Abstract
We tested the hypothesis that regular aerobic exercise reverses arterial inflammation with aging. When compared with young controls (6.2 ± 0.4 mo; n = 7), old (31.3 ± 0.5 mo; n = 11) male B6D2F1 cage-restricted mice demonstrated increased arterial activation of the proinflammatory transcription factor NF-κB, as indicated by greater aortic phosphorylation of both the inhibitor of NF-κB kinase (IKK) and the p65 subunit of NF-κB (both P < 0.05). Similarly, aortic expression of the proinflammatory cytokines IL-1 and IL-6, IFN-γ, and TNF-α were greater in the old mice (all P < 0.05). Macrophage and T lymphocyte abundance was unchanged with age in the aortic intima and media but was markedly increased in the adventitia and perivascular fat tissue of old mice (all P < 0.05). This proinflammatory arterial phenotype with aging was associated with vascular dysfunction, as reflected by impaired nitric oxide-mediated endothelium-dependent dilation. Voluntary wheel running (10–14 wk) normalized aortic IKK-NF-κB activation, cytokine expression, adventitial and perivascular macrophage infiltration, and vascular function in old mice (32.4 ± 0.3 mo; n = 8) while having no consistent effects in young mice. Short-term voluntary wheel running started late in life reverses arterial inflammation with aging in mice possibly via outside-in actions. These anti-inflammatory effects may play an important role in the amelioration of age-associated vascular dysfunction by regular aerobic exercise.
Keywords: nuclear factor-κB, cytokines, macrophages, vascular dysfunction
cardiovascular diseases (CVD) remain the leading causes of morbidity and mortality in modern societies, and age is the major risk factor for CVD (28, 31). This effect of aging on CVD risk is primarily the result of pathophysiological changes to arteries that lead to vascular dysfunction and disease (28).
Although the mechanisms are incompletely understood, one of the key pathological changes to arteries with age is the development of chronic, low-grade inflammation (5, 6). This inflammation involves phosphorylation/activation of the proinflammatory transcription factor NF-κB via phosphorylation of inhibitor of NF-κB kinase (IKK) and increased expression of proinflammatory cytokines including IL-1 and IL-6, IFN-γ, and TNF-α (6, 8, 47). Although the source of the inflammation is unclear, immune cell (macrophages, T lymphocytes) infiltration of the arterial intima (from the lumen) or the arterial adventitia (from surrounding perivascular tissue) may contribute to arterial inflammation in experimental atherosclerosis and hypertension (23, 53). Relatively little is known about the effects of primary aging on arterial immune cell infiltration. It is clear, however, that the inflammatory state that develops in arteries with age is associated with vascular dysfunction, as indicated by impaired endothelium-dependent dilation (EDD) (5, 20, 47).
Regular aerobic exercise is associated with a reduced risk of CVD (2, 42) and preservation of arterial function (10, 43, 44, 48) with aging in humans. It has been hypothesized that aerobic exercise exerts anti-inflammatory effects that could contribute to its vascular-protective influence (34, 38, 39). That regular exercise is associated with lower circulating concentrations of inflammatory proteins in some adults is consistent with this idea (18, 21, 24, 52). However, to our knowledge there is little or no direct evidence that aerobic exercise has anti-inflammatory effects on arteries.
In the present study, we sought to determine whether voluntary wheel running, as a model of aerobic exercise, ameliorates large artery inflammation in old mice, defined as reductions in the expression of proinflammatory cytokines and infiltration of macrophages and lymphocytes to levels not different from those observed in young control mice. To do so, we assessed IKK-NF-κB activation, inflammatory cytokines, region-specific macrophage and T lymphocyte infiltration, and vascular function in large elastic arteries from young and old B6D2F1 cage-restricted mice, as well as mice studied after 10–14 wk of wheel running, a model of voluntary aerobic exercise (17, 20, 29).
METHODS
Ethical approval.
All animal procedures conformed to the Guide for the Care and Use of Laboratory Animals [National Institutes of Health (NIH) Publication No. 85-23, Revised 1996] and were approved by the University of Colorado at Boulder Animal Care and Use Committee.
Animals.
