Abstract
This study investigated the sex differences in the contribution of nitric oxide (NO) and prostaglandins (PGs) to flow-mediated dilation (FMD). Radial artery (RA) FMD, assessed as the dilatory response to 5-min distal cuff occlusion, was repeated after three separate brachial artery infusions of saline (SAL), NG-monomethyl-l-arginine (l-NMMA), and ketorolac (KETO) + l-NMMA in healthy younger men (M; n = 8) and women (W; n = 8). In eight subjects (4 M, 4W) RA FMD was reassessed on a separate day with drug order reversed (SAL, KETO, and l-NMMA + KETO). RA FMD was calculated as the peak dilatory response observed relative to baseline (%FMD) and expressed relative to the corresponding area under the curve shear stress (%FMD/AUC SS). l-NMMA reduced %FMD similarly and modestly (P = 0.68 for sex * trial interaction) in M and W (all subjects: 10.0 ± 3.8 to 7.6 ± 4.7%; P = 0.03) with no further effect of KETO (P = 0.68). However, all sex * trial and trial effects on %FMD/AUC SS for l-NMMA and KETO + l-NMMA were insignificant (all P > 0.20). There was also substantial heterogeneity of the magnitude and direction of dilator responses to blockade. After l-NMMA infusion, subjects exhibited both reduced (n = 14; range: 11 to 78% decrease) and augmented (n = 2; range: 1 to 96% increase) %FMD. Following KETO + l-NMMA, seven subjects exhibited reduced dilation (range: 10 to 115% decrease) and nine subjects exhibited augmented dilation (range: 1 to 212% increase). Reversing drug order did not change the nature of the findings. These findings suggest that RA FMD is not fully or uniformly NO dependent in either men or women, and that there is heterogeneity in the pathways underlying the conduit dilatory response to ischemia.
Keywords: shear stress, nitric oxide, prostaglandins, vasodilation
brachial artery flow-mediated dilation (FMD), the shear-evoked conduit artery dilatory response to a period of tissue ischemia, is a standard noninvasive technique for assessing endothelial function and has been correlated with coronary artery endothelial function (3). FMD is predictive of future cardiovascular events (21, 60), providing independent prognostic value to cardiovascular risk assessment in post menopausal women (48) and predicting future cardiac events in patients with established coronary artery disease (7).
Reductions in FMD are widely assumed to reflect diminished nitric oxide (NO) dilator function as several pivotal human studies have concluded that brachial and radial artery FMD are dependent on a NO pathway (12, 28, 29, 40). In these studies, blockade of NO by administration of the endothelial nitric oxide synthase (eNOS) inhibitor [NG-monomethyl-l-arginine (l-NMMA)] in young, healthy humans abolished the conduit dilatory response to a 5-min period of distal occlusion. By contrast, a recent study (44) was unable to reduce radial FMD with a large dose of l-NMMA and concluded that there may be heterogenous vasodilator phenotypes which affect the contribution of NO to FMD.
The aforementioned studies investigating the NO dependence of FMD were either conducted solely in young men (12, 35, 44) or included too few women to investigate sex differences (29, 40). Prostaglandins (PGs) and endothelium-derived hyperpolarizing factor (EDHF) also contribute to endothelium-dependent relaxation in animal models, and there are sex differences in the magnitude of the dilatory response and pathways activated by increased shear stress (26, 41, 51). For example, shear stress-induced dilations in rat tail arteries are fully NO dependent in male rats but NO and EDHF dependent in female rats (41). In NO and PG knockout mice, females but not males maintain mesenteric artery endothelium-dependent dilation, suggesting a greater compensatory role of EDHF in female mice (51). As brachial artery FMD is greater in young women than men (33, 43), and young women generally exhibit greater vascular responsiveness to other vasodilator stimuli (10, 31, 42), it is possible that the dilator mechanisms underlying FMD differ in men vs. women.
Understanding the pathways underlying FMD is the critical first step for developing interventions aimed at correcting deficits in endothelium-dependent dilator function observed with aging and disease. Accordingly, the purpose of the present investigation was to 1) confirm recent findings that FMD is not solely NO dependent and 2) determine sex differences in the contribution of NO and PG to radial artery endothelium-dependent dilation in healthy young men and women by measuring FMD after brachial artery infusion of single and combined doses of NO and PG inhibitors (l-NMMA and ketorolac, respectively).
