Abstract
Gonadotropin-regulated testicular RNA helicase (GRTH/DDX25) is a testis-specific gonadotropin-regulated RNA helicase that is present in Leydig cells (LCs) and germ cells and is essential for spermatid development and completion of spermatogenesis. Normal basal levels of testosterone in serum and LCs were observed in GRTH null (GRTH−/−) mice. However, testosterone production was enhanced in LCs of GRTH−/− mice compared with WT mice by both in vivo and in vitro human chorionic gonadotropin stimulation. LCs of GRTH−/− mice had swollen mitochondria with a significantly increased cholesterol content in the inner mitochondrial membrane. Basal protein levels of SREBP2, HMG-CoA reductase, and steroidogenic acute regulatory protein (StAR; a protein that transports cholesterol to the inner mitochondrial membrane) were markedly increased in LCs of GRTH−/− mice compared with WT mice. Gonadotropin stimulation caused an increase in StAR mRNA levels and protein expression in GRTH−/− mice versus WT mice, with no further increase in SREBP2 and down-regulation of HMG-CoA reductase protein. The half-life of StAR mRNA was significantly increased in GRTH−/− mice. Moreover, association of StAR mRNA with GRTH protein was observed in WT mice. Human chorionic gonadotropin increased GRTH gene expression and its associated StAR protein at cytoplasmic sites. Taken together, these findings indicate that, through its negative role in StAR message stability, GRTH regulates cholesterol availability at the mitochondrial level. The finding of an inhibitory action of GRTH associated with gonadotropin-mediated steroidogenesis has provided insights into a novel negative autocrine molecular control mechanism of this helicase in the regulation of steroid production in the male.
Keywords: Cholesterol, Gene Regulation, Hormones, RNA Helicase, Steroid Hormone, GRTH/DDX25 Null Mice, Leydig Cell, Androgen, Autocrine Regulation, Steroidogenic Acute Regulatory Protein (StAR)
Introduction
Gonadotropin-regulated testicular RNA helicase (GRTH/DDX25)3 is a testis-specific member of the DEAD-box family of RNA helicases present in Leydig and germ cells of pubertal and adult testes. It is the only member of the RNA helicase family of proteins to be hormonally and developmental regulated (1). In Leydig cells, GRTH is transcriptionally up-regulated by gonadotropin via a second messenger (cAMP) by the actions of androgen through androgen receptors present in Leydig cells (2). An atypical functional androgen response element (TGTCCC) half-site identical to the human secretory component gene (3) located at −827 bp in the 5′-flanking region of the GRTH gene (4) plays a key role in the androgen regulation of GRTH gene transcription (5). GRTH is a multifunctional protein and a component of the ribonucleoprotein complex involved in gene-specific mRNA transport from the nucleus to the cytoplasm and protein translation in germ cells (6). GRTH also has an essential role in maintenance of the chromatoid body structure and function in spermatids, where it transports relevant messages presumably for storage/degradation/processing during spermatogenesis (7).
Male mice lacking GRTH are sterile due to lack of sperm resulting from failure of round spermatids to elongate, and the chromatoid body in spermatids is markedly reduced in size (8). The normal basal serum levels of luteinizing hormone, FSH, and testosterone observed in null mice exclude abnormal steroidogenesis as responsible for the arrest of spermiogenesis. Leydig cells from null mice have reduced lipid droplets and swollen mitochondria without normal central cristae. Abundant lipid droplets and mitochondria with lamellar cristae are morphological characteristics of the Leydig cell for its steroidogenic function (9). The mitochondria are the sites where cholesterol (supplied by lipid droplets) is converted to pregnenolone by cytochrome P450 cholesterol side chain cleavage (CYP11A1, P450scc) as the first step in the steroid biosynthetic pathway. However, no difference in the basal protein levels of P450scc and the downstream key enzyme 17α-hydroxylase was observed in the testes of GRTH null mice (8). The abnormal ultrastructural change in Leydig cells in the absence of GRTH could reflect hyperstimulation of the early steroidogenic pathway prior to pregnenolone formation with depletion of lipid droplets and changes in mitochondrial structure.
Gonadotropin-induced steroidogenesis in the gonads is mediated by increases in intracellular cAMP, followed by activation of PKA activity. The specific PKA-anchoring protein AKAP121 tethers PKA type II into the vicinity of steroidogenic acute regulatory protein (StAR), a key transport protein that regulates cholesterol transfer from the outer to the inner mitochondrial membrane (10) in response to cAMP signaling. To explore further the impact of the morphological changes in Leydig cells of GRTH knock-out animals at the functional level, the steroidogenic responses of these cells to gonadotropin was studied in GRTH null mice and compared with WT mice. Because cholesterol is the essential precursor for all steroid hormones, genes involved in the uptake/transport of cholesterol and in de novo cholesterol biosynthesis were analyzed to gain insights into the role of GRTH in the early steps of steroidogenesis in Leydig cells.
EXPERIMENTAL PROCEDURES
Animals
Wild-type (C57BL/6-SV129J, Charles River) and GRTH null (GRTH−/−) male mice (8) were housed under pathogen-free, temperature- and light-controlled conditions (20 °C; alternating light/dark cycle with 14 h of light and 10 h of darkness). All animal studies were approved by the NICHD Animal Care and Use Committee. Animals were killed by asphyxiation with CO2 and decapitated. Testes were removed and decapsulated for Leydig cell purification. Animals were treated with a single subcutaneous (sc) injection of human chorionic gonadotropin (hCG; 5 units of Pregnyl, 0.5 μg/mouse) or vehicle (0.1 ml of PBS) and were killed 24 h after hCG or PBS injection. Serum was collected for testosterone measurement. Leydig cells were isolated from testes by collagenase dispersion and purified by centrifugal elutriation (9).
