Abstract
Femoral artery occlusion augments the sympathetic nerve and pressor responses to muscle contraction and muscle metabolites injected into the arterial blood supply of the hindlimb muscles in rats. The underlying mechanism by which these reflex responses are enhanced after muscle vascular insufficiency is unclear. Purinergic P2X3 receptor has been reported to contribute to the metabolic component of the exercise pressor reflex. Thus the purpose of this study was to examine if chronic femoral occlusion would alter the expression of P2X3 in dorsal root ganglion (DRG) neurons of rats. Also, P2X3-mediated sympathetic responsiveness was examined after femoral occlusion. In addition, the role played by nerve growth factor (NGF) in regulating the expression and response of P2X3 was examined. Western blot analysis showed that 24 h of femoral ligation increased the levels of P2X3 (optical density: 0.93 ± 0.07 in control and 1.37 ± 0.10 after occlusion; P < 0.05 vs. control). The fluorescence immunohistochemistry further demonstrated that the occlusion elevated P2X3 expression in DRG neurons (percentage of P2X3-positive cells: 33 ± 3% in control and 51 ± 3% in occlusion; P < 0.05 vs. control). Furthermore, the results showed that responses of renal sympathetic nerve activity and blood pressure to stimulation of P2X were greater in occluded rats than responses in control rats by injection of α,β-methylene ATP into the arterial blood supply of the hindlimb muscle. Finally, infusion of NGF in the hindlimb muscles of healthy rats increased P2X3 (optical density: 0.98 ± 0.12 in control and 1.37 ± 0.16 with NGF; P < 0.05 vs. control). The pressor response to injection of α,β-methylene ATP was increased in the rats with NGF infusion. Likewise, blocking NGF attenuated exaggeration of the reflex response induced by α,β-methylene ATP in occluded rats. The findings of this study suggest that the levels of P2X3 in primary afferent neurons are upregulated as the blood supply to the hindlimb is deficient under ischemic conditions, leading to augmentation of the muscle reflex. NGF is closely related to increases in P2X3 receptor expression and response.
Keywords: purinergic receptor, muscle afferents, peripheral artery disease, adenosine 5′-triphosphate, blood pressure
the exercise pressor reflex is a neural control mechanism responsible for the cardiovascular responses to exercise (35, 37). Group III and IV muscle afferents represent the sensory arm of this reflex. As exercise is initiated, group III and IV thin fiber nerves are mechanically and metabolically stimulated leading to reflex increases in arterial blood pressure and heart rate (HR) primarily through activation of sympathetic nerve activity (23–26). Group III afferents are thought to be predominantly mechanosensitive, whereas group IV afferents are thought to be a predominantly metabosensitive (23–26). Prior studies (40, 47–50) have indicated that the exercise pressor reflex is altered in cardiovascular diseases.
Peripheral arterial disease (PAD) is one of the most common and important public health problems that affect the lifestyles in 20% of adults who are older than 55 yr (13, 41, 46). The main cause of this disease is the narrowing of blood vessels in the lower limbs, predominantly due to atherosclerotic vascular disease (2). The first clinical sign of PAD is usually intermittent claudication during exercise (46). Prior studies (3, 4) reported that autonomic responses are enhanced during exercise in PAD patients.
A rat model of femoral artery occlusion used to study intermittent claudication seen in human PAD has been well established (52). This model exhibits impaired limb blood flow reserve capacity with exercise but normal flow at rest (58, 59). A previous study (50) using this model has illustrated that the pressor response to static muscle contraction is increased in rats with femoral occlusion. Furthermore, the reflex sympathetic nerve and pressor responses to stimulation of metabolically sensitive receptors, such as transient receptor potential vanilloid type 1 (TRPV1) and acid-sensing ion channels (ASICs), are augmented in occluded rats (33, 56). The upregulation of these metabolites receptor in sensory nerves (neurons) is considered a mechanism that contributes to the augmented responses following femoral occlusion (33, 56).
Several prior studies (17–19, 27, 29, 30, 32, 36) suggest that ATP-sensitive purinergic P2X receptors contribute to the metabolic component of the exercise pressor reflex in cat and rat models. First, the concentration of ATP in the muscle interstitium is increased during muscle contraction and stretch (29, 30). Second, injection of α,β-methylene ATP (α,β-me-ATP), a P2X receptor agonist, into the arterial blood supply of the hindlimb muscle increases blood pressure via reflex mechanism (17, 32). Third, blocking P2X receptors (i.e., P2X2/3 and P2X3) attenuates discharge of group IV afferent fibers and blood pressure response during static muscle contraction (18, 19, 27, 36).
On the basis of these data, it was hypothesized that femoral artery occlusion increases P2X3 receptors in primary afferent neurons/dorsal root ganglion (DRG) neurons and thereby leads to the enhanced reflex responses to stimulation of P2X3. Western blotting and immunohistochemistry were employed to examine P2X3 in DRG neurons of control rats and those with femoral artery occlusion. To determine P2X responsiveness, sympathetic and cardiovascular responses to injections of α,β-me-ATP into the arterial blood supply of the hindlimb muscles were further examined in both groups.
Nerve growth factor (NGF) is a secreted signaling protein that plays an important role in sympathetic and sensory neuron survival. Femoral artery occlusion elevates the levels of NGF in the hindlimb muscles and DRG neurons of rats (57). Also, previous reports (44) suggest that NGF can evoke P2X receptor expression in the DRG neurons. Based on these data, it was postulated that NGF is involved in upregulation of P2X3 in sensory nerves and contributes to the exaggeration of the muscle reflex after femoral occlusion by increasing the expression of P2X3. Thus the levels of P2X3 protein in DRG neurons and the cardiovascular responses to arterial injections of α,β-me-ATP were examined after infusion of NGF into hindlimb muscles through a microosmotic pump. Also, the pressor and heart rate responses to α,β-me-ATP were examined after NGF antibody was administered into the hindlimb muscles.
METHODS
All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Pennsylvania State College of Medicine and complied with the National Institutes of Health guidelines.
Femoral artery occlusion.
A rat model of femoral artery occlusion has been well described to induce hindlimb muscle vascular insufficiency/muscle ischemia (52). The surgical procedures were performed in sixty-four male Sprague-Dawley rats (5–7 wk old) as previously described (33). While the rats were under inhalation of an isoflurane-oxygen mixture (2–5% isoflurane in 100% oxygen), their femoral artery on one limb was surgically exposed, dissected, and ligated ∼3 mm distal to the inguinal ligament. The same procedures were performed on the other limb except that a suture was placed below the femoral artery but was not tied; this served as the control. The postoperative care of the animals was taken. Buprenorphine hydrochloride (0.05 mg/kg sc) was given as analgesic after the surgery and then every 12 h before the experiments as needed. In addition, after the surgery each rat received penicillin potassium (100,000 IU/kg im twice daily) and lactated ringers (5 ml/kg sc). To better examine rats' healthy status, the body weights of rats were measured before experiments. If the rats were found to lose 10% of their body weight after the surgery, they would not be included in the experiment. Then, recovery times of 6, 24, and 72 h were allowed before the experiments began.
Administration of NGF.
The microosmotic pump (Alzet model 1003D, 3-day delivery; length: 1.5 cm and diameter: 0.6 cm) containing NGF and saline was implanted subcutaneously in the hindlimbs of 12 healthy rats under anesthesia (57). Note that the pumps were placed in the femoral triangle region and outlet of the pump was 2–3 mm distal to the inguinal ligament. Then, NGF was delivered at a rate of 0.25 μg/h to one leg. A total of 18 μg NGF was delivered over 72 h. Saline in the same volume was delivered at the same infusion rate on another leg as the control. Previous experiments (57) have shown that delivered NGF in this manner can increase responsiveness of sensory nerve receptors (i.e., TRPV1).
Western blot analysis.
Eighteen rats were used to examine expression of P2X3 protein in lumbar (L4–6) DRGs of control and occluded limbs. Six rats were used to examine P2X3 in L4–6 DRGs of control limb with saline infusion and experimental limb with NGF infusion. Western blot methods were performed as previously described (33, 34). In brief, DRGs of the rats were removed. All DRGs tissues from individual rats were sampled for Western blot analysis. Total protein was then extracted by homogenizing DRG sample in ice-cold radioimmunoprecipitation assay buffer containing 25 mM Tris·HCl (pH 7.6), 150 mM NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, and 0.1% SDS with protease inhibitor cocktail kit (Sigma-Aldrich, St. Louis, MO). The lysates were centrifuged at 15,000 g for 15 min at 4°C; the supernatants were collected for measurements of protein concentrations using a bicinchoninic acid assay reagent kit (Pierce Biotechnology, Rockford, IL) and then stored in −80°C for later use.
After being denatured by heating at 95°C for 5 min in an SDS sample buffer (Cell Signaling Technology, Danvers, MA), the supernatant samples containing 20 μg of protein was loaded onto 10–12% SDS-polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA) and then electrically transferred to a polyvinylidene fluoride membrane (GE Water & Process Tech, Trevose, PA). The membrane was blocked in 5% nonfat milk in 0.1% Tween-TBS buffer for 1 h and was then incubated overnight with primary antibody:rabbit anit-P2X3 at 1:1,000 dilutions (Santa Cruz Biotechnology, Santa Cruz, CA).
After being fully washed, the membrane was incubated with horseradish peroxidase-linked anti-rabbit secondary antibody at 1:1,000 dilutions and visualized for immunoreactivity using an enhanced chemiluminescence system (Cell Signaling Technology). The membrane was stripped and incubated with mouse anti-β-actin (Sigma-Aldrich) to show equal loading of the protein in the Western blot analysis. The densities of P2X3 and β-actin bands were determined using the NIH Scion Image Software.
Fluorescence immunohistochemistry.
Six rats were used to examine P2X3 immunostaining in L4–6 DRGs of control and occluded limbs. After anesthetization with inhalation of an isoflurane-oxygen mixture, the rats were transcardially perfused sequentially with 200 ml of ice-cold saline containing 1,000 U heparin, 500 ml of 4% freshly prepared ice-cold paraformaldehyde in PBS (pH 7.4), and 200 ml of 10% sucrose. L4–6 DRGs of control and occluded limbs were immediately removed once perfusion was complete. DRGs were further fixed in 4% paraformaldehyde for 2 h and stored in 30% sucrose overnight. The processed DRGs were embedded in Tissue-Tek (Sakura Finetek, Torrance, CA) onto dry ice and cut on a cryostat to obtain 10-μm sections. The sections were then mounted onto microscope slides and dried at room temperature.
The sections were fixed with 4% paraformaldehyde for 10 min, permeabilized, and blocked with 0.3% Triton X-100 in PBS supplemented with 5% goat serum for 1 h and then incubated with rabbit anti-P2X3 (1: 250; Neuromics, Edina, MN) primary antibody overnight at 4°C. After being thoroughly washed in PBS, the sections were incubated with goat anti-rabbit secondary antibody conjugated to Alexa fluor 594 (Invitrogen, Molecular Probes, Carlsbad, CA) for 2 h at room temperature. The sections were coverslipped and then examined under a fluorescence microscope (Nikon Eclipse 80i, Tokyo, Japan).
After all sections of each lumbar DRG were processed for P2X3, every fourth section was chosen in a consecutive fashion to obtain at least five sections per animal for immunostaining analysis. The images were obtained with an attached digital camera, stored on a computer, and then analyzed using the Nis-Elements software (Nikon). The methods to analyze immunostaining were described in the previous report (33). Cells with >1.75 times of background intensity were considered to be positive (33). The number of P2X3-positive cells and the total cells was counted in each section. The percentage of P2X3-positive cells vs. total cells was calculated. Note that the majority of DRG cells showed a clear nucleus and perimeter in the sections, and only neurons with a clear nucleus and perimeter were selected for analysis of the total cells and P2X-positive cells in the current study.
Measurements of NGF.
Three subgroups of rats were used in this experiment: 1) five rats served as control; 2) five rats were infused with NGF in one leg and with saline in another leg; and 3) five rats who received 24 h of femoral ligation on both legs and 10 μg of NGF antibody were given in one leg and saline was given in the contralateral leg. These rats were anesthetized by inhalation of an isoflurane oxygen mixture (2–5% isoflurane in 100% oxygen) and killed by decapitation. The L4-L6 DRGs and ventrolateral medulla were removed quickly, weighed, and frozen at −80°C for NGF measurements. NGF levels in DRG tissues were determined using a two-site immunoenzymatic assay (ELISA) as previously described (61). Note that the ELISA measurements were made on L4-L6 DRGs/medulla from individual animals. Briefly, polystyrene 96-well microtitel immunoplates were coated with affinity-purified polyclonal goat anti-NGF antibody (Promega). Parallel wells were coated with purified goat IgG for evaluation of nonspecific signal. After overnight incubation at room temperature and 2 h of incubation with the coating buffer (50 mM carbonate buffer, pH 9.5, in 2% BSA) plates were washed with 50 mM Tris·HCl (pH 7.4; 200 mM NaCl, 0.5% gelatin, and 0.1% Triton X-100). After extensive washing, the diluted samples and the NGF standard solutions (Promega), ranging from 0 to 500 pg/ml, were distributed in each plate and left at room temperature overnight. The plates were then washed and incubated with 4 mU of anti β-NGF-galactosidase per well (Boehringer Mannheim). After a 2-h incubation at 37°C, the plates were washed and then incubated with 100 μl of substrate solution (4 mg of chlorophenol red per ml of substrate buffer: 100 mM HEPES, 150 mM NaCl, 2 mM MgCl2, and 0.1% sodium azidey 1% BSA) that was added to each well. After an incubation of 2 h at 37°C, the optical density was measured at 575 nm using an ELISA reader (BioTek).
In addition, blood samples were taken from the control rats and rats who received NGF infusion for measurements of plasma NGF. First, blood samples were centrifuged to obtain plasma. In a similar way, a NGF ELISA kit (Abcam) was used to determine the level of plasma NGF. Polystyrene 96-well microtitel immunoplates was previously coated with anti-rat NGF antibody. After incubation and being washed, plasma samples and standard NGF solutions were added in each plate and incubated with biotinylated antibody and horseradish perioxidase-conjugated streptavidin. Then, the optical density was measured after the plates were washed and incubated with substrate solution.
Examination of reflex sympathetic and cardiovascular responses.
The eight control rats and nine rats with 24 h of femoral occlusion were anesthetized with a mixture of 2–5% isoflurane and oxygen and ventilated as described previously (33, 56). The right jugular vein and common carotid artery were cannulated to deliver fluids and to connect a pressure transducer for measurement arterial blood pressure, respectively. A catheter (PE10) was then inserted into the femoral artery for injection of drugs into the arterial blood supply of the hindlimb muscles. A bundle of the renal nerves on the left side were carefully dissected and placed on laboratory film, followed by a bipolar electrode being placed under the isolated renal nerves and embedded in a silicone gel fixed to the surrounding tissue. The skin on the back was used to form a pool that was filled with warm (37°C) mineral oil. The renal sympathetic nerve activity (RSNA) signal was amplified with an amplifier (P511; Grass Instruments) and recorded.
Decerebration was performed as previously described (33, 56) to avoid the confounding effects of anesthesia on the reflex pressor response. A transverse section was made anterior to the superior colliculus and extending ventrally to the mammillary bodies. All brain tissues from rostral to the section were removed. Following this procedure, the anesthesia was withdrawn from the rats. A recovery period of 60 min was allowed before the experiment began.
During the experiments, baseline blood pressure and fluid balance were maintained with a continuous infusion of saline. Body temperature was continuously monitored and maintained at 37.5–38.5°C with a heating pad and external heating lamps.
α,β-me-ATP (Sigma) was dissolved in saline and made at 0.0625, 0.125, and 0.25 mM. Then, they were administered into the femoral artery of control limb and occluded limb. The injection volume was adjusted to 0.15–0.25 ml according to the rat's body weight. The duration of the injection was 1 min. The interval between the two injections was ≥20 min. On the completion of three injections, the sciatic nerve was severed and 0.25 mM of α,β-me-ATP was repeated to confirm that the response was due to stimulation of afferents within the hindlimb.
In another group of animals, 0.125 mM of α,β-me-ATP were arterially injected into the hindlimb muscles of six rats that received previous infusion of NGF and saline.
In an additional group, the femoral occlusion was performed on one leg and sham procedure was performed on another leg as control in eight rats. Ten micrograms of NGF antibody (Abcam) were administered into the hindlimb muscles (the superficial white portion of the gastrocnemius muscle) of each leg 24 h before experiments. Then, 0.125 mM of α,β-me-ATP were arterially injected to examine effects of blocking NGF on pressor and heart rate responses to stimulation of P2X.
All measured data of RSNA, blood pressure, and HR were continuously recorded and stored on a computer with PowerLab system (Ad instruments, Castle Hill, Australia). Mean arterial pressure (MAP) was obtained by integrating the arterial signal with a time constant of 4 s. HR was calculated on a basis of beat to beat from the arterial pressure pulse. The peak responses of MAP and HR were determined by the peak change from the control value. RSNA signals were transformed into absolute values, integrated over a 1-s interval, and subtracted by 1 s of integrated background noise. To quantify RSNA response to α,β-me-ATP injection, baseline values were obtained by taking the mean value for the 30 s immediately before each injection and by ascribing the mean value of 100%, and relative change from baseline during the injection was then evaluated.
Data analysis.
All experimental data are presented as means ± SE. Comparisons of variables for P2X3 optical density, NGF, MAP, and RSNA responses were performed using one-way ANOVA followed by Tukey's post hoc test as appropriate. Comparisons of variables for percentage of P2X3-positive neurons were made using Student's t-test. A value of P < 0.05 was considered significant. All statistic analysis was implemented using the SAS 9.13 for Windows (SAS Institute, Cary, NC).
RESULTS
Levels of P2X3 protein in DRG of occluded and control limbs.
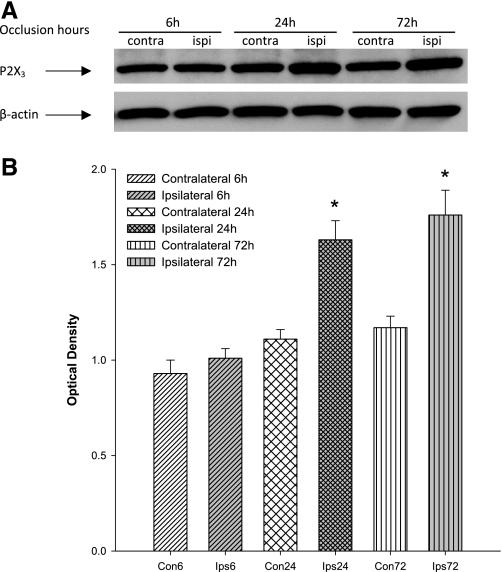
Western blot analysis was used to examine the levels of P2X3 protein in the lumbar DRG of occluded limbs and control limbs of rats 6, 24, and 72 h following femoral occlusion (n = 6 in each group). There were no significant differences in the levels of P2X3 in the DRG of control limbs of rats 6, 24, and 72 h after occlusion (P > 0.05, shown in Fig. 1). The data demonstrated that 24 and 72 h of femoral occlusion significantly elevated the levels of P2X3 protein in the lumbar DRG (Fig. 1). However, 6 h of femoral occlusion had no significant effects on the expression of P2X3 (Fig. 1). The intensity of P2X3 signal in the DRG tissues was ∼1.47-fold greater in the limb with 24 h of occlusion than that in the control limb. There were no significant differences in the elevated levels of P2X3 protein between 24 and 72 h of occlusion (Fig. 1).
Fig. 1.
Effects of femoral artery occlusion on purinergic P2X3 receptor expression in dorsal root ganglion (DRG) neurons at different time courses. Western blot assay was employed to examine P2X3 proteins in L4-L6 DRG at 6, 24, and 72 h following femoral artery occlusion. Ligation was performed on the 1 hindlimb [ipsilateral (ipsi)]. Sham-operated procedure was performed on the contralateral limb [contralateral (contra)] of the same rats, and this served as control. A: representative bands of P2X3 expression. Bands of β-actin are used as control for an equal protein loading. B: average data. Optical density is expressed in arbitrary units normalized against a control sample. Data in histograms represent means ± SE; n = 6 animals per group. *P < 0.05 vs. control.
P2X3 immunostaining in DRG neurons after femoral occlusion.
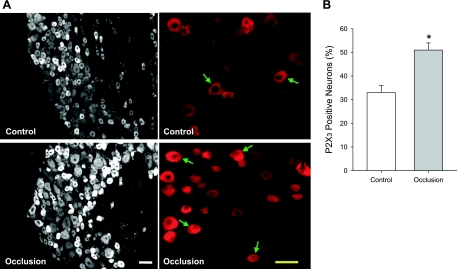
Fluorescence immunohistochemistry was used to examine P2X3-positive DRG neurons of control and occluded limbs of rats (n = 6). Consistent with previous findings (38, 39), the present experiments also illustrated that immunoreactivity of P2X3 receptor was seen in small to medium diameter (<35 μm) of DRG neurons. Furthermore, Fig. 2, A (typical images) and B (average data), shows that the number of P2X3-positive DRG neurons was >24 h after femoral artery occlusion compared with control. The percentage of P2X3-positive cells is 33 ± 3% in control and 51 ± 3% in occlusion (P < 0.05 vs. control).
Fig. 2.
Localization of P2X3 in lumbar DRG neurons 24 h following femoral artery occlusion. Fluorescence immunohistochemistry was employed to examine expression of P2X3 in DRG neurons of control limb and occluded limb. A: representative photomicrographs show P2X3 staining at a lower power (left) and a higher power (right). Arrows indicate P2X3-positive cells. Scale bar = 50 μM. B: histograms show that percentage of P2X3-positive neurons is greater in DRG neurons of femoral artery occlusion (n = 6) than that in control (n = 6). *P < 0.05, compared with control.
Sympathetic and cardiovascular responses to arterial injection of α,β-me-ATP.
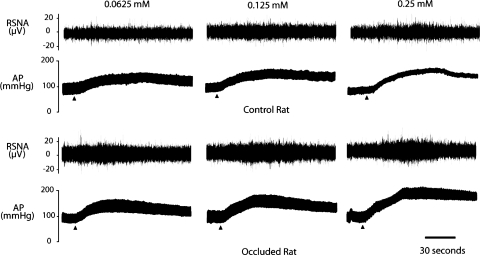
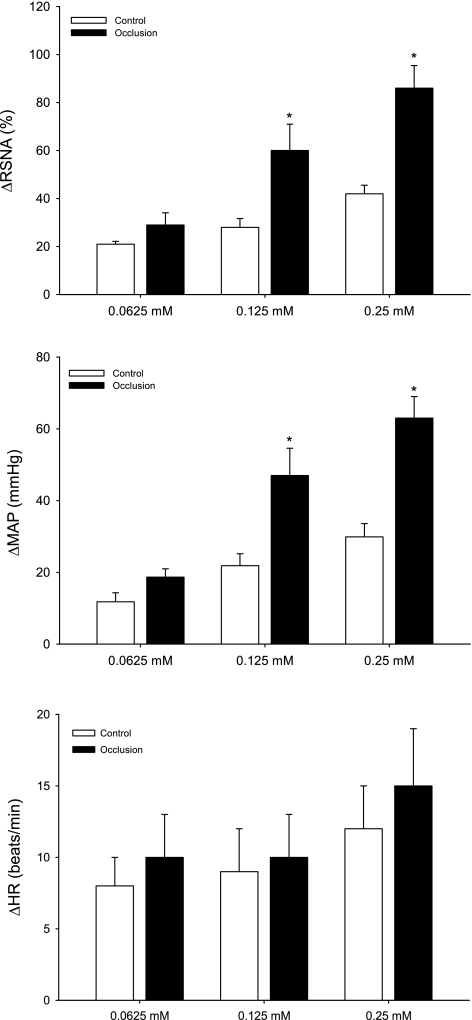
Table 1 shows baseline values for MAP and HR before arterial injections of α,β-me-ATP in control rats (n = 8) and in rats with 24 h of femoral occlusion (n = 9). There were no significant differences in baseline MAP and HR before each of the injections. Both Figs. 3 (typical recording) and 4 (average data) illustrate the effects of increasing concentrations (0.0625, 0.125, and 0.25 mM) of α,β-me-ATP injected into the hindlimb muscles on RSNA and MAP in occluded and control groups. Arterial injection of α,β-me-ATP evoked dose-related increases in RSNA and MAP in both groups. However, the responses induced by 0.125 and 0.25 mM of α,β-me-ATP were significantly amplified in occluded rats compared with responses in control rats (Figs. 3 and 4). Note that there was no significant difference in HR responses evoked by an arterial injection of α,β-me-ATP in both groups.
Table 1.
Basal MAP and HR before arterial injection of α,β-me-ATP
| Baseline MAP, mmHg |
Baseline HR, beats/min |
|||
|---|---|---|---|---|
| α,β-me-ATP | Control | Occluded | Control | Occluded |
| 0.0625 mM | 96 ± 6 | 100 ± 9 | 378 ± 12 | 388 ± 17 |
| 0.125 mM | 97 ± 9 | 103 ± 10 | 385 ± 15 | 390 ± 18 |
| 0.25 mM | 95 ± 5 | 100 ± 6 | 395 ± 20 | 387 ± 20 |
Values are means ± SE. Number of animals = 8 in control and 9 in occluded rats. α,β-me-ATP, α,β-methylene ATP; MAP, mean arterial pressure; HR, heart rate. There is no significant difference among basal values.
Fig. 3.
Typical recording of renal sympathetic nerve activity (RSNA) and arterial blood pressure (AP) responses to stimulation of P2X. Arrowheads indicate a start of injections. Three dosages of α,β-methylene ATP (α,β-me-ATP) were injected into arterial blood supply of the hindlimb muscles in sham control and occluded rats.
Fig. 4.
Changes in RSNA and mean arterial pressure (MAP) in response to stimulation of afferent nerve P2X receptors. Three dosages of α,β-methylene ATP were injected into arterial blood supply of the hindlimb muscles of control rats (n = 8) and rats with 24 h of femoral artery occlusion (n = 9). Values are means ± SE. HR, heart rate. *P < 0.05, compared with control.
In addition, the pressor response induced by 0.25 mM of α,β-me-ATP was examined before and after the sciatic nerve was severed. When the nerve was intact, MAP responses were 28 ± 5 mmHg in control rats (n = 5) and 56 ± 8 mmHg in occluded rats (n = 5). The responses were significantly attenuated following severing the sciatic nerve. MAP responses were 5 ± 2 mmHg in control rats and 8 ± 3 mmHg in occluded rats. These data suggest that the pressor response was due to stimulation of afferents within the hindlimb.
Effects of NGF on expression of P2X3 and its responsiveness.
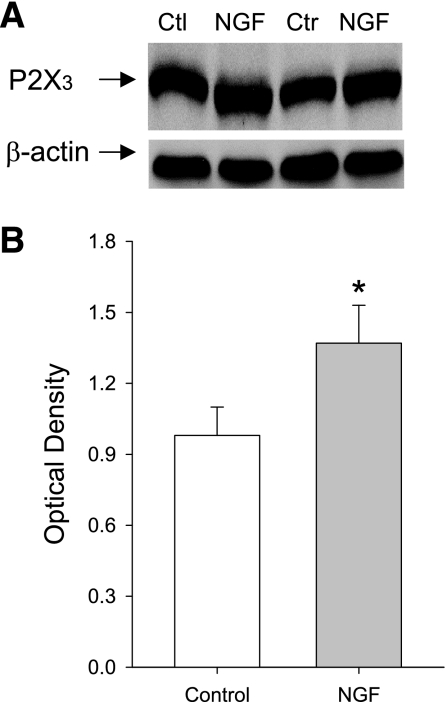
To determine the role of NGF in modulating afferent nerves P2X3 responses, NGF was continually infused into the hindlimb muscles of healthy rats (n = 6) through a microosmotic pump. Western blot analysis was then used to determine the levels of P2X3 protein in DRG tissues of legs infused with NGF and in DRGs of control legs infused with saline. Figure 5 shows that NGF infused into the hindlimb muscles significantly increased expression of P2X3 in DRG neurons compared with those in controls. The intensity of P2X3 seen in DRGs of experimental legs is ∼1.39-fold greater than that in control legs.
Fig. 5.
Effects of nerve growth factor (NGF) infusion on expression of P2X3 proteins in DRG neurons. Exogenous NGF was continually infused at a rate of 0.25 μg/h into the hindlimb of healthy rats with a microosmotic pump for 72 h. Saline was infused into the contralateral limb as control. Once deliveries of NGF and saline were complete, the levels of P2X3 proteins were examined in bilateral DRGs (L4–6) using western blot analysis. A: representative bands of P2X3 expression. Bands of β-actin are used as control for an equal protein loading. Note that the repetitive control (Ctl, Ctr) and NGF expressions are shown. B: average data. Optical density is expressed in arbitrary units normalized against a control sample. Data in histograms represent means ± SE; n = 6 animals per group. *P < 0.05, compared with control.
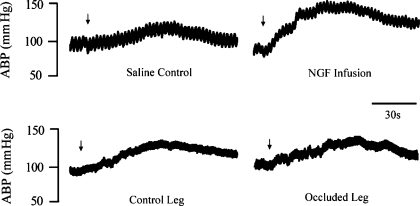
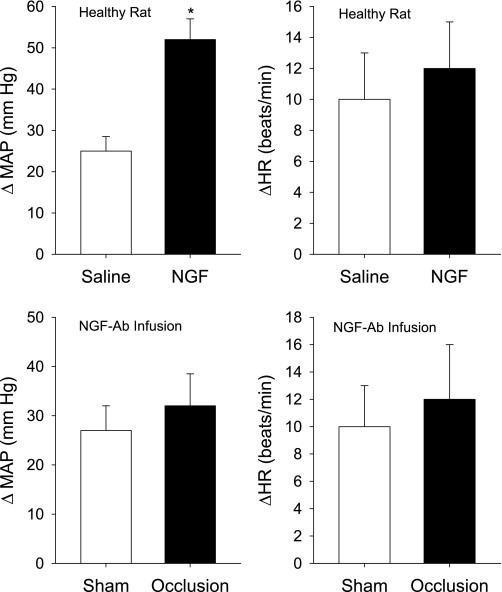
In addition, to examine responsiveness of P2X after infusion of NGF into the muscles, 0.125 mM of α,β-me-ATP were injected into the femoral arteries of experimental and control legs of rats (n = 6). Figures 6 and 7 demonstrate that the same dose α,β-me-ATP evoked a greater MAP response in legs infused with NGF compared with the response in control legs infused with saline. Increases in MAP after injection of α,β-me-ATP were 25 ± 3 mmHg in control legs and 52 ± 4 mmHg in legs infused with NGF (P < 0.05 vs. control).
Fig. 6.
Typical recording of arterial blood pressure (ABP) response to arterial injection of α,β-me-ATP; 0.125 mM of α,β-me-ATP were injected. Arrows indicate a start of injections. Top: effects of NGF infusion on pressor response to stimulation of P2X. α,β-me-ATP was injected into arterial blood supply of the muscles of saline control and experimental legs infused with NGF (0.25 μg/h for 72 h) in healthy rats. Bottom: effects of NGF neutralization on the pressor response to stimulation of P2X in sham control leg and occluded leg. NGF-Ab (10 μg each leg) was injected into both legs 24 h before experiments. Then, α,β-me-ATP was arterially injected to examine the pressor response to stimulation of P2X.
Fig. 7.
Top: effects of NGF infusion on blood pressure and heart rate responses to stimulation of P2X. α,β-me-ATP at 0.125 mM was injected into arterial blood supply of the muscles of control leg and experimental legs in 6 health rats. Control legs were infused with saline and experimental legs were infused with NGF (0.25 μg/h for 72 h of continuous delivery) in the same rats. Values are means ± SE. *P < 0.05, compared with control. Note that baseline MAP is 98 ± 5 mmHg in control group and 96 ± 6 mmHg in experimental group (P > 0.05). Bottom: effects of NGF neutralization on pressor and heart rate responses to stimulation of P2X in sham control and occluded leg of 8 rats. α,β-me-ATP at 0.125 mM induced no significant difference in pressor and heart rate responses in both legs. Ten micrograms of NGF-Ab were injected into each leg 24 h before experiments. Baseline MAP is 99 ± 8 mmHg in control rats and 95 ± 5 mmHg in occluded (P > 0.05). Values are means ± SE.
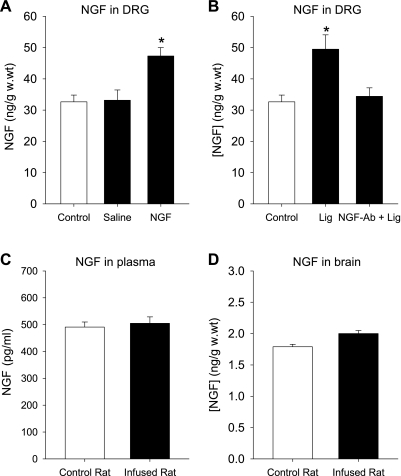
To determine effectiveness of NGF infusion, the levels of NGF in DRG tissues of control rats and rats infused with NGF were examined (shown in Fig. 8). Consistent with a prior study (57), the levels of NGF in the DRG of control rats were 32.7 ± 2.2 ng/g wet weight (n = 5). After NGF was infused into the hindlimb muscles, NGF was significantly increased in the DRG of infused leg (n = 5). However, this did not significantly alter the levels of NGF in the DRG of the control leg infused with saline in the same rats (n = 5). The levels of NGF were 33.2 ± 3.3 ng/g wet weight in control and 47.3 ± 2.7 ng/g wet weight with NGF infusion (P < 0.05, vs. control).
Fig. 8.
A: levels of NGF in the DRG tissues of 5 control rats and 5 rats infused with NGF. In the infused rats, 1 limb was infused with NGF; and the contralateral limb was infused with saline. [NGF], NGF concentration. *P < 0.05 vs. control rats and control legs infused with saline. There was no significant difference in NGF levels between control rats and control legs infused with saline. B: levels of NGF in the DRG tissues of 5 rats who received 24 h of femoral ligation (Lig) on both hindlimbs. In those rats, saline was given in 1 limb and NGF in another limb. *P < 0.05 vs. femoral ligation with application of NGF antibody. C and D: levels of NGF in plasma and brain tissues of 5 control rats and 5 rats infused with NGF. No significant differences in NGF in plasma and in brain were seen in control rats and infused rats.
In addition, the levels of NGF in plasma and in the ventrolateral medulla were examined in control rats and infused rats. Figure 8 shows that no significant difference in NGF was seen in both groups.
Effects of blocking NGF on pressor response to arterial injection of α,β-me-ATP.
NGF antibody was administered into the hindlimb muscles of each leg 24 h before experiments. Then, α,β-me-ATP at 0.125 mM was arterially injected into the hindlimb muscles of control leg and occluded leg of eight rats. Figures 6 and 7 show that α,β-me-ATP induced no significant difference in pressor and heart rate responses in control leg and occluded leg. Increases in MAP after injection of α,β-me-ATP were 27 ± 5 mmHg in control legs and 32 ± 7 mmHg in occluded legs (P > 0.05 vs. control).
Also, effectiveness of NGF antibody was examined in this experiment. Twenty four hours of femoral ligation were performed on both legs (n = 5). Ten micrograms of NGF antibody were given in one leg, and saline was given in the contralateral leg to serve as control. Similar to the results previously reported (57), femoral occlusion significantly increased the levels of NGF in the DRG; however, application of NGF antibody attenuated occlusion-induced NGF (shown in Fig. 8). After femoral ligation, the levels of NGF in the DRG were 49.5 ± 4.6 ng/g wet weight in control and 34.5 ± 2.7 ng/g wet weight with NGF antibody (P < 0.05 vs. control with saline).
DISCUSSION
The purpose of the present study was to determine whether P2X3 receptor on primary afferent nerves contributes to the enhanced sympathetic responsiveness elicited by femoral artery ligation. Also, this study was to examine whether NGF was engaged in the role of sensory nerve P2X3 in augmented responses evoked by the hindlimb vascular insufficiency. First, the levels of P2X3 protein expression were detected in the DRG of control leg and leg with femoral artery ligation. Western blot analysis demonstrated that 24 and 72 h of femoral artery occlusion significantly elevated P2X3 expression in lumbar DRGs. Twenty hours following the ligation surgery, the level of P2X3 was 1.47-fold greater in occluded rats than in control animals. Fluorescence immunohistochemistry further confirmed that femoral occlusion increased P2X3 immunoreactivity in small to medium diameter of DRG neurons. In addition, injection of α,β-me-ATP into the arterial blood supply of the hindlimb muscles evoked greater increases in RSNA and MAP in occluded rats than in control rats. The findings of this study suggest that there is a close linkage in increased P2X3 receptors on afferent nerves and augmented sympathetic responsiveness to stimulation of muscle afferent nerves under conditions of femoral occlusion.
Furthermore, infusion of NGF into the hindlimb of healthy rats through a microosmotic pump induced a 1.39-fold increase in P2X3 protein of the DRGs of the infused leg compared with the control leg. Also, NGF infused into the hindlimb significantly enhanced the pressor response to arterial injection of α,β-me-ATP. On the other hand, blocking NGF attenuated exaggeration of the reflex response induced by α,β-me-ATP in occluded rats. These findings suggest that NGF is closely related to upregulation of P2X3 expression in DRG neurons and to augmentation in the sympathetic and blood pressure responses to activation of P2X3 as the hindlimb vascular insufficiency occurs.
All P2X subtypes, except P2X7, are found in sensory neurons; however, the most prominent subtype is P2X3 (8, 9). For instance, the extent of P2X3 mRNA expression is two times greater than the expression of P2X2 receptor mRNA (53) in sensory neurons. P2X3 expression in DRG neurons is upregulated in the process of pathological responses (i.e., peripheral nerve injury and inflammation, etc.; Refs. 1, 5, 55, 60). Therefore, in the present study, a rat model of intermittent claudication induced by femoral artery ligation was used to examine the levels of P2X3 protein expression in the DRG tissues of occluded and control limbs. The data demonstrate that P2X3 was increased in DRG neurons of occluded limb 24 and 72 h after the femoral ligation surgery.
ATP is the endogenous ligand for P2X3 and P2X2/3 receptors, and these subtypes of P2X receptors have been recognized to play a major role in mediating the primary sensory effects of ATP (8, 9). In adults, P2X3 and P2X2/3 receptors are predominantly localized on small to medium diameter sensory neurons within DRG (7, 10, 12, 28, 51). Consistent with these previous findings, the results of the present study using fluorescence immunohistochemistry have also demonstrated that P2X3 receptors appeared in the lumbar DRG neurons with <35 μm in diameter. Furthermore, femoral occlusion selectively increased immunoreactivity of P2X3 in small to medium diameter of DRG neurons.
Previous studies (29, 30) demonstrated that contraction of skeletal muscle leads to a significant increase in the levels of ATP in the interstitium of muscles, and the elevation of ATP concentration is linearly related to magnitude of muscle tension. Also, there is strong evidence that ATP elicits the exercise pressor reflex by stimulation of muscle afferent P2X. First, injection of α,β-me-ATP into the arterial blood supply of the hindlimb muscles or directly into the gastrocnemius muscle stimulates over two-thirds of the group IV afferents in cats and rats (21, 45). Second, stimulation of P2X receptors on muscle afferents increases blood pressure via a reflex mechanism (17, 32). Third, blocking P2X receptors attenuates discharge of group IV afferent fibers as well as blood pressure response during static muscle contraction (18, 19, 27, 36). In a recent study (36), blocking P2X3 and P2X2/3 receptors by injecting A-317491 and RO-3, two structurally different P2X3 and P2X2/3 receptor antagonists, into the arterial circulation of the muscles showed that the pressor response to arterial injection of α,β-me-ATP was significantly attenuated. This prior study further demonstrated that the pressor response to postcontraction circulatory occlusion, a stimulus of metaboreceptors, is attenuated after blocking of P2X3 and P2X2/3, which suggest that P2X3 and P2X2/3 receptors contribute to the metabolic component of the exercise pressor reflex.
Under conditions of muscle vascular insufficiency induced by femoral artery ligation, the exercise pressor reflex is amplified in rats (50). Furthermore, prior studies (3, 4) reported that autonomic responses during exercise are enhanced in PAD patients. The underlying mechanism at receptor levels by which these reflex responses are enhanced after intermittent claudication needs to be determined. In the current study, femoral occlusion not only augments expression of P2X3 in afferent nerves but also exaggerates sympathetic and blood pressure responses to arterial administration of α,β-me-ATP. The data indicate that the augmented muscle reflex may be related, at least in part, to upregulation of P2X3 receptors following femoral occlusion. However, engagement of P2X2/3 receptor in enhanced reflex cannot be ruled out since α,β-me-ATP used in the present experiments is not selective to P2X3.
Nevertheless, evidence has suggested that ATP and P2X receptors contribute to the metabolic component of the exercise pressor reflex (metaboreflex) after femoral occlusion. Also, it is likely that P2X plays a role in sensitizing the mechanoreflex in states of the hindlimb muscle vascular insufficiency. For example, activation of P2X receptors enhances blood pressure response to passive muscle stretch, a stimulus of mechanoreflex (32). P2X-mediated muscle mechanoreceptor responses are augmented in rats with heart failure compared with a control group (31). Moreover, expression of P2X is increased in DRG of rats with heart failure (15). Thus upregulation of P2X3 receptors in DRG neurons may also enhance the exercise pressor reflex by facilitating the mechanoreflex in PAD.
NGF is synthesized in various cells including normal skeletal muscle tissue (54). Previous data (14) indicate that hindlimb ischemia induces an increase in endogenous NGF production in skeletal muscle. NGF is taken up by nerve terminals and retrogradely transported to the cell bodies once it is released into the muscle interstitium. Generally, the accumulated neurotrophin in the cell bodies is regarded as a requirement for physiological or pathological responsiveness in neurons (11). In addition, measurement of NGF in DRG tissue of rats with femoral artery occlusion revealed that 24 and 48 h of occlusion significantly increased NGF levels in DRG sensory neurons (57). Notably, exogenous application of NGF has been reported to lead to expression of P2X3 in sensory neurons (44), although it is unclear if endogenous NGF induces P2X3 expression in sensory nerves. Nevertheless, administration of NGF antibody to neutralize endogenous NGF attenuated amplification in the pressor response evoked by arterial injection of α,β-me-ATP in occluded rats, suggesting that NGF plays a role in regulating P2X3 response after femoral occlusion.
In the present study, the results further demonstrated that infusion of NGF into the hindlimb muscle of healthy rats induced a 1.39-fold increase in expression of P2X3 protein of DRG neurons. Also, infusion of NGF enhanced blood pressure response to arterial injection of α,β-me-ATP as it was seen in occluded rats. Taken together, the experimental findings suggest that NGF is engaged in upregulation of P2X3 in sensory nerves and then contributes to the exaggeration of the muscle reflex after femoral occlusion by increasing the expression of P2X3.
Note that in addition to the P2X3 receptor, NGF plays a role in regulating a number of ligand-gated ion channels including TRPV1, ASIC3, and bradykinin B2 receptor (16, 43). These receptors are responsive to ischemia. For example, femoral artery ligation elevates expression of chemically sensitive TRPV1 and ASIC3 and then induces greater increases in sympathetic nerve activity and blood pressure as their respective receptor agonist is injected in the arterial blood supply of the hindlimb muscles (33, 56). Among these receptors, ASIC3 and bradykinin B2 have been reported to modulate the exercise pressor reflex (20, 22, 42). Thus elevation of NGF is likely to affect a number of afferent nerve receptors in modulating the exercise pressor reflex after femoral occlusion.
Moreover, the levels of NGF in the DRG tissues of control rats and rats infused with NGF were examined to determine effectiveness of NGF infusion. Consistent with the data from a prior study (57), the similar levels of NGF were seen in the DRG of control rats. After NGF was infused into the hindlimb muscles, NGF was significantly increased in the DRG of infused leg. The level of elevated NGF in the DRG was close to that seen under 24 h of ischemic conditions. However, infusion of NGF did not significantly alter the levels of NGF in the DRG of the control leg infused with saline in the same rats. The data suggest that the dosage of NGF infused into the hindlimb muscles was effective and reasonable in this experiment.
Likewise, the effectiveness of NGF antibody was examined in this experiment. Similar to the results previously reported (57), 24 h of femoral occlusion significantly increased the levels of NGF in the DRG. However, application of NGF antibody attenuated occlusion-elevated NGF.
In addition, the levels of NGF in plasma and in the medulla were examined in control rats and rats infused with NGF. No significant difference in NGF was seen in both groups. This result suggests that the infusion of NGF into the hindlimb muscles had negligible systemic effects. Nevertheless, it cannot be ruled out if the infusion of NGF affected afferent nerves other than muscle.
Prior studies (6) demonstrate that the levels of extracellular ATP are increased in ischemic tissues. Increased ATP is likely to upregulate P2X3 and thereby augment receptor activity. Thus additional investigations need to be performed to examine interstitial ATP of the hindlimb muscles at different time points following femoral occlusion to clarify the effects of ATP. It is speculated that following femoral occlusion the concentration of ATP is elevated in the muscle interstitium of occluded leg, and this increases expression of P2X3 engaged in augmented sympathetic nerve activity. In addition, another limitation of this study is that arterial injection of α,β-me-ATP may not mimic the exercise pressor reflex in a physiologic way. A better way to address this issue is to determine the effect of a P2X antagonist on the pressor response to muscle contraction in sham and occluded rats. If P2X antagonist had more inhibitory effects on the exercise pressor reflex in occluded rats than that in sham rats, it would greatly support the role played by P2X.
In summary, the data of the present study have shown that the femoral artery occlusion significantly increases the levels of P2X3 protein in lumbar DRG neurons. The enhanced expression of P2X3 is specifically seen in small to medium diameter of sensory neurons. Stimulation of afferent P2X3 receptors with α,β-me-ATP evokes greater increases in sympathetic nerve activity and arterial blood pressure in rats with femoral occlusion. Furthermore, infusion of NGF into the hindlimb muscle of rats increases expression of P2X3 in DRG neurons as well as pressor response induced by stimulation of P2X3. These findings suggest that P2X3 in afferent nerves plays an important role in augmented sympathetic responsiveness via a reflex pathway when blood supply to the hindlimb muscles is insufficient as seen in PAD.
GRANTS
Support for this study was provided by National Heart, Lung, and Blood Institute Grants R01-HL-090720 and P01-HL-096570 and American Heart Association Established Investigator Award 0840130N.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We express gratitude to Chunying Yang for technical assistance.
REFERENCES
- 1. Ambalavanar R, Moritani M, Dessem D. Trigeminal P2X3 receptor expression differs from dorsal root ganglion and is modulated by deep tissue inflammation. Pain 117: 280–291, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Aslam F, Haque A, Foody J, Lee LV. Peripheral arterial disease: current perspectives and new trends in management. South Med J 102: 1141–1149, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology 50: 361–374, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Bakke EF, Hisdal J, Jorgensen JJ, Kroese A, Stranden E. Blood pressure in patients with intermittent claudication increases continuously during walking. Eur J Vasc Endovasc Surg 33: 20–25, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Banerjee B, Medda BK, Schmidt J, Zheng Y, Zhang Z, Shaker R, Sengupta JN. Altered expression of P2X3 in vagal and spinal afferents following esophagitis in rats. Histochem Cell Biol 132: 585–597, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borst MM, Schrader J. Adenine nucleotide release from isolated perfused guinea pig hearts and extracellular formation of adenosine. Circ Res 68: 797–806, 1991 [DOI] [PubMed] [Google Scholar]
- 7. Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci 12: 256–268, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Burnstock G. Current status of purinergic signalling in the nervous system. Prog Brain Res 120: 3–10, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci 22: 182–188, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol 240: 31–304, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Campenot RB, MacInnis BL. Retrograde transport of neurotrophins: fact and function. J Neurobiol 58: 217–229, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature 377: 428–431, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA 290: 86–97, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Emanueli C, Salis MB, Pinna A, Graiani G, Manni L, Madeddu P. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation 106: 2257–2262, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Gao Z, Xing J, Sinoway L, Li J. P2X receptor-mediated muscle pressor reflex in myocardial infarction. Am J Physiol Heart Circ Physiol 292: H939–H945, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Giniatullin R, Nistri A, Fabbretti E. Molecular mechanisms of sensitization of pain-transducing P2X3 receptors by the migraine mediators CGRP and NGF. Mol Neurobiol 37: 83–90, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Hanna RL, Hayes SG, Kaufman MP. α,β-Methylene ATP elicits a reflex pressor response arising from muscle in decerebrate cats. J Appl Physiol 93: 834–841, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Hanna RL, Kaufman MP. Activation of thin-fiber muscle afferents by a P2X agonist in cats. J Appl Physiol 96: 1166–1169, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Hanna RL, Kaufman MP. Role played by purinergic receptors on muscle afferents in evoking the exercise pressor reflex. J Appl Physiol 94: 1437–1445, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Hayes SG, Kindig AE, Kaufman MP. Blockade of acid sensing ion channels attenuates the exercise pressor reflex in cats. J Physiol 581: 1271–1282, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hayes SG, McCord JL, Kaufman MP. Role played by P2X and P2Y receptors in evoking the muscle chemoreflex. J Appl Physiol 104: 538–541, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Hayes SG, McCord JL, Rainier J, Liu Z, Kaufman MP. Role played by acid-sensitive ion channels in evoking the exercise pressor reflex. Am J Physiol Heart Circ Physiol 295: H1720–H1725, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory, and airway responses to exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Control of Respiratory and Cardiovascular Systems. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, pt. II, chapt. 10, p. 381–447 [Google Scholar]
- 24. Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983 [DOI] [PubMed] [Google Scholar]
- 25. Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984 [DOI] [PubMed] [Google Scholar]
- 26. Kaufman MP, Waldrop TG, Rybicki KJ, Ordway GA, Mitchell JH. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovasc Res 18: 663–668, 1984 [DOI] [PubMed] [Google Scholar]
- 27. Kindig AE, Hayes SG, Kaufman MP. Purinergic 2 receptor blockade prevents the responses of group IV afferents to post-contraction circulatory occlusion. J Physiol 578: 301–308, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature 377: 432–435, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Li J, King NC, Sinoway LI. ATP concentrations and muscle tension increase linearly with muscle contraction. J Appl Physiol 95: 577–583, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Li J, King NC, Sinoway LI. Interstitial ATP and norepinephrine concentrations in active muscle. Circulation 111: 2748–2751, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Li J, Sinoway A, Gao Z, Maile M, Pu M, Sinoway L. Muscle mechanoreflex and metaboreflex responses after myorcardial infarction in rats. Circulation 110: 3049–3054, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Li J, Sinoway LI. ATP stimulates chemically sensitive and sensitizes mechanically sensitive afferents. Am J Physiol Heart Circ Physiol 283: H2636–H2643, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Liu J, Gao Z, Li J. Femoral artery occlusion increases expression of ASIC3 in dorsal root ganglion neurons. Am J Physiol Heart Circ Physiol 299: H1357–H1364, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu J, Mao W, Ding B, Liang CS. ERKs/p53 signal transduction pathway is involved in doxorubicin-induced apoptosis in H9c2 cells and cardiomyocytes. Am J Physiol Heart Circ Physiol 295: H1956–H1965, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCord JL, Tsuchimochi H, Kaufman MP. P2X2/3 and P2X3 receptors contribute to the metaboreceptor component of the exercise pressor reflex. J Appl Physiol 109: 1416–1423, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983 [DOI] [PubMed] [Google Scholar]
- 38. Norenberg W, Illes P. Neuronal P2X receptors: localisation and functional properties. Naunyn Schmiedebergs Arch Pharmacol 362: 324–339, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Novakovic SD, Kassotakis LC, Oglesby IB, Smith JA, Eglen RM, Ford AP, Hunter JC. Immunocytochemical localization of P2X3 purinoceptors in sensory neurons in naive rats and following neuropathic injury. Pain 80: 273–282, 1999 [DOI] [PubMed] [Google Scholar]
- 40. O'Leary DS. Altered reflex cardiovascular control during exercise in heart failure: animal studies. Exp Physiol 91: 73–77, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Ouriel K. Peripheral arterial disease. Lancet 358: 1257–1264, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Pan HL, Stebbins CL, Longhurst JC. Bradykinin contributes to the exercise pressor reflex: mechanism of action. J Appl Physiol 75: 2061–2068, 1993 [DOI] [PubMed] [Google Scholar]
- 43. Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci 29: 507–538, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Ramer MS, Bradbury EJ, McMahon SB. Nerve growth factor induces P2X(3) expression in sensory neurons. J Neurochem 77: 864–875, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Reinohl J, Hoheisel U, Unger T, Mense S. Adenosine triphosphate as a stimulant for nociceptive and non-nociceptive muscle group IV receptors in the rat. Neurosci Lett 338: 25–28, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Rejeski WJ, Tian L, Liao Y, McDermott MM. Social cognitive constructs and the promotion of physical activity in patients with peripheral artery disease. J Cardiopulm Rehabil Prev 28: 65–72, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Sinoway LI, Li J. A perspective on the muscle reflex: implications for congestive heart failure. J Appl Physiol 99: 5–22, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol 91: 89–102, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Smith SA, Williams MA, Leal AK, Mitchell JH, Garry MG. Exercise pressor reflex function is altered in spontaneously hypertensive rats. J Physiol 577: 1009–1020, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol 299: H106–H113, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vulchanova L, Riedl MS, Shuster SJ, Buell G, Surprenant A, North RA, Elde R. Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology 36: 1229–1242, 1997 [DOI] [PubMed] [Google Scholar]
- 52. Waters RE, Terjung RL, Peters KG, Annex BH. Preclinical models of human peripheral arterial occlusive disease: implications for investigation of therapeutic agents. J Appl Physiol 97: 773–780, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Wirkner K, Sperlagh B, Illes P. P2X3 receptor involvement in pain states. Mol Neurobiol 36: 165–183, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Wu C, Erickson MA, Xu J, Wild KD, Brennan TJ. Expression profile of nerve growth factor after muscle incision in the rat. Anesthesiology 110: 140–149, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xiang Z, Xiong Y, Yan N, Li X, Mao Y, Ni X, He C, LaMotte RH, Burnstock G, Sun J. Functional up-regulation of P2X 3 receptors in the chronically compressed dorsal root ganglion. Pain 140: 23–34, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xing J, Gao Z, Lu J, Sinoway LI, Li J. Femoral artery occlusion augments TRPV1-mediated sympathetic responsiveness. Am J Physiol Heart Circ Physiol 295: H1262–H1269, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xing J, Lu J, Li J. Contribution of nerve growth factor to augmented TRPV1 responses of muscle sensory neurons by femoral artery occlusion. Am J Physiol Heart Circ Physiol 296: H1380–H1387, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yang HT, Feng Y. bFGF increases collateral blood flow in aged rats with femoral artery ligation. Am J Physiol Heart Circ Physiol 278: H85–H93, 2000 [DOI] [PubMed] [Google Scholar]
- 59. Yang HT, Feng Y, Allen LA, Protter A, Terjung RL. Efficacy and specificity of bFGF increased collateral flow in experimental peripheral arterial insufficiency. Am J Physiol Heart Circ Physiol 278: H1966–H1973, 2000 [DOI] [PubMed] [Google Scholar]
- 60. Zhang C, Li G, Liang S, Xu C, Zhu G, Wang Y, Zhang A, Wan F. Myocardial ischemic nociceptive signaling mediated by P2X3 receptor in rat stellate ganglion neurons. Brain Res Bull 75: 77–82, 2008 [DOI] [PubMed] [Google Scholar]
- 61. Zettler C, Bridges DC, Zhou XF, Rush RA. Detection of increased tissue concentrations of nerve growth factor with an improved extraction procedure. J Neurosci Res 46: 581–594, 1996 [DOI] [PubMed] [Google Scholar]