathetic system resulting in epinephrine and norepinephrine release, and the mobilization of endogenous opioid peptides (12, 13). Although acute activation may be a protective coping mechanism, chronic activation of these stress responses is believed to cause dysfunction in many systems, including the immune system (14). Activation of the HPA axis, characterized by circulating elevated levels of glucocorticoids, has been shown to be a potent modulator of the immune system. Morphine withdrawal has been shown to activate the HPA axis with a resultant transient increase in corticosterone (15–17). Although chronic morphine-induced elevations in corticosterone levels are mediated by the μ-opioid receptor, as evidenced by a loss of corticosterone increase in μ-opioid receptor knock-out (MORKO) mice, the role of MOR in MW withdrawal has not been investigated (18). More recently, we have shown that morphine withdrawal stress resulted in skewing the Th cell polarization toward the Th2 lineage (19). These studies support previous studies by the Eisenstein group (9, 10, 19) showing that mice subjected to morphine withdrawal stress in the context of LPS stimulation displayed decreased levels of IFN-γ and IL-12. The protective role of IL-12 in human infectious diseases, including leprosy (20), tuberculosis (21), and leishmaniasis (22), has been well characterized. On the contrary, overexpression of IL-12 may contribute to the development of chronic inflammatory disorders (23), including Crohn disease and rheumatoid arthritis. Thus, the regulated expression of IL-12 in antigen-presenting cells is a critical event in the pathogenesis of infectious and inflammatory diseases.
Although several studies using dexamethasone as a surrogate for stress in vitro reported inhibition of IL-12p40 production in LPS-stimulated monocytic cells, thus far, to our knowledge, there have been no studies that have systematically investigated the role of corticosterone in MW-induced immunosuppression and specifically IL-12p40 synthesis.
In the current investigation, we studied the effects of MW in vivo in WT and MORKO mice and in vitro in the presence of corticosterone, to simulate stress, in primary murine macrophage cells and macrophage cell lines to delineate the role of corticosterone in MW-induced inhibition of IL-12p40 expression in LPS-stimulated cells.
EXPERIMENTAL PROCEDURES
Animals
8–10-week-old B6129SF2 and B6129PFI male mice and MORKO male mice were used in the experiments described herein. Animals were housed 4 animals/cage under controlled conditions of temperature and lighting (12-h light/dark cycle) and given free access to standard food and tap water. All animals were allowed to acclimate to their environment for at least 7 days prior to any experimental manipulations. Mice were sacrificed by carbon dioxide asphyxiation, and spleen tissues were harvested aseptically. Discomfort, distress, and injury to the animals were minimized. The Institutional Animal Care and Use Committee at the University of Minnesota has approved all protocols in use, and all procedures are in agreement with the guidelines set forth by the National Institute of Health Guide for the Care and Use of Laboratory Animals.
In Vivo Withdrawal Model
Mice were subjected to a well established model for both generating morphine dependence and producing withdrawal (24). Animals were anesthetized by inhaling isoflurane (3%), followed by implantation with morphine pellets (75 mg each) or placebo pellets (kindly provided by NIDA, National Institutes of Health, Rockville, MD), depending on the experiment. The implantation procedure consisted of making a small incision on the dorsal side of the animal and inserting a pellet (placebo or morphine) into the subcutaneous space created by the incision. Pellets were wrapped with nylon mesh and secured with surgical thread to facilitate easy removal. The incision was closed with the use of stainless steel wound clips. Following the morphine exposure period (72 h), the pellets were removed by opening the wound clips and taking out the pellets wrapped in nylon mesh. The wound was again closed with a wound clip. Removal of the pellets initiated spontaneous withdrawal in these animals; this technique is a widely utilized and accepted model for eliciting withdrawal (3). Classic withdrawal symptoms, including diarrhea, wet dog shakes, tremors, lack of grooming, increased agitation, and up to a 5% reduction in body weight occurred in morphine-withdrawn mice. The morphine withdrawal period consisted of either 4, 8, or 24 h, and at the initiation of withdrawal, animals were administered 20 μg of LPS intraperitoneally (Sigma). At the conclusion of all procedures, animals were returned to their home cages, separated by experimental groups, and not housed more than 4 animals/cage. Following the withdrawal period, animals were sacrificed by CO2 asphyxiation, and spleens were harvested as described below. Prior to sacrifice, blood was collected via the retro-orbital plexus or cardiac puncture.
Preparation of Murine Macrophages
Primary peritoneal macrophages were aseptically collected by flushing the peritoneal cavity with PBS with a 10-ml syringe. Collected cells were pelleted by low speed centrifugation and maintained in RPMI 1640 (Invitrogen) supplemented with 10% FBS and 1% penicillin/streptomycin. Spleens were removed aseptically, and suspensions were prepared by forcing the tissue through a cell strainer (70 μm) with a sterile syringe plunger. Cell suspensions were maintained in culture dishes with RPMI 1640 but without FBS to facilitate macrophage attachment. Following attachment, cells were washed to remove contaminating cell populations. Cells were collected and counted and were plated in 24-well culture plates at a concentration of 2 × 106 cells/ml in triplicate. Cells were then stimulated with LPS and incubated overnight at 37 °C, 5% CO2.
Cell Culture
The mouse alveolar macrophage cell line, CRL2019 (American Type Culture Collection, Manassas, VA) was used for in vitro experiments. Murine peritoneal macrophage cell line J774.1 was also used for EMSA experiments. Cells were maintained in RPMI 1640 (CRL2019) and DMEM (J744.1) supplemented with 10% FBS and 1% penicillin/streptomycin. Cells were plated at a concentration of 0.5 × 106 cells/ml in 10-cm culture plates. Cells were subjected to the in vitro withdrawal method described below in triplicate and incubated at 37 °C, 5% CO2.
In Vitro Withdrawal
To replicate conditions tested in vivo, either primary cells or the cell lines were plated as described above. Following plating, cells were treated with 100 nm morphine sulfate (NIDA, National Institutes of Health, Rockville, MD) once per day for three consecutive days. On the fourth day, the cells were washed 3–5 times with PBS to simulate withdrawal. The cells were incubated in serum-free RPMI 1640 medium for 24 h, followed by LPS (100 ng/ml) treatment for 6 h. In in vitro studies, before the end of LPS treatment, cells were also treated with corticosterone (300 ng/ml) for 30 min.
Corticosterone Radioimmunoassay
WT and MORKO mice were sacrificed following MW experiments, and plasma samples were collected and stored at −70 °C until analyzed. Plasma concentrations of corticosterone in WT and MORKO mice were analyzed using a 125I-coupled, double antibody radioimmunoassay (ICN Biochemicals, Costa Mesa, CA) according to the manufacturer's instructions. The concentrations were expressed as ng/ml.
RNA Extraction and RT-PCR Analysis
Cells (CRL2019 and J774.1) following treatments were collected in 1 ml of TRIzol reagent (Invitrogen), and total RNA was extracted as per the manufacturer's protocol. Total RNA was quantified and frozen at −80 °C until used. RNA was reverse transcribed using Moloney murine leukemia virus RT (New England Biolabs, Ipswich, MA) together with random hexamers (GeneLink, Hawthorne, NY). One hundred nanograms of cDNA was used for real-time PCR and gel-based PCR to study the expression profile of mouse IL-12p40 and β-actin or GAPDH. Sense and antisense oligonucleotide primers were designed for RT-PCR using DNA sequence information obtained from the Genome Data base (National Center for Biotechnology Information) and were synthesized at the Bio-Medicine Genomic Center facility, University of Minnesota. The following specific primers were used for real-time as well as gel-based PCR: for IL-12p40, sense (5′-TCATCAGGGACATCATCAAAC-3′) and antisense (5′-TGAGGGAGAAGTAGGAATGGG-3′); for β-actin, sense (5′-ATATCGCTGCGCTGGTCGTC-3′) and antisense (5′-AGGATGGCGTGAGGGAGAGC-3′); and for GAPDH, sense (5′-CGACTTCAACAGCAACTCCCACTCT-3′) and antisense (5′-TGGGTGGTCCAGGGTTTCTTACTC-3′). The real-time PCR analysis was performed using SYBR Green master mix (Applied Biosystems, Carlsbad, CA) on a 7500 real-time PCR station (Applied Biosystems). Primers for β-actin/GAPDH were used as internal controls. The results for real-time PCR were calculated by using the ΔcT-ΔcT method and were expressed as -fold expression.