Testosterone Measurement
The steroidogenic function of Leydig cells (testosterone measurement) was analyzed in the medium of cells for 3 h in the presence or absence of hCG (100 ng). Testosterone in both serum and medium was measured using an enzyme immunoassay kit (ALPCO Diagnostics, Windham, NH) following the manufacturer's protocol.
EM Analysis
Testicular tissue were fixed in 2.5% glutaraldehyde at 4 °C overnight, post-fixed in 1% osmium tetroxide, and en bloc-stained in Spurr's epoxy. Thin sections were post-stained with lead citrate and examined under a transmission electron microscope.
Preparation of Inner Mitochondrial Membrane and Cholesterol Measurement
Mitochondria from Leydig cells (106 cells) were extracted using a mitochondrial isolation kit (Thermo Scientific). The inner mitochondrial membrane was obtained as described previously (11). Briefly, mitochondria were resuspended in isolation buffer (210 mm mannitol, 70 mm sucrose, 0.1 mm EDTA, and 1 mm Tris-HCl buffer (pH 7.4)) and sonicated for 5 min at 40 watts. The inner mitochondrial membrane was obtained by centrifugation at 105,000 × g for 60 min at 4 °C. The pellet containing the inner mitochondria was resuspended in PBS, and protein concentration was determined by the method of Bradford (23) using BSA as a standard. To determine the degree of contamination of the inner mitochondrial membrane with the outer membrane, Western blotting with antibodies against cytochrome c oxidase IV (a marker for inner mitochondrial membranes) was performed. Cholesterol content was measured using an Amplex® red cholesterol assay kit (A12216, Molecular Probes) following the manufacturer's protocol.
Western Blot Analysis
Protein samples were extracted from Leydig cells using mammalian protein extraction reagent (Pierce) in the presence of protease inhibitor mixture (Roche Applied Science). The extracts (25 μg) were subjected to separation on a 4–20% SDS-polyacrylamide gel and transferred to nitrocellulose membrane for Western analysis with GRTH peptide (amino acids 465–477)-specific rabbit polyclonal antibody (2) purified using protein A-Sepharose (Amersham Biosciences) or antibodies (including anti-rabbit antisera) obtained from the indicated commercial sources: anti-HMG-CoA reductase (HMGCR; sc-33827), anti-StAR (sc-25806), anti-ApoA1 (sc-30089), and mouse anti-SREBP2 (sterol regulatory element-binding protein 2) monoclonal (sc-13552) antibodies (Santa Cruz Biotechnology) and anti-cytochrome c oxidase IV antibody (4844, Cell Signaling). β-Actin antisera (monoclonal; Sigma) were used for normalization. Antibodies were used at dilutions of 1:200 to 1:1000, and the appropriate secondary antibody was employed (horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse IgG at 1:10,000 and horseradish peroxidase-conjugated donkey anti-goat IgG 1:1000 to 1:2000). Immunosignals were detected by the SuperSignal chemiluminescence system (Pierce). The signals were recorded on x-ray film and subsequently quantitated by densitometry using a Bio-Rad GS-700 imaging densitometer. The value of each individual immunoreactive band was normalized to the corresponding β-actin immunoreactive band.
Co-immunoprecipitation and RT-PCR Analysis
Testicular extracts (1 mg) or cytoplasmic compartment extracts (500 μg) from WT or GRTH−/− mice isolated utilizing a Pierce extraction kit were initially subjected to preclearing by incubation with 40 μl of protein A-agarose (50% slurry) and 2 μg of normal rabbit IgG in immunoprecipitation assay buffer (50 mm NaCl, 50 mm Tris-Cl, 1% Nonidet P-40, 0.5% deoxycholate, and 0.1% SDS) with gentle agitation. The recovered supernatant was incubated with affinity-purified GRTH peptide (amino acids 465–477)-specific rabbit polyclonal antibody (2 μg) (2) for 2 h at 4 °C in the presence of 1× protease inhibitor mixture to co-immunoprecipitate the GRTH-ribonucleoprotein complex. 50 μl of protein A-agarose in a 50% slurry was added, and incubation was continued overnight at 4 °C. Protein A-precipitated GRTH-ribonucleoprotein complex was recovered by brief centrifugation, followed by washing three times with immunoprecipitation assay buffer. The RNA from the complexes was extracted by phenol/chloroform/isoamyl alcohol (25:24:1, v/v; Invitrogen) and subjected to RT-PCR analysis. First-strand cDNA reverse-transcribed using a SuperScript III first-strand synthesis kit (Invitrogen) was further amplified by PCR with specific primer sets for the genes of interest (see below).