METHODS
Study Population
Sixteen healthy, nonsmoking young adults (8 men and 8 women, ages 20–35), with no diagnosed cardiovascular, pulmonary, metabolic, or chronic diseases were enrolled in the study. Exclusion criteria included stage I or higher blood pressure (>140/90 mmHg), cardiac abnormalities evidenced during a Balke treadmill test to maximal exertion, use of medications affecting hemodynamic variables (cholesterol-lowering medications, blood pressure lowering medications, antioxidant supplementation, use of hormone therapy or oral contraceptives in the last 12 mo), hypercholesterolemia (LDL cholesterol >160 mg/dl, total cholesterol >240 mg/dl, and triglycerides >150 mg/dl), anemia (hemoglobin <11 g/dl), and regular exercise >2 days/wk or maximal oxygen uptake (V̇o2max) >80th percentile for age-group norms (2). Subjects provided written, informed consent to participate as approved by the Institutional Review Board at Hartford Hospital in agreement with the guidelines set forth by the Declaration of Helsinki.
Screening Visit
Data for inclusion/exclusion criteria as described above were assessed on the first screening visit. After a 12-h fast, subjects underwent a venous blood draw to analyze whole blood viscosity, blood lipid levels, and hemoglobin (Clinical Laboratory Partners, Hartford, CT). Vital signs (blood pressure and heart rate), height, weight, and waist circumference were measured, and forearm volume was determined by water displacement.
Subjects also underwent a metabolic treadmill exercise test using the modified Balke protocol (2) to document cardiorespiratory fitness (V̇o2max). Expired oxygen, carbon dioxide, and ventilatory volume were determined using a Parvomedics TrueOne 2400 metabolic cart (ParvoMedics, Sandy, UT) and a breath-by-breath method. Maximal oxygen uptake was determined by averaging the two highest consecutive 30-s values.
Testing Procedures
Subjects participated in two or three testing sessions: a noninvasive familiarization visit, an invasive catheterization visit, and, in a subset of eight subjects, an additional catheterization visit to test the effect of drug order. For all testing visits, young female subjects were studied in days 1–7 of their menstrual cycle to standardize the influence of female hormones. In addition, subjects were fasted for ≥8 h and were asked to refrain from exercise, migraine medicine, pain medications (aspirin, ibuprofen, and other nonsteroidal anti-inflammatories), or herbal supplements for ≥24 h before testing. All study procedures were performed in a quiet, temperature-controlled room.
Familiarization visit.
A noninvasive study visit was performed to determine the reproducibility of the FMD technique and establish comparative values for brachial artery endothelium-dependent and -independent dilation in the study population. Brachial FMD of the nondominant arm of each subject was measured twice (with a rest period of 10 min separating each test) with the subject lying supine with the arm extended 80° from the torso at heart level. Blood pressure in the dominant arm was measured every minute with an automated blood pressure monitor (Vital Signs Monitor; WelchAllyn, Skaneateles, NY). A rapid-inflation/deflation pneumatic cuff (Hokanson, Bellevue, WA) was placed around the forearm immediately distal to the olecranon process (45). The artery was imaged 1–3 in. proximal to the olecranon process using a 5- to 12-MHz multifrequency linear-array transducer attached to a high-resolution ultrasound machine (Terason t3000; TeraTech, Burlington, MA). Once a satisfactory image using optimal B-mode imaging was obtained, the placement of the probe was fixed on the upper arm to ensure that the site of measurement did not change during the two trials. Doppler velocity was also measured continuously with the Terason using a 60° angle of insonation held constant throughout the study. After the subject rested in the supine position for 10 min, resting brachial artery diameter and velocity were measured for 1 min before inflation of the pneumatic cuff. The cuff was then inflated to suprasystolic pressure (300 mmHg) for 5 min; diameter and velocity recordings resumed 30 s before cuff deflation and continued for 3 min after deflation.
After another 10-min rest period, endothelium-independent dilation of the arm was assessed with administration of 0.4 mg sublingual nitroglycerin (NTG: Nitrostat, Parke-Davis, Morris Plains, NJ). After 1 min of baseline diameter measurements, diameters were measured continuously for 10 min after administration of NTG.
Catheterization visit.
A 20 -gauge Teflon catheter (Arrow International, Reading, PA) was inserted into the brachial artery of the left arm at the level of the antecubital fossa using 1–2 ml of 2% lidocaine hydrochloride (Fujisawa, Deerfield, IL) to anesthetize the overlying skin. Saline and study drugs were administered via the brachial artery catheter using a three-port connector in series with a catheter-transducer system. One port was attached to the pressure transducer and GE Dash 4000 Monitor (GE Health Care, United Kingdom) to allow measurement of arterial pressure. The remaining two ports were connected to an Abbott Plum XL3 Triple Channel Infusion Pump (Abbott Labs, Abbott Park, IL) for drug infusions and continuous saline administration for flushing (2 U/ml at 3 ml/h).