Enzyme-linked Immunosorbent Assays
Quantikine ELISA kits were obtained from R&D Systems (Minneapolis, MN) and were performed according to the manufacturer's directions. Briefly, 50 μl of sample supernatant or serum was assayed in triplicate per experimental condition. Following the incubation period, plates were washed, and 100 μl of detection antibody conjugated to horseradish peroxidase was added to the wells. Finally, 100 μl of substrate solution was added, and absorbance was read at 450 nm using a plate reader (Flostar, BMG Labtech). Optical density measurements for the standards were used to generate a standard curve, and the concentration of the particular cytokine in each of the samples was extrapolated from this standard curve.
EMSAs
Transcription factor interactions with DNA response elements were assessed using EMSAs. Nuclear extracts were prepared with an NXTract nuclear extraction kit (Sigma). Briefly, cells were resuspended in lysis buffer containing dithiothreitol (DTT) and protease inhibitors. Cells were then lysed using mild detergent and centrifuged to separate cytoplasmic protein pool and pellet. The pellet was dissolved in extraction buffer to extract the nuclear protein pool. Activator Protein-1 (AP-1), NFκB, and C/EBP consensus oligonucleotides were synthesized and labeled with IR700 at the 5′-end, and both strands were duplexed at the Bio-Medical Genomics Center, University of Minnesota. The sequence for the probes were as follows: for probe AP-1, sense (IR700–5′-CGCTTGATGACTCAGCCGGAA-3′ and antisense (5′-TTCCGGCTGAGTCATCAAGCG-3′); for probe NFκB, sense (IR700–5′-AGTTGAGGGGACTTTCCCAGGC-3′) and antisense (5′-GCCTGGGAAAGTCCCCTCAACT-3′); and for probe C/EBP, sense (IR700–5′-TGCAGATTGCGCAATCTGCA-3′) and antisense (5′-TGCAGATTGCGCAATCTGCA-3′). Mutant probes for NFκB, AP-1, and C/EBP were also synthesized from the same facility (AP-1, 5′-CGCTTGATGACTTGGCCGGAA-3′; C/EBP, 5′-TGCAGAGACTAGTCTCTGCA-3′; NFκB, 5′-AGTTGAGGCGACTTTCCCAGG-3′). Underlined bases are mutated bases. Unlabeled probes were purchased from Promega and were used at a 70-fold excess of labeled probe. EMSA was performed using an Odyssey Infrared EMSA kit (LiCor Biosciences, Lincoln, NE) according to the manufacturer's instructions. Approximately 10 μg of nuclear extracts were incubated with 50 fmol of labeled probe in binding buffer. The probe and nuclear proteins were incubated for 30 min at room temperature. DNA-protein complexes were resolved on 4.5% non-denaturing acrylamide gels. Gels were then scanned directly in an Odyssey scanner (LiCor Biosciences) to visualize DNA-protein interaction.
Transient Transfections
The hIL-12p40 promoter-luciferase reporter plasmid was kindly provided by Dr. A. Kumar (University of Ottawa), and the construct has been described previously (25). CRL2019 cells were transfected with plasmids using Effectene reagent (Qiagen, Valencia, CA) according to the manufacturer's instructions. Briefly, 10 μg of IL-12p40 promoter-firefly luciferase reporter plasmid and 0.5 μg of pRL-TK-Renilla reniformis luciferase reporter internal control plasmid (Promega, Madison, WI) were incubated for 10 min with Effectene reagent in standard RPMI medium to allow formation of complexes. Complexes were added directly to each well of a 6-well plate, and cells were maintained in 37 °C, 5% CO2 culture conditions. Transfections were performed on day 3 of the 5-day experiment. After treatment, cells were lysed, and luciferase activity was measured using a Dual-Luciferase reporter assay system (Promega) and a Turner Biosystems TD 20/20 luminometer according to the manufacturer's instructions. Data are presented as standardized luciferase activity, determined by the ratio between firefly luciferase and R. reniformis luciferase.
Western Blot Analysis
Cells were treated with LPS (100 ng/ml, 6 h) after MW treatment and, at the end of LPS treatment, treated with corticosterone (300 ng/ml, 30 min). In experiments with PD98059, calyculin A, and/or wedelolactone, cells were treated with different inhibitors for 1 h prior to LPS treatment. Total cell lysate was extracted for MAPK experiments, whereas proteins were isolated from nuclear and cytosolic cell extracts in glucocorticoid receptor translocation experiments. Fifty micrograms of total protein was loaded on a 7.5% denaturing gel, electrophoresed, and then transferred to a PVDF membrane. The antibodies used were glucocorticoid receptor (Millipore, Billerica, MA), β-actin, α-tubulin, and RNA polymerase II (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), pERK1/2, ERK1/2, p-p38, p38, pSAPK-JNK, and SAPK-JNK (Cell Signaling, Danvers, MA). PD98059 and calyculin A were purchased from Cell Signaling (Danvers, MA), and wedelolactone was purchased from Sigma. β-Actin, α-tubulin, and RNA polymerase II antibodies were used as loading controls for cytosolic and nuclear extract, respectively.
Statistics
Each cytokine supernatant protein concentration was expressed as the percentage change versus placebo ± S.D., and comparisons between group means were assessed using an unpaired Student's t test. Significance was defined as p < 0.05.
RESULTS
Morphine Withdrawal Inhibits LPS-induced IL-12p40 Cytokine Expression, and This Effect Is Abrogated in MORKO Mice
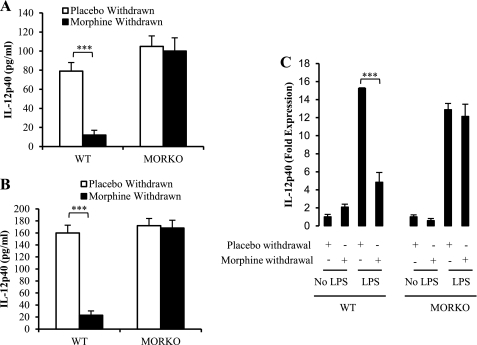
Wild type (WT) mice were subjected to placebo withdrawal (sham withdrawal) or morphine withdrawal by the removal of either placebo or morphine pellet in the presence of LPS stimulation. Following the withdrawal periods, the animals were sacrificed, and serum samples were collected to assess IL-12p40 protein levels by ELISA. MW significantly decreased LPS-induced IL-12p40 production at 8 h postwithdrawal. It was interesting to observe that although in the MORKO mice MW did not result in significant inhibition in IL-12p40, the base-line IL-12p40 levels in the placebo group were significantly greater in the MORKO animals compared with the WT animals. The increased base-line levels in the MORKO group may be attributed to endogenous opioids that may be binding to MOR in the WT animals to modulate IL-12p40. These data further support the role of MOR in MW-induced inhibition of IL-12p40. (Fig. 1A). Peritoneal macrophages were also collected from these animals and cultured overnight and restimulated ex vivo with LPS. Results demonstrated a significant decrease in IL-12p40 production in peritoneal macrophages harvested from morphine-withdrawn animals (24 h) when compared with placebo-withdrawn animals. When the same manipulations were performed in MORKO mice, there was no significant difference between any of the groups examined, indicating that morphine-induced decrements in IL-12p40 production involved the classical μ-opioid receptor (Fig. 1B).