Real-time PCR Quantification of mRNA
Total RNA from Leydig cells obtained from adult mouse testes (GRTH+/+ and GRTH−/−) was isolated using an RNeasy mini kit (Qiagen). Prior to reverse transcription reaction, total RNA was treated with DNase I to remove any possible co-purified genomic DNA. 1 μg of RNA was reverse-transcribed using the SuperScript III first-strand synthesis system containing a mixture of oligo(dT)20. The first-strand cDNA was used as a template in real-time PCR with SYBR Green Master Mix using an ABI 7500 sequence detection system (Applied Biosynthesis). The cycling program was set as follows: denaturing at 95 °C for 10 min, followed by 45 cycles at 95 °C for 15 s and 60 °C for 1 min. Specific primers for gene of interests were designed accordingly: HMGCR (NM_008255.2), TTA ATG GAA GCC AGT GGT CC (forward) and GCC ATC ACA GTG CCA CAT AC (reverse); Srebp2 (NM_033218.1), GCG GAG GAG AAA ATC CTA CC (forward) and GTA CAG GCT CTC CTT GGC TG (reverse); StAR (NM_011485.4), GTT CCT CGC TAC GTT CAA GC (forward) and GCT TTC CTT CTT CCA GCC TT (reverse); β-actin (NM_007393.3), GGT ACC ACC ATG TAC CCA GG (forward) and GAA AGG GTG TAA AAC GCA GC (reverse); and GRTH (NM_013932.4), TCA AAA TCC AAG AGA TGG C (forward) and GAA CTT GCC CAT CCT TTC AA (reverse). The specificity of the PCR products was verified by melting curve analyses at the end of the PCR and agarose gel analysis. Results presented are from three individual experiments; each sample was assayed in triplicate and normalized to the level of β-actin mRNA and is expressed as -fold change from WT controls.
mRNA Stability and Nuclear Run-on of StAR and Other Genes
Leydig cells of WT and GRTH−/− mice were cultured in Medium 199 containing 0.1% BSA for 1 h, followed by incubation with 10 μg/ml actinomycin D for 0–10 h. Total RNA was isolated, reverse-transcribed, and quantitated by real-time PCR as described above. For transcription analysis, nuclei were isolated from Leydig cells by adding lysis buffer (10 mm Tris-HCl, 10 mm NaCl, 3 mm MgCl2, and 0.5% Nonidet P40), and run-on assays were performed as described previously with minor modifications (12). Nuclei were resuspended in 200 μl of storage buffer (50 mm Tris-HCl (pH 8.3), 40% glycerol, 5 mm MgCl2, and 0.1 mm EDTA). Nuclear RNA was labeled by in vitro transcription with 200 μl of nuclear extract by incubating with 20 μl of [32P]UTP (3000 Ci/mmol, 10 mCi/ml; PerkinElmer Life Sciences) and 200 μl of 2× reaction buffer containing 10 mm Tris-HCl (pH 8), 5 mm MgCl2, 0.2 mm KCl, and 1 mm each unlabeled ATP, CTP, and GTP for 30 min at 30 °C. Reactions were stopped by adding RNeasy lysis buffer, followed by total RNA extraction using RNeasy mini kits. Linearized full-length mouse StAR, SREBP2, and β-actin subcloned into the pCR4-TOPO vector (Invitrogen) were immobilized on a GeneScreen Plus membrane (PerkinElmer Life Sciences) using a slot-blot apparatus. The labeled RNAs were then hybridized to the membranes for 36 h at 60 °C. The membranes were washed with 2× SSC and visualized using a PhosphorImager. Signals were quantitated by ImageQuant 2 software and normalized to β-actin as a nascent StAR transcript (Molecular Dynamics).
Immunofluorescence
Purified Leydig cells were fixed in 4% paraformaldehyde for 20 min at room temperature. Cells were spin down for 30 s, and cell pellets were resuspended in 100 μl of PBS. Cell suspensions were spread on slides and dried in a humidified box. The slides were permeabilized with 0.2% Triton X-100 for 5 min and wash three with PBS (3 × 5 min each). Antigen retrieval procedures were performed by incubating slides in 10 mm sodium citrate and 0.05% Tween (pH 6) for 20 min at 95–100 °C, followed by cooling off at room temperature and washing with PBS and 0.1% Tween 20. Nonspecific signals were blocked with 0.2% Triton X-100 and 10% normal goat serum in PBS for 45 min at room temperature. Slides were incubated with primary antibodies in dilution buffer (0.2% Triton X-100, 1% BSA, and 1% normal goat serum in PBS) with GRTH-specific rabbit polyclonal antibody (1:200) purified by peptide affinity chromatography (2) and goat anti-StAR polyclonal antibody (1:200; sc-23523, Santa Cruz Biotechnology) for 1 h at 22 °C. Rabbit or goat IgG was used as the negative control. Subsequently, slides were incubated with secondary antibodies (Alexa Fluor 647 donkey anti-rabbit and Alexa Fluor 568 donkey anti-goat; 1:1000) for 45 min at room temperature. Slides were mounted in ProLong Gold antifade reagent and stained with DAPI (4′,6′-diamidino-2-phenylindole) (Invitrogen). Samples were visualized using an Axioplan 2 imaging fluorescence microscope (Carl Zeiss, Thornwood, NY), and digitized images were taken and processed with AxioVision Version 4.5 software (Carl Zeiss).
Statistical Analysis
The significance of differences in the expression of mRNAs, proteins, and testosterone between groups (WT and knock-out (KO)) was determined by Dunnett's multiple-comparison test (one-way analysis of variance).
