To the Editor: Confocal endomicroscopy is emerging as a safe, minimally-invasive means of improving the accuracy of endoscopic screening and surveillance for Barrett’s esophagus (1) and colorectal cancer (2). When used alongside “red-flag” techniques such as autofluorescence imaging (AFI) or narrow-band imaging (NBI), endomicroscopy enables the gastroenterologist to evaluate the gastric or colonic mucosa with cellular level resolution in a real-time “optical biopsy” (3). Both endoscope-based and probe-based platforms have demonstrated improved diagnostic yield and very high preliminary accuracy compared to standard endoscopy in prospective, double-blind studies (4,5). However, the cost and learning curve associated with these systems limits their usage to a select number of academic centers (3). To enable widespread translation of this promising technology, we developed a high-resolution microendoscope (HRME) in prototype form for under $5,000 (6,7). The instrument uses a 1-mm diameter probe which passes through the working channel of a standard gastroscope or colonoscope; images are displayed at 15 frames-per-second at 400× magnification. A light-emitting diode (LED) provides illumination at 445 nm which is delivered through the probe to the tissue surface. The spatial resolution of the probe is 4.4 µm and the field-of-view 720 µm in diameter. In this Letter, we present the first in vivo clinical applications of this technology in the upper and lower GI tracts. We present a case involving surveillance and guided therapy of Barrett’s esophagus, followed by three cases illustrating the characteristic features of normal, inflammatory, and adenomatous tissue in the colon. The study was approved by the Institutional Review Boards of Mount Sinai Hospital and Rice University.
Similar to several confocal endomicroscopy studies (3), the HRME uses fluorescent contrast agents, typically either topical acriflavine or proflavine. In the esophagus, this enables the discrete, evenly spaced nuclei of normal squamous mucosa to be distinguished from the glandular appearance of columnar metaplasia (fig. 1a–d). Barrett’s metaplasia has previously been characterized on HRME by large glands with intact nuclear polarity, whereas high-grade dysplasia (HGD) exhibits crowded, irregular glands and loss of nuclear polarity (6,8).
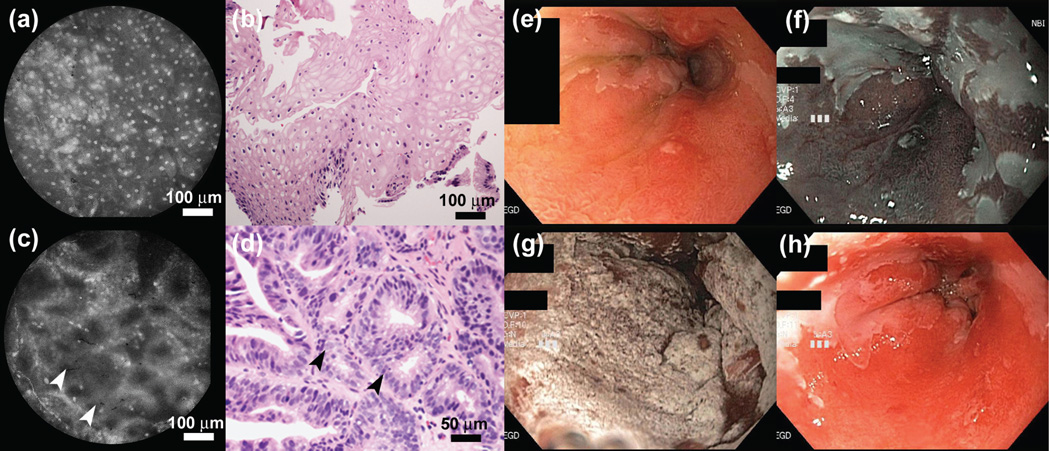
Figure 1.
HRME imaging in the esophagus. (a) Normal squamous mucosa and (c) Barrett’s metaplasia with focal HGD (arrows) in a 47-year old male undergoing endoscopic evaluation. (b,d) Histopathology sections (H&E) corresponding to the locations imaged in panels (a) and (c) respectively. (e) High-definition white light, and (f) NBI view of the esophagus in the same patient. (g) White light view during, and (h) post cryotherapy treatment.