After a 30-min rest period, saline was infused (to establish a control condition for any effects on FMD from administration of infusate) at 2 ml/min for 10 min. Radial FMD was then performed using identical procedures as those described above, although the ultrasound probe was placed ∼10 cm distal to the antecubital fossa and the occlusion cuff placed around the subject's wrist. After a 10-min rest period, l-NMMA (Clinalfa, Laufelfingen, Switzerland) was infused at 5 mg/ml at 2 ml/min for 10 min to block NO production. Radial FMD was then repeated. After another 10-min rest period, ketorolac (Toradol; Abbott Labs) was infused at 600 μg/ml at 2 ml/min for 5 min, followed by a maintenance dose of l-NMMA (5 mg/ml at 2 ml/min) for an additional 5 min. Radial FMD was then repeated a third time. Drug doses were designed to equal or exceed those used in previous studies successfully blocking NO and PG in the forearm (11, 29, 40, 49, 50, 58).
Subset catheterization visit.
Four women and four men repeated the above-described protocol on a separate day with the order of l-NMMA and ketorolac administration reversed. Following an identical saline infusion, a radial FMD test, and a 10-min rest period, ketorolac was infused at 600 ug/ml at 2 ml/min for 5 min. Radial FMD was then performed followed by a 10-min rest period. l-NMMA was then infused at 5 mg/ml at 3 ml/min for 10 min (to infuse the same total dose as given in the combined initial and maintenance doses from the first catheterization visit), and radial FMD was performed a third time. In a subset of five subjects (3 men and 2 women), the efficacy of the PG blockade was confirmed by taking an arterial blood sample prior to the saline infusion and again after the final FMD test (after ketorolac/l-NMMA infusion) to measure thromboxane B2, an indicator of platelet cyclooxygenase activity during whole blood clotting. Sample analysis was performed by ELISA by Oxford Biomedical Research (Oxford, MI).
FMD Data Analysis
Ultrasound images were recorded at five frames per second using Camtasia software (TechSmith, Okemos, MI) and saved as AVI files. Diameters and velocities were analyzed using Brachial Analyzer software (Medical Imaging Applications, Coralville, IA).
Diameter analysis.
A technician, blinded to any subject information, selected a region of interest along the arterial wall, and the edge of the intima-lumen interface was detected by pixel density and represented by a line of best fit. Multiple perpendicular lines were fit between the near and far walls and averaged to report a composite diameter measurement for each frame. Diameter analyses were triggered by the corresponding Doppler wavefrom to capture only end diastolic diameters. Resting diameters were calculated as the average of images taken over the 1-min baseline. The peak diameter was calculated as the highest 3-s average observed postocclusion. FMD was expressed as the percent dilation (%FMD) relative to the baseline measurement for each trial.
Velocity analysis.
A region of interest was selected around the Doppler waveform, and the trace of the velocity-time integral was used to calculate mean velocity for each cardiac cycle. Velocity was matched to the corresponding diameter and shear stress was calculated in dyn/cm2 using the formula 4 μ·V/D, where μ is blood viscosity in mPA·s at 60 rpm, D is artery diameter (cm), and V is in cm/s. The shear stress postocclusion area under the curve (AUC SS) was then calculated from cuff release up until the time of peak diameter (4, 55), and FMD was normalized to the AUC SS with the latter divided by an arbitrary value of 1,000 to simplify data presentation.
Endothelium-independent dilation.
For NTG measurements, diameters were analyzed as described above, with the resting diameter calculated as the average of images taken over the 1-min baseline and the peak diameter calculated as the highest 3-s average observed post-NTG administration. NTG dilation (%NTG) was calculated as the percent change in dilation relative to baseline.
Arterial blood pressure and vascular conductance (catheterization visits).
Radial artery blood flow (RBF) was calculated as blood velocity * π * (radial diameter/2)2 * 60, where the RBF is in ml/min, the blood velocity is in cm/s, the radial diameter is in cm, and 60 is used to convert from ml/s to ml/min. Intraarterial blood pressure at the baseline of each trial was used to calculate radial vascular conductance as radial blood flow/arterial pressure *100.
Statistical Analyses
All statistical analyses were performed with SPSS 15.0 (SPSS, Chicago, IL). A one-way ANOVA was used to compare baseline differences between men and women. A repeated-measures ANOVA was used to compare dilatory responses between groups following drug infusions. FMD variables (%FMD, AUC SS, and %FMD/AUC SS) were analyzed with trial (drug infusion) as the within-subject factor, sex as the between-subject factor and a condition-by-trial interaction. Significance was determined at P < 0.05 and a Tukey post hoc adjustment was applied to significant individual and interaction comparisons.
RESULTS
Subject Characteristics
Subject characteristics are shown in Table 1. The coefficient of variation calculated for two repeated trials of brachial FMD normalized to AUC SS was 3.5% for men and 3.7% for women.
Table 1.