Male B6D2F1 mice were obtained from the National Institute on Aging rodent colony. All mice were housed in an animal care facility at the University of Colorado at Boulder on a 12-h:12-h light/dark cycle. Young (6.3 ± 0.4 mo; n = 7) and old (31.3 ± 0.5 mo; n = 11 mo; ∼50% survival) mice were fed normal rodent chow ad libitum and housed in standard mouse cages. Separate groups of young (4.8 ± 0.2 mo; n = 5) and old (32.4 ± 0.3 mo; n = 8) mice were housed in cages fitted with running wheels for 10–14 wk before euthanization. Running distance was monitored daily. The young and old mice averaged 8.7 ± 1.0 and 2.1 ± 0.2 km/day, respectively, consistent with previous observations from our laboratory (17). Mice were euthanized via exsanguination by cardiac puncture while under isoflurane anesthesia (17, 29, 36). To determine the efficacy of voluntary wheel running as a stimulus capable of producing exercise-training effects, skeletal muscle citrate synthase activity was determined in quadriceps muscle homogenate as previously described (9, 17, 40).
Aortic protein expression/phosphorylation.
To provide adequate tissue for measurement of proteins, the thoracic aorta was excised and cleared of perivascular adipose tissue, while maintained in 4°C PSS and frozen in liquid nitrogen. Whole artery lysates were prepared as previously described (17, 29, 36). Protein expression was assessed by standard Western blot procedures using primary antibodies against IKK (1:200; 87 kDa; Santa Cruz Biotechnology, Santa Cruz, CA), phosphorylated IKK (p-IKK; 1:200; 87 kDa; Cell Signaling, Danvers, MA), the p65 subunit of NF-κB (p65, 1:1,000; 65 kDa; Cell Signaling), phosphorylated p65 NF-κB (pp65; 1:1,000; 65 kDa, Cell Signaling), and a horseradish peroxidase-conjugated secondary antibody (Jackson Immunological, West Grove, PA) and Supersignal ECL (Pierce, Rockford, IL). Bands were visualized using a digital acquisition system (ChemiDoc-It; UVP, Upland, CA) and quantified using ImageJ 1.42 software (NIH, Bethesda, MD). To account for differences in protein loading, expression is presented normalized to GAPDH (1:1,000; 37 kDa; Cell Signaling) expression. Representative images show bands from the same blot and exposure. Phosphorylated and total proteins were assessed in the same lysates run on two identical gels per blots. The quantifications of total protein are normalized to GAPDH of the same band after stripping and reprobing and expressed relative to the mean of the young control bands on the given blot. The ratio of phosphorylated to total protein is calculated from the bands for a given sample lysate run on the two gels per membranes, with each normalized to its own GAPDH. These data are also expressed as a ratio of the mean of the young control group within a given set of lysates.
Aortic cytokine/chemokine concentrations.
Cytokine concentrations were measured in aortic lysates by multiplex ELISA (Searchlight Mouse Inflammatory Cytokine Kit; Aushon Biosystems, Billerica, MA) according to manufacturer instructions as described previously (36).
Immunohistochemistry for infiltrating macrophages, lymphocytes, and TNF-α.
A portion of the thoracic aortas (∼1 to 2 mm) was embedded in optimum cutting temperature (OCT; Sakura) without removing the adventitia or perivascular fat and frozen and 10-μm sections were mounted on glass microscope slides. Immunohistochemistry was performed using primary antibodies against macrophages and T lymphocytes, F4/80 (1:1,000; ab6640; Abcam), and CD3 (1:500; ab5690; Abcam), respectively. Positive staining was visualized with 3,3′-diaminobenzidine (DAB; K4011; Dako), and sections were counterstained with Mayer's hematoxylin (S3309; Dako) to identify nuclei. Multiple images of each section were digitally captured at 100× (Nikon Eclipse TS100; Nikon) and combined (Photoshop Elements 6.0) to produce a single high-resolution image of an entire section. Using ImageJ 1.42, a technician blinded to group identity outlined the adventitia with a pen tablet (Bamboo Fun, Wacom) and counted the total number of nuclei and the number of nuclei with positive staining for either macrophages or T cells. Infiltration was expressed as the percentage of total cells (nuclei). For each animal, three serial sections were analyzed, and the results were averaged. Because macrophages or T cells were rarely observed in the intima or media, infiltration in these regions was not quantified. Immunohistochemistry for TNF-α (1:50; No. 3053; Biovision) also was performed on a subset of aortas from old cage-restricted and old voluntary running mice (n = 3/group) to gain initial insight into the localization of proinflammatory signals.
Vascular function.
Because the entire thoracic aorta was utilized to obtain measures of cytokines and immunohistochemistry, endothelial function was assessed in the carotid arteries of these mice as described previously (17, 20, 29). This approach has been used previously by our laboratory (14, 17, 29) and other investigators (1, 11), since EDD responses are similar in carotid arteries and aorta of mice (3).
To assess endothelial function, EDD and the nitric oxide (NO) contribution to dilation were measured in response to the cumulative addition of ACh (1 × 10−9 to 1 × 10−4 mol/l) in the absence or presence of the NO synthase inhibitor N-nitro-l-arginine methyl ester (l-NAME; 0.1 mmol/l; 30 min). Endothelium-independent dilation was assessed in response to sodium nitroprusside (1 × 10−10 to 1 × 10−4 mol/l). Vessel diameters were measured by MyoView software (DMT, Atlanta, GA). All dose response data are presented as percentages of possible dilation after preconstriction to phenylephrine (2 μmol/l), and NO bioavailability was determined from the maximal EDD in the absence or presence of l-NAME as described previously (17, 29). Sensitivity to ACh and sodium nitroprusside was defined as the concentration that elicited 50% of the maximal response (IC50).
Statistics.
To assess group differences in endothelium-dependent and -independent dilation, the maximal vasodilation and sensitivity to ACh (IC50) was used to characterize the overall responses. To determine NO bioavailability, the maximal vasodilation to ACh after l-NAME inhibition of NO synthase was subtracted from the maximal ACh-induced dilation in the same artery. For other animal and vessel characteristics, group differences were determined by one-way ANOVA with least squares differences post hoc test where appropriate. Bivariate correlations were performed to examine the relation between maximal vasodilation and variables of interest. Data are presented as means ± SE. Significance was set at P < 0.05.
RESULTS
Animal characteristics.
Body mass and epididymal adipose tissue mass did not differ with age in cage control mice but were lower in young and old animals that underwent voluntary wheel running (P < 0.05; Table 1). Aging was associated with an increase in heart mass (P < 0.01) that tended to be blunted by voluntary wheel running (P = 0.06; Table 1). Gastrocnemius, but not soleus, muscle mass was decreased in old cage-restricted (P < 0.01) and voluntary wheel running young (P = 0.05) and old (P < 0.01) mice relative to young controls. Citrate synthase activity did not differ between young and old cage control mice but was higher in the groups that underwent voluntary wheel running (P < 0.05; Table 1).
Table 1.
Animal characteristics
| Young | Old | Young VR | Old VR | |
|---|---|---|---|---|
| Age, mo | 6.2 ± 0.4 | 31.3 ± 0.5* | 4.8 ± 0.2 | 32.4 ± 0.3* |
| Mass, g | ||||
| Body | 35.0 ± 1.9 | 35.8 ± 0.7 | 28.2 ± 0.5* | 31.4 ± 1.7† |
| Heart | 0.178 ± 0.007 | 0.236 ± 0.012* | 0.167 ± 0.010 | 0.202 ± 0.012 |
| Epididymal white adipose | 0.797 ± 0.156 | 0.613 ± 0.086 | 0.435 ± 0.020* | 0.268 ± 0.037*† |
| Soleus muscle | 0.014 ± 0.001 | 0.012 ± 0.001 | 0.012 ± 0.002 | 0.013 ± 0.001 |
| Gastrocnemius muscle | 0.193 ± 0.003 | 0.154 ± 0.005* | 0.160 ± 0.002* | 0.149 ± 0.014* |
| Quadriceps muscle citrate Synthase activity, mol·min−1·g wet wt−1 | 19.3 ± 1.0 | 19.2 ± 1.3 | 23.5 ± 0.7* | 25.2 ± 1.0*† |
Values are means ± SE. VR, voluntary wheel running.
Difference from young;
difference from old, P < 0.05.
Aerobic exercise reverses age-associated increases in arterial proinflammatory IKK/NF-κB signaling.
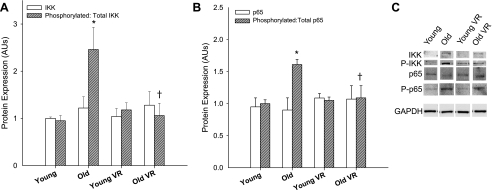
Total IKK and NF-κB protein expression did not differ with age, but the ratios of phosphorylated to total IKK and NF-κB (Fig. 1; P < 0.01) were increased in aortas of old compared with young cage-restricted mice. Voluntary wheel running normalized the phosphorylated-to-total ratio for IKK and NF-κB (Fig. 1) in old mice to that observed in young controls, without affecting total protein expression, whereas no effects were observed in young mice.
Fig. 1.
Aortic expression of total (white bars) and the ratio of phosphorylated (p) to total (hatched bars) expression of the inhibitor of NF-κB kinase (IKK; n = 5–11/group; A) and the p65 subunit of NF-κB (n = 5–11/group; B) in young and old cage-restricted and voluntary wheel running (VR) mice with representative Western blots (C). Protein expression was normalized to own GAPDH and presented as a ratio of young control mean. GAPDH expression was similar between blots, and 1 set of representative bands is presented. Values are means ± SE. *P < 0.05 vs. young; †P < 0.05 vs. old. AU, arbitrary units.
Aerobic exercise normalizes age-associated increases in arterial proinflammatory cytokines.
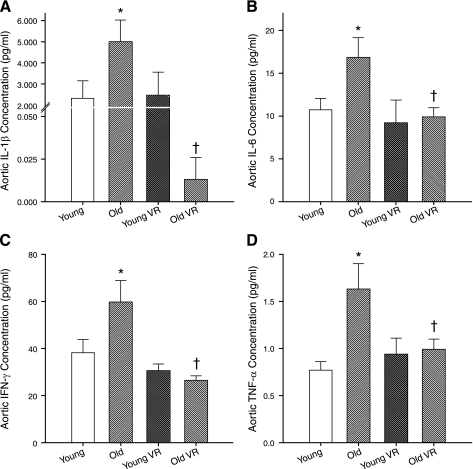
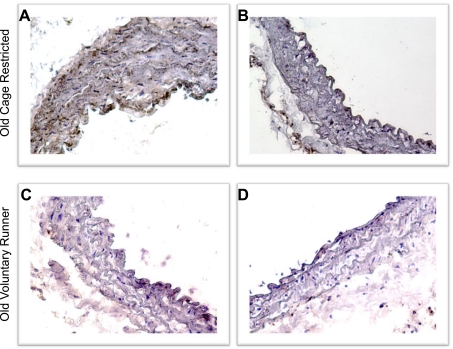
Aortic concentrations of IL-1β, IL-6, IFN-γ, and TNF-α were increased in old compared with young cage-restricted mice (Fig. 2; P < 0.05). Voluntary wheel running reversed the age-related increases in aortic cytokine concentrations in old mice to levels at or below those of young controls, without affecting expression in young mice (Fig. 2). In histological preparations of aortas from subsets of old cage-restricted and voluntary wheel running mice (n = 3/group), TNF-α appeared to be expressed diffusely in the medial and endothelial layers (Fig. 3A) and was associated with nuclei in the adventitial space (Fig. 3B). Voluntary wheel running appeared to reduce TNF-α expression in all arterial compartments (Fig. 3, C and D).
Fig. 2.
Aortic concentrations (all n = 5–11/group) of IL-1β (A), IL-6 (B), IFN-γ (C), and TNF-α (D) in young and old cage-restricted and VR mice. *P < 0.05 vs. young; †P < 0.05 vs. old.
Fig. 3.
Expression of TNF-α in aortas from old cage-restricted (A and B) and VR (C and D) mice. Images are from 2 mice per group. The color brown indicates positive staining for TNF-α with blue nuclear counterstain.
Aerobic exercise ameliorates age-associated increases in arterial macrophage infiltration.
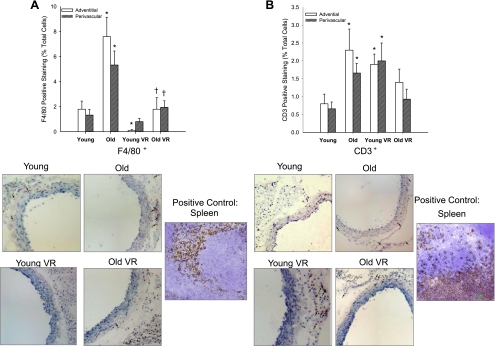
There was no detectable infiltration of macrophages or lymphocytes into either the media or intima of the aorta of young or old mice. However, macrophage (F4/80+ cells; P < 0.01) and T lymphocyte (CD3+ cells; P < 0.05) infiltration of the aortic adventitia and surrounding perivascular tissue was greater in old cage- compared with young cage-restricted mice (Fig. 4). In old mice, voluntary wheel running reversed the infiltration of macrophages in the adventitia and perivascular tissue to levels not different than young controls and tended to reduce perivascular T lymphocyte infiltration (P = 0.10; Fig. 4). Young mice that performed voluntary running showed modestly lower macrophage infiltration in the adventitia (Fig. 4A; P < 0.01) but higher infiltration of lymphocytes in the adventitia (P < 0.05) and perivascular tissues (P = 0.06; Fig. 4B) compared with young cage restricted.
Fig. 4.
Adventitial (white bars) and perivascular (hatched bars) macrophage (F4/80; A) and T lymphocyte (CD3; B) infiltration of aortas from young and old cage-restricted and VR mice (n = 5–9/group). Representative images are shown below summary graph, and arrows denote positive staining. *P < 0.05 vs. young; †P < 0.05 vs. old.
Aerobic exercise rescues age-associated vascular dysfunction.
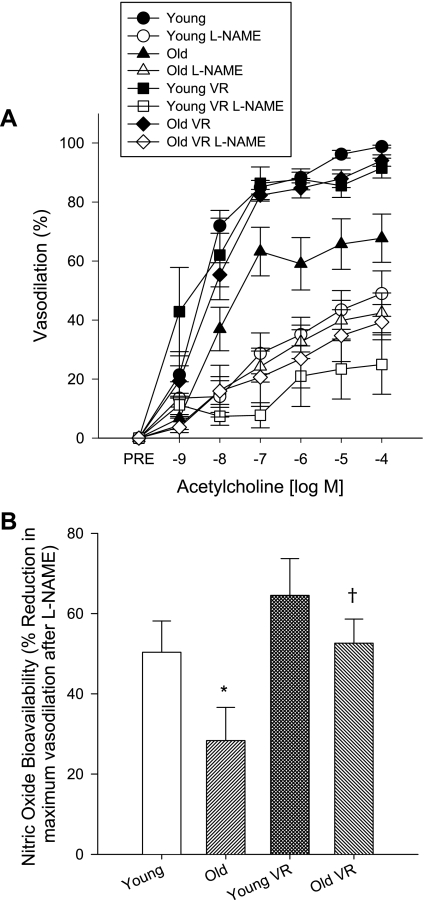
Maximal carotid artery luminal diameter was greater in old compared with young cage-restricted mice (P < 0.05) and was unaffected by exercise (Table 2). Carotid artery dilation to ACh (EDD; P < 0.01) and NO-mediated EDD (P < 0.05) were impaired in the old cage-restricted mice compared with the young controls, whereas dilation to the NO donor sodium nitroprusside did not differ (data not shown). Voluntary wheel running restored EDD and NO-mediated EDD (P < 0.05) in old mice to levels observed in the young controls, while having no effect in young mice (Fig. 5).
Table 2.
Carotid artery maximal diameter and phenylephrine-induced preconstriction in young, old, VR, and old VR mice in the absence or presence of the nitric oxide synthase inhibitor l-NAME
| Young | Old | Young VR | Old VR | |
|---|---|---|---|---|
| Maximal diameter, μm | 411 ± 6 | 432 ± 5* | 404 ± 7 | 427 ± 5* |
| Preconstriction, % | ||||
| Alone | 19 ± 1 | 19 ± 3 | 18 ± 3 | 16 ± 2 |
| l-NAME | 27 ± 3† | 27 ± 2† | 34 ± 4† | 23 ± 3† |
Values are means ± SE.
Difference from young;
difference after N-nitro-l-arginine methyl ester (l-NAME), P < 0.05.
Fig. 5.
Carotid artery endothelium-dependent dilation in the absence or presence of N-nitro-l-arginine methyl ester (l-NAME; A) and nitric oxide bioavailability (B) in young and old cage-restricted and VR mice (n = 5–10/group). Values are means ± SE. *P < 0.05 vs. young; †P < 0.05 vs. old.
Correlations.
Among all animals, maximal EDD was inversely related to macrophage accumulation in both the adventitial (R = −0.69; P = 0.04) and perivascular (R = −0.70; P = 0.03) layers. Maximal EDD was not related to any other variable. There were no significant relations between any other variables (all P > 0.15).
DISCUSSION
The overall novel finding of the present study is that regular aerobic exercise ameliorates arterial inflammation with aging in mice. Specifically, our results show that the anti-inflammatory effects of aerobic exercise on aging arteries include normalization of IKK-NF-κB activation, proinflammatory cytokines, and adventitial-perivascular macrophage infiltration. These anti-inflammatory effects are associated with amelioration of age-associated vascular dysfunction.
The present findings of increased vascular IKK-NF-κB activation with aging are in agreement with previous observations in both rodents (6, 55) and humans (12, 13), suggesting a primary role of this proinflammatory transcription factor signaling pathway in arterial inflammation with aging (6, 16). Phosphorylation of IKK leads to disinhibition of NF-κB in the cytosol with subsequent phosphorylation and translocation of NF-κB subunits p65 and p50 to the nucleus, inducing transcription and expression of several inflammatory cytokines in the arterial wall and overall promotion of a proinflammatory state (8, 22, 25). The results of the present study showing increases in aortic expression of the inflammatory cytokines IL-1β, IL-6, IFN-γ, and TNF-α in the old mice are consistent with this process.
Our study extends these prior findings by showing that regular aerobic exercise exerts potent anti-inflammatory actions in aged arteries, including inhibition of IKK-NF-κB activation and reductions in expression of proinflammatory cytokines to levels at or below those observed in healthy young controls. Aerobic exercise reduces circulating concentrations of inflammatory proteins in some middle-aged and older adults (18, 21, 24, 52). To our knowledge, this is the first direct evidence for anti-inflammatory effects of aerobic exercise in arterial tissues per se in the context of aging or any other state associated with vascular dysfunction. Preliminary results of immunohistochemical staining of aortas from old cage-restricted and voluntary running mice in the present study suggest that exercise-mediated reductions in proinflammatory cytokines may occur in multiple sites within the vascular wall, because TNF-α expression appeared to be decreased diffusely in both endothelial and medial layers, as well as in areas surrounding nuclei in the adventitial and perivascular layers.
One mechanism by which proinflammatory activation and cytokine production could be mediated in arteries with aging is increased immune cell infiltration, including macrophages and T lymphocytes. These immune cells produce inflammatory cytokines that can, in turn, initiate and sustain vascular inflammation (53). Circulating immune cells could infiltrate the intima and other layers of the artery from the lumen (inside-out) or, alternatively, infiltrate the adventitia initially from the surrounding perivascular tissues (outside-in). The little available evidence on primary aging is somewhat inconsistent. Sporadic clustering of macrophages in the aortic wall is more common in older compared with young human donors (51), and there is evidence of increased accumulation of polymorphonuclear leukocytes in aorta of old F344 rats (55). Others have reported little or no accumulation of macrophages or T lymphocytes in the intima or media with aging in rodents (30, 33) and increased T cell, but not macrophage, density with aging in adventitia of C57Bl/6 mice (33).
In the present study, we found increased macrophages and T lymphocytes in aortic adventitia and perivascular tissue, but not in the arterial intima or media, with aging in B6D2F1 mice, a first generation hybrid cross of C57Bl/6 and DBA mice. The densities of the cells were less than that observed with some experimental models of atherosclerosis (53), but similar to that observed in angiotensin-infused mice (45). These results suggest that immune cell infiltration and inflammation of arteries with primary aging may be mediated at least in part via outside-in mechanisms targeting the perivascular tissue and adventitial layer of arteries, as hypothesized previously in settings of CVD (37, 41, 50). Most importantly, here we report for the first time that wheel running normalizes macrophage infiltration of the aortic adventitia of old mice to levels observed in young mice, although less of an effect was observed in T lymphocyte density in old mice. To our knowledge, this is the first evidence that voluntary aerobic exercise can reverse macrophage accumulation within the normal arterial wall. An earlier study reported that forced swimming in apo E-deficient mice fed a high-fat diet reduced macrophage and CD4+ cell accumulation within fatty streak lesions in aortic lumens (35).
The results of the present study suggest that macrophages may be the more important physiological change affecting vascular function in settings of aging and aerobic exercise. First, the increase in the number of lymphocytes observed in the adventitial and perivascular layers with aging was relatively small compared with macrophages. Second, wheel running produced a significant reduction in macrophage infiltration, but only a trend for decreases in lymphocytes in old mice. Third, lymphocyte infiltration was paradoxically increased in young mice that underwent wheel running in the face of unchanged EDD, dissociating these events. Finally, a significant negative relation was observed between maximal EDD and macrophage, but not lymphocyte, infiltration in the overall group. Together, these observations are consistent with the concept that voluntary aerobic exercise may exert its anti-inflammatory influence on aging arteries, at least in part, by inhibiting macrophage infiltration of perivascular tissue and the adventitia.
We reported recently that this model of voluntary aerobic exercise reverses age-associated reductions in NO-mediated endothelium-dependent dilation in carotid arteries of B6D2F1 mice, while having no effects in young animals (17, 20). Our laboratory also found evidence for a role of reduced superoxide-associated vascular oxidative stress in mediating the effects of wheel running on endothelial function in old mice (17). The results of the present study showing amelioration of endothelial dysfunction in old mice by wheel running are consistent with these previous observations. Our findings here extend these earlier data by showing that the restoration of endothelial function by wheel running in old mice is associated with amelioration of arterial inflammation. It is well established that oxidative stress and inflammation are reciprocally amplifying biological processes (26, 46, 47). As such, the present findings along with the prior report from our laboratory (17) provide support for the idea that regular aerobic exercise may reverse age-related arterial dysfunction via a combination of antioxidant and anti-inflammatory effects.
Previous work has linked T lymphocyte activation to the development of hypertension and angiotensin-induced vascular endothelial dysfunction (23). We did not measure arterial pressure in the present study, but previously have shown impaired endothelial function with aging in this model in the absence of significant increases in mean arterial pressure (17, 29). Moreover, although baseline mean pressure was lower in wheel running compared with cage-restricted old mice in an earlier study from our laboratory (17), blood pressure and maximal EDD were not related. Given the lack of consistent associations between lymphocyte infiltration and endothelial function described above for our current study, and for blood pressure and endothelial function in earlier investigations from our laboratory (17, 29), these events do not appear to be mechanistically related in the present model of aging and aerobic exercise.
The mechanisms that underlie the anti-inflammatory effects of exercise on arteries with aging are not known but may include a direct effect of increases in laminar shear at the endothelium (as a result of increases in blood flow and/or pressure during running) or via changes in circulating and/or locally produced factors (38, 39). Although anti-inflammatory signaling from endothelium in response to increases in laminar shear is appealing, it is unclear how this signal would be transduced through the arterial media to influence macrophage infiltration in the adventitial and perivascular spaces. Candidate molecules for systemic or local factors that can influence both endothelial function and inflammation with aging and exercise include ANG II (4, 7, 27, 54) and endothelin-1 (4, 15, 19). Both have potent proinflammatory effects (19, 27), and vascular reactivity to these molecules is influenced by aging and exercise (4, 15, 32, 49). Age and aerobic exercise could influence such factors in the circulation or locally in the adventitia (e.g., vasovasorum) or the perivascular tissue to affect inflammatory signaling in arteries.
In conclusion, voluntary aerobic exercise reverses arterial inflammation with aging in mice. This may be mediated in part via inhibition of macrophage infiltration of perivascular tissue and arterial adventitia. These anti-inflammatory actions may play an important role in the beneficial effects of voluntary aerobic exercise on vascular function observed in middle-aged and older adults (39).
GRANTS
This work was supported by NIH Awards AG000279, AG033196, AG033755, AG029337, and AG013038.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Eric Chung for his assistance with animal care and monitoring and Alexander Vanengelenburg for technical assistance with immunohistochemistry. All experiments were performed in the Integrative Physiology Laboratory of Dr. Douglas Seals at the University of Colorado at Boulder. L. A. Lesniewski and A. J. Donato contributed to all aspects of the study including the conception and design of the experiments, collection, analysis, interpretation of data, and drafting and revision of the manuscript. D. R. Seals contributed to the conception and design of the experiments, interpretation of data, and drafting and revision of the manuscript. J. R. Durrant, M. L. Connell, G. D. Henson, and A. D. Black contributed to the collection and analysis of data and revision of the manuscript.
REFERENCES
- 1. Blackwell KA, Sorenson JP, Richardson DM, Smith LA, Suda O, Nath K, Katusic ZS. Mechanisms of aging-induced impairment of endothelium-dependent relaxation: role of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol 287: H2448–H2453, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 262: 2395–2401, 1989 [DOI] [PubMed] [Google Scholar]
- 3. Bonthu S, Heistad DD, Chappell DA, Lamping KG, Faraci FM. Atherosclerosis, vascular remodeling, and impairment of endothelium-dependent relaxation in genetically altered hyperlipidemic mice. Arterioscler Thromb Vasc Biol 17: 2333–2340, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Bunker AK, Laughlin MH. Influence of exercise and perivascular adipose tissue on coronary artery vasomotor function in a familial hypercholesterolemic porcine atherosclerosis model. J Appl Physiol 108: 490–497, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-alpha treatment in aging. Am J Pathol 170: 388–398, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-kappaB. J Appl Physiol 105: 1333–1341, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Cavanagh EM, Inserra F, Ferder L. Angiotensin II blockade: a strategy to slow ageing by protecting mitochondria? Cardiovasc Res 89: 31–40, 2011 [DOI] [PubMed] [Google Scholar]
- 8. de Winther MP, Kanters E, Kraal G, Hofker MH. Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol 25: 904–914, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol 80: 261–270, 1996 [DOI] [PubMed] [Google Scholar]
- 10. DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Didion SP, Ryan MJ, Didion LA, Fegan PE, Sigmund CD, Faraci FM. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circ Res 91: 938–944, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NF kappa B, reduced I kappa B alpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell 7: 805–812, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 100: 1659–1666, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol 297: H425–H432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Donato AJ, Lesniewski LA, Delp MD. The effects of aging and exercise training on endothelin-1 vasoconstrictor responses in rat skeletal muscle arterioles. Cardiovasc Res 66: 393–401, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Donato AJ, Pierce GL, Lesniewski LA, Seals DR. Role of NFkappaB in age-related vascular endothelial dysfunction in humans. Aging (Albany NY) 1: 678–680, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol 587: 3271–3285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elosua R, Bartali B, Ordovas JM, Corsi AM, Lauretani F, Ferrucci L. Association between physical activity, physical performance, and inflammatory biomarkers in an elderly population: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 60: 760–767, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Finsnes F, Lyberg T, Christensen G, Skjonsberg OH. Effect of endothelin antagonism on the production of cytokines in eosinophilic airway inflammation. Am J Physiol Lung Cell Mol Physiol 280: L659–L665, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-beta1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol 588: 3971–3982, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol 153: 242–250, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Guzik TJ, Harrison DG. Endothelial NF-kappaB as a mediator of kidney damage: the missing link between systemic vascular and renal disease? Circ Res 101: 227–229, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jankord R, Jemiolo B. Influence of physical activity on serum IL-6 and IL-10 levels in healthy older men. Med Sci Sports Exerc 36: 960–964, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Karin M. The beginning of the end: IkappaB kinase (IKK) and NF-kappaB activation. J Biol Chem 274: 27339–27342, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov 3: 73–80, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Kim JM, Heo HS, Ha YM, Ye BH, Lee EK, Choi YJ, Yu BP, Chung HY. Mechanism of Ang II involvement in activation of NF-kappaB through phosphorylation of p65 during aging. Age (Dordr). In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ, Seals DR. B6D2F1 mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. J Gerontol A Biol Sci Med Sci 64: 9–20, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li Z, Froehlich J, Galis ZS, Lakatta EG. Increased expression of matrix metalloproteinase-2 in the thickened intima of aged rats. Hypertension 33: 116–123, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 121: e46–e215, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Maeda S, Tanabe T, Miyauchi T, Otsuki T, Sugawara J, Iemitsu M, Kuno S, Ajisaka R, Yamaguchi I, Matsuda M. Aerobic exercise training reduces plasma endothelin-1 concentration in older women. J Appl Physiol 95: 336–341, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Moos MP, John N, Grabner R, Nossmann S, Gunther B, Vollandt R, Funk CD, Kaiser B, Habenicht AJ. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 25: 2386–2391, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation 116: 2110–2118, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Okabe TA, Shimada K, Hattori M, Murayama T, Yokode M, Kita T, Kishimoto C. Swimming reduces the severity of atherosclerosis in apolipoprotein E deficient mice by antioxidant effects. Cardiovasc Res 74: 537–545, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell 9: 304–312, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J 153: 907–917, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Seals DR, Desouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol 105: 1323–1332, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seals DR, Walker AE, Pierce GL, Lesniewski LA. Habitual exercise and vascular ageing. J Physiol 587: 5541–5549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Srere PA, Brooks GC. The circular dichroism of glucagon solutions. Arch Biochem Biophys 129: 708–710, 1969 [DOI] [PubMed] [Google Scholar]
- 41. Stern N, Marcus Y. Perivascular fat: innocent bystander or active player in vascular disease? J Cardiometab Syndr 1: 115–120, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Sui X, LaMonte MJ, Laditka JN, Hardin JW, Chase N, Hooker SP, Blair SN. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA 298: 2507–2516, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101: 2896–2901, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Tieu BC, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, Brasier AR. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest 119: 3637–3651, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci 65: 1028–1041, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-κB activation in aged rat arteries. Am J Physiol Heart Circ Physiol 293: H37–H47, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Vaitkevicius PV, Fleg JL, Engel JH, O′Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 88: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 49. Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension 50: 403–409, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Verhagen SN, Visseren FL. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis 214: 3–10, 2011 [DOI] [PubMed] [Google Scholar]
- 51. Wang M, Zhang J, Jiang LQ, Spinetti G, Pintus G, Monticone R, Kolodgie FD, Virmani R, Lakatta EG. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension 50: 219–227, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Wannamethee SG, Lowe GD, Whincup PH, Rumley A, Walker M, Lennon L. Physical activity and hemostatic and inflammatory variables in elderly men. Circulation 105: 1785–1790, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol 8: 802–815, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Wray DW, Nishiyama SK, Harris RA, Richardson RS. Angiotensin II in the elderly: impact of angiotensin II type 1 receptor sensitivity on peripheral hemodynamics. Hypertension 51: 1611–1616, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Zou Y, Yoon S, Jung KJ, Kim CH, Son TG, Kim MS, Kim YJ, Lee J, Yu BP, Chung HY. Upregulation of aortic adhesion molecules during aging. J Gerontol A Biol Sci Med Sci 61: 232–244, 2006 [DOI] [PubMed] [Google Scholar]