RESULTS
Steroidogenic Response (Testosterone) to Gonadotropin Stimulation in WT and GRTH KO Mice
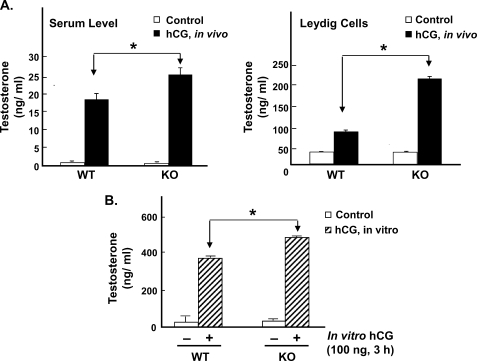
In GRTH KO mice, basal circulating levels of testosterone were normal, and basal testosterone levels in Leydig cells from KO mice were comparable to those in WT mice (Fig. 1A). Upon in vivo hCG stimulation, testosterone serum levels and testosterone production by Leydig cells were significantly increased in both WT and KO mice. However, in GRTH null mice, the steroid increases induced by hCG were significantly higher than those observed in WT mice (Fig. 1A, right panel). Similar to the in vivo hCG response, Leydig cells from GRTH null mice showed enhanced increases in testosterone production upon in vitro hCG stimulation compared with WT mice (p < 0.05) (Fig. 1B). Taken together, these results indicate that GRTH has a negative influence on steroidogenesis that is revealed by the gonadotropin treatment. Because our previous studies demonstrated no changes in steroidogenic enzymes (cholesterol side chain cleavage enzyme and 17α-hydroxylase) in KO mice (8), it is likely that GRTH may affect early steps in either the biosynthesis of cholesterol and/or its transport and availability at mitochondrial sites.
FIGURE 1.
Steroidogenic response to gonadotropin stimulation in WT and GRTH KO mice. A, testosterone response (serum and Leydig cell) to an in vivo single dose of hCG (0.5 μg, sc, 24 h) in WT and GRTH KO mice. B, testosterone response to in vitro hCG (100 ng, 3 h) stimulation in purified Leydig cells prepared from WT and KO mice. Data are presented as the mean ± S.E. of three independent experiments performed in triplicate. *, p < 0.05.
Abnormal Mitochondrial Morphology in Leydig Cells of GRTH KO Mice Associated with High Cholesterol Content in the Inner Mitochondrial Membrane
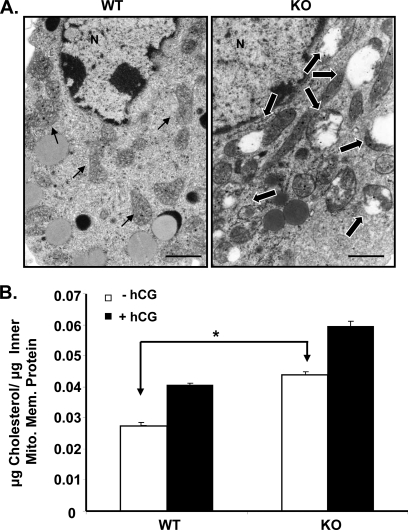
Because earlier EM analysis of the ultrastructural morphology of Leydig cells from GRTH null mice showed the presence of significantly swollen mitochondria without normal central cristae compared with WT mice (Fig. 2A) (8), we further investigated whether the increased testosterone response to hCG in GRTH KO mice was related to the cholesterol content in the mitochondria of Leydig cells. The highly enriched inner mitochondrial membrane preparation reflected by the presence of cytochrome c oxidase IV (data not shown) showed significantly increased accumulation of cholesterol in GRTH KO mice compared with WT mice (p < 0.01) (Fig. 2B). hCG caused increases in cholesterol content in Leydig cells from WT and GRTH KO mice. The cholesterol levels attained by gonadotropin stimulation were significantly higher than those observed in WT mice. These results suggest the involvement of GRTH in the local transport of cholesterol to the inner mitochondrial membrane in the Leydig cell.
FIGURE 2.
Abnormal mitochondrial morphology in Leydig cells of GRTH KO mice associated with high cholesterol content in the inner mitochondrial membrane. A, EM analysis of Leydig cells in WT and GRTH KO mice. Small arrows, mitochondria with normal morphology; large arrows, swollen mitochondria without normal central cristae. N, nuclear. Scale bars = 1 μm. B, cholesterol content in the inner mitochondrial membrane (Mito. Mem.) of purified Leydig cells from WT and KO mice. Animals were treated with a single dose of hCG (0.5 μg, sc, 24 h). Data are presented as the mean ± S.E. of three independent experiments performed in triplicate. *, p < 0.05.
mRNA and Protein Expression of Genes Involved in Cholesterol Synthesis/Transfer in Leydig Cells from WT and GRTH KO Mice
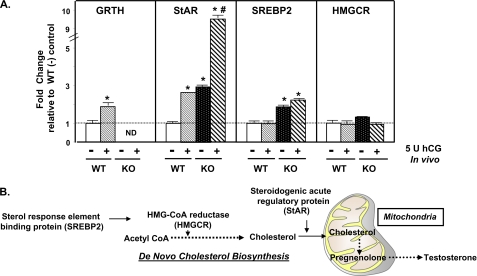
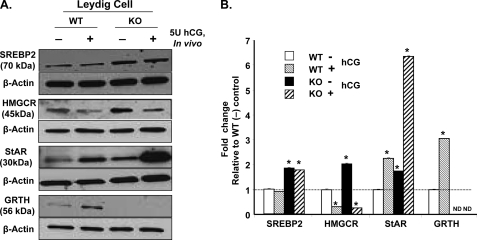
To investigate the mechanism associated with increased metabolic available cholesterol in the mitochondria of Leydig cells from GRTH KO mice, a panel of genes involved in cholesterol synthesis and transfer was analyzed (Fig. 3B). The expression of SREBP2, HMGCR, and StAR at both the mRNA and protein levels was determined in Leydig cells prepared from WT and KO mice injected with hCG in vivo. Basal mRNA levels of StAR and SREBP2 but not HMGCR were significantly increased in Leydig cells of GRTH KO mice compared with WT mice (Fig. 3A). hCG stimulation increased the levels of StAR in WT mice to those observed in basal KO mice. The StAR mRNA levels induced by gonadotropin treatment were 4-fold higher in KO mice than in WT mice. hCG did not have an effect on SREBP2 and HMGCR mRNA expression in both WT and GRTH KO mice. A significant increase in the basal protein level of mature SREBP2 (70 kDa; key regulator for genes involved in cholesterol metabolism) was observed in Leydig cells of GRTH null mice compared with WT mice. The SREBP2 precursor of 125 kDa was not detected in this study, suggesting that it is completely processed in WT and KO mice. HMGCR (a rate-limiting enzyme of cholesterol synthesis) and StAR (which mediates the cholesterol transfer from the outer to the inner mitochondrial membrane) were also significantly increased in Leydig cells of GRTH null mice (Fig. 4). These findings indicate that GRTH has an inhibitory effect on the expression of genes of relevance in the biosynthesis and accessibility of cholesterol at mitochondrial sites and hence participates in the modulation of gonadal steroidogenesis. Protein levels of GRTH and StAR were further increased in response to in vivo hCG stimulation in Leydig cells of WT mice (Fig. 4). Moreover, the increase in StAR protein by in vivo hCG treatment was magnified in Leydig cells of KO mice compared with WT mice. No effect was noted in SREBP2, whereas hCG induced down-regulation of HMGCR, and this inhibitory effect was also apparent in KO mice. The increase in GRTH mRNA and protein is due to the transcriptional up-regulation induced by hCG/cAMP/androgen/androgen receptor observed previously (1, 5). These results point to a gene-specific effect of GRTH on an early step in steroidogenesis.
FIGURE 3.
Expression of mRNAs (basal and hCG treatment) of genes involved in cholesterol synthesis/transfer in Leydig cells from WT and GRTH KO mice. A, mRNA levels were assessed in purified Leydig cells prepared from adult mice (WT and GRTH KO) 24 h after in vivo injection of hCG (+; 5 units, subcutaneous) or vehicle in the control group (−). Gene expression was analyzed by real-time RT-PCR and normalized to β-actin. Results are presented as -fold change relative to the WT control (−; dotted line). Data are presented as the mean ± S.E. of three independent experiments performed in triplicate. *, p < 0.05 compared with the WT control; #, p < 0.05 compared with the KO control. ND, not detectable. B, general scheme highlighting genes involved in the classical SREBP2-regulated pathway for cholesterol biosynthesis and transfer to mitochondria, followed by testosterone production.
FIGURE 4.
Effect of hCG on the expression of proteins involved in cholesterol synthesis/transfer in Leydig cells from WT and GRTH KO mice. A, Western blotting was used to analyze protein levels in purified Leydig cells prepared from adult mice (WT and GRTH KO) 24 h after in vivo injection of hCG (+; 5 units, sc) or vehicle in the control group (−). β-Actin was used for normalization. Data are representative of three independent experiments. B, diagram presentation of -fold change relative to the WT control (dotted line). Signals were quantitated and normalized to β-actin. Values are means ± S.E. *, p < 0.05. ND, not detectable.
StAR mRNA Associates with GRTH Protein, Which Acts as a Negative Regulator of StAR mRNA Stability
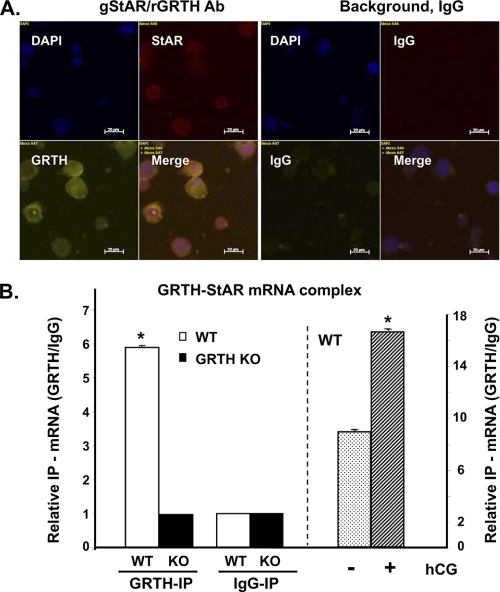
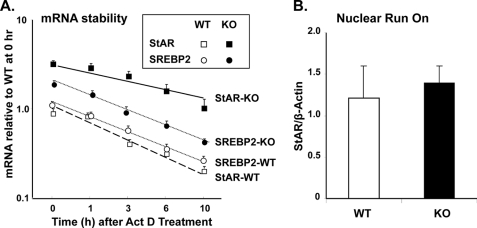
Immunofluorescence analysis showed that GRTH and StAR were colocalized in mouse Leydig cells (Fig. 5A). Reverse transcription and real-time PCR analysis of immunoprecipitated testicular GRTH complexes revealed GRTH association with StAR mRNA transcripts (Fig. 5B, left panel). No association of GRTH protein with either SREBP2 or HMGCR mRNA was observed (data not shown). Protein extracts from GRTH null mice or IgG immunoprecipitation was used as a negative control to validate the findings. A significant increase (1-fold) in StAR mRNA message associated with GRTH was observed at cytoplasmic sites in testes from WT mouse after in vivo treatment with hCG (Fig. 5B, right panel). Increases in the half-life of StAR were observed in KO mice compared with WT mice (6.0 ± 1.3 versus 2.2 ± 0.2 h, p < 0.01) (Fig. 6A). In contrast, no change in the half-life of SREBP2 (2.42 ± 0.17 versus 2.18 ± 0.21 h, p > 0.05) was observed. Run-on studies demonstrated no change in nascent StAR transcripts (Fig. 6B). Taken together, these results indicate a negative role of GRTH in StAR expression associated with StAR mRNA stability.
FIGURE 5.
Colocalization of GRTH and StAR in Leydig cells (A) and StAR mRNA association with GRTH protein (B). A, purified mouse Leydig cells were immunostained with rabbit anti-GRTH (rGRTH) and goat anti-StAR (gStAR) polyclonal antibodies, followed by Alexa Fluor 647 donkey anti-rabbit and Alexa Fluor 568 donkey anti-goat secondary antibodies. IgG were used as the background control. DAPI indicates nuclear staining. The merged images show DAPI-stained StAR and GRTH. B, real-time PCR analysis of the StAR message associated with immunoprecipitated (IP) total testicular GRTH complexes from WT and KO mice (left) or at the testicular cytoplasmic site of WT animals treated with in vivo hCG (+; 5 units, sc, 24 h) or vehicle in the control group (−) (right). Data are expressed as a relative ratio of messages from immunoprecipitated GRTH (GRTH-IP) to the immunoprecipitated IgG negative control group (IgG-IP). Data are representative of three independent experiments. *, p < 0.05.
FIGURE 6.
GRTH as a negative regulator of StAR mRNA stability. A, real-time PCR analysis of StAR and SREBP2 mRNAs in Leydig cells from WT and GRTH KO mice. Cells were incubated with 10 μg/ml actinomycin D (Act D) for 1–10 h. Data are presented as relative to WT mice at 0 h. Values are means ± S.E. of three independent experiments performed in triplicate. B, isolated nuclei of Leydig cells from WT and KO mice were used for nuclear run-on analysis of the nascent StAR RNA transcript. Data were normalized to β-actin as the mean ± S.E.
DISCUSSION
The availability of cholesterol in the Leydig cell as a precursor of steroid biosynthesis is critical to testicular steroidogenesis and male reproduction. This study presents the first evidence for the involvement of an RNA helicase, GRTH/DDX25, in the regulation of this process. The abnormal swollen structure of the mitochondria and the marked reduction in lipid droplets noted in Leydig cells from GRTH null mice coincided with the marked accumulation of cholesterol in the inner mitochondrial membrane and an increase in the expression of genes involved in cholesterol synthesis/transfer (HMGCR, Srebp2, and StAR). An additional increase in StAR expression and also testosterone production were observed following gonadotropin stimulation in GRTH null mice. The evidence of the association of GRTH with StAR mRNA and its inhibitory effect on StAR message stability indicates that GRTH has a major role in cholesterol homeostasis to limit overproduction of androgen upon stimulation of steroidogenic enzymes by gonadotropin in the Leydig cell.
Mitochondria are the sites for the rate-limiting step of cholesterol conversion to pregnenolone, which is required for androgen production. Changes in the state of the mitochondria play a central role in Leydig cell steroidogenesis (13). The abnormal swollen mitochondria with intact outer and inner membranes in the absence of GRTH (Fig. 2A) (8) could be reflective of a hyperstimulatory mechanism to maintain normal androgen production in GRTH null mice. The observed increased cholesterol content in the inner mitochondrial membrane of Leydig cells from GRTH null mice (Fig. 2B) with no change in the level of P450scc for pregnenolone synthesis (8) and enzymes involved in the distal steps of the steroidogenic pathway (3β-hydroxysteroid dehydrogenase, 17α-hydroxylase, and others) with normal basal testosterone production (Fig. 1) indicate a regulatory role of GRTH at steps prior to pregnenolone synthesis. Despite the higher cholesterol levels observed in GRTH KO mice at mitochondrial sites, basal testosterone levels were comparable in WT and GRTH null mice. However, an increase in testosterone levels became apparent in GRTH KO mice upon hCG stimulation compared with WT mice. This likely results from the availability of basal accumulated mitochondrial cholesterol as the substrate of side chain cleavage enzyme (P450scc) in the initial step of pregnenolone production upon hormonal stimulation of P450scc and other distal enzymes of the androgen biosynthetic pathway. Dysregulation of cholesterol homeostasis in Leydig cells of GRTH null mice was further evident by the significant increase in the expression of HMGCR and StAR (Fig. 4), known as key factors in cholesterol biosynthesis and transfer from cytoplasmic sites to the inner mitochondrial membrane, respectively. Taken together, our findings demonstrate an exacerbation of HMGCR and StAR function and build-up of the cholesterol pool at the inner mitochondrial membrane in the absence of GRTH.
Growing evidence suggests that steroidogenic tissue does not depend primarily on extracellular cholesterol uptake to regulate cholesterol homeostasis when intracellular cholesterol is depleted in either normal rat Leydig cells (14) or tumor mouse Leydig cells (MA10) (15). Thus, it is generally agreed that cholesterol biosynthesis in Leydig cells derives mainly from endogenous precursors (14–16). Although SREBP2 as a transcription factor is known to activate genes involved in the cholesterol biosynthetic pathway and receptor-mediated cholesterol uptake (17) in most mammalian cells, this may not be the case for gonadal steroidogenesis. Studies in mouse Leydig cells revealed that cAMP-stimulated expression of genes involved in cholesterol synthesis is independent of the nuclear recruitment of SREBP2 (16). In the case of GRTH null mice, the significantly up-regulated SREBP2 expression (Figs. 3 and 4) could result from a negative feedback response to the depletion of cytosolic levels of cholesterol with reduced lipid droplets (8). The finding of no change in the SREBP2-targeted HMGCR gene expression at the mRNA level (Fig. 3) supports early studies showing the lack of a regulatory role of SREBP2 in cholesterol synthesis in Leydig cells (16). However, the role of SREBP2 in cholesterol formation in Leydig cells require further studies, and the GRTH−/− model may be appropriate for this purpose. Although the mRNA level of HMGCR was not affected in the absence of GRTH, HMGCR protein, required for conversion of HMG-CoA to mevalonate in the early step of cholesterol synthesis (Fig. 4), was significantly increased in GRTH KO mice. It is likely that there is a direct negative effect of GRTH on the expression of HMGCR at the post-transcriptional level. On the other hand, hCG caused down-regulation of HMGCR protein in both WT and GRTH KO mice without a significant change in its mRNA level. The decrease in HMGCR protein is consistent with earlier findings of a decrease in HMGCR enzyme activity upon hCG stimulation in the rat, which is likely due initially to negative feedback by products of androgen biosynthesis and subsequently to the loss of the luteinizing hormone receptor upon in vivo hCG treatment (14).
On the other hand, StAR is crucial in cholesterol transport to the inner mitochondrial membrane. StAR synthesis is tightly regulated by PKA in response to gonadotropic hormone-stimulated cAMP signaling. Transport of StAR to the mitochondria was thought to be facilitated by AKAP proteins (18). However, comparative gene expression profiling4 showed no change in AKAP message in Leydig cells from GRTH KO mice, in which StAR mRNA was significantly increased (Fig. 3). StAR protein associates with cholesterol (19) and a multiple-protein complex, which in turn further facilitates the transit of StAR/cholesterol to the inner mitochondrial membrane to serve as substrate for P450scc (20). In this study, we have provided evidence for a negative role of GRTH in the regulation of cholesterol homeostasis in the Leydig cell through its modulation of StAR mRNA stability. The half-life of StAR was significantly reduced in WT mice compared with KO mice (2.2 versus 6 h). The changes in mRNA half-life could result from its association with GRTH protein (Fig. 6A). GRTH is a multifunctional protein that regulates germ cell development (6) through its association with specific mRNAs. GRTH participates in the transport of messages between and within cell compartments and in translational events in germ cells (21, 22). Together, the evidence that GRTH protein associates with StAR mRNA (Fig. 5B) and the finding of an enhanced half-life of the StAR message in the absence of GRTH (Fig. 6A) led us to propose a post-transcriptional aspect of GRTH regulation of StAR expression through the small RNA regulatory pathway to control StAR mRNA degradation. Lack of GRTH prevents StAR mRNA degradation and enhances StAR protein synthesis. Consequently, the increase in cholesterol delivery by StAR enhances the androgen production upon gonadotropin stimulation observed in GRTH KO mice (8).
GRTH expression is transcriptionally up-regulated by gonadotropin via cAMP and androgen/androgen receptor in the Leydig cell (1). This rise in GRTH could in turn increase StAR mRNA association and StAR mRNA degradation. The fact that only StAR showed a marked increase upon gonadotropic hormone (hCG) stimulation in the absence of GRTH indicates the central role of GRTH-regulated StAR in the process of androgen biosynthesis. A working model of GRTH action on the homeostasis of androgen formation in Leydig cells can be related to cholesterol formation and StAR message stability, synthesis, and cholesterol transfer to the mitochondria (Fig. 7).
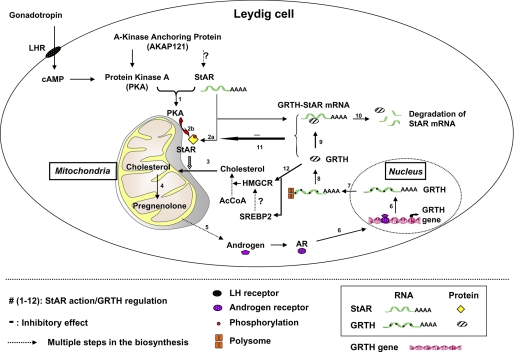
FIGURE 7.
Postulated model for GRTH negative autocrine molecular mechanism of regulation of androgen synthesis in Leydig cells. Steps 1–12 indicate the sequence of gonadotropic hormone stimulation of androgen synthesis through PKA-regulated StAR action and GRTH negative regulation (see below). Dotted arrows, multiple steps of cholesterol and steroidogenesis; AcCoA, acetyl-CoA; ?, not determined; bent arrow, transcriptional initiation. After gonadotropin binds to the luteinizing hormone receptor (LHR) at the cell surface of Leydig cells, cAMP-activated PKA type II (step 1) facilitates StAR mRNA tethering, protein synthesis (step 2a), and activation (phosphorylation; step 2b) in the vicinity of mitochondria (10). In turn, StAR enhances the transfer of cholesterol to the inner mitochondrial membrane (step 3) to initiate pregnenolone synthesis (step 4) as the rate-limiting step for the consequent androgen production (step 5). Androgen transcriptionally activates GRTH gene expression through binding to the androgen receptor (AR; step 6) (1, 5). GRTH transport its own RNA message from the nucleus (step 7) to the cytosol to be translated at polysome sites (step 8) (6). GRTH further associates with StAR mRNA as a GRTH-StAR mRNA complex (step 9) to enhance StAR message degradation presumably through the small RNA pathway system (step 10). This negative regulatory action of GRTH on the fate of StAR mRNA (step 11) controls androgen homeostasis in the Leydig cell. In the absence of GRTH, increased HMGCR protein levels through a yet-to-be-identified mechanism increase de novo cholesterol synthesis (step 12) as a compensatory reaction to restore the intracellular cholesterol pool. The prolonged StAR mRNA half-life and enhanced protein levels facilitate cholesterol accumulation at the inner mitochondria and the increased androgen production observed in GRTH null mice upon gonadotropin stimulation.
In conclusion, the depletion of lipid vesicles and the hyperactive mitochondrial structural changes found in Leydig cells of GRTH null mice are reflective components of cholesterol transport and synthesis with enhanced function of HMGCR, SREBP2, and StAR under basal conditions. This regulation by StAR is more apparent following gonadotropin stimulation. Increases in androgen production result from stimulation of steroidogenic enzymes by gonadotropin in the Leydig cell, where cholesterol is highly increased at mitochondrial sites in the absence of GRTH. The increase in StAR mRNA and protein expression in the absence of GRTH points to a key role of GRTH in the regulation of StAR in steroidogenesis. The inhibitory action of GRTH associated with gonadotropin-mediated steroidogenesis provides insights into a new negative autocrine molecular control mechanism and the role of this RNA helicase in the regulation of steroid production in the male.
This work was supported by the NICHD Intramural Research Program, National Institutes of Health.
H. Sato, C.-H. Tsai-Morris, and M. L. Dufau, unpublished data.
- GRTH
- gonadotropin-regulated testicular RNA helicase
- StAR
- steroidogenic acute regulatory protein
- hCG
- human chorionic gonadotropin
- HMGCR
- HMG-CoA reductase
- KO
- knock-out
- sc
- subcutaneous.
REFERENCES
- 1. Tang P. Z., Tsai-Morris C. H., Dufau M. L. (1999) J. Biol. Chem. 274, 37932–37940 [DOI] [PubMed] [Google Scholar]
- 2. Sheng Y., Tsai-Morris C. H., Dufau M. L. (2003) J. Biol. Chem. 278, 27796–27803 [DOI] [PubMed] [Google Scholar]
- 3. Haelens A., Verrijdt G., Schoenmakers E., Alen P., Peeters B., Rombauts W., Claessens F. (1999) Mol. Cell. Endocrinol. 153, 91–102 [DOI] [PubMed] [Google Scholar]
- 4. Tsai-Morris C. H., Lei S., Jiang Q., Sheng Y., Dufau M. L. (2004) Gene 331, 83–94 [DOI] [PubMed] [Google Scholar]
- 5. Villar J., Tsai-Morris C. H., Dufau M. L. (2010) FASEB J. 24, 833.2619917671 [Google Scholar]
- 6. Sheng Y., Tsai-Morris C. H., Gutti R., Maeda Y., Dufau M. L. (2006) J. Biol. Chem. 281, 35048–35056 [DOI] [PubMed] [Google Scholar]
- 7. Sato H., Tsai-Morris C. H., Dufau M. L. (2010) Biochim. Biophys. Acta 1803, 534–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsai-Morris C. H., Sheng Y., Lee E., Lei K. J., Dufau M. L. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 6373–6378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aquilano D. R., Dufau M. L. (1984) Endocrinology 114, 499–510 [DOI] [PubMed] [Google Scholar]
- 10. Dyson M. T., Jones J. K., Kowalewski M. P., Manna P. R., Alonso M., Gottesman M. E., Stocco D. M. (2008) Biol. Reprod. 78, 267–277 [DOI] [PubMed] [Google Scholar]
- 11. Tsai-Morris C. H., Aquilano D. R., Dufau M. L. (1985) Endocrinology 116, 31–37 [DOI] [PubMed] [Google Scholar]
- 12. Tang P. Z., Tsai-Morris C. H., Dufau M. L. (1998) Endocrinology 139, 4496–4505 [DOI] [PubMed] [Google Scholar]
- 13. Allen J. A., Shankara T., Janus P., Buck S., Diemer T., Hales K. H., Hales D. B. (2006) Endocrinology 147, 3924–3935 [DOI] [PubMed] [Google Scholar]
- 14. Charreau E. H., Calvo J. C., Nozu K., Pignataro O., Catt K. J., Dufau M. L. (1981) J. Biol. Chem. 256, 12719–12724 [PubMed] [Google Scholar]
- 15. Mascaró C., Nadal A., Hegardt F. G., Marrero P. F., Haro D. (2000) Biochem. J. 350, 785–790 [PMC free article] [PubMed] [Google Scholar]
- 16. Eacker S. M., Agrawal N., Qian K., Dichek H. L., Gong E. Y., Lee K., Braun R. E. (2008) Mol. Endocrinol. 22, 623–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Horton J. D., Goldstein J. L., Brown M. S. (2002) J. Clin. Invest. 109, 1125–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manna P. R., Huhtaniemi I. T., Stocco D. M. (2009) Endocrinology 150, 3308–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barbar E., Lehoux J. G., Lavigne P. (2009) Mol. Cell. Endocrinol. 300, 89–93 [DOI] [PubMed] [Google Scholar]
- 20. Midzak A., Rone M., Aghazadeh Y., Culty M., Papadopoulos V. (2011) Mol. Cell. Endocrinol. 336, 70–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dufau M. L., Tsai-Morris C. H. (2007) Trends Endocrinol. Metab. 18, 314–320 [DOI] [PubMed] [Google Scholar]
- 22. Tsai-Morris C. H., Sheng Y., Gutti R. K., Tang P. Z., Dufau M. L. (2010) J. Androl. 31, 45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bradford M. M. (1976) Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]