Subject characteristics
| Men (n = 8) | Women (n = 8) | |
|---|---|---|
| Age, yr | 29 ± 4 | 28 ± 5 |
| V̇o2max, ml · kg−1 · min−1 | 44.2 ± 7.5 | 34.0 ± 7.3* |
| Resting SBP, mmHg | 113 ± 8 | 99 ± 7* |
| Resting DBP, mmHg | 69 ± 8 | 61 ± 3* |
| Resting HR, beats/min | 56 ± 9 | 60 ± 9 |
| LDL cholesterol, mg/dl | 103 ± 36 | 84 ± 19 |
| HDL cholesterol, mg/dl | 56 ± 16 | 68 ± 10* |
| BMI, kg/m2 | 25.4 ± 3.0 | 24.7 ± 2.5 |
| Forearm volume, ml | 1046 ± 132 | 873 ± 134* |
| Whole blood viscosity, mPA · s | 5.4 ± 0.5 | 4.5 ± 0.6* |
| Hemoglobin, g/dl | 15.4 ± 1.2 | 12.9 ± 1.2* |
| %FMD | 8.7 ± 3.1 | 7.8 ± 1.7 |
| %FMD/AUC SS | 0.070 ± 0.036 | 0.079 ± 0.038 |
| %NTG | 23.5 ± 6.8 | 32.0 ± 8.8* |
Data are group means ± SD. V̇o2max, maximal oxygen uptake; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; LDL, low-density lipoprotein; HDL, high-density lipoprotein; BMI, body mass index; %FMD, brachial artery flow-mediated dilatory response, averaged between 2 trials; %FMD/AUC SS, brachial FMD normalized to area-under-the-curve shear stress, averaged between 2 trials; %NTG, brachial dilatory response to nitroglycerin.
P < 0.05, significant difference between men and women.
Baseline Hemodyamic Parameters Across Infusion Trials
Group means for resting radial diameter, RBF, and radial vascular conductance measured immediately following infusions of saline, l-NMMA and ketorolac + l-NMMA (the main catheterization visit) as well as saline, ketorolac, and l-NMMA + ketorolac (reverse drug order visit) are shown in Table 2. All trial * sex interactions were insignificant (all P > 0.08) and thus male and female data are combined.
Table 2.
Baseline hemodynamics across infusion trials
| Saline | l-NMMA | KETO + l-NMMA | Trial | Interaction | |
|---|---|---|---|---|---|
| All subjects (n = 16) | |||||
| Diameter, mm | 2.1 ± 0.4 | 2.1 ± 0.3 | 2.1 ± 0.4 | 0.68 | 0.56 |
| RBF, ml/min | 16.4 ± 14.2 | 11.7 ± 7.5 | 11.7 ± 5.4 | 0.05 | 0.87 |
| RVC, ml · min−1 · 100 mmHg−1 | 20.9 ± 17.7 | 14.5 ± 9.6* | 14.0 ± 6.3* | 0.03 | 0.86 |
| Saline | KETO | l-NMMA + KETO | Trial | Interaction | |
|---|---|---|---|---|---|
| Reverse Drug (n =8) | |||||
| Diameter, mm | 2.3 ± 0.3 | 2.2 ± 0.3 | 2.2 ± 0.4 | 0.03 | 0.08 |
| RBF, ml/min | 26.3 ± 21.4 | 18.1 ± 12.0 | 9.6 ± 5.6* | 0.03 | 0.15 |
| RVC, ml · min−1 · 100 mmHg−1 | 34.2 ± 26.2 | 23.1 ± 13.9 | 12.1 ± 6.9* | 0.02 | 0.16 |
Data are means ± SD. Trial, P value for trial effect for each variable; interaction, P value for sex * trial interaction for each variable; l-NMMA, NG-monomethyl-l-arginine; KETO, ketorolac; RBF, radial blood flow; RVC, radial vascular conductance.
P < 0.05, significant difference from saline condition.