A 47-year-old male with longstanding reflux symptoms and Barrett’s esophagus was referred for endoscopic evaluation. The patient underwent high-definition white light endoscopy (fig. 1e) with narrow-band imaging (fig. 1f) which revealed a 6-cm segment of Barrett’s (Prague Classification C5M6) with several “NBI-abnormal” areas showing a distorted mucosal and vascular pattern. These areas were sprayed with 1–2 ml of 0.01% (w/v) proflavine and imaged with the HRME probe (see Supplementary Video 1 online). HRME imaging revealed non-neoplastic epithelium in the majority of NBI-abnormal areas. However, focal HGD was noted in two separate areas in the proximal portion of the segment, evidenced by a proliferation of small irregular glandular structures (arrows in Fig. 1c,d). The patient opted for and subsequently underwent endoscopic cryoablation of the Barrett’s segment (Fig. 1g,h).
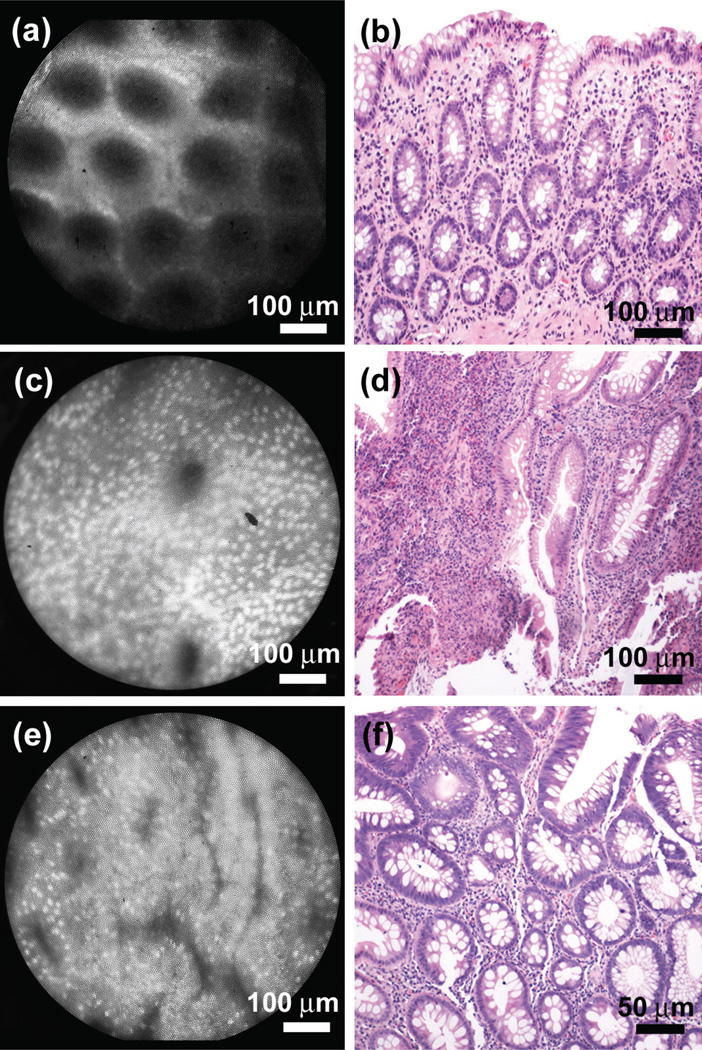
We have also used the HRME during screening colonoscopy, where the ability to rapidly stratify benign polyps and precancerous adenomas is essential. In normal colonic mucosa, small uniformly spaced circular crypts, absent of glandular distortion or atrophy are readily apparent under HRME imaging (fig. 2a), and also seen in the corresponding histopathology (fig. 2b). Small, basally oriented nuclei are also apparent under HRME imaging. With inflammatory polyps, a dense population of inflammatory cells is commonly observed on HRME (fig. 2c), with only a few irregularly shaped and variably sized glands visible. Tubular adenomas (fig. 2e) are easily distinguished; glands appear as irregular structures with heterogeneous orientation, involving elongated, crowded cells, and enlarged nuclei.
Figure 2.
HRME imaging in the colon. (a) HRME image of the normal colon in vivo, with corresponding histopathology from the same site (b). Note the appearance of small, uniformly spaced circular crypts, with small, basally oriented nuclei. (c) HRME image and (d) corresponding histopathology of an inflammatory polyp, presenting a dense population of inflammatory cells and few irregularly shaped glands. (e) HRME image of a tubular adenoma, revealing highly irregular and heterogeneously oriented glands, with elongated, crowded cells, and enlarged nuclei.
Endomicroscopy has the potential to become an important clinical tool (9), but its overall impact on patient care may ultimately be limited by cost. HRME imaging provides real-time “optical biopsies” with many of the same diagnostically relevant features established for confocal endomicroscopy, yet costs significantly less than probe and endoscope based confocal platforms. Low-cost endomicroscopy may prove to be a more widely accessible adjunct to standard endoscopy for managing conditions including Barrett’s metaplasia and screening colonoscopy, by assisting the endoscopist in selecting sites for biopsy and/or guiding treatment.
Supplementary Material
Supplementary Video 1: Video capture from the endoscopic procedure illustrated in Figure 1. The fluorescent contrast agent (proflavine) is topically administered via spray catheter, followed by HRME imaging, revealing regions of small irregular glandular structures indicative of HGD. The patient subsequently underwent endoscopic cryoablation of the Barrett’s segment.
Acknowledgments
Financial support: This work was funded by the National Institutes of Health, grants R01 EB007594, and R01 CA140257.
Human Subjects: This study was approved by the Institutional Review Boards of Mount Sinai Hospital and Rice University.
Footnotes
Specific author contributions:
MP: Designed and assembled HRME instrumentation. Drafted the manuscript. MP has approved the final draft submitted.
PMV: Operated HRME instrumentation. PV has approved the final draft submitted.
AP: Evaluated histopathology and HRME image data. AP has approved the final draft submitted.
RRK: Designed the study. Interpreted image data. RRK has approved the final draft submitted.
SA: Designed the study. Performed endoscopic image collection. Interpreted image data. SA has approved the final draft submitted.
Potential competing interests: RRK holds patents related to endomicrosopy devices. MCP, PMV, ADP, SA have no potential competing interests.
References
- 1.Kiesslich R, Gossner L, Goetz M, et al. In vivo histology of Barrett's esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2006;4:979–987. doi: 10.1016/j.cgh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Kiesslich R, Goetz M, Vieth M, et al. Technology insight: confocal laser endoscopy for in vivo diagnosis of colorectal cancer. Nat Clin Pract Oncol. 2007;4:480–490. doi: 10.1038/ncponc0881. [DOI] [PubMed] [Google Scholar]
- 3.Goetz M, Kiesslich R. Advances of endomicroscopy for gastrointestinal physiology and diseases. Am J Physiol Gastrointest Liver Physiol. 2010;298:G797–G806. doi: 10.1152/ajpgi.00027.2010. [DOI] [PubMed] [Google Scholar]
- 4.Dunbar KB, Okolo P, Montgomery E, et al. Confocal laser endomicroscopy in Barrett’s esophagus and endoscopically inapparent Barrett's neoplasia: a prospective, randomized, double-blinded, controlled, crossover trial. Gastrointest Endosc. 2009;70:645–654. doi: 10.1016/j.gie.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallace MB, Sharma P, Lightdale C, et al. Preliminary accuracy and interobserver agreement for the detection of intraepithelial neoplasia in Barrett's esophagus with probe-based confocal laser endomicroscopy. Gastrointest Endosc. 2010;72:19–24. doi: 10.1016/j.gie.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muldoon TJ, Anandasabapathy S, Maru D, et al. High-resolution imaging in Barrett’s esophagus: a novel, low-cost endoscopic microscope. Gastrointest Endosc. 2008;68:737–744. doi: 10.1016/j.gie.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pierce MC, Yu D, Richards-Kortum R. High-resolution fiber-optic microendoscopy for in situ cellular imaging. JoVE. 2011;47 doi: 10.3791/2306. http://www.jove.com/index/Details.stp?ID=2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muldoon TJ, Thekkek N, Roblyer D, et al. Evaluation of quantitative image analysis criteria for the high-resolution microendoscopic detection of neoplasia in Barrett’s esophagus. J Biomed Opt. 2010;15 doi: 10.1117/1.3406386. 026027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bisschops R, Bergman J. Probe-based confocal laser endomicroscopy: scientific toy or clinical tool? Endoscopy. 2010;42:487–489. doi: 10.1055/s-0029-1244196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Video 1: Video capture from the endoscopic procedure illustrated in Figure 1. The fluorescent contrast agent (proflavine) is topically administered via spray catheter, followed by HRME imaging, revealing regions of small irregular glandular structures indicative of HGD. The patient subsequently underwent endoscopic cryoablation of the Barrett’s segment.