Abstract
Obese individuals exhibit impaired functional vasodilation and exercise performance. We have demonstrated in obese Zucker rats (OZ), a model of morbid obesity, that insulin resistance impairs functional vasodilation via an increased thromboxane receptor (TP)-mediated vasoconstriction. Chronic treadmill exercise training improves functional vasodilation in the spinotrapezius muscle of the OZ, but the mechanisms responsible for the improvement in functional vasodilation are not clear. Based on evidence that exercise training improves insulin resistance, we hypothesized that, in the OZ, exercise training increases functional vasodilation and exercise capability due to decreases TP-mediated vasoconstriction associated with improved insulin sensitivity. Six-week-old lean Zucker rats (LZ) and OZ were exercised on a treadmill (24 m/min, 30 min/day, 5 days/wk) for 6 wk. An oral glucose tolerance test was performed at the end of the training period. We measured functional vasodilation in both exercise trained (spinotrapezius) and nonexercise trained (cremaster) muscles to determine whether the improved functional vasodilation following exercise training in OZ is due to a systemic improved insulin resistance. Compared with LZ, the sedentary OZ exhibited impairments in glucose tolerance and functional vasodilation in both muscles. The TP antagonist SQ-29548 improved the vasodilator responses in the sedentary OZ with no effect in the LZ. Exercising training of the LZ increased the functional vasodilation in spinotrapezius muscle, with no effect in the cremaster muscle. Exercising training of the OZ improved glucose tolerance, along with increased functional vasodilation, in both the spinotrapezius and cremaster muscles. SQ-29548 treatment had no effect on the vasodilator responses in either cremaster or spinotrapezius muscles of the exercise-trained OZ. These results suggest that, in the OZ, there is a global effect of exercising training to improve insulin resistance and increase functional vasodilation via a decreased TP-mediated vasoconstriction.
Keywords: functional vasodilation, insulin resistance, thromboxane
chronic exercise training has been reported to improve metabolic disorders (11, 17) and exercising capability in obese subjects (18, 35, 38). We have shown that 6 wk of exercise training improves not only endothelium function, but also the functional vasodilation (the vasodilator response to muscle contraction) in the spinotrapezius muscle (exercising muscle) in obese Zucker rats (OZ), a model of morbid obesity (42). These results suggest that an increased endothelial derived relaxing factor(s) could be responsible for the enhanced functional vasodilation in OZ. However, the mechanisms responsible for the training-induced improvement in functional vasodilation and exercise capability in obese subjects or OZ are not clear.
Arachidonic acid (AA) and its metabolites are important for functional vasodilation (16, 28, 30, 32). The exercise-induced increase in vasodilator prostanoid(s), such as PGI2, could be released from multiple sites (9, 26, 29, 47), with the endothelium considered as the major source (15). During exercise, PGI2 production is the dominant pathway of AA metabolism leading to vasodilation, while thromboxane A (TxA2) generation has been found to decrease (24). However, in diabetics and obesity, the insulin resistance and metabolic disorders are associated with an impaired AA metabolism and resultant accumulation of PGH2 and/or TxA2 (22, 46). This increase in PGH2 and/or TxA2 would attenuate functional hyperemic responses via an enhanced thromboxane receptor (TP)-mediated vasoconstriction. Indeed, our laboratory's published work has demonstrated that insulin resistance and the resultant hyperglycemia in the OZ impair the functional vasodilation via an increased TP activation (41). Since exercise training has been reported to improve insulin sensitivity and endothelial dysfunction in diabetic humans and rodents (19, 40), we hypothesized that, in the OZ, exercise training increases functional vasodilation and exercise capability due to a decreased TP-mediated vasoconstriction associated with improved insulin sensitivity. Although our laboratory's previous study has shown exercise leads to improved functional vasodilation in spinotrapezius muscle of OZ (42), the involvement of insulin resistance and AA metabolism has not been determined. In addition, in the present study, we measured functional vasodilation in both an exercising (spinotrapezius) and a nonexercising (cremaster) muscle to determine whether there was a global effect from effect training.
METHODS
Animals.
Male lean Zucker rats (LZ) and OZ were acquired from Harlan Laboratories. Experimental protocols for this study were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center and were carried out according to both the National Institutes of Health Guide for the Care and Use of Laboratory Animals and guidelines of the Animal Welfare Act. Rats were separated one rat per cage at 22°C (12:12-h light-dark cycle) with free access to food and water. Food intake was measured every day using an electronic balance. One-half of the LZ and OZ were placed in the exercise protocol, while the other one-half were kept without exercise to represent the sedentary group.
Exercise training protocol.
Six- to seven-week-old LZ and OZ were trained on a treadmill, 30 min/day, 5 days/wk, for 6 wk. The speed was started at 14 m/min and was increased by 2 m/min every minute, until a maximum speed of 24 m/min was reached. The rats were exercised in a temperature (22°C) and humidity- (55%) controlled room using a Columbus Instruments (Columbus, OH) treadmill.
Maximal oxygen consumption and workload.
After 6 wk of training, the rats were allowed to rest for 24 h. A Columbus Instruments metabolic cage was used to measure the oxygen consumption capacity of the rats. Rats ran in the metabolic cage with a 15° inclined treadmill. The treadmill speed was started at 12 m/min and increased by 2 m/min at each 30 s, until a stable peak oxygen consumption level was achieved. An endurance test was determined using the same exercise training protocol. The speed was started at 14 m/min and was increased by 2 m/min every minute until a maximum speed of 24 m/min was reached. The rats ran on the treadmill until exhaustion. Rats that refused to run and stayed on the shock pad for 30 s were considered to be exhausted. The total distance that each rat ran was recorded. Workload was calculated by distance × body weight.
Oral glucose tolerance test.
An oral glucose tolerance test (OGTT) (50% dextrose solution, 3 ml/kg) was performed in LZ and OZ, one-half sedentary and one-half exercised. The rats were fasted 12 h before starting the OGTT. Blood samples were withdrawn immediately before glucose application by gavages and then at 20, 50, 160, and 240 min. Blood samples were collected from the tail vein (12–13 wk), and glucose levels were measured using a Beckman Clinical Chemistry Analyzer (Beckman Instruments, Fullerton, CA).
Functional and AA-induced vasodilation.
The functional and AA-induced vasodilation was determined in an exercising (spinotrapezius) and nonexercising muscle (cremaster), as previous described (13, 42). The right spinotrapezius muscle or the right cremaster muscle was prepared for experimental observation. In brief, rats were anesthetized with pentobarbital sodium (65 mg/kg ip), and the trachea was intubated. Animals spontaneously breathed a gas mixture containing 30% oxygen and 70% nitrogen. The left jugular vein was cannulated for the supplemental addition of anesthetic. At all times during the surgery and subsequent experiments, the spinotrapezius muscle or the cremaster muscle was kept at in situ dimensions and continuously superfused with physiological saline solution (PSS). The composition of PSS for spinotrapezius muscle and cremaster muscle was applied according to previous studies (in mM): 118.07 NaCl, 6.17 KCl, 2.55 CaCl2, and 25 NaHCO3 for spinotrapezius muscle (42); or 131.9 NaCl, 4.7 KCl, 2.0 CaCl2, 1.2 MgSO4, and 20 NaHCO3 for cremaster muscle (34). The PSS was aerated with a 5% CO2-95% N2 gas mixture (pH 7.4, 35°C) to ensure that the oxygen supply was only from blood. A segment of the arcade arterioles in spinotrapezius muscle or a third-order arteriole in the cremaster muscle were randomly chosen for study. At the completion of the experiments, animals were euthanized by a cardiac injection of pentobarbital sodium or 10% KCl. Death was confirmed by a lack of a heartbeat and spontaneous breathing. The microcirculation of the spinotrapezius muscle or the cremaster muscle was transilluminated and observed with a Nikon microscope fitted with ×10 water-immersion objective (numerical aperture = 0.3). The microscopic image was televised with a Dage closed-circuit television camera and displayed on a Sony monitor. Vessel diameter was measured using a Texas A&M video analyzer modified to function as a video micrometer. The resolution of this system was ±1 μm. Hooked silver-silver chloride electrodes (Grass Instruments) were placed at each end of the spinotrapezius muscle or the cremaster and connected to a Grass S44 stimulator. Diameters of the vessels were obtained in the resting muscle and immediately after 2 min of electrical stimulation (4–5 V, 1 Hz).
Arteriolar diameter was obtained in the resting muscle and immediately after 2 min of electrical muscle stimulation. After a 15-min recovery period, AA (1 μM) was added to superfusion solution, and steady-state vasodilatory responses were measured. After the arteriole had returned to its resting diameter (∼15–20 min), the tissue was pretreated by the TP antagonist SQ-29548 (1 μM) for 30 min, and then the muscle stimulation and AA treatment protocols were repeated. At the end of the experiment, adenosine (100 μM) and sodium nitroprusside (100 μM) were applied to determine the maximal luminal diameter.
Drugs and vasoactive agents.
AA and SQ-29548 (TP antagonist) were purchased form Cayman Chemical and stored in ethanol as stock solutions. During the experiments, the final concentration of ethanol in the superfusion solution was <0.1%.
Data analysis and statistical methods.
Arteriolar diameter data were collected at 1 Hz to a personal computer and stored to disk for later analysis. The effects of SQ-29548 on vasodilatory responses were analyzed using two-way repeated-measures ANOVA. All of the other data were analyzed using two-way ANOVA. Where significant main effects occurred, individual groups were compared using the Holm-Sidak method. All data are means ± SE (Figures) or SD (Tables). Probability values of P < 0.05 were accepted as statistically significant for all comparisons.
RESULTS
Body weight, food intake, and OGTT.
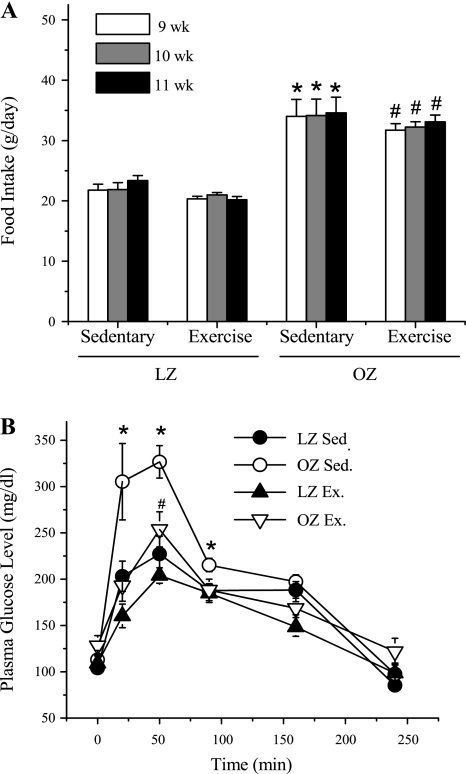
As shown in Table 1, OZ exhibited significantly higher body weight compared with LZ, and chronic exercise training slightly decreased the bodyweight in OZ, with no effect in LZ. The body weight is the average of all of the rats used in the present study. Figure 1A presents food intake throughout the last 3-wk period of training for the four groups (sedentary LZ, sedentary OZ, exercised LZ, and exercised OZ). The OZ exhibited an increased food intake compared with the age-matched LZ in both sedentary and exercised groups. Exercise training had no significant effect on the food intake in either LZ or OZ group. At the end of the training period (11–12 wk), the fasting glucose levels were not different among groups (Fig. 1B). Plasma glucose levels increased in all groups following treatment with dextrose and returned back to the fasting levels after 240 min. Sedentary OZ exhibited an elevated postprandial glucose level compared with the LZ. Exercise training normalized the postprandial hyperglycemia in the OZ group with no effect in the LZ.
Table 1.
Body weight, V̇o2max, and workload in the sedentary and exercise-trained LZ and OZ
| LZ Sed | LZ Ex | OZ Sed | OZ Ex | |
|---|---|---|---|---|
| Body weight (11-12 wk), g | 311 (26) | 325 (20) | 478 (31)* | 448 (33)+# |
| V̇o2max, ml · kg−1 · min−1 | 63 (2) | 71 (3)* | 49 (8)* | 55 (3)+# |
| Workload, m × kg | 375 (56) | 755 (69)* | 145 (23)* | 465 (74)+# |
Values are means (SD); n = 6 rats/group, except for workload LZ Sed, where n = 5 rats/group. V̇o2max, maximum oxygen consumption; Sed, sedentary; Ex, exercised; LZ, lean Zucker rats; OZ, obese Zucker rats. The body weight is the sum of that of all of the rats used in the present study.
Significant difference vs. LZ Sed (body weight: P = 0.009; V̇o2max: P = 0.009 vs. LZ Ex, P = 0.001 vs. OZ Sed; workload: P = 0.001 vs. LZ Ex, P = 0.001 vs. OZ Sed; two-way ANOVA).
Significant difference vs. LZ Ex (body weight: P = 0.017; V̇o2max: P = 0.001; workload: P = 0.001; two-way ANOVA). + Significant difference vs. OZ Sed (body weight: P = 0.025; V̇o2max: P = 0.033; workload: P = 0.001; two-way ANOVA).
Fig. 1.
The daily food intake and postprandial plasma glucose levels in sedentary (Sed) and exercised (Ex) animals. A: obese Zucker rats (OZ) exhibit significantly higher daily food intake compared with age-matched (9–11 wk) lean Zucker rats (LZ) in either the Sed (*significant difference: 9 wk, P = 0.001; 10 wk, P = 0.001; 11 wk, P = 0.001, two-way ANOVA) or Ex group (#significant difference: 9 wk, P = 0.001; 10 wk, P = 0.001; 11 wk, P = 0.001, two-way ANOVA). Exercise training did not change food intake significantly in LZ or OZ. B: the fasting glucose levels were not significantly different between Sed and Ex rats in both LZ and OZ groups. OZ exhibited significantly higher postprandial hyperglycemia compared with LZ in Sed group (*significant difference: 20 min, P = 0.013; 50 min, P = 0.001; 90 min, P = 0.025, two-way ANOVA; #significant difference: P = 0.04 vs. LZ Ex). Exercise normalized the enhanced postprandial glucose levels in OZ, which has no effect on LZ. Values are means ± SE (LZ Sed, n = 5; OZ Sed, n = 6; LZ Ex, n = 6; OZ Ex, n = 5).
Work load and maximum oxygen consumption.
As shown in Table 1, OZ exhibited a significantly lower maximum oxygen consumption (V̇o2max) than LZ. Chronic exercise significantly enhanced the V̇o2max, both in LZ and OZ groups, with the V̇o2max remaining lower in trained OZ than in trained LZ. OZ exhibited significant impaired workload compared with LZ. Chronic exercise training doubled the workload in LZ, while enhancing the workload threefold in OZ. However, the workload of the trained OZ was still significantly lower than that of trained LZ.
Vasodilator responses in spinotrapezius and cremaster muscle.
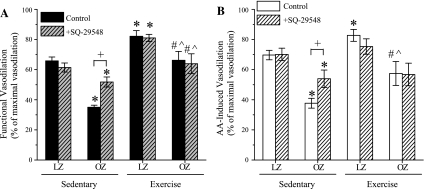
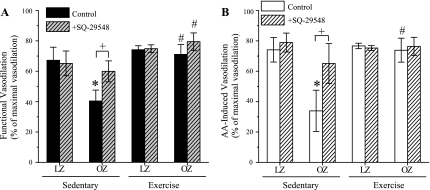
The basal and the maximal arterial diameters in sedentary and exercise-trained LZ and OZ are shown in Table 2. There were no significant differences in the basal and maximal arteriolar diameters between the groups. SQ-29548 had no effect on the basal arteriolar diameters in all of the animal groups. Vasodilator responses to muscle stimulation and AA application are shown in Fig. 2, A and B (spinotrapezius muscle) and Fig. 3, A and B (cremaster muscle), respectively. In both spinotrapezius and cremaster muscles, functional and AA-induced vasodilations were significantly blunted in sedentary OZ group compared with sedentary LZ. Chronic exercise training significantly improved the vasodilator responses to muscle stimulation and AA in both spinotrapezius and cremaster muscle in OZ. Notably, in the LZ, exercise training significantly enhanced the vasodilation induced by muscle stimulation and AA in spinotrapezius muscle, but had no effect in the cremaster muscle. SQ-29548 (1 μM) treatment improved the functional and AA-induced vasodilation in spinotrapezius and cremaster muscle of sedentary OZ, with no effect in the sedentary LZ, exercise-trained LZ, or exercise-trained OZ.
Table 2.
The basal and the maximal arterial diameters in sedentary and exercise-trained LZ and OZ
| Spinotrapezius Muscle |
Cremaster Muscle |
|||||||
|---|---|---|---|---|---|---|---|---|
| LZ Sed | LZ Ex | OZ Sed | OZ Ex | LZ Sed | LZ Ex | OZ Sed | OZ Ex | |
| Basal diameter | 16 (2) | 17 (2) | 17 (1) | 19 (1) | 16 (2) | 14 (2) | 17 (3) | 14 (2) |
| SQ-29548 | 16 (2) | 17 (3) | 17 (2) | 19 (1) | 16 (2) | 14 (2) | 17 (3) | 14 (2) |
| Maximum diameter | 37 (3) | 44 (9) | 40 (3) | 43 (10) | 42 (10) | 35 (4) | 44 (10) | 39 (11) |
Values are means (SD) in μm. The basal diameter before and after SQ-29548 treatment and the maximal diameter in all the animal groups in both spinotrapezius and cremaster muscles are shown. No significant differences between all of these groups were found.
Fig. 2.
The effect of exercise training on functional and arachidonic acid (AA)-induced vasodilation in the spinotrapezius muscle, with or without SQ-29548 pretreatment. A: OZ exhibited significantly blunted functional vasodilation compared with LZ in the Sed group (*P = 0.001, two-way ANOVA). Chronic exercise training significantly enhanced functional vasodilatory response both in LZ (*P = 0.004, two-way ANOVA) and OZ (#P = 0.001, two-way ANOVA). However, Ex OZ failed to reach the same level of functional vasodilation compared with Ex LZ (∧ P = 0.006, two-way ANOVA). SQ-29548 pretreatment significantly enhanced functional vasodilation vs. OZ Sed control [+ P = 0.001, two-way repeated-measures (RM) ANOVA]. SQ-29548 partially restored the functional vasodilatory response vs. LZ (*P = 0.02, two-way RM ANOVA). SQ-29548 had no effect on vasodilation in Sed LZ, Ex OZ, or LZ induced by functional stimulation compared with the control group. B: AA-induced vasodilations are similar to the results of functional vasodilation. *Significant difference vs. LZ Sed (P = 0.001 vs. OZ Sed; P = 0.028 vs. LZ Ex, two-way ANOVA). #Significant difference vs. OZ Sed (P = 0.007, two-way ANOVA). ∧ Significant difference vs. LZ Ex (P = 0.002, two-way ANOVA). SQ-29548 pretreatment significantly enhanced AA-induced vasodilation vs. OZ (+ P = 0.004, two-way RM ANOVA). SQ-29548 partially restored the AA-induced vasodilatory response vs. LZ with SQ-29548 pretreatment (*P = 0.016, two-way RM ANOVA). SQ-29548 had no effect on vasodilation in Ex OZ induced by AA compared with the control group. Values are means ± SE (LZ Sed, n = 6; OZ Sed, n = 7; LZ Ex, n = 5; OZ Ex, n = 5).
Fig. 3.
The effect of exercise training on functional and AA-induced vasodilation in the cremaster muscle. A: OZ exhibited significantly blunted functional vasodilation compared with LZ in the Sed group (*P = 0.01, two-way ANOVA). Chronic exercise training significantly enhanced functional vasodilatory response in OZ (#P = 0.006, two-way ANOVA). SQ-29548 pretreatment significantly enhanced functional vasodilation vs. OZ control (+ P = 0.003, two-way RM ANOVA). SQ-29548 had no effect on vasodilation in Sed LZ, Ex OZ, or LZ induced by functional stimulation compared with the control group. B: AA-induced vasodilations are similar to the results of functional vasodilation. *Significant difference vs. LZ Sed (P = 0.003, two-way ANOVA). #Significant difference vs. OZ Sed (P = 0.003, two-way ANOVA). SQ-29548 has a similar effect on AA-induced vasodilation as on functional vasodilation. Values are means ± SE (LZ Sed, n = 6; OZ Sed, n = 5; LZ Ex, n = 6; OZ Ex, n = 5).
DISCUSSION
The major findings of this study are that: 1) OZ exhibited impaired functional vasodilation in both spinotrapezius and cremaster muscles, with administration of TP antagonist SQ-29548 improving the vasodilator responses; 2) exercise training improved the insulin resistance and the vasodilator responses to muscle stimulation and AA in both spinotrapezius and cremaster muscles of OZ, with SQ-29548 having no further effect on the vasodilator responses; 3) in the LZ, exercise training increased the vasodilator responses in spinotrapezius muscles, with no effect in the cremaster muscle; and 4) exercise training resulted in a larger percent increase in workload in the OZ compared with the LZ.
The improvement of vascular responses in the OZ was not limited to the spinotrapezius muscle that was activated during exercise training. An important consequence of these results is that the improved functional vasodilation in the cremaster muscle following exercise training in OZ suggests a global effect of exercise to improve vascular responses. In addition, exercise training increased the vasodilator responses in spinotrapezius muscle, but not in the cremaster muscle of LZ, suggesting a local effect of exercise training to improve vascular responses in spinotrapezius muscle. Since there was no TP activation during vasodilator responses in the LZ, the enhanced AA-induced vasodilation in spinotrapezius muscle following exercise training in the LZ suggests that an increased release and/or response to vasodilator prostanoids contribute, at least in part, to the elevated functional vasodilation in the exercising muscles.
The increase in blood flow in skeletal muscle during exercise (functional hyperemia) is reduced in obese humans and animal models of obesity (8, 10). Consistent with our laboratory's previous studies (41, 45, 46), the impaired vasodilator responses to muscle contraction and AA in OZ were improved following TP inhibition using SQ-29548. We have shown in vivo that vasoconstrictor response to the thromboxane analog U-46619 was not different between LZ and OZ (41), consistent with our laboratory's in vitro study (31) in gracilis arteries. In addition, the thromboxane synthase inhibitor 1-benzylimidazole did not alter vasodilatory responses in OZ (41), similar to findings in the diabetic rat (33). Therefore, the enhanced TP activation during muscle contraction in OZ is likely due to accumulation of PGH2, rather than increased TxA2 synthesis, or TP expression, or its downstream signaling. Neither SQ-29548 nor 1-benzylimidazole decreased basal arteriolar diameters, suggesting that the accumulation of TP activator(s) only occurs in response to stimuli, such as muscle contraction. Further studies are needed to definitely determine which factor is responsible for the enhanced TP activator(s) in OZ during the hyperemic response.
Hyperglycemia is known to be associated with a shift of AA metabolism to vasoconstrictors and impaired vascular function (2, 25, 27, 37). Zou et al. (49) reported that hyperglycemia alters AA metabolism, leading to accumulation of PGH2 (a TP activator) in human aortic endothelium. Although the fasting glucose levels may be normal in obesity, postprandial hyperglycemia, instead of increased fasting glucose levels, has been suggested as a risk factor for cardiovascular disease (36). Our laboratory has demonstrated that insulin resistance and resultant postprandial hyperglycemia in OZ are responsible for increased vascular reactive oxygen species and the impaired functional vasodilation in spinotrapezius muscle due to TP activation (41). In the present study, we found that the functional and AA-induced vasodilations were also impaired due to elevated TP activation in the nonexercise trained (cremaster) muscle of OZ (Figs. 2 and 3), with the impaired vasodilation improved along with increased insulin sensitivity. Together, these results suggest a systemic impairment in the AA metabolism and vascular responses associated with insulin resistance in this model of obesity.
Consistent with our laboratory's previous findings, treatment with SQ-29548 only partially restored the functional and AA-induced vasodilation in the spinotrapezius muscle of the sedentary OZ (43). Our laboratory has shown that this partial restoration is due to both impaired production and response to vasodilator prostanoids, such as PGI2 (20, 21, 45). Alternatively, the lack of a full return of functional vasodilation following TP inhibition in OZ may be due to impaired nitric oxide (NO) synthesis and/or sensitivity, as observed in obese and type 2 diabetic patients and animals (3, 14, 23). Our laboratory has shown that NO plays a role in functional vasodilation (44). However, in the cremaster muscle of sedentary OZ, we found that SQ-29548 treatment normalized both the functional and AA-induced vasodilator responses, suggesting a minimal role for NO. Although the mechanisms responsible for the different effects of SQ-29548 between spinotrapezius and cremaster muscles are not clear, the elevated TP activation in both tissues suggests a global consequence of the obesity as contributing to an impaired functional vasodilation and exercise capability.
It has been demonstrated that acute or chronic exercise improves insulin resistance and whole body glucose homeostasis in obese subjects and animals through an increased translocation and/or expression of glucose transporter-4 (1, 4, 5, 7). We have shown that minimizing the hyperglycemia in streptozotocin diabetic LZ increased the impaired functional vasodilation via a decreased TP-mediated vasoconstriction (43). More specifically, we have demonstrated that insulin resistance and resultant hyperglycemia in OZ contribute to the elevated TP activation and impaired functional vasodilation via increased vascular reactive oxygen species (41). Since a causative relationship between insulin resistance and the impaired functional vasodilation in OZ has already been established, we believe that the improved glucose tolerance in the trained OZ (Fig. 1) following exercise training contributes, at least in part, to minimize TP activation and increase functional vasodilation. In addition, the current results show that an increased functional and AA-induced vasodilation in the cremaster muscle in OZ is due to a global effect from exercise training, with this beneficial effect absent in LZ. These results suggest a mechanism that is distinct in trained OZ to globally modulate functional vasodilation, such as improved insulin resistance.
Similar with our laboratory's previous findings (42), the present study showed that exercise training increased the functional vasodilation in the spinotrapezius muscle of both LZ and OZ. However, in the LZ, the functional and AA-induced vasodilations were only increased in the spinotrapezius muscle, but not in the cremaster muscle following exercise training (Figs. 2 and 3). These results suggest a local effect of exercise training on increasing the vasodilator responses in the exercising muscles. We have demonstrated that TP-mediated vasoconstriction is minimal in LZ during functional and AA-induced vasodilation. Therefore, the increased AA-induced vasodilation in the spinotrapezius muscle of trained LZ (Fig. 2B) suggests that an enhanced vasodilator prostanoids synthesis and/or sensitivity is responsible, at least in part, for the increased functional vasodilation. There is evidence that chronic exercise training in nonobese humans increases prostacyclin synthesis, along with an increased V̇o2max and exercise capability (48). In the OZ, both the functional and AA-induced vasodilations in the spinotrapezius muscle were partially restored with treatment of SQ-29548 (Fig. 4, A and B), but were normalized following exercise training (Fig. 4C). These results also suggest an increased vasodilator prostanoid(s) production or sensitivity as a local effect of exercise training in OZ. Other vasodilator(s) that are involved in local functional vasodilation, such as NO (44), may also be improved following exercising training. For example, NO synthesis and sensitivity have been shown to be elevated following exercise training in both lean and obese subjects (9, 12). In addition, the basal and the maximal diameters were not altered by exercise training, suggesting that the increased vasodilator responses are not due to an altered maximal vasodilator capability or vascular anatomy (Table 2).
V̇o2max and workload are determined by the blood flow and oxygen supply to exercising muscles (39). It should be realized that, due to the different skeletal muscle-to-fat mass ratio, the comparison of V̇o2max between lean and obese rats is difficult. Since exercise training did not change body weight in either LZ or OZ (Table 1), the increased V̇o2max following exercise training in either the LZ or OZ indicates an improved functional hyperemic response. However, it is based on the assumption that exercise training did not significantly alter the muscle-to-fat mass ratio in either LZ or OZ. Therefore, we used workload as another index of blood flow and oxygen supply during exercise. Based on the current and previous studies (11, 41), the impaired V̇o2max and workload in the sedentary OZ may be due to decreased functional vasodilation and/or microvascular density in the exercising muscle (6). Following exercise training, there was an increased V̇o2max and workload in both LZ and OZ, which correlates with the enhanced functional vasodilation in the spinotrapezius muscle, as observed in both groups.
The V̇o2max and workload in the exercise-trained OZ were still lower compared with the exercise-trained LZ. This difference could be due to the lower functional vasodilation (Fig. 2A), reduced skeletal microvascular density, and/or a possible blunted vasodilator property in the upstream vessels of the exercise-trained OZ compared with the trained LZ. There is evidence that a 10-wk exercise training protocol fails to normalize a decreased microvessel density in exercising muscles of OZ (11). Moreover, the exercise trained OZ exhibited a larger percent increase in functional vasodilation in spinotrapezius muscle (∼90% for OZ vs. ∼20% for LZ) (Fig. 2A) and workload (∼220% for OZ vs. ∼101% for LZ) (Table 1) compared with the exercised LZ. In addition to a local effect of exercise training, the improved insulin resistance and hyperglycemia in the OZ following exercise training could also improve functional vasodilation in the spinotrapezius muscle. Therefore, the larger percent increase in workload and functional vasodilation in spinotrapezius muscles in exercise-trained OZ may be due to a combination of both local and global effects of exercise training on the vascular responses in the spinotrapezius muscle.
Conclusions.
Exercise training improved insulin sensitivity and increased functional vasodilation via a decreased TP-mediated vasoconstriction in the OZ. The improved functional vasodilation occurred in a muscle that was activated during exercise, the spinotrapezius, and a muscle that is not activated with exercise, the cremaster. These results suggest that exercise training leads to a global effect to improve vascular responses in OZ. Based on the present results and our published work (41, 42), we believe that the vascular improvements are due to improved insulin sensitivity. These results provide evidence of the benefit of exercise training on improved vascular responses in nonexercising tissue.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-89581 and HL-51971.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Elizabeth K. Jones for the animal training.
REFERENCES
- 1. Banks EA, Brozinick JT, Jr, Yaspelkis BB, III, Kang HY, Ivy JL. Muscle glucose transport, GLUT-4 content, and degree of exercise training in obese Zucker rats. Am J Physiol Endocrinol Metab 263: E1010–E1015, 1992 [DOI] [PubMed] [Google Scholar]
- 2. Beckman JA, Goldfine AB, Gordon MB, Creager MA. Ascorbate restores endothelium-dependent vasodilation impaired by acute hyperglycemia in humans. Circulation 103: 1618–1623, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Bradley SJ, Kingwell BA, Canny BJ, McConell GK. Skeletal muscle neuronal nitric oxide synthase micro protein is reduced in people with impaired glucose homeostasis and is not normalized by exercise training. Metabolism 56: 1405–1411, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Brozinick JT, Jr, Etgen GJ, Jr, Yaspelkis BB, III, Kang HY, Ivy JL. Effects of exercise training on muscle GLUT-4 protein content and translocation in obese Zucker rats. Am J Physiol Endocrinol Metab 265: E419–E427, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Burstein R, Zissholtz A, Zick-Bachar Y, Epstein Y, Shapiro Y, Karnieli E. Glucose uptake by adipocytes of obese rats: effect of one bout of acute exercise. Can J Physiol Pharmacol 70: 1473–1476, 1992 [DOI] [PubMed] [Google Scholar]
- 6. Duscha BD, Kraus WE, Keteyian SJ, Sullivan MJ, Green HJ, Schachat FH, Pippen AM, Brawner CA, Blank JM, Annex BH. Capillary density of skeletal muscle: a contributing mechanism for exercise intolerance in class II-III chronic heart failure independent of other peripheral alterations. J Am Coll Cardiol 33: 1956–1963, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Etgen GJ, Jr, Jensen J, Wilson CM, Hunt DG, Cushman SW, Ivy JL. Exercise training reverses insulin resistance in muscle by enhanced recruitment of GLUT-4 to the cell surface. Am J Physiol Endocrinol Metab 272: E864–E869, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Fossum E, Hoieggen A, Moan A, Rostrup M, Nordby G, Kjeldsen SE. Relationship between insulin sensitivity and maximal forearm blood flow in young men. Hypertension 32: 838–843, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Frandsen U, Bangsbo J, Langberg H, Saltin B, Hellsten Y. Inhibition of nitric oxide synthesis by systemic N(G)-monomethyl-l-arginine administration in humans: effects on interstitial adenosine, prostacyclin and potassium concentrations in resting and contracting skeletal muscle. J Vasc Res 37: 297–302, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Frisbee JC. Impaired skeletal muscle perfusion in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 285: R1124–R1134, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Frisbee JC, Samora JB, Peterson J, Bryner R. Exercise training blunts microvascular rarefaction in the metabolic syndrome. Am J Physiol Heart Circ Physiol 291: H2483–H2492, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Gordon MB, Jain R, Beckman JA, Creager MA. The contribution of nitric oxide to exercise hyperemia in the human forearm. Vasc Med 7: 163–168, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Gray SD. Rat spinotrapezius muscle preparation for microscopic observation of the terminal vascular bed. Microvasc Res 5: 395–400, 1973 [DOI] [PubMed] [Google Scholar]
- 14. Grijalva J, Hicks S, Zhao X, Medikayala S, Kaminski PM, Wolin MS, Edwards JG. Exercise training enhanced myocardial endothelial nitric oxide synthase (eNOS) function in diabetic Goto-Kakizaki (GK) rats. Cardiovasc Diabetol 7: 34, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gryglewski RJ, Botting RM, Vane JR. Mediators produced by the endothelial cell. Hypertension 12: 530–548, 1988 [DOI] [PubMed] [Google Scholar]
- 16. Hammer LW, Ligon AL, Hester RL. ATP-mediated release of arachidonic acid metabolites from venular endothelium causes arteriolar dilation. Am J Physiol Heart Circ Physiol 280: H2616–H2622, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Hansen D, Dendale P, van Loon LJ, Meeusen R. The impact of training modalities on the clinical benefits of exercise intervention in patients with cardiovascular disease risk or type 2 diabetes mellitus. Sports Med 40: 921–940, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Heath GW, Brown DW. Recommended levels of physical activity and health-related quality of life among overweight and obese adults in the United States, 2005. J Phys Act Health 6: 403–411, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Henriksen EJ. Invited review: Effects of acute exercise and exercise training on insulin resistance. J Appl Physiol 93: 788–796, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Hodnett BL, Dearman JA, Carter CB, Hester RL. Attenuated PGI2 synthesis in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 296: R715–R721, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hodnett BL, Xiang L, Dearman JA, Carter CB, Hester RL. K(ATP)-mediated vasodilation is impaired in obese Zucker rats. Microcirculation 15: 485–494, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ishizuka T, Matsui T, Kurita A. Ramatroban, a TP receptor antagonist, improves vascular responses to acetylcholine in hypercholesterolemic rabbits in vivo. Eur J Pharmacol 468: 27–35, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Kalani M. The importance of endothelin-1 for microvascular dysfunction in diabetes. Vasc Health Risk Manag 4: 1061–1068, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karamouzis M, Karamouzis I, Vamvakoudis E, Ampatzidis G, Christoulas K, Angelopoulou N, Mandroukas K. The response of muscle interstitial prostaglandin E2 (PGE2), prostacyclin I2 (PGI2) and thromboxane A2 (TXA2) levels during incremental dynamic exercise in humans determined by in vivo microdialysis. Prostaglandins Leukot Essent Fatty Acids 64: 259–263, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Kim SH, Park KW, Kim YS, Oh S, Chae IH, Kim HS, Kim CH. Effects of acute hyperglycemia on endothelium-dependent vasodilation in patients with diabetes mellitus or impaired glucose metabolism. Endothelium 10: 65–70, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Klumpp G, Schildknecht S, Nastainczyk W, Ullrich V, Bachschmid M. Prostacyclin in the cardiovascular system: new aspects and open questions. Pharmacol Rep 57, Suppl: 120–126, 2005 [PubMed] [Google Scholar]
- 27. Komers R, Zdychova J, Cahova M, Kazdova L, Lindsley JN, Anderson S. Renal cyclooxygenase-2 in obese Zucker (fatty) rats. Kidney Int 67: 2151–2158, 2005 [DOI] [PubMed] [Google Scholar]
- 28. McKay MK, Gardner AL, Boyd D, Hester RL. Influence of venular prostaglandin release on arteriolar diameter during functional hyperemia. Hypertension 31: 213–217, 1998 [DOI] [PubMed] [Google Scholar]
- 29. McLennan IS, Macdonald RE. Prostaglandin synthetase and prostacyclin synthetase in mature rat skeletal muscles: immunohistochemical localisation to arterioles, tendons and connective tissues. J Anat 178: 243–253, 1991 [PMC free article] [PubMed] [Google Scholar]
- 30. Murrant CL, Duza T, Kim MB, Cohen KD, Sarelius IH. Arteriolar dilations induced by contraction of hamster cremaster muscle are dependent on changes in endothelial cell calcium. Acta Physiol Scand 180: 231–238, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Naik JS, Xiang L, Hester RL. Enhanced role for RhoA-associated kinase in adrenergic-mediated vasoconstriction in gracilis arteries from obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 290: R154–R161, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Nuttle LC, Ligon AL, Farrell KR, Hester RL. Inhibition of phospholipase A2 attenuates functional hyperemia in the hamster cremaster muscle. Am J Physiol Heart Circ Physiol 276: H1289–H1294, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Quilley J, McGiff JC. Renal vascular responsiveness to arachidonic acid in experimental diabetes. Br J Pharmacol 100: 336–340, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saito Y, Eraslan A, Lockard V, Hester RL. Role of venular endothelium in control of arteriolar diameter during functional hyperemia. Am J Physiol Heart Circ Physiol 267: H1227–H1231, 1994 [DOI] [PubMed] [Google Scholar]
- 35. Sothern MS. Exercise as a modality in the treatment of childhood obesity. Pediatr Clin North Am 48: 995–1015, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care 22: 920–924, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Vitale C, Mercuro G, Cornoldi A, Fini M, Volterrani M, Rosano GM. Metformin improves endothelial function in patients with metabolic syndrome. J Intern Med 258: 250–256, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Walberg JL. Aerobic exercise and resistance weight-training during weight reduction. Implications for obese persons and athletes. Sports Med 7: 343–356, 1989 [DOI] [PubMed] [Google Scholar]
- 39. Wilmore JH, Costill DL. Physiology of Sport and Exercise (3rd Ed.). Champaign, IL: Human Kinetics, 2005 [Google Scholar]
- 40. Wojtaszewski JF, Nielsen JN, Richter EA. Invited review: Effect of acute exercise on insulin signaling and action in humans. J Appl Physiol 93: 384–392, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Xiang L, Dearman J, Abram SR, Carter C, Hester RL. Insulin resistance and impaired functional vasodilation in obese Zucker rats. Am J Physiol Heart Circ Physiol 294: H1658–H1666, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Xiang L, Naik J, Hester RL. Exercise-induced increase in skeletal muscle vasodilatory responses in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 288: R987–R991, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Xiang L, Naik JS, Abram SR, Hester RL. Chronic hyperglycemia impairs functional vasodilation via increasing thromboxane-receptor-mediated vasoconstriction. Am J Physiol Heart Circ Physiol 292: H231–H236, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Xiang L, Naik JS, Hester RL. Functional vasodilation in the rat spinotrapezius muscle: role of nitric oxide, prostanoids and epoxyeicosatrienoic acids. Clin Exp Pharmacol Physiol 35: 617–624, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xiang L, Naik JS, Hodnett BL, Hester RL. Altered arachidonic acid metabolism impairs functional vasodilation in metabolic syndrome. Am J Physiol Regul Integr Comp Physiol 290: R134–R138, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Yamada M, Omata K, Abe F, Ito S, Abe K. Changes in prostacyclin, thromboxane A2 and F2-isoprostanes, and influence of eicosapentaenoic acid and antiplatelet agents in patients with hypertension and hyperlipidemia. Immunopharmacology 44: 193–198, 1999 [DOI] [PubMed] [Google Scholar]
- 47. Yu H, Gallagher AM, Garfin PM, Printz MP. Prostacyclin release by rat cardiac fibroblasts: inhibition of collagen expression. Hypertension 30: 1047–1053, 1997 [DOI] [PubMed] [Google Scholar]
- 48. Zoladz JA, Majerczak J, Duda K, Chlopicki S. Exercise-induced prostacyclin release positively correlates with VO(2max) in young healthy men. Physiol Res 58: 229–238, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Zou MH, Shi C, Cohen RA. High glucose via peroxynitrite causes tyrosine nitration and inactivation of prostacyclin synthase that is associated with thromboxane/prostaglandin H(2) receptor-mediated apoptosis and adhesion molecule expression in cultured human aortic endothelial cells. Diabetes 51: 198–203, 2002 [DOI] [PubMed] [Google Scholar]