FMD Responses Across Infusion Trials
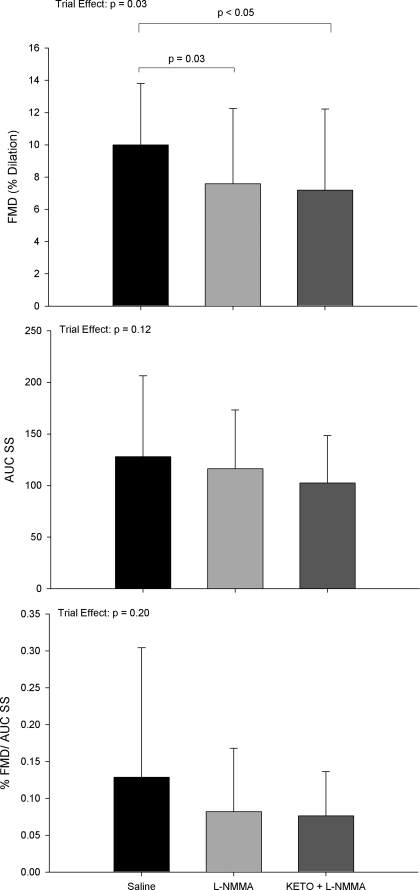
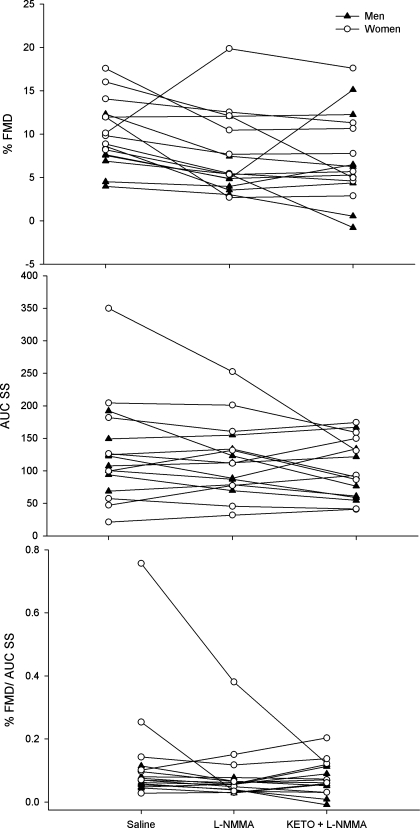
For repeated measures analyses associated with the main catheterization visit, all trial * sex interactions were insignificant (all P > 0.30). Consequently data are presented as group means with men and women combined for %FMD, AUC SS, and %FMD/AUC SS following l-NMMA and ketorolac infusions (Fig. 1). Individual responses to drug infusions are shown in Fig. 2.
Fig. 1.
Group means ± SD for all subjects in the main catheterization study [saline followed by NG-monomethyl-l-arginine (l-NMMA) followed by ketorolac (KETO) + l-NMMA] are shown for percent flow-mediated dilation (%FMD; top), area under the curve shear stress (AUC SS; middle), and %FMD/AUC SS (bottom) with P values for trial effects and any significant differences between conditions on each graph.
Fig. 2.
Individual %FMD (top), AUC SS (middle), and %FMD/AUC SS (bottom) responses to saline, l-NMMA, and ketorolac (KETO) + l-NMMA infusion are shown for men (▴) and women (○).
Calculating the percent change in %FMD after infusions of l-NMMA and ketorolac + l-NMMA again yielded no sex differences (P = 0.63 and 0.55, respectively). On average, l-NMMA infusion reduced %FMD by 23.7 ± 37.0%, with 14 subjects exhibiting reduced dilation (range: 11 to 78% decrease) and 2 subjects exhibiting augmented dilation (range: 1 to 96% increase). On average, ketorolac + l-NMMA infusion increased %FMD relative to l-NMMA alone by 1.0 ± 70.1%, although seven subjects (3 men, 4 women) exhibited reduced dilation (range: 10 to 115% decrease) and the remaining nine subjects (5 men, 4 women) exhibited augmented dilation (range: 1 to 212% increase).
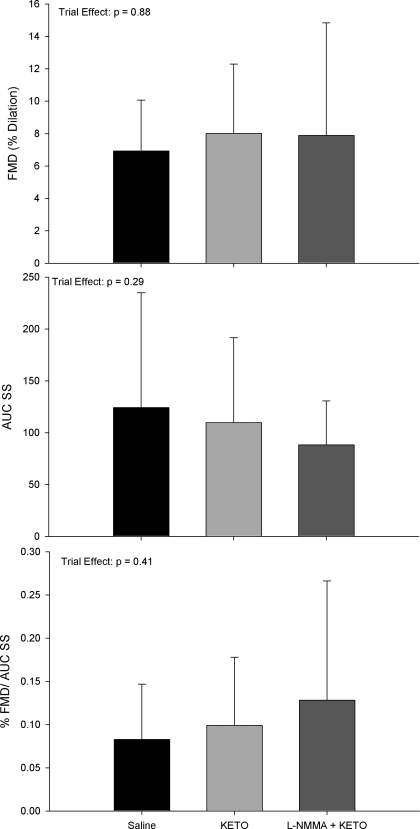
Effect of Drug Order on Dilator Responses
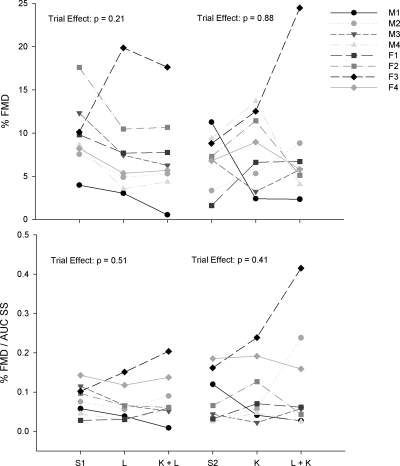
For repeated-measures analyses associated with the subset catheterization visit, again all trial * sex interactions were insignificant (all P > 0.21). Consequently, data are presented as group means with men and women combined for % FMD, AUC SS, and % FMD/AUC SS following ketorolac and combined l-NMMA + ketorolac infusions (Fig. 3). Similar to heterogeneity observed in the main catheterization visit, ketorolac infusion alone increased %FMD relative to saline condition in six subjects (range: 32 to 310% increase) and decreased %FMD/AUC SS in two subjects (range: 53 to 79% decrease). Combined l-NMMA + ketorolac infusion evoked augmented dilation relative to ketorolac alone in four subjects (range: 2 to 96% increase) and reduced dilation in four subjects (range: 2 to 70% decrease). Finally, a comparison of the effects of l-NMMA and ketorolac between the main visit and reverse drug order visit for %FMD and %FMD/AUC SS is shown in Fig. 4.
Fig. 3.
Group means ± SD for the 8 subjects who participated in the reverse drug order catheterization study [saline, followed by ketorolac (KETO) followed by l-NMMA + KETO] are shown for %FMD (top), AUC SS (middle), and % FMD/AUC SS (bottom) with P values for trial effects and any significant differences between conditions on each graph.
Fig. 4.
Individual responses in %FMD (top) and %FMD/AUC SS (bottom) in the 8 subjects [4 males (M), 4 females (F)] who participated in both the main and reverse drug order catheterization visits. S1, saline on first visit; L, l-NMMA alone on first visit; K + L, ketorolac + l-NMMA on first visit; S2, saline on second visit; K, ketorolac alone on second visit; L + K, l-NMMA + ketorolac on second visit. P values for trial effects in the 8 subjects are shown on each graph.
Effectiveness of Ketorolac Blockade
Thromboxane B2 immediately before saline infusion was 15.6 ± 26.6 ng/ml and decreased in all five subjects to 0.7 ± 0.9 ng/ml following combined ketorolac/l-NMMA infusion (P = 0.04 using Wilcoxon-Signed Ranks test for paired comparison of nonparametric data).
DISCUSSION
The current investigation represents what is, to the best of our knowledge, the first study to assess the effects of NO and PG inhibition on conduit FMD in equal numbers of men and women. Major findings are that there is no evidence of systematic sex differences in the dilator pathways underlying radial FMD but rather substantial between-subject heterogeneity in these pathways such that blocking NO and PG evokes subject-specific increases or decreases in the conduit vasodilator response to increased shear stress. Moreover, in agreement with recent findings in healthy young men, NO does not appear obligatory for radial FMD in either men or women.
Presence of Different Dilator Phenotypes?
It has long been assumed that conduit FMD is NO dependent, based on several pivotal studies, which largely abolished radial (28, 29, 40, 52), brachial (12) or femoral (32) FMD with l-NMMA infusion. Recently, Pyke et al. (44) attempted to replicate these studies and were unable to block radial FMD with high-dose l-NMMA infusion in healthy young volunteers. Although studies in animals (15, 17) have established that the contribution of NO to vasodilation differs in the conduit vs. resistance vasculature, all of the human studies have assessed conduit FMD (with the majority focused on radial FMD) and thus regional vascular specificity does not explain the discrepant results. Rather, Pyke et al. (44) postulated that there may be different dilator phenotypes underlying conduit FMD in humans. The current data support this hypothesis. While there was a small but significant (∼25%) reduction in %FMD with l-NMMA alone (Fig. 1), the magnitude of this effect varied considerably among subjects, as NOS inhibition reduced %FMD by a range of 11–78% in 14 out of 16 subjects. In addition, there was substantial heterogeneity displayed by subjects in response to blockades of both NO and PGs when %FMD was considered alone or normalized to the shear stimulus (Figs. 2 and 4). Furthermore, Mayahi et al. (37) recently found that l-NMMA infusion did not block brachial FMD in patients with BH4 deficiency caused by a genetic mutation in GTP cyclohydrolase, an enzyme regulating BH4; similar results have been published in patients with hypertension (19). Fischer et al. (16) also found that cytochrome P450 2C metabolites contribute to radial FMD, as blocking this pathway with sulphenazole reduces radial FMD from 11.5 to 7.4%. Notably, they found that blocking NO with l-NMMA alone also reduced FMD by an equivalent amount (from 11.5 to 6% dilation), with combined blockade further lowering FMD to 3.9%, suggesting multiple, redundant pathways underlying FMD.