FIGURE 1.
Morphine withdrawal decreases serum levels, protein levels, and message levels of LPS-induced IL-12p40 in WT but not in MORKO mice. Blood was collected by cardiac puncture from WT mice and MORKO mice (A) that underwent 8 h of withdrawal in the presence of LPS (n ≥ 3/group). Cytokine-specific ELISAs were performed on the serum samples to assess levels of IL-12p40. Peritoneal macrophages were collected from WT mice and MORKO mice (B) that underwent 24 h of withdrawal in the presence of LPS (n ≥ 3/group). Cytokine-specific ELISAs were performed on the cell supernatants to assess the protein levels of IL-12p40. Data are presented as pg/ml ± S.D. (error bars) and are representative of at least three independent experiments. C, splenocytes extracted from WT and MORKO mice (n ≥ 3/group) were either placebo- or morphine-withdrawn in the presence or absence of LPS (100 ng/ml). Total RNA was extracted from splenocytes and reverse transcribed and used for real-time PCR. Data are presented as -fold expression of IL-12p40 ± S.E. (error bars) and are representative of three independent experiments. ***, p < 0.001.
IL-12p40 Message Levels in Primary Splenic Macrophage Cells Are Also Inhibited in Morphine-withdrawn Samples
Primary splenic macrophage cells were extracted from LPS-stimulated morphine-withdrawn and placebo-withdrawn WT and MORKO mice (n ≥ 3). The message level of IL-12p40 was measured using quantitative RT-PCR (Fig. 1C). Our results show that MW decreased LPS-induced message levels of IL-12p40 almost 5-fold, whereas this decrease was abolished in MORKO mice, indicating that an intact μ-opioid receptor is essential for withdrawal-mediated LPS-induced IL-12p40 production and that modulation of IL-12p40 following morphine withdrawal is transcriptionally regulated.
Morphine Withdrawal Stress Induces a Transient Increase in Plasma Corticosterone in WT Mice through a MOR-dependent Pathway
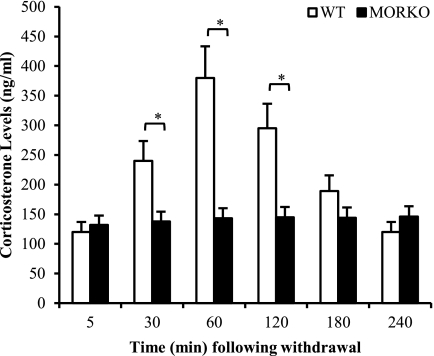
To determine if morphine withdrawal resulted in HPA activation and corticosterone release, WT and MORKO mice were morphine-withdrawn as described under “Experimental Procedures.” Corticosterone levels in plasma were evaluated at varying time points. We show that MW resulted in an increase in corticosterone levels, which peaked at around 60 min following withdrawal and returned back to base line at around 240 min following withdrawal (Fig. 2). The MW-induced increase in plasma corticosterone levels was completely abolished in the MORKO mice (Fig. 2), once again implicating the role of MOR in MW-induced activation of the HPA axis.
FIGURE 2.
Morphine withdrawal induced a transient increase in corticosterone levels in WT but not in MORKO mice. Plasma samples were collected from WT and MORKO mice (n ≥ 3/group) at different time points just following MW, and corticosterone levels were measured using the 125I double antibody radioimmunoassay method. Mean concentrations are expressed as ng/ml ± S.D. (error bars) and represent three independent experiments. *, p < 0.05.
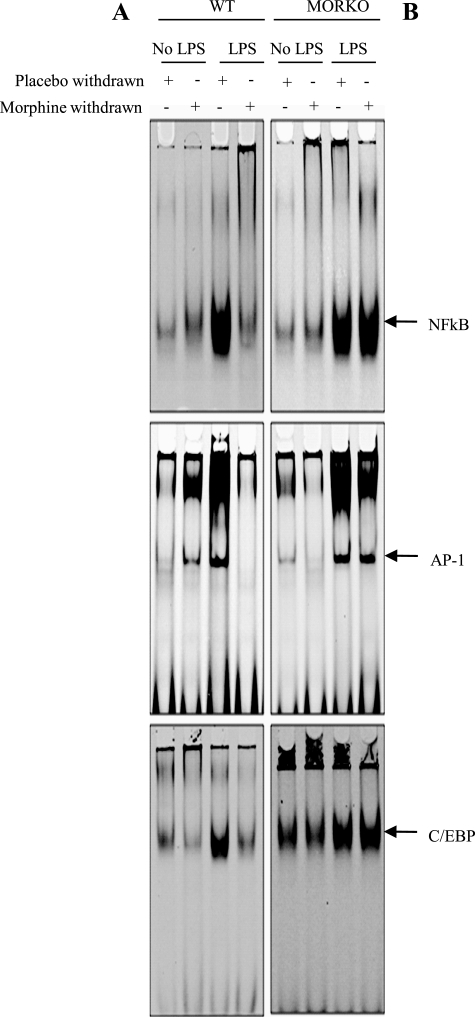
Morphine Withdrawal Inhibits LPS-induced Consensus Sequence Binding to NFκB, C/EBP, and AP-1 in Splenocytes Derived from WT Mice but Not in MORKO Mice
Spleen-derived macrophages were harvested from WT and MORKO mice and used in the following experiments, and nuclear protein extracts from these samples were subjected to EMSA analysis. The transcription factors tested were selected because they were all known to have binding sites on the IL-12p40 promoter. LPS treatment resulted in a significant increase in the binding of NFκB, C/EBP, and AP-1 to their respective consensus binding sequences when compared with untreated samples. MW alone did not result in a significant change in consensus oligonucleotide binding; however, MW in the presence of LPS resulted in a significant decrease in LPS-induced binding to all three consensus oligonucleotides (Fig. 3A). MW-mediated inhibition of LPS-induced binding of transcription factors to the consensus oligonucleotide was completely abolished in macrophages harvested from MORKO splenocytes (Fig. 3B) because there were no differences in interaction between LPS and LPS plus morphine-withdrawn samples. These results implicate MW modulation of LPS-induced transcriptional regulation of IL-12p40 as the mechanism by which MW inhibits IL-12p40 protein levels.
FIGURE 3.
Morphine withdrawal decreased consensus sequence binding of NFκB, AP-1, and C/EBP in WT mice but not in MORKO mice. Splenocytes were isolated from WT (A) and MORKO (B) mice (n ≥ 3/group) that underwent 8 h of withdrawal in the absence or presence of LPS, and the cells were subsequently treated overnight with 100 ng/ml LPS. Nuclear extracts were prepared and incubated with IR700-labeled DNA probes for NFκB, AP-1, and C/EBP transcription binding sites. DNA-protein complexes were run on 4.5% non-denaturing acrylamide gels and visualized on an Odyssey scanner (LiCor Biosciences). Each scanned figure is representative of three independent experiments.
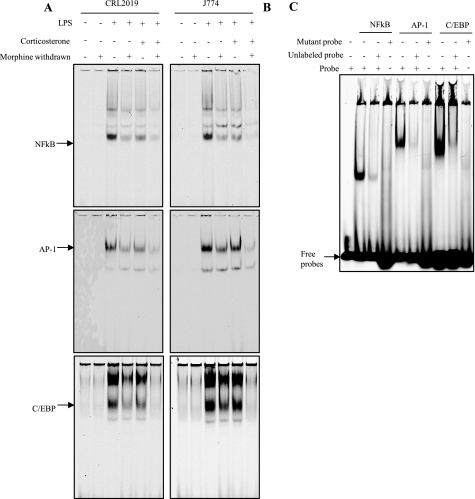
Morphine Withdrawal Reduces Consensus Sequence Binding of LPS-induced Interaction of NFκB, C/EBP, and AP-1 in Macrophage Cells in Vitro, and Corticosterone Treatment Further Attenuates It
The consequences of withdrawal were also investigated in vitro in murine peritoneal macrophage cell line J774.1 and the alveolar macrophage cell line CRL2019. Similar to in vivo MW, in vitro MW in the presence of LPS also resulted in a significant decrease in the binding of the transcription factors NFκB, C/EBP, and AP-1 to their respective consensus oligonucleotide in both CRL2019 cells (Fig. 4A) and J774.1 cells (Fig. 4B). To determine the contribution of corticosterone to MW-induced modulation of IL-12p40 transcription, cells were subjected to morphine withdrawal in the presence or absence of corticosterone. The corticosterone groups were treated with 300 ng/ml (a concentration that was attained in mice following morphine withdrawal) corticosterone 30 min prior to lysis. Although corticosterone treatment alone resulted in a decrease in LPS-induced binding of NFκB, C/EBP, and AP-1 to their respective consensus oligonucleotides, the effect was more dramatic when cells were subjected to both MW and corticosterone treatment. Interestingly, the inhibition observed with corticosterone treatment was more dramatic in the CRL2019 cells when compared with J774.1 cells. LPS-induced NFκB binding was dramatically and consistently higher in both cell lines when compared with AP-1 and C/EBP, underscoring its role in the transcriptional regulation of IL-12p40. MW in the presence of LPS and corticosterone reduced the NFκB binding to basal levels. Although treatment with corticosterone alone did not dramatically decrease LPS-induced binding of AP-1 as well as C/EBP to their consensus oligonucleotide, the combination of MW and corticosterone treatment reduced binding of these transcription factors following LPS stimulation to base-line levels. These data show that NFκB is a strong modulator of transcriptional regulation of IL-12p40 in addition to AP-1 and C/EBP.
FIGURE 4.
Corticosterone attenuated LPS-induced decrease in consensus sequence binding of NFκB, AP-1, and C/EBP in CRL2019 cells and J774.1 cells. CRL 2019 (A) and J774.1 (B) cells were treated with corticosterone and/or LPS following morphine withdrawal. Nuclear extracts were incubated with IR700-labeled DNA probes for NFκB, AP-1, and C/EBP. DNA-protein complexes were run on 4.5% non-denaturing acrylamide gels and visualized on an Odyssey scanner (LiCor Biosciences). Each scanned figure is representative of three independent experiments. C, specificity of probe binding. Nuclear extracts from CRL 2019 cells treated with LPS and/or corticosterone following morphine withdrawal were incubated with IR700-labeled consensus sequence probes only, with probes, with mutated probes, and with cold probes with no labeling to validate specific binding of probes. DNA-protein complexes were run on 4.5% non-denaturing acrylamide gels and visualized on an Odyssey scanner (LiCor Biosciences). Each scanned figure is representative of three independent experiments.
To determine the specificity of the binding interactions, labeled probes were competed with either excess unlabeled consensus oligonucleotide or mutated consensus oligonucleotide (Fig. 4C). Cold probe competed with the labeled probe and decreased LPS-induced shift in the oligonucleotides. Mutated probes failed to induce a shift in the binding, validating the specificity of the binding to the consensus oligonucleotides.
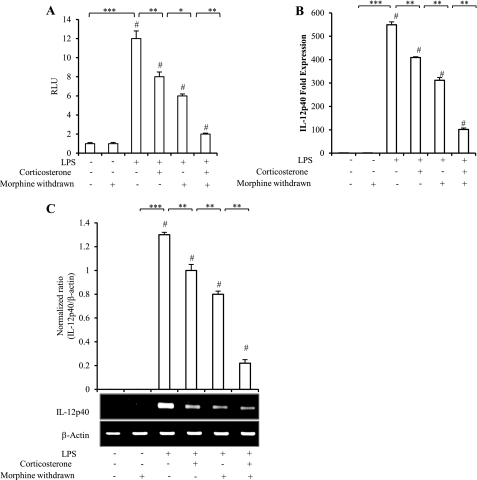
In Vitro Morphine Withdrawal in the Presence of Corticosterone Potentiates a Decrease in IL-12p40 Promoter Activity
In order to understand in more detail how MW inhibits LPS-induced IL-12p40 mRNA expression, a previously established in vitro MW model was used (24). In this set of experiments, CRL2019 macrophages were transfected with the IL-12p40 promoter-luciferase constructs and treated according to the in vitro withdrawal methodology described under “Experimental Procedures.” Cell lysates were prepared, and luciferase activity, an indicator of IL-12p40 activation, was measured. As expected (Fig. 5A), LPS treatment resulted in a significant increase in IL-12p40 promoter activity when compared with no treatment controls. Cells subjected to MW showed a significant decrease in LPS-induced IL-12p40 promoter activity when compared with LPS alone treatment groups, although MW by itself had no effect on IL-12p40 promoter activity. Corticosterone-treated cells also showed a significant decrease in LPS-induced IL-12 promoter activity. Interestingly, when cells were subjected to both MW and corticosterone treatment, an additive decrease in IL-12p40 promoter activity was observed. These results suggest that corticosterone may contribute to the decreased promoter activity but, more importantly, that MW can also act independently of corticosterone and inhibit LPS-induced IL-12p40 promoter activity. These results were interesting, given that in many cases, it was the subsequent production of corticosterone caused by either morphine treatment or withdrawal that resulted in decrements of several physiological functions.
FIGURE 5.
In vitro morphine withdrawal and corticosterone treatment decreased IL-12p40 promoter activity and message levels in LPS-stimulated CRL2019 macrophages. CRL2019 macrophages were transfected with IL-12p40 promoter construct followed by morphine withdrawal paradigm and stimulated for 6 h with 100 ng/ml LPS. Promoter activity (A) is presented as standardized luciferase activity. Total RNA was extracted, and message levels were analyzed using real-time PCR (B) and gel-based PCR (C) for IL-12p40. Housekeeping gene β-actin was amplified as an internal control. The graph was plotted against data normalized to β-actin expression. Each treatment group was tested in triplicate, and results are representative of at least three independent experiments ± S.D. (error bars). #, p < 0.01 versus control samples; *, p < 0.01; **, p < 0.05; ***, p < 0.001. RLU, Relative Luciferase Units.
We further investigated the role of corticosterone on MW-induced modulation of LPS-induced IL-12p40 message levels in CRL2019 cells using real-time PCR and gel-based PCR. LPS treatment resulted in a 550-fold induction in IL-12p40 message levels (Fig. 5B). In cells subjected to MW, LPS-induced IL-12p40 message levels were significantly decreased (46% reduction). Corticosterone treatment also resulted in a significant reduction in LPS-induced IL-12p40 mRNA levels (27%), but the effects were less than those observed with MW. However, when cells were subjected to both MW and corticosterone treatment, there was an additive decrease (82%) in IL-12p40 mRNA levels. Similar results were obtained with gel-based PCR (Fig. 5C). These data clearly indicate that MW-mediated modulation of LPS-induced IL-12p40 transcriptional regulation at the message level is independent of corticosterone, but the presence of corticosterone potentiates MW effects.
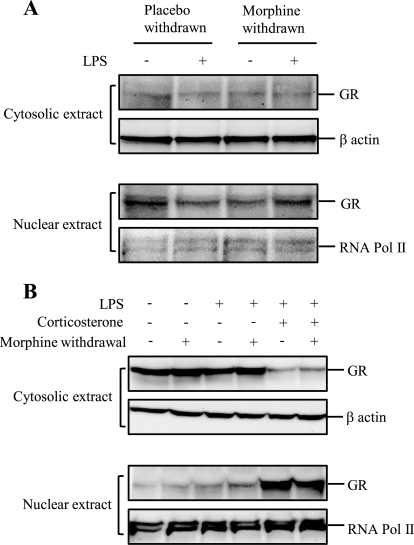
In Vivo Morphine Withdrawal Has No Effect on Glucocorticoid Receptor Translocation in WT Mice
Corticosterone, a glucocorticoid, binds to cytosolic glucocorticoid receptors. Ligand-activated receptors then translocate to the nucleus in order to bind to the glucocorticoid receptor response element to modulate gene transcription. To further determine if modulation of glucocorticoid receptor nuclear translocation is a possible mechanism for the potentiating effect of corticosterone, we investigated the effect of MW on glucocorticoid receptor translocation in morphine-withdrawn samples. WT mice were treated with LPS or saline for 8 h following the morphine withdrawal paradigm. The nuclear and cytosolic proteins were extracted from splenic macrophages. The proteins were electrophoresed and transferred to PVDF membrane. The membranes were probed with antibody against glucocorticoid receptor. LPS did not induce glucocorticoid receptor translocation in either placebo-withdrawn or morphine-withdrawn splenocyte-derived macrophages (Fig. 6A), indicating that HPA activation with the release of glucocorticoids does not act at the transcriptional level to modulate MW-induced inhibition of LPS-induced IL-12p40 expression.
FIGURE 6.
Morphine withdrawal did not increase the glucocorticoid receptor (GR) translocation to nucleus in WT mice and CRL2019 macrophage cells. Splenic macrophages from WT mice (A) (n ≥ 3/group) or CRL2019 cells (B) were treated with the morphine withdrawal paradigm as discussed under “Experimental Procedures.” Nuclear extract and cytosolic extract were prepared, and 50 μg of total proteins was loaded onto 7.5% denaturing gel. The membranes were probed for glucocorticoid receptor. Blots were reprobed with β-actin and RNA polymerase II antibody as loading controls for cytosolic extract and nuclear extract, respectively. Each blot is representative of at least three independent experiments.
When the effects of MW and corticosterone treatment were tested in an in vitro MW model, as expected, corticosterone treatment alone resulted in a significant translocation of glucocorticoid receptor into the nucleus in glucocorticoid receptor to nucleus in CRL2019 cells (Fig. 6B). However, similar to in vivo studies, nuclear translocation of glucocorticoid receptor was not observed in morphine-withdrawn samples, and no additive effect on nuclear translocation was observed in MW samples that were treated with corticosterone. These data clearly indicate that the potentiating effects of corticosterone on morphine withdrawal were not mediated through modulation of gene transcription through glucocorticoid receptor translocation but may be mediated through a non-genomic pathway.
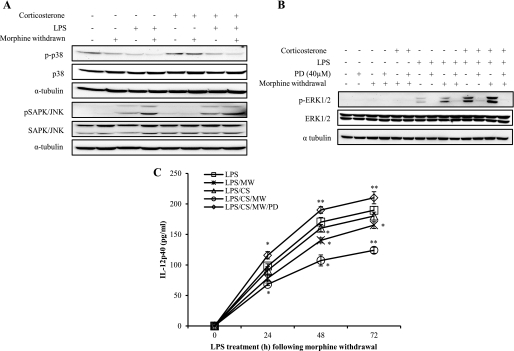
Morphine Withdrawal Results in LPS-induced Hyperactivation of ERK1/2 and Induction of SAPK/JNK Activation, Which Is Potentiated in the Presence of Corticosterone
Macrophage activation by microbial components involves cascades of intracellular signaling pathways, including those that lead to activation of different MAPKs like ERK1/2, p38, and SAPK/JNK. LPS-induced activation of SAPK/JNK was observed in the MW samples and corticosterone-treated samples. However, we did not observe an augmented activation when cells were subjected to both MW and corticosterone (Fig. 7A), suggesting that the augmented IL-12p40 response that we observed when both insults were present may not be mediated by SAPK/JNK activation. Interestingly, LPS treatment did not result in any significant activation of p38MAPK, and the effect was not dramatically modulated by MW or corticosterone (Fig. 7A). We then determined if ERK1/2 kinases play a role in MW-induced inhibition of LPS-induced IL-12p40 expression. Our result show that MW alone in the presence of LPS results in significant activation of ERK1/2 when compared with vehicle-withdrawn samples. Interestingly, a dramatic hyperactivation was observed in MW samples that were treated with corticosterone (Fig. 7B). From these data, we conclude that hyperactivation of ERK1/2 may be a potential mechanism by which MW and corticosterone may negatively regulate LPS-induced IL-12p40 expression.
FIGURE 7.
Morphine withdrawal hyperactivates ERK1/2 with corticosterone treatment in LPS-stimulated CRL2019 cells, and ERK1/2 activation is inhibited by PD98059. CRL2019 cells were treated with PD98059 for 1 h before treating with 100 ng/ml LPS (6 h) and/or 300 ng/ml corticosterone (30 min) before the end of LPS treatment following the morphine withdrawal paradigm as discussed under “Experimental Procedures.” Total cell extract was prepared, and 50 μg of total proteins were loaded onto 10% denaturing gel. A, the membranes were probed with phosphorylated isoforms and total isoforms of p38 and SAPK/JNK MAPKs. The blots were reprobed with α-tubulin as a loading control. B, the membranes were probed with phosphorylated isoforms and total isoforms of ERK1/2 MAPK antibodies. The blots were reprobed with α-tubulin as a loading control. C, cells were treated with PD98059 (40 μm) for 1 h before treating with LPS (100 ng/ml) for 0, 24, 48, and 72 h following morphine withdrawal. Corticosterone treatment was done for 30 min at the end of LPS treatment, and the cultured medium was collected to perform ELISA for IL-12p40. IL-12p40 protein was expressed as pg/ml concentration for LPS alone (□), LPS with morphine withdrawal (×), LPS with corticosterone (CS) (△), LPS with corticosterone in MW samples (○), or LPS plus corticosterone with MW in the presence of PD98059 (PD) (♢). Each experiment is a representation of at least three independent experiment ± S.D. (error bars). *, p < 0.05; **, p < 0.01 compared with LPS alone samples.
Treatment with ERK1/2 Inhibitor PD98059 Rescues Morphine Withdrawal-mediated Suppression of LPS-induced IL-12p40 Expression
To determine if ERK1/2 hyperactivation is the mechanism underlying MW modulation of LPS-induced IL-12p40 expression, we tested if the ERK1/2 inhibitor PD98059 (40 μm) will rescue MW induced modulation of LPS-induced IL-12p40. CRL 2019 cells were pretreated with either PD98059 or vehicle and subjected to MW alone or MW in the presence of corticosterone. As shown before, LPS treatment induced IL-12p40 protein levels in a time-dependent manner, and MW and MW plus corticosterone significantly inhibited LPS-induced IL-12p40 protein levels. However, when cells were pretreated with PD98059 (40 μm) and then subjected to MW or MW plus corticosterone, the effect of MW- or MW plus corticosterone-induced inhibition was completely reversed at every time point tested (Fig. 7C). These data clearly indicated that MW- or MW plus corticosterone-induced inhibition of LPS-induced IL-12p40 expression suppression was mediated through a mechanism that involved ERK1/2 activation.
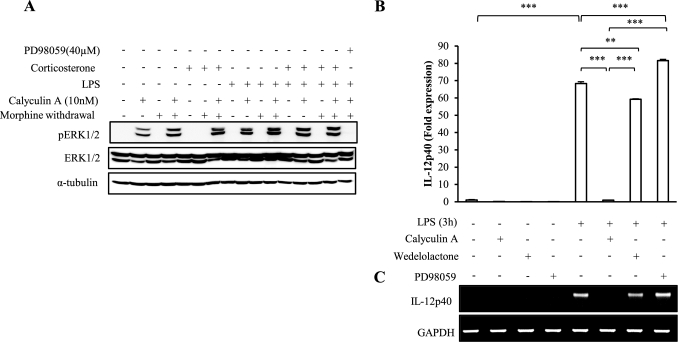
Treatment with Phosphatase PP2A Inhibitor Hyperactivates ERK1/2 Phosphorylation and Further Attenuates IL-12p40 Message Levels
In pursuit of what regulates ERK1/2 hyperactivation, cells were treated with phosphatase PP2A inhibitor calyculin A prior to LPS treatment following the MW paradigm. PP2A is a positive modulator of cellular IKK activity and interacts with regulatory subunit IKKγ. Our data show that MW inhibits NFκB activation, implying inhibition of IKK activation. We further demonstrate that calyculin A treatment led to greater ERK1/2 phosphorylation in the MW and corticosterone-treated samples, suggesting a role for PP2A in modulating ERK1/2 activation (Fig. 8A). Calyculin A treatment further hyperactivated ERK1/2 phosphorylation even in the resting cells beyond basal phosphorylation. In LPS and corticosterone-treated MW samples, calyculin A treatment phosphorylated ERK1/2 to a greater extent compared with no calyculin A treatment, so much so that the basal hyperactivation was not seen in these samples. Basal ERK1/2 phosphorylation in samples not treated with calyculin A was not visible because of a masking effect due to a very high ceiling effect of ERK1/2 phosphorylation in calyculin A-treated samples. This overhyperactivation of ERK1/2 kinase due to calyculin A treatment was inhibited when ERK1/2 inhibitor PD98059 was used, establishing that PP2 activation is upstream to ERK1/2 activation (lane 16). To further establish that ERK1/2 hyperactivation was the potential mechanism involved in MW-induced IL-12p40 inhibition, we investigated the effect of calyculin A and the IKK inhibitor, wedelolactone, on IL-12p40 expression. CRL 2019 cells were treated with the inhibitors, followed by LPS treatment for 3 h. Our data show that calyculin A treatment completely suppressed LPS-induced IL-12p40 message levels as early as at 3 h of LPS treatment (Fig. 8, B and C). Although significant inhibition in LPS-induced IL-p40 was observed in wedelolactone-treated samples, the effect was not as dramatic as calyculin A treatment. Both the effect of calyculin A and that of wedelolactone were inhibited by PD98059, indicating that the final common downstream signal is ERK1/2 activation. This confirms that LPS plus corticosterone treatment in the presence of MW inhibits the IKK-NFκB signal transduction pathway, and this dysregulation of IKK activity means that less PP2A is available for repressing the MEK1/2 and ERK1/2 pathway, therefore leading to hyperactivation of ERK1/2.
FIGURE 8.
Treatment with PP2A inhibitor calyculin A hyperactivates basal ERK1/2 phosphorylation and inhibits message levels of IL-12p40. A, CRL2019 cells were treated with PP2A inhibitor calyculin A (10 nm) and ERK1/2 inhibitor PD98059 (40 μm) for 1 h before treating with LPS (100 ng/ml) for 6 h followed by corticosterone treatment (300 ng/ml) for 30 min following morphine withdrawal. Total protein was extracted, and 50 μg was loaded onto 10% denaturing gel. The membranes were probed with phosphorylated isoforms and total isoforms of ERK1/2 MAPK antibodies. The blots were reprobed with α-tubulin antibody as a loading control. Each blot is representative of three independent experiments. B and C, CRL 2019 cells were treated with PP2A inhibitor calyculin A (10 nm), IKK inhibitor wedelolactone (20 μm), and ERK1/2 inhibitor PD98059 (40 μm) for 1 h, followed by LPS (100 ng/ml) treatment for 3 h. Following treatment, cells were washed, and total RNA was extracted. cDNA was used to analyze message levels of IL-12p40 using real-time PCR (B) and gel-based PCR (C). Housekeeping gene GAPDH was amplified as internal control. Error bars, S.D.
DISCUSSION
The aim of the current investigations was to delineate the mechanism underlying MW-induced inhibition of IL-12p40, a key cytokine that is produced by phagocytic macrophages for the regulation of antigen-presenting cells and effector lymphocytes during an immune response to pathogens. We demonstrate, using an in vivo model of MW, a significant decrease in LPS-induced IL-12p40 production in the plasma of MW animals. Ex vivo stimulation of peritoneal macrophages harvested from MW mice with LPS also showed a significant decrease in IL-12p40 production. MW-mediated modulation of LPS-induced IL-12p40 was completely abolished in the MORKO animals, implicating the role of μ-opioid receptors in MW-induced changes.
To delineate the molecular mechanism underlying MW effects on LPS-induced IL-12p40, mRNA and promoter activity of IL-12p40 were investigated following MW. Our data show that LPS-induced IL-12p40 mRNA levels were significantly inhibited in peritoneal macrophages harvested from MW animals when compared with placebo withdrawal animals. Because the promoter of the IL-12p40 gene contains functional cis-acting sequences, including response elements for NFκB, AP-1, and C/EBP (26–29), which are key transcription factors in inflammation, we used EMSA to understand how MW modulated IL-12p40 production at the transcriptional level. We demonstrate that MW resulted in a significant blunting of LPS-induced binding of transcription factors NFκB, C/EBP, and AP-1 to DNA consensus oligonucleotide sequences. These results suggest that MW may result in the modulation of signal transduction pathways that converge on LPS/TLR4 signaling to post-translationally modify transcription factors, leading to disruption in their nuclear translocation.
The role of stress in drug addiction is well established. The noradrenergic system and the HPA axis comprise two major adaptive mechanisms to stress. Like stressors, morphine withdrawal activates the HPA axis in rats (16), which results in the release of adrenocorticotropin from the pituitary with subsequent increase in corticosterone secretion (16, 30, 31). Similarly, we show a significant but transient increase in corticosterone following MW in the WT animals. The role of corticosterone as an immunosuppressor has been well documented. At the molecular level, at least three mechanisms have been proposed to mediate glucocorticoid effects on immunity and inflammation. Glucocorticoid receptor-induced transactivation and transrepression are the “classical” mechanisms whereby ligand-activated glucocorticoid receptors bind to glucocorticoid receptor response elements and either activate or repress transcription of the targeted gene. Transactivation of genes encoding inhibitory proteins and transrepression of inflammatory genes have been described. However, the majority of anti-inflammatory effects are due to so-called cross-talk, in which glucocorticoid-bound glucocorticoid receptors interact with transcription factor proteins, such as NFκB and AP-1, interfering with their ability to activate transcription of target genes. To determine the role of corticosterone in MW-mediated modulation of LPS-induced IL-12p40, macrophage cell lines, CRL2019 and J774.1, were subjected to MW in the absence and presence of corticosterone. Interestingly, MW in the absence of corticosterone was able to significantly inhibit LPS-induced binding of the transcription factors NFκB, C/EBP, and AP-1 to DNA consensus oligonucleotide sequences, suggesting a mechanism that is independent of corticosterone. However, a significant and a more than additive effect is seen when corticosterone is present at the time of MW, suggesting a potentiating effect of corticosterone. Although glucocorticoids have also been shown to decrease IL-12p40 production through strong inhibition of NFκB and AP-1, there are no reports of glucocorticoid receptor response element sites in murine IL-12p40 promoter (25, 27). However, mutation in these transcription factor binding sites in the promoter of human IL-12p40 abrogated the luciferase assay, indicating the importance of these transcription factors in regulating IL-12p40 gene following glucocorticoid treatment (25). As a complement to the classic genomic theory, a non-genomic mechanism has been proposed for the rapid action of glucocorticoids (32, 33). It is speculated that glucocorticoids might affect the expression of genes by modulating cell signaling pathways that are not activated via glucocorticoid receptor activation (i.e. non-genomic pathways). In our study, when glucocorticoid receptor translocation into the nucleus was investigated, although corticosterone treatment resulted in a significant increase in glucocorticoid receptor nuclear translocation, MW treatment resulted in no significant increase in glucocorticoid receptor translocation with MW either in vivo or in vitro, suggesting that corticosterone may be potentiating MW effects through a non-genomic pathway.
Previous studies have reported that inhibition of p38, ERK, or JNK in primary monocytes results in enhanced binding of AP-1 and Sp1 to IL-12p40 promoter (34). In contrast, p38 and ERK inhibition had essentially no effect on NFκB binding. Contrary to that, in THP-1 cells, inhibition of p38, ERK, and JNK significantly enhanced LPS-induced binding of NFκB and Sp1 to IL-12p40 promoter, whereas AP-1 binding decreased (34). In another report, IL-12p40 production is regulated by NFκB and AP-1 through the activation of upstream calcium and PI3K pathways (35). In macrophages, C/EBP is involved in the inducible expression of several genes that are important for inflammation and immunity, including IL-12p40. In our experiments, MW decreased LPS-induced C/EBP binding with consensus sequence. The activity and expression of three C/EBP members (α, β, and δ) are regulated by a number of inflammatory signals, including LPS and a range of cytokines (36, 37). Interestingly, a recent study showed that triptolide-induced inhibition of IL-12p40 transcription was preceded by sustained phosphorylation of ERK1/2 and that blocking the activity of ERK1/2 but not those of p38 and JNK can substantially rescue triptolide-induced inhibition of IL-12p40 production (38). Our data show that MW resulted in a dramatic and significant increase in LPS-induced ERK1/2 activation. A similar LPS-induced ERK1/2 activation was also observed with corticosterone treatment alone. Interestingly, LPS-induced ERK1/2 was significantly hyperactivated in the presence of MW and corticosterone. ERK is a family of serine/threonine protein kinases that have been functionally linked to addiction through phosphorylation of transcription factors leading to changes in target gene expression. ERK phosphorylates various substrates, including many enzymes, transcription factors, and proteins. Following activation, ERK dissociates from cytoplasmic anchors, such as MEK, and translocates to the nucleus, where it phosphorylates its nuclear substrates. Activated ERK does not always localize to the nucleus. Several transcription factors are activated by ERK in the cytoplasm, and they translocate to the nucleus after phosphorylation (39, 40). MW have been shown to up-regulate ERK1/2 phosphorylation in neuronal cells. Naloxone-precipitated withdrawal in morphine-dependent rats, resulting in an intense behavioral reaction (41), induced a robust stimulation of MEK1/2 in the cerebral cortex and corpus striatum. Other studies have shown activation of the spinal ERK1/2 pathway may contribute to the development of morphine dependence and withdrawal, and the function of pERK1/2 is partly accomplished via the CREB-dependent gene expression (42). Our present findings further attribute ERK1/2 activation as a potential mechanism in MW-induced IL-12p40 inhibition. We show that MW-induced increase in ERK activity was due to an enhancement in the phosphorylation state of the enzyme, without changes in total ERK immunoreactivity. This suggests that the effects of MW that may be mediated by ERK1/2 are likely to be affected through the activation (via phosphorylation) of ERKs. This conclusion was further supported by the use of an inhibitor (PD98059) of MEK1/2, upstream signaling proteins of ERK1/2, which rescued MW-induced inhibition of IL-12p40 expression. LPS stimulation in macrophages triggers cascades of intracellular signaling events, including those that lead to activation of IKK. IKK activation leads to the activation of the phosphatase PP2A, thereby dephosphorylating ERK1/2. This was further supported by real-time PCR data where calyculin A hyperactivated ERK1/2 phosphorylation but at the same time suppressed IL-12p40 message levels as early as 3 h following LPS treatment. Wedelolactone, the inhibitor of IKK that is upstream of PP2A, also suppressed IL-12p40 message levels, albeit to a lesser degree when compared with calyculin A. This inhibition of IL-12p40 expression was reversed when cells were treated with the ERK1/2 inhibitor PD98059. Therefore, we speculate that MW inhibits LPS-induced IKK activity, thereby inactivating PP2A activity. This leads to persistent activation of MEK1/2, leading to hyperactivation of ERK1/2 and suppression of IL-12p40 expression (Fig. 9).
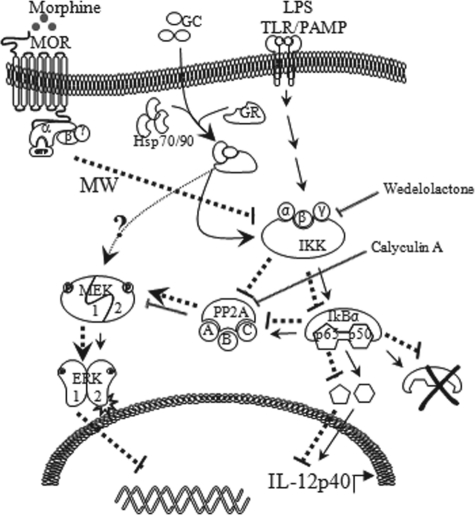
FIGURE 9.
Proposed mechanism for negative regulation of IL-12p40 expression by hyperactivation of ERK1/2 in the presence of LPS, corticosterone, and MW. Key mediators downstream of the LPS signaling pathway lead to expression of IL-12p40 (solid lines). MW in the presence of corticosterone inhibits LPS-induced IKK activation, and subsequent phosphorylation of IkBα therefore decreases translocation of p65 and p50. At the same time, inhibited IKK inactivates PP2A recruitment and its phosphatase activity, thereby promoting hyperactivation of MEK1/2 and subsequently ERK1/2 hyperactivation (dotted lines). Inhibition of PP2A activity hyperactivates ERK1/2 phosphorylation and down-regulates IL-12p40 message levels. GC, glucocorticoid.
The unique role of IL-12p40 in the regulation of IL-12 suggests that it is critically involved in the immunopathogenesis of Th1-mediated inflammatory and autoimmune disorders. Our investigations regarding the mechanism underlying the inhibitory effects of MW on IL-12p40 production have revealed the role of different transcription factors (in particular NFκB, AP-1, and C/EBP) in regulating IL-12 production. At the same time, this is also regulated by the ERK1/2 pathway via a feedback mechanism thereby controlling the system in check. These results suggested that MW can disrupt the normal immune function and can possibly lead to enhanced susceptibility to infection. Understanding mechanisms underlying it can be a driving force to understand host responses and cellular immune functions to HIV and drug abusers and relapsed patients undergoing withdrawal phases.
Acknowledgment
We thank Dr. A. Kumar (University of Ottawa) for the human IL-12p40 promoter.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 DA 12104, RO1 DA 022935, KO2 DA 015349, and P50 DA11806 (to S. R.).
- HPA
- hypothalamic-pituitary-adrenal
- MW
- morphine withdrawal
- MOR
- μ-opioid receptor
- MORKO
- μ-opioid receptor knock-out
- SAPK
- stress-activated protein kinase
- C/EBP
- CCAAT/enhancer-binding protein.
REFERENCES
- 1. Roy S., Wang J., Kelschenbach J., Koodie L., Martin J. (2006) J. Neuroimmune Pharmacol. 1, 77–89 [DOI] [PubMed] [Google Scholar]
- 2. Sacerdote P. (2008) Curr. Opin. Support Palliat. Care 2, 14–18 [DOI] [PubMed] [Google Scholar]
- 3. Rahim R. T., Adler M. W., Meissler J. J., Jr., Cowan A., Rogers T. J., Geller E. B., Eisenstein T. K. (2002) J. Neuroimmunol. 127, 88–95 [DOI] [PubMed] [Google Scholar]
- 4. Bhargava H. N., Thomas P. T., Thorat S., House R. V. (1994) Brain Res. 642, 1–10 [DOI] [PubMed] [Google Scholar]
- 5. Eisenstein T. K., Rahim R. T., Feng P., Thingalaya N. K., Meissler J. J. (2006) J. Neuroimmune Pharmacol. 1, 237–249 [DOI] [PubMed] [Google Scholar]
- 6. Rahim R. T., Feng P., Meissler J. J., Rogers T. J., Zhang L., Adler M. W., Eisenstein T. K. (2004) J. Neuroimmunol. 147, 114–120 [DOI] [PubMed] [Google Scholar]
- 7. Rahim R. T., Meissler J. J., Jr., Adler M. W., Eisenstein T. K. (2005) J. Leukocyte Biol. 78, 1185–1191 [DOI] [PubMed] [Google Scholar]
- 8. Rahim R. T., Meissler J. J., Zhang L., Adler M. W., Rogers T. J., Eisenstein T. K. (2003) J. Neuroimmunol. 144, 16–27 [DOI] [PubMed] [Google Scholar]
- 9. Feng P., Wilson Q. M., Meissler J. J., Jr., Adler M. W., Eisenstein T. K. (2005) Infect. Immun. 73, 7953–7959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feng P., Meissler J. J., Jr., Adler M. W., Eisenstein T. K. (2005) J. Neuroimmunol. 164, 57–65 [DOI] [PubMed] [Google Scholar]
- 11. Stewart J. (2003) Am. J. Addict. 12, 1–17 [PubMed] [Google Scholar]
- 12. Bryant H. U., Story J. A., Yim G. K. (1988) Psychosom. Med. 50, 576–585 [DOI] [PubMed] [Google Scholar]
- 13. Bryant H. U., Kuta C. C., Story J. A., Yim G. K. (1988) Biochem. Pharmacol. 37, 3777–3780 [DOI] [PubMed] [Google Scholar]
- 14. Yang E. V., Glaser R. (2002) Int. Immunopharmacol. 2, 315–324 [DOI] [PubMed] [Google Scholar]
- 15. Milanés M. V., Laorden M. L., Angel E., Tankosic P., Burlet A. (2002) Neurosci. Lett. 334, 58–62 [DOI] [PubMed] [Google Scholar]
- 16. Laorden M. L., Castells M. T., Milanés M. V. (2002) Br. J. Pharmacol. 136, 67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kishioka S., Nishida S., Fukunaga Y., Yamamoto H. (1994) Jpn. J. Pharmacol. 66, 257–263 [DOI] [PubMed] [Google Scholar]
- 18. Roy S., Wang J. H., Balasubramanian S., Sumandeep Charboneau R., Barke R., Loh H. H. (2001) J. Neuroimmunol. 116, 147–155 [DOI] [PubMed] [Google Scholar]
- 19. Kelschenbach J., Barke R. A., Roy S. (2005) J. Immunol. 175, 2655–2665 [DOI] [PubMed] [Google Scholar]
- 20. Sieling P. A., Modlin R. L. (1994) Immunobiology 191, 378–387 [DOI] [PubMed] [Google Scholar]
- 21. Zhang M., Gately M. K., Wang E., Gong J., Wolf S. F., Lu S., Modlin R. L., Barnes P. F. (1994) J. Clin. Invest. 93, 1733–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pirmez C., Yamamura M., Uyemura K., Paes-Oliveira M., Conceição-Silva F., Modlin R. L. (1993) J. Clin. Invest. 91, 1390–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seder R. A., Kelsall B. L., Jankovic D. (1996) J. Immunol. 157, 2745–2748 [PubMed] [Google Scholar]
- 24. Way E. L., Loh H. H., Shen F. (1968) Science 162, 1290–1292 [DOI] [PubMed] [Google Scholar]
- 25. Ma W., Gee K., Lim W., Chambers K., Angel J. B., Kozlowski M., Kumar A. (2004) J. Immunol. 172, 318–330 [DOI] [PubMed] [Google Scholar]
- 26. Ma X., Chow J. M., Gri G., Carra G., Gerosa F., Wolf S. F., Dzialo R., Trinchieri G. (1996) J. Exp. Med. 183, 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murphy T. L., Cleveland M. G., Kulesza P., Magram J., Murphy K. M. (1995) Mol. Cell Biol. 15, 5258–5267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Plevy S. E., Gemberling J. H., Hsu S., Dorner A. J., Smale S. T. (1997) Mol. Cell Biol. 17, 4572–4588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhu C., Gagnidze K., Gemberling J. H., Plevy S. E. (2001) J. Biol. Chem. 276, 18519–18528 [DOI] [PubMed] [Google Scholar]
- 30. Núñez C., Földes A., Pérez-Flores D., García-Borrón J. C., Laorden M. L., Kovács K. J., Milanés M. V. (2009) Endocrinology 150, 3118–3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cleck J. N., Blendy J. A. (2008) J. Clin. Invest. 118, 454–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haller J., Mikics E., Makara G. B. (2008) Front. Neuroendocrinol. 29, 273–291 [DOI] [PubMed] [Google Scholar]
- 33. Makara G. B., Haller J. (2001) Prog. Neurobiol. 65, 367–390 [DOI] [PubMed] [Google Scholar]
- 34. Boucher J. G., Parato K. A., Frappier F., Fairman P., Busca A., Saxena M., Blahoianu M. A., Ma W., Gajanayaka N., Parks R. J., Kumar A., Angel J. B. (2010) Viral Immunol. 23, 17–28 [DOI] [PubMed] [Google Scholar]
- 35. Ma W., Mishra S., Gee K., Mishra J. P., Nandan D., Reiner N. E., Angel J. B., Kumar A. (2007) J. Biol. Chem. 282, 13351–13362 [DOI] [PubMed] [Google Scholar]
- 36. Tengku-Muhammad T. S., Hughes T. R., Ranki H., Cryer A., Ramji D. P. (2000) Cytokine 12, 1430–1436 [DOI] [PubMed] [Google Scholar]
- 37. Cardinaux J. R., Allaman I., Magistretti P. J. (2000) Glia 29, 91–97 [PubMed] [Google Scholar]
- 38. Zhang Y., Ma X. J. (2010) J. Immunol. 184, 3866–3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Casar B., Pinto A., Crespo P. (2009) Cell Cycle 8, 1007–1013 [DOI] [PubMed] [Google Scholar]
- 40. Casar B., Arozarena I., Sanz-Moreno V., Pinto A., Agudo-Ibáñez L., Marais R., Lewis R. E., Berciano M. T., Crespo P. (2009) Mol. Cell Biol. 29, 1338–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cao J. L., He J. H., Ding H. L., Zeng Y. M. (2005) Pain 118, 336–349 [DOI] [PubMed] [Google Scholar]
- 42. Cao J. L., Liu H. L., Wang J. K., Zeng Y. M. (2006) Neuropharmacology 51, 315–326 [DOI] [PubMed] [Google Scholar]