Abstract
Objective
To create a functional status outcome measure for large outcome studies that is well defined, quantitative, sufficiently rapid, reliable, minimally dependent on subjective assessments, and applicable to hospitalized pediatric patients across a wide spectrum of ages and inpatient environments.
Patients and Methods
The Functional Status Scale (FSS) was developed by a multidisciplinary consensus process. Domains of functioning included mental status, sensory, communication, motor, feeding, and respiratory categorized from normal (1) to very severe dysfunction (5). The Adaptive Behavior Assessment System (ABAS) II established construct validity and calibration within domains.
Seven institutions provided pediatric intensive care unit (PICU) patients within 24 hours of PICU discharge, high-risk non-PICU patients within 24 hours of admission, and technology-dependent children. Primary care nurses completed the ABAS II based on patient’s functioning when the FSS was completed. Patients from 10% of the study days were used to evaluate inter-rater reliability. Data were randomly split into estimation and validation sets. Statistical analyses included Pearson correlations, construct validity, linear regression analysis, receiver operating characteristic (ROC) curve analysis for discriminant validity, and the intraclass correlation for inter-rater reliability.
Results
A total of 836 children with a mean FSS of 10.3 (standard deviation 4.4) were studied. Eighteen percent had the minimum possible FSS = 6, 44% had FSS ≥ 10, 14% had a FSS ≥ 15, and 6% had FSS scores ≥ 20. Each FSS domain was associated with mean ABAS II (p<.0001). Cells in each domain were collapsed and reweighted, which improved correlations with ABAS II from −0.58 to −0.62 in the estimation sample, and −0.60 to −0.63 in the validation sample (p<0.001 for improvements). Discrimination was very good for moderate and severe dysfunction (ABAS II categories) and improved with FSS weighting (area under the ROC curve > 0.8). Intraclass correlations of original and weighted total FSS were 0.95 and 0.94 respectively.
Conclusions
The FSS met our objectives and is well suited for large outcome studies.
Keywords: Pediatrics, functional status, outcome assessment (health care), activities of daily living, adaptive behavior, health status indicators, health utilities index, treatment outcome, child
INTRODUCTION
A major challenge of pediatrics and its subspecialties is to develop a functional outcome measure that is well defined, quantitative, sufficiently rapid and reliable, minimally dependent on subjective assessments, applicable to as full an age spectrum as possible, and pertinent to hospitalized patients in as many inpatient environments as possible. Since existing measures available for children are either excessively time consuming to conduct (1, 2), available or validated for a limited age spectrum (3), or simply require too much subjective assessment and future projection by raters (4,5), a new functional outcome measure fulfilling these criteria is especially desirable to enable large outcome studies.
We wanted to be able to measure the changing functional status of children such as motor or major cognitive deficits during hospitalization. While not necessarily being predictive of long-term outcome, we wanted to enable a large Pediatric ICU outcome study. The conceptual framework for development of this measure was the activities of daily living scale used in outcome studies of adults. In children, however, the expected performance of activities of daily living changes with developmental stages. A similar conceptual characterization for children is adaptive behavior, but the formal assessment of adaptive behavior is time consuming and requires specific expertise.
The aim of this study was to develop a quantitative, rapid, and reliable scale of functional status for children conceptually similar to activities of daily living, and to compare the performance of the scale against a validated, more extensive measure of adaptive behavior.
PATIENTS and METHODS
This study was conducted at the seven PICUs participating in the National Institute of Child Health and Human Development (NICHD) Collaborative Pediatric Critical Care Research Network (CPCCRN). The Institutional Review Board at each site approved the protocol.
Functional Status Scale (FSS)
The FSS was developed by a formal consensus process of health professionals from 11 institutions within and outside the research network including pediatricians, pediatric neurologists, pediatric developmental psychologists, pediatric physiatrists, pediatric nurses, pediatric intensivists, and pediatric respiratory therapists. A prior, single institution pilot study had demonstrated the utility of using the primary nurse as a suitable observer for functional status, the potential of a simple scale to accurately reflect functional status as measured by adaptive behavior, and the potential to correctly categorize patients by functional status. The pilot study showed very good inter-rater reliability between two data collectors, and supported the use of adaptive behavior to establish external validity. Domains of functioning selected during the consensus process included mental status, sensory functioning, communication, motor functioning, feeding, and respiratory status (Table 1). Functional status for each domain was categorized from normal (1) to very severe dysfunction (5). FSS scores ranged from 6 to 30. The definitions of the domain cells are shown in the Appendix.
Table 1.
FSS Domains of Functioning in the Functional Status Scale (FSS)
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| NORMAL | MILD DYSFUNCTION | MODERATE DYSFUNCTION | SEVERE DYSFUNCTION | VERY SEVERE DYSFUNCTION | |
| MENTAL STATUS | Normal sleep/wake; appropriate responsivity | Sleepy but arousable to noise/touch/movement and/or periods of social nonresponsivity | lethargic and/or irritable | Minimal arousal to stimulus (stupor) | Unresponsive and/or Coma and/or Vegetative |
| SENSORY | Intact hearing and vision and responsive to touch | Suspected hearing or Suspected vision loss. | Not reactive to auditory stimuli or Not reactive to visional stimuli | Not reactive to auditory stimuli and Not reactive to visional stimuli | Abnormal response to pain or touch |
| COMMUNICATION | Appropriate non-crying vocalizations, interactive facial expressiveness, or gestures | Diminished Vocalization Diminished Facial Expression and/or social responsiveness | Absence of attention getting behavior | No demonstration of discomfort | Absence of communication |
| MOTOR FUNCTION | Coordinated body movements and Normal muscle control and Awareness of action and why it’s being done | 1 limb functionally impaired | 2 or more limbs functionally impaired | Poor head control | Diffuse Spasticity, Paralysis, Decerebrate/Decorticate Posturing |
| FEEDING | All food taken by mouth with age appropriate help | NPO or need for age-inappropriate help with feeding | Oral and tube feedings | Parenteral Nutrition with oral or tube feedings | All parenteral nutrition |
| RESPIRATORY | Room air and no artificial support or aids | Oxygen and/or Suctioning | Tracheostomy | CPAP for all or part of the day and/or Mechanical ventilator support for part of the day | Mechanical ventilatory support for all of the day and night |
Adaptive Behavior Assessment System II (ABAS II) (6)
The ABAS II, a validated questionnaire to assess adaptive behavior, was utilized to establish construct validity and to provide calibration of the FSS scores within each domain. We selected adaptive behavior as a similar but not identical measure of function, recognizing that correlation between adaptive behavior scores purporting to measure the same functions such as ABAS II and the Vineland Adaptive Behavior Score (1) is only moderate. For example, the correlations between the ABAS II skill areas and the Vineland Adaptive Behavior Scale domains ranged from 0.17 to 0.79, and the overall correlation between the scores, both of which are in-depth assessments of adaptive behavior, was 0.75. Correlations between ABAS II and the Scales of Independent Behavior – Revised (SIB-R), a screening test, was only 0.18 (7). Since functional status and adaptive behavior are not identical concepts, we expected only modest correlation between the FSS and ABAS II scores.
The ABAS II has 10 skill areas that are scaled to age-normalized performance, with each scaled skill area having a mean ± standard deviation of 10 ± 3. Since not all skill areas were relevant to hospitalized children, we administered only selected skill areas. For children aged 0 – 6 years, we administered the communication, pre-academics, health and safety, leisure, self-care, self-direction, social, and motor skill areas. For children 6 through 18 years of age, we administered the communication, health and safety, leisure, self-care, self-direction, and social skill areas. The “gold standard” selected for establishing acceptable correlation of the FSS was the “mean partial ABAS II”, calculated as the mean of all skill area scores available for a participating child. In this report, all “ABAS II” values reported refer to this “partial” application of the full instrument.
Study Data Collection
Patients were eligible if they were greater than 38 weeks gestation and less than 18 years of age, and additionally met criteria for one of three study groups of patients at high risk for functional disabilities (for example, anatomic abnormalities of the brain, metabolic conditions, chronic respiratory disease, etc). These groups were PICU patients within 24 hours of discharge, high-risk non-PICU patients within 24 hours of hospital admission, and technology dependent children. The study sample was selected to achieve a final distribution of 40% PICU discharges, 40% high-risk admissions, and 20% technology-dependent children. High risk hospitalizations were based on, but not limited to, a preselected set of diagnoses including spina bifida, mental retardation, seizure disorders, other neurological disorders and chromosomal abnormalities. Technology dependent patients were studied either during their acute hospitalization, at long-term care facilities, or during a clinic visit. If during a given day of the study period, more eligible patients were available within a study group than could be assessed, study patients were randomly selected. Patients were enrolled on weekdays from July 28, 2006 through March 1, 2007.
Data included subject age, gender, acute diagnosis, major clinical events resulting in, or likely to result in major decrements in functional status, emergency/elective status on admission, operative status at the time of inclusion, use of sedatives, narcotics, sleeping aids or other therapies that could potentially interfere with functional status including restraints, arm boards, bandages, casts, and other devices.
The FSS was collected by a research coordinator (nurse or respiratory therapist) or physician-investigator at each site. All individuals collecting data were trained by the data coordinating center at the same training session. The ABAS II was completed by the primary care nurse within 4 hours of the collection of the FSS data based on his/her understanding of the patients functioning at the time the FSS was completed. Nurses were oriented to the study prior to its onset.
We randomly selected 10% of study days to evaluate inter-rater reliability. On these days, both a study physician and research coordinator independently completed the FSS evaluation within 4 hours of each other.
Statistical Methods
The mean ABAS II value among all available skill areas was selected as the “gold standard” for comparison to the FSS (see above). A more complex skill area weighting approach was not implemented, as the covariance structures of the ABAS II subscales in the population used to derive and validate the ABAS were not available.
We recognized a priori that the cells of dysfunction within each domain were ordered by the experts, but were not necessarily assigned appropriate relative weights. To assess this possibility, we first randomly allocated two thirds of the sample for use in the estimation set and one third for the validation set within each study site and study arm (PICU, high-risk hospital, and technology dependent). We kept all FSS domains since they were thought to be important during the expert consensus process. Our approach evaluated the relationship between each of the FSS domains separately versus the mean ABAS II using univariable linear regression to establish a new relative weighting system for the cells of each domains. These scores were scaled to a value of 1 representing normal function (weighted FSS). Cells in each FSS domain were collapsed (combined) if their coefficients and their standard errors indicated significant overlap, there was lack of appropriate ordering of the coefficient values, and the clinical significance of the cells could be meaningfully combined. The stability of the observations and changes in the estimation set was confirmed in the validation set.
Face validity and logical content validity of the FSS were established through the expert consensus process. Construct validity was established by correlating the performance of the FSS with adaptive behavior as measured by the ABAS-II. Discriminant validity was established by receiver operating characteristic (ROC) curve analysis using dysfunction groups classified by the ABAS-II. Association between the FSS (original and weighted) and ABAS II was assessed using Pearson correlations. The significance of the difference between these two (dependent) correlation coefficients was assessed via t-test, using an asymptotic approach of Hotelling (8). The significance of the difference between two ROC curves was assessed using the nonparametric approach of DeLong (9), implemented in SAS via the %ROC macro.
Inter-rater reliability was assessed via the weighted kappa statistic for the various components of the FSS (10). The total FSS was treated as a continuous variable for the reliability analysis, and thus the intraclass correlation coefficient (ICC) was used to assess reliability of this score. The research coordinator and study physician assessments were treated as separate observations. An intercept-only linear mixed model was fitted with the total FSS as the outcome, and a random intercept for each subject. ICC was then calculated as the ratio of the estimated variance attributable to subject, divided by the sum of this variance plus the residual variance.
RESULTS
Characteristics of Enrolled Subjects
A total of 836 patients in the 3 groups were enrolled in the 7 hospitals (Table 2). Table 3 shows the descriptive data. A total of 32% of PICU and high-risk hospitalizations were post-operative with neurosurgery being the predominant surgery and 65% were emergency admissions. The primary systems of dysfunction were neurological, respiratory, cardiovascular, gastrointestinal, oncological and orthopedic. Only 2% of patients had limitations of care orders at the time of the assessment. The comparison of the estimation and validation samples (Table 3) shows that these samples were very similar. Medications and physical restraints influencing or potentially influencing the FSS were common (Table 4).
Table 2.
Location of Enrollment for Study Patients
| All | Estimation Set | Validation Set | |
|---|---|---|---|
| (n=836) | (n=570) | (n=266) | |
| PICU | 345 (41%) | 235 (41%) | 110 (41%) |
| High-Risk Acute | 340 (41%) | 230 (40%) | 110 (41%) |
| Technology-dependent | 151 (18%) | 105 (18%) | 46 (17%) |
Table 3.
Characteristics of Study Patients
| All | Estimation Set | Validation Set | |
|---|---|---|---|
| All Study Arms (n) | 836 | 570 | 266 |
| Age (years, mean ± standard deviation) | 6.8 ± 5.5 | 6.7 ± 5.4 | 6.9 ± 5.5 |
| Female Gender (n (%)) | 331 (40%) | 218 (38%) | 113 (42%) |
| Race | |||
| African American (n (%)) | 212 (25%) | 142 (25%) | 70 (26%) |
| White (n (%)) | 561 (67%) | 387 (68%) | 174 (65%) |
| Asian (n (%)) | 31 (4%) | 19 (3%) | 12 (5%) |
| Other/Unknown (n (%)) | 32 (4%) | 22 (4%) | 10 (4%) |
| Hispanic Ethnicity (n (%)) | 140 (17%) | 91 (16%) | 49 (18%) |
| Post-Operative* (n=685, n(%)) | 218 (32%) | 142 (31%) | 76 (35%) |
| Neurosurgery (n) | 82 | 54 | 28 |
| Orthopedic (n) | 34 | 24 | 10 |
| Cardiac (n) | 30 | 20 | 10 |
| Other (n) | 72 | 44 | 28 |
| Emergency Admission* (n=685, n (%)) | 443 (65%) | 301 (65%) | 142 (65%) |
| Primary Systems of Dysfunction | |||
| Cardiovascular (n (%)) | 91 (11%) | 65 (11%) | 26 (10%) |
| Gastrointestinal (n (%)) | 59 (7%) | 44 (8%) | 15 (6%) |
| Neurological (n (%)) | 284 (34%) | 203 (36%) | 81 (30%) |
| Oncology (n (%)) | 62 (7%) | 42 (7%) | 20 (8%) |
| Orthopedics (n (%)) | 61 (7%) | 42 (7%) | 19 (7%) |
| Respiratory (n (%)) | 155 (19%) | 91 (16%) | 64 (24%) |
| Other (n (%)) | 124 (15%) | 83 (15%) | 41 (15%) |
| Limitations of Care Orders | 13 (2%) | 10 (2%) | 3 (1%) |
| PICU length of stay (days) (mean ± standard deviation) | (n=345) 4.5 ± 10.4 |
(n=235) 4.6 ± 10.7 |
(n=110) 4.3 ± 9.7 |
This characteristic was not applicable to technology-dependent patients.
Table 4.
Patient Care Factors Potentially Influencing Functional Status.*
| All (n=836) | Estimation Set (n=570) | Validation Set (n=266) | |
|---|---|---|---|
| Sedative(s) within 24 hours of the assessment | 77 (9%) | 55 (10%) | 22 (8%) |
| Paralytics in the PICU | 94 (11%) | 63 (11%) | 31 (12%) |
| Narcotic(s) during hospitalization prior to the assessment | 153 (18%) | 104 (18%) | 49 (18%) |
| Movement restricting arm board(s) | 190 (23%) | 123 (22%) | 67 (25%) |
| Restraint(s) | 17 (2%) | 16 (3%) | 1 (0.4%) |
| Movement restricting bandages or casts | 34 (4%) | 29 (5%) | 5 (2%) |
Three subjects in the estimation set had missing data for the arm board, restraint, and bandages/cast factors.
FSS Distribution and Association with the ABAS-II
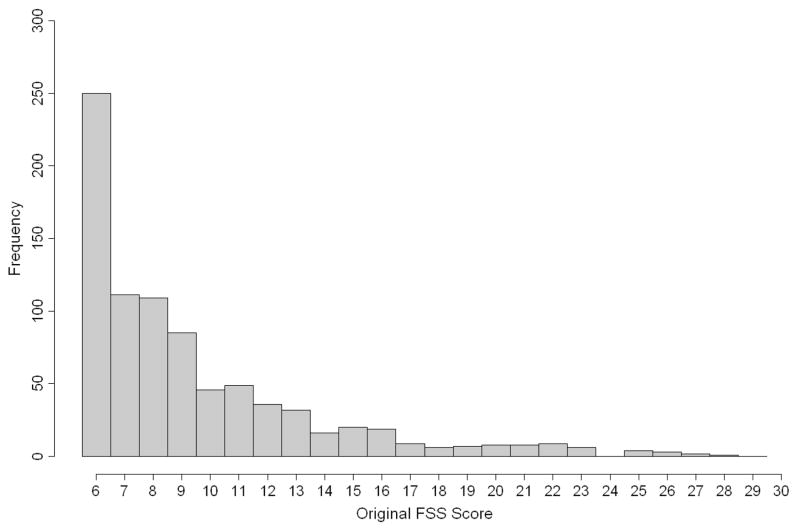
Figure 1 shows the relative frequencies of the FSS scores in the total sample. There was a wide range of functioning ranging from 6 (normal in all domains) to very severe dysfunction (maximum score = 30 for very severe dysfunction in all domains). The average FSS was 10.3 ± 4.4. A total of 18% of patients had a FSS of 6, while 6% had scores ≥ 20, with the highest score being 29 in these subjects.
Figure 1.
Distribution of original FSS scores in the overall studied population of 836 patients.
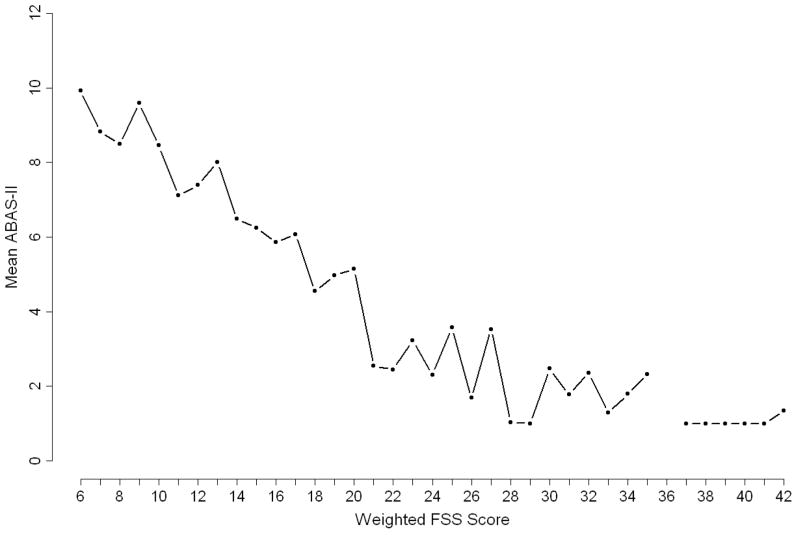
For purposes of assessing association with ABAS II and appropriate reweighting, the FSS scores were next compared to the mean ABAS II for the estimation set. The average ABAS II in the 570 estimation sample subjects was 6.8 ± 4.3. Figure 2 shows the relationship between the mean ABAS II and the original FSS in the estimation set. The Pearson correlation between mean ABAS II and FSS was −0.58 (p<0.001). Each FSS domain was highly significantly associated with the mean ABAS II (p<.0001 for each of the six FSS domains).
Figure 2.
Mean values of ABAS II, according to original FSS value for the 570 patients in the estimation population.
The relative importance of the cells in each FSS domain is shown as mean ABAS II for each cell (Table 5). According to these estimates, cells in the estimation set in each domain were reweighted (Table 6). The analysis resulted in collapsing two cells (severe and very severe) in the mental status domain, and collapsing 3 cells (moderate, severe, and very severe) in the sensory, communications, and respiratory domains. In the feeding domain we collapsed the very severe and mild cells and also the moderate and severe cells. All cells were kept intact in the motor domain. For each domain, the cells collapsed were consistent with the concept of adaptive behavior as assessed by the ABAS II. For example, the cells in mental status (stupor and coma) affect adaptive behavior to a similar extent. The collapsing of mild and very severe in the feeding domain also was consistent with inpatient hospital practice of providing TPN to NPO patients.
Table 5.
ABAS Values (Average ± Standard Error of Estimated Population Mean) for Cells of the Original FSS Domains. Adjacent categories subsequently collapsed in the weighted FSS are shown without line cell dividers in this table.
| FSS Domain\Level | None | Mild | Moderate | Severe | Very Severe |
|---|---|---|---|---|---|
| Mental | 8.3 ± 0.2 | 6.0 ± 0.3 | 4.1 ± 0.5 | 1.5 ± 0.9 | 1.1 ± 1.2 |
| Sensory | 7.5 ± 0.2 | 4.6 ± 0.5 | 2.1 ± 0.9 | 1.4 ± 1.2 | 1.0 ± 1.6 |
| Communication | 8.7 ± 0.2 | 4.9 ± 0.3 | 1.6 ± 0.5 | 1.4 ± 1.1 | 1.3 ± 1.0 |
| Motor | 8.5 ± 0.2 | 7.1 ± 0.3 | 5.5 ± 0.4 | 3.0 ± 0.7 | 1.5 ± 0.6 |
| Feeding | 8.7 ± 0.2 | 6.1 ± 0.3 | 3.7 ± 0.3 | 4.8 ± 1.0 | 6.7 ± 1.0 |
| Respiratory | 7.5 ± 0.2 | 6.4 ± 0.5 | 2.9 ± 0.8 | 3.9 ± 1.0 | 3.7 ± 0.7 |
Table 6.
Relative Values for FSS Domain Cells Based on Univariate Analysis
| FSS Domain \ Level | None | Mild | Moderate | Severe | Very Severe |
|---|---|---|---|---|---|
| Mental | 1.0 | 3.3 | 5.2 | 8.0 | 8.0 |
| Sensory | 1.0 | 3.9 | 6.8 | 6.8 | 6.8 |
| Communication | 1.0 | 4.8 | 8.2 | 8.2 | 8.2 |
| Motor | 1.0 | 2.4 | 4.1 | 6.6 | 8.0 |
| Feeding | 1.0 | 3.5 | 5.9 | 5.9 | 3.5 |
| Respiratory | 1.0 | 2.1 | 5.0 | 5.0 | 5.0 |
The collapsed and reweighted domains then had weights assigned according to the mean ABAS values in each cell referenced to a score of 1 for normal. The “weighted FSS” scale is shown in Table 6, with scores ranging from 6 to 41.9. Due to the collapsing of the mild and very severe feeding domain scores, the maximum score is achieved from the very severe scores in all domains except feeding, where the highest score is in the severe category.
The performance of the original FSS and the weighted FSS is shown in Table 7 for both the estimation and validation data sets. Figure 3 shows the relationship between the mean ABAS II and the weighted FSS in the estimation set. The correlations between ABAS II and FSS improved with the weighting from −0.58 to −0.62 in the estimation sample, and −0.60 to −0.63 in the validation sample. The differences between these two correlation coefficients for the original and weighted correlations were highly statistically significant (p<0.001) in both datasets. The ability of the FSS to discriminate between functional and dysfunctional patients was also assessed with ROC analysis, using two different classification cutpoints representing 1 and 2 standard deviations below the general population ABAS II mean. The area under the ROC curve improved from the original FSS to the weighted FSS in both the estimation and validation samples. The weighted FSS showed significantly (p<0.001) higher areas under the ROC curve in the estimation sample for both cutpoints, and trends for higher area under the ROC curve (p=0.11 for ABAS II ≤ 7 and p=0.19 for ABAS II ≥ 4) in the smaller validation sample.
Table 7.
Correlations of Original and Weighted FSS with the ABAS II, and Area Under the ROC Curve for Predicting ABAS II Cutpoints
| Correlation with ABAS II | ROC - Cutpoint ABAS II <=4 | ROC - Cutpoint ABAS II <=7 | |
|---|---|---|---|
| Original FSS – estimation sample | −0.58 | 0.83 | 0.79 |
| Original FSS - validation sample | −0.60 | 0.82 | 0.86 |
| Weighted FSS – estimation sample | −0.62 | 0.85 | 0.81 |
| Weighted FSS - validation sample | −0.63 | 0.83 | 0.87 |
Figure 3.
Mean values of ABAS II, according to weighted FSS value rounded to the nearest integer for the 570 patients in the estimation population.
Additionally, the FSS showed a consistent, moderate to strong association with ABAS II across levels of other patient factors examined: age, elective/emergency status, operative status, patient type (PICU, high-risk hospital, technology dependent), and study site.
Reliability of the FSS
Table 8 shows the weighted kappa values for the original FSS and the weighted FSS for 97 subjects in the reliability analysis. The intraclass correlation of the original total FSS was 0.95, while the intraclass correlation was 0.94 for the weighted total FSS indicating overall high reproducibility. Domain-specific unweighted kappa values ranged from 0.54 to 0.88 for the original FSS and 0.52 and 0.89 for the weighted FSS components. Some of the lack of agreement between the observers was undoubtedly due to real differences in patient status since the coordinator and physician observations could have been performed up to 4 hours apart. For example, the worst kappa values were noted for mental status, which corresponds in part to actual mental status changes associated with medications, time of day, and improving patient status.
Table 8.
Weighted Kappa Values for Reliability of FSS Components
| Weighted Kappa Value | ||
|---|---|---|
| Domain | Original | Weighted FSS |
| Mental | 0.54 | 0.52 |
| Sensory | 0.76 | 0.74 |
| Communication | 0.81 | 0.79 |
| Motor | 0.78 | 0.80 |
| Feeding | 0.87 | 0.88 |
| Respiratory | 0.88 | 0.89 |
DISCUSSION
Our goal was to develop a scale that could measure functional status at any time, reflecting the dynamic state of disease and recovery, but not specifically intended to predict long-term outcome. The FSS is conceptually based on activities of daily living used in adult studies to characterize functioning, disability, and dependency. (11, 12) Since expectations for activities of daily living in children change with developmental stages, we transitioned to using adaptive behavior. These measures approximate activities of daily living with developmental adjustments and there is a wide overlap in the skill sets identified by both methods. For example, activities of daily living consist of personal self-care (feeding oneself, bathing, toileting), mobility (movement from bed to a standing position or to a chair, walking with or without assistance, or using a wheelchair) and continence (urine, feces) (13). Adaptive skills include a repertoire of skills to meet the daily demands and expectations of the environment including eating, dressing, expressing needs, communicating, and behavior control, as well as more advanced skills (1, 6). Since adaptive behavior assessment methods may not be appropriate for large outcome studies because of the time and expertise required for test administration, we developed and validated the FSS to correlate but not duplicate adaptive behavior.
Our consensus process used the input from 7 types of health professionals from 11 institutions to create the FSS domains and their gradations of dysfunction. The 7 institution study of 836 patients included a wide range of patients at high risk of functional disability. The correlation coefficients between the FSS and adaptive behavior measured by the ABAS II were ≥ −0.58, similar to the correlations between different measures of adaptive behavior (7). We considered these correlations to be appropriate since the FSS was not designed to duplicate adaptive behavior scores. Additionally, each the six FSS domains were associated with the mean ABAS II (p<.0001 for each of the six FSS domains). Recognizing that the experts creating the FSS developed functional domains with a series of dysfunctional states that were not appropriately scaled, we statistically created these relative weights with improved correlation between the weighted FSS and ABAS II. Perhaps the most important aspect of performance was the FSS’ discrimination for moderate and severe decrements in adaptive behavior (area under the ROC curve of 0.81 and 0.85 respectively for the weighted FSS). Performance was also stable in the estimation and validation sets, an important score characteristic for study use. Finally, the reliability of the FSS was very good. The worst kappa values were seen in the assessment of mental status, a difference that we believe was real due to the time delay between the two raters.
Even though the FSS performed well, it is possible that subgroup-specific FSS versions could be constructed that would more accurately predict ABAS II, and better summarize functional status within specific subgroups. For example, it is probable that the relationship between the FSS and the ABAS II is not linear across all degrees of dysfunction. A larger sample might have enabled us to better focus on subgroups and to better describe the relationship between ABAS II and the FSS. Most notably, the study focused only on a specific group of hospitalized patients and should not be immediately generalized to other populations without further study. Nevertheless, our study demonstrates that a single FSS instrument is strongly correlated with adaptive behavior across the spectrum of our patient population, and that this instrument is an appropriate method to characterize functional status. Future studies may demonstrate that the FSS at specific points in time correlates with long-term patient mortality and morbidity.
The FSS will expand the perspective of pediatric outcome studies. Until now, large studies primarily used mortality as the outcome. Other outcome measures including functional status or quality of life have been generally too time consuming for large studies, available or validated for only a limited age spectrum, or require too much subjective assessment and future projection by raters (1–5). Additionally, functional status is a very relevant intensive care and hospital outcome. While physiologic status has often been used to predict mortality (14), it may also result in functional status changes. For example, cardiovascular compromise may cause neurological injury and/or be associated with long-term cardiac compromise as well as death. A functional status assessment will enable studies of quality of care similar to those accomplished by methods such as PRISM (15) but using functional status as well as mortality as an outcome.
Appropriate outcome measures are often the limiting methodology for important and needed outcome studies (16, 17). Outcome measures must not only be relevant to the study question, but also applicable to age range of the study, reliable within that age range, and measure what it is purported to measure. The FSS is a significant advance for pediatric outcome studies. It is a well-defined, quantitative, rapid and reliable measure of functional status. Importantly, it is not dependent on subjective assessments. It is applicable to the pediatric age spectrum from full-term newborns to adolescents, and it is pertinent to hospitalized patients. The FSS is well suited for use in large pediatric outcome studies.
Acknowledgments
Children’s Hospital of Michigan, Detroit, MI: Sabrina Heidemann, MD, Maureen Frey, PhD, RN; Children’s National Medical Center, Washington DC: Michael Bell, MD, Jean Reardon, BSN, RN; Arkansas Children’s Hospital, Little Rock, AR: Parthak Prodhan, MD, Glenda Hefley, MNSc, RN; Seattle Children’s Hospital, Seattle, WA: Thomas Brogan, MD, Ruth Barker, RRT; Children’s Hospital of Pittsburgh, Pittsburgh, PA: Shekhar T. Venkataraman, MD, Alan Abraham, BA; Children’s Hospital Los Angeles, Los Angeles, CA: J. Francisco Fajardo, CLS (ASCP), RN, MD; Mattel Children’s Hospital at University of California Los Angeles, Los Angeles, CA; University of Utah (Data Coordinating Center), Salt Lake City, UT: Amy Donaldson, MS, Jeri Burr, BS, RNC, CCRC, Devinder Singh, BS, Rene Enriquez, BS; National Institute of Child Health and Human Development, Bethesda, MD: Tammara Jenkins, MSN RN University of Texas Health Sciences Center, Houston, TX: Linda Ewing Cobb, PhD,; University of Minnesota, Minneapolis, MN: Elizabeth Gilles, MD; Children’s Healthcare of Atlanta, Atlanta, GA: Maurice Sholas, MD, PhD: University of Colorado at Denver and Health Sciences Center, Devner CO: Dennis Matthews, MD
This work was supported by cooperative agreements from the National Institute of Child Health and Human Development and the Department of Health and Human Services (U10HD050096, U10HD049981, U10HD500009, U10HD049945, U10HD049983, U10HD050012 and U01HD049934).
Abbreviations
- FSS
Functional Status Scale
- ABAS
Adaptive Behavior Assessment System
- PICU
pediatric intensive care unit
- ROC
receiver operating characteristic
- NICHD
National Institute of Child Health and Human Development
- CPCCRN
Collaborative Pediatric Critical Care Research Network
- SIB-R
Scales of Independent Behavior – Revised
- ICC
intraclass correlation coefficient
- CPAP
Continuous positive airway pressure
Appendix. Functional Status Scale - Definitions
MENTAL STATUS
Mental Status: Normal
Normal Sleep and wake periods;
Appropriate social responsivity
Sleep refers to a restful state without over-reaction (crying, agitation) to noises in the environment. Awake refers to awareness with behavior appropriate for age. Infants and children in this state should be appropriately aware, alert and responsive of self and environment.
Mental Status: Mild Dysfunction
-
Sleepy but arousable to noise or touch or movement
and/or
Periods of reduced social responsivity
Sleeps more of the time than is age appropriate; will sleep much of time if left alone but is able to be aroused with stimulation such as noise, if touched or position changes.
and/or
Decreased responsiveness to social overtures
and/or
Does not consistently focus or follow on a person or object crossing line of vision.
Mental Status: Moderate Dysfunction
-
Lethargic
and/or
Irritable
Lethargic infants and children are drowsy, sluggish, or have an unusual lack of energy. They are arousable, but become less responsive or return to a sleep-like state without frequent stimulation. Irritable infants and children are inconsolable often with an increased sensitivity to stimulation. Infants often react to stimuli with a high-pitched cry.
Mental Status: Severe Dysfunction
Minimal arousal to stimulus (stupor)
Stuporous infants and children have decreased or impaired consciousness marked by diminution in reactions to environmental stimuli. They may open eyes and focus, but do not maintain any meaningful reaction to physical environment. They make little or no eye contact. They will respond to noxious stimuli with semi-purposeful (i.e. poorly organized) movements or withdrawal.
Mental Status: Very Severe Dysfunction
-
Unresponsive
and/or
-
Coma
and/or
Vegetative
These infants and children are unconscious. Coma is a deep or profound state of unconsciousness from which they cannot be aroused. They do not sense or respond to external stimuli or internal needs. Vegetative infants and children have no evidence of awareness of self or environment. They may have intermittent wakefulness manifested by sleep- wake cycles. There is no evidence of sustained, reproducible, purposeful or voluntary behavioral responses to visual, auditory, tactile, or noxious stimuli.
SENSORY
Sensory: Normal
-
Intact hearing
and
Intact vision
Intact hearing is demonstrated by individuals localizing/moving eyes and/or head toward sound stimulus in room. Intact vision is evidenced by individuals turning gaze to focus on person or object that crosses his visual field.
Sensory: Mild Dysfunction
-
Suspected hearing loss
or
Suspected vision loss
There is suspicion of hearing or vision loss as evidenced by inconsistent focusing or localization of sound. Responsiveness to touch is not impaired.
Sensory: Moderate Dysfunction
-
Not reactive to auditory stimuli
or
Not reactive to visual stimuli
There is lack of evidence for hearing or vision as demonstrated by lack of focusing, or localization of sound. Responsiveness to touch is not impaired.
Sensory: Severe Dysfunction
-
Not reactive to auditory stimuli
and
Not reactive to visual stimuli
There is lack of evidence of hearing or vision as evidenced by lack of tracking, and localization of sound. Responsiveness to touch is not impaired.
Sensory: Very Severe Dysfunction
Abnormal response to touch
Infant/child has abnormal response to touch or pain as evidenced by the absence of purposeful, or semi-purposeful movements. There may be a withdrawal or spinal response.
COMMUNICATION
Communication: Normal
-
Vocalization appropriate for age
and
Interactive facial expressions or gestures.
Infants make sounds to make presence known. Children use words to convey needs. Interactive facial expressions and gesture are a process of non-verbal communication, often closely associated with emotions.
Communication: Mild Dysfunction
Diminished Vocalization
Diminished social expression (facial or verbal)
There is a decrease in socialization and social expression.
Communication: Moderate Dysfunction
Absence of attention-getting behavior
Infants and children who do not demonstrate behavior that “says” “look at me, here I am”. Children may initiate attention-getting behavior, but cannot communicate their needs.
Communication: Severe Dysfunction
No demonstration of discomfort
Infants and children do not cry or cry very little with painful procedures or if uncomfortable
Communication: Very Severe Dysfunction
Absence of communication.
There is no communication using facial expressions, body posture, or voice. There is no communication regarding physiological or psychological needs.
MOTOR FUNCTIONING
Motor Functioning: Voluntary movements: Normal
-
Coordinated body movements
and
-
Normal muscle control
and
Awareness of action
Infants and children have coordinated movements with normal muscle control. They are aware of the action and its purpose. e.g., infant kicks limbs, vocalizes when parent enters. Infant can hold rattle and transfer it from one hand to another. Toddler carries object, holds onto stuffed animal, sucks thumb. Child writes or plays with toys.
Motor Functioning: Mild Dysfunction
1 limb functionally impaired
There is a partial or complete loss of functionality of the limb. Impairment may be from medical devices such as restraints, IV boards, bandages, or casts, or due to physical issues such as deformities, weakness, stiffness, spasticity, and/or movement disorders. Weakness is demonstrated when infants and children are able to move limb off a surface (against gravity) while holding an object or against resistance. They may be able to perform normal age appropriate activities but with increased effort. Stiffness is demonstrated when one or more limbs have increased resistance to passive motion but are still held in normal position or postures. Stimulation does not result in flexion, extension or arching.
Motor Functioning: Moderate Dysfunction
2 or more limbs functionally impaired
There is a partial or complete loss of functionality of 2 or more limbs. Impairment may be from medical devices such as restraints, IV boards, bandages, or casts, or due to physical issues such as deformities, weakness, stiffness, spasticity, and movement disorder. Weakness is demonstrated when infants and children are able to move limb off a surface (against gravity) while holding an object or against resistance. They may be able to perform normal age appropriate activities but with increased effort. Stiffness is demonstrated when one or more limbs have increased resistance to passive motion but are still held in normal position or postures. Stimulation does not result in flexion, extension or arching. Spasticity is abnormally increased muscle tone with involuntary movement. Limb(s) feels tight, rigid and limb reflexes are exaggerated. There is resistance to bending and the neck is hyperextended.
Motor Functioning: Severe Dysfunction
Poor Head Control
Head control is poor with decreased ability to hold head upright at 90 degrees. Unable or cannot hold head still when less than 90°. If trunk is supported head will fall back, to side or front and he/she is unable to bring head to the upright position if sitting or midline if supine or prone.
Motor Functioning: Very Severe Dysfunction
Paralyzed
Decerebrate/Decorticate Posturing
Paralysis is the loss of voluntary motor function. There is abnormal muscle tone. Mental Status may be preserved or altered. Decerebrate posture consists of rigid extension of all extremities with internal rotation. There is downward pointing of toes. Decorticate posture consists of rigid flexion of upper extremities with clenched fists and extension of lower extremities.
FEEDING
Feeding: Normal
All food taken PO with age appropriate help.
There is no parenteral or gavage feeding. Feeding methods are age appropriate. The number of calories is not part of this category.
Feeding: Mild Dysfunction
NPO
Oral feedings with increased caloric density/food
There is no parenteral or tube feeding. Dextrose solutions of 5% or less are not included in parenteral feeding. Increased oral density feeds are special formulas and/or additions to diet.
Feeding: Moderate Dysfunction
Need for age-inappropriate help with feeding
This consists of feeding by caretaker when independent feeding is expected or use of feeding aid is used at an inappropriate age (e.g., bottle).
Feeding: Severe Dysfunction
Feeding tube with or without parenteral nutrition.
Parenteral nutrition includes intravenous nutrition via a peripheral or central vein with a dextrose concentration greater than 5%. It usually includes fat and protein. Tube feedings include nasogastric, oral-gastric, and nutrition via small bowel tubes.
Feeding: Very Severe Dysfunction
All parenteral nutrition
Parenteral nutrition includes intravenous nutrition via a peripheral or central vein with a dextrose concentration greater than 5%. It usually includes fat and protein.
RESPIRATORY STATUS
Respiratory Status: Normal
Room air and no artificial support or aids
The infant or child is breathing in room air without the need for artificial help including suctioning, oxygen, or mechanical support.
Respiratory Status: Mild Dysfunction
-
Oxygen
and/or
Suctioning
Oxygen is given via any apparatus including blow-by, cannula, face mask, etc. Suctioning includes any oral or tracheal suctioning.
Respiratory Status: Moderate Dysfunction
Tracheostomy
Respiratory Status: Severe Dysfunction
-
CPAP for all or part of the day
and/or
Mechanical ventilator support for part of the day
CPAP (Continuous positive airway pressure) may be administered through a facemask or tracheostomy. Mechanical support includes positive or negative pressure ventilation devices such as bipap, and positive pressure mechanical ventilation.
Respiratory Status: Very Severe Dysfunction
Mechanical ventilatory support for all day and night.
Mechanical support includes positive or negative pressure ventilation devices such as bipap, and positive pressure mechanical ventilation.
References
- 1.Sparrow SS, Bulla DA, Cicchetti DV. Vineland Adaptive Behavior Scales–II. Circle Pines, Minn: Pearson Educational Inc; 2006. [Google Scholar]
- 2.Bayley N. Manual for the Bayley Scales of Infant Development. 2. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 3.Guide for the Uniform Data Set for Medical Rehabilitation for Children (WeeFIM), version 4.0. Buffalo, NY: State University of New York at Buffalo; 1993. [Google Scholar]
- 4.Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28:2616–20. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- 5.Fiser DH, Tilford JM, Roberson PK. Relationship of illness severity and length of stay to functional outcomes in the pediatric intensive care unit: a multi-institutional study. Critical Care Medicine. 2000;28:1173–1179. doi: 10.1097/00003246-200004000-00043. [DOI] [PubMed] [Google Scholar]
- 6.Harrison PL, Oakland T. PsychCorp. 2. 2003. ABAS II. Adaptive Behavior Assessment System, second edition. [Google Scholar]
- 7.Harrison PL, Oakland T. PsychCorp. 2. 2003. ABAS II. Adaptive Behavior Assessment System, second edition; pp. 122–137. [Google Scholar]
- 8.Hotelling H. The selection of variates for use in prediction with some comments on the general problem of nuisance parameters. Ann Math Statist. 1940;11:271–283. [Google Scholar]
- 9.Delong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated received operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 10.Cicchetti DV, Allison T. A new procedure for assessing reliability of scoring EEG sleep recordings. Amer J of EEG Technology. 1971;11:101–109. [Google Scholar]
- 11.Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51:451–458. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- 12.Amemiya T, Oda K, Ando M, et al. Activities of daily living and quality of life of elderly patients after elective surgery for gastric and colorectal cancers. Ann Surg. 2007;246:222–2008. doi: 10.1097/SLA.0b013e3180caa3fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindeboom R, Vermeulen M, Holman R, et al. Activities of daily living instruments: Optimizing scales for neurologic assessments. Neurology. 2003;60:738–742. doi: 10.1212/01.wnl.0000044402.16315.fc. [DOI] [PubMed] [Google Scholar]
- 14.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated pediatric risk of mortality score. Crit Care Med. 1996;24:743–52. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Pollack MM, Cuerdon TC, Patel KM, et al. Impact of quality-of-care factors on pediatric Intensive Care Unit mortality. J Amer Med Ass. 1994;272:941–6. [PubMed] [Google Scholar]
- 16.Harmony R, Hochman J. Cardiogenic shock: Current concepts and improving outcomes. Circulation. 2008;117:686–697. doi: 10.1161/CIRCULATIONAHA.106.613596. [DOI] [PubMed] [Google Scholar]
- 17.Marcin JP, Pollack MM. Triage scoring systems, severity of illness measures, and mortality prediction models in pediatric trauma. Crit Care Med. 2002;30:S457–S467. doi: 10.1097/00003246-200211001-00011. [DOI] [PubMed] [Google Scholar]