Compensatory responses to reduced NO bioavailability in both human and animal models also indicate that there are additive and/or alternative pathways contributing to FMD. For example, adults with hypertension and heart failure utilize EDHF to maintain endothelium-dependent dilation when NO is compromised (30, 53). Similarly, aged male and female rats upregulate PG and EDHF, respectively, to maintain gracilis muscle flow-induced arteriolar dilation following chronic treatment with Nω-nitro-l-arginine methyl ester (59). In the current study, we speculate that the relatively robust %FMD observed following NOS inhibition signals an intact compensatory dilator system in healthy young humans that is advantageous for endothelial health; however, studies in adults with endothelial dysfunction are necessary to confirm this hypothesis.
Taken collectively, these results suggest that there is substantial heterogeneity in the pathways underlying FMD. These pathways may be influenced by factors such as age, cardiovascular health, and genetic predisposition, although the current data do not indicate a systematic influence of sex in healthy adults. Previous findings regarding the NO dependence of FMD thus may have been specific to the subject population studied, and therefore the assumption that conduit FMD is largely NO dependent may not be as widely applicable as previously assumed.
Effectiveness of NO and PG Blockade?
An alternative explanation for the current observations is that NO and PGs were not fully blocked by the infusion of l-NMMA and ketorolac, respectively. Direct measurements of thromboxane B2, as well as infusion of equivalent or greater doses of ketorolac as used in previous studies to block PGs (11, 49, 50), support the efficacy of the PG block. We did not directly infuse acetylcholine in the current study to test the efficacy of the NO block. However, the dose of l-NMMA used in the current study (83–143 mg/l forearm volume) equals or exceeds the dose used in most previous studies exploring the contribution of NO to either FMD or forearm blood flow (11, 29, 40, 49, 50). Moreover, we used an infusion dose (10 mg/min) similar to that utilized by Wray et al. (58) (8.6 ± 0.9 mg/min), who assessed efficacy of the NO block with a dose-response curve showing a clear plateau in resting brachial vasoconstriction in response to increasing doses of l-NMMA. Finally, the reduction in radial vascular conductance (∼30%) that we observed with l-NMMA infusion is comparable to that observed in related studies using l-NMMA (13, 27, 29, 44, 52, 58). Collectively, these studies support that eNOS was effectively blocked by l-NMMA in the current investigation.
Methodological Differences Between Studies?
One additional confounding factor that deserves mention is the evolving methodology used to assess FMD in humans (24, 55). Considerations such as the relationship between the shear stimulus and the conduit dilation, the time course of dilation, principles of ultrasonography, the time at which peak conduit dilation is observed, the intrasubject variability of FMD, and the standardization of testing conditions have been advanced since the original guidelines for measuring FMD were established (9). Accordingly, the techniques used to measure FMD are discrepant between studies, making direct comparisons between investigations difficult. Thus the emerging data regarding heterogenous dilator pathways underlying FMD may also reflect in part the effect of improving accuracy in evaluating shear-mediated dilatory responses. Additionally, previous studies investigating NO dependency of FMD have utilized sample sizes of 8–10 subjects, whereas the current sample size comprised 16 subjects. Therefore, it is possible that studying larger numbers of subjects may amplify the number of unique vasodilator phenotypes in the study population and consequently increase the observed heterogeneity of pathways underlying FMD.
Interaction Between NO and PGs in FMD Response?
An additional finding of the study is that reversing drug order in a subset of subjects did not elicit fully identical effects on the dilatory response to single and combined NO and PG blockade (Fig. 4). For example, for the eight subjects who completed both catheterization visits, we compared 1) the directional (i.e., increase or decrease) effect of l-NMMA (alone or in combination with ketorolac) and 2) the directional effect of ketorolac (alone or in combination with l-NMMA) on FMD between trials. This yielded 16 possible comparisons (2 for each subject). In 12 of these comparisons (5/8 for l-NMMA and 7/8 for ketorolac), there was agreement (e.g., if a subject exhibited reduced FMD with l-NMMA the first trial, the same effect was repeated in the second trial when l-NMMA was infused following ketorolac infusion). However, in four of these comparisons (25% of cases; 2 in men and 2 in women), there was discrepancy between the trials such that, for example, a subject who exhibited reduced FMD with l-NMMA alone responded to l-NMMA infusion superimposed on previous ketorolac infusion with an augmentation in FMD.
This finding may be a consequence of day-to-day variability in FMD, and future studies are necessary to establish the reproducibility of within-subject variability in the response to NO and PG inhibitors alone and in combination. Alternatively, this observation could also indicate an effect of crosstalk between vasoactive substances released by the endothelium. For example, PGs may inhibit NO (56, 57) and endothelin (34) whereas NO stimulates PG production (8, 47) and inhibits endothelin secretion (6). In humans, Taddei et al. (54) found that the inhibitory influence of l-NMMA as well as the potentiating effects of l-arginine on the forearm dilatory response to acetylcholine was abolished in hypertensive adults and could only be restored through inhibition of cylcooxygenase. Thus blocking a specific vasoactive pathway and/or eliciting compensation by an alternative pathway may change the interaction between endothelial-derived substances and result in disparate effects on FMD when drugs are infused alone or in combination.
Importance of Understanding the Pathways Underlying FMD
The assumption that FMD is solely an assessment of NO function is so prevalent that most studies reporting reduced FMD end with the conclusion that findings represent dysfunction in some portion of the NO signaling cascade (25, 38). For example, a recent review (1) on the topic states that “when performed by experienced personnel in a standardized setting…brachial artery FMD is a useful measure of NO-dependent endothelial function.” The interpretation of FMD as NO dependent in men and women of varying ages and health conditions has accordingly shaped NO-dependent interventional strategies such as administering l-arginine (the substrate for eNOS; Ref. 18), BH4 (an eNOS cofactor; Ref. 14), or antioxidants (which enhance endothelial NO bioavailability; Ref. 46) to improve FMD. However, if FMD does in fact involve additional and/or compensatory dilator pathways, which differ substantially between individuals, then therapeutic or interventional strategies aimed at improving this important cardiovascular risk factor by targeting NO may be ineffective.
Interestingly, recent evidence suggests that the distal vs. proximal cuff occlusion methodology used to assess FMD is equally predictive of future cardiovascular effects (22), despite evidence suggesting that the latter method evokes non-NO-mediated dilation (12). Therefore, our findings likely do not alter the utility of FMD for assessment of cardiovascular disease risk and progression. However, if evaluating NO bioavailability is the goal of an investigation, then our results do suggest that an alternative method, such as the dilatory response to handgrip exercise (58), or measurement of NO metabolites (5), may be more valid. By contrast, in the current study, %FMD/AUC SS was not significantly reduced by l-NMMA infusion, and therefore normalization of FMD to the shear stimulus may provide an index of vascular function that is less reliant on NO.
Methodological Limitations
It should be noted that we measured blood viscosity (used in our calculations of shear stress) on the first study visit and not repeatedly during the infusion visit. While it is possible that blood viscosity may have changed throughout the repeated infusion trials, this is unlikely due to the published intraindividual stability of blood viscosity (36) as well as the small total volume of infusate.
In addition, ketorolac is a nonspecific inhibitor of cyclooxygenase (COX) I and II and therefore cannot be used to distinguish between contributions of vasoconstrictors such as PG endoperoxides (20) and thromboxane A2 (23) and vasodilators such as PG prostacyclin (39) to FMD. The nonspecific nature of ketorolac on the arachidonic acid pathway may thus explain our observations that approximately equal numbers of subjects responded to ketorolac infusion with augmented vs. reduced FMD, as it is likely that there is substantial inter-individual variability in the production of vasoconstrictor and vasodilator prostanoids through this pathway. However, this does not alter our conclusion that pathways underlying FMD in healthy young humans are heterogenous.
Conclusions
The current investigation evaluated the effect of blocking NO and PGs on radial FMD in humans. While systematic sex differences in the contributions of these dilators to FMD were not observed, heterogenous responses to infusions of l-NMMA and ketorolac indicate that there is substantial variability in the pathways underlying FMD. Moreover, results confirm recent evidence that conduit FMD is not fully or uniformly NO dependent.
GRANTS
Financial support was provided by a Hartford Hospital Open Competition Grant, the 2010-0089 CT DPH Biomedical Research Award, and National Heart, Lung, and Blood Institute Grants 1R01-HL-098085-01 (to B. A. Parker) and 1R01-HL-081893-04 (to P. D. Thompson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge the assistance of Jeffrey Capizzi, William Roman, Lindsay Lorson, Dr. David Proctor, Candy Johnson, and Nicole Chomek.
REFERENCES
- 1. Al-Qaisi M, Kharbanda RK, Mittal TK, Donald AE. Measurement of endothelial function and its clinical utility for cardiovascular risk. Vasc Health Risk Manag 4: 647–652, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American College of Sports Medicine ACSM's Guidelines for Exercise Testing and Prescription (7th ed.), edited by Whaley ME, Brubaker PH, Otto RM. Philadelphia, PA: Lippincott Williams and Wilkins, 2006, p. 99–102 [Google Scholar]
- 3. Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol 26: 1235–1241, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Black MA, Cable NT, Thijssen DH, Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension 51: 203–210, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Bryan NS, Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med 43: 645–657, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cao WB, Zeng ZP, Zhu YJ, Luo WC, Cai BQ. Inhibition of nitric oxide synthesis increases the secretion of endothelin-1 in vivo and in cultured endothelial cells. Chin Med J (Engl) 107: 822–826, 1994 [PubMed] [Google Scholar]
- 7. Chan SY, Mancini GB, Kuramoto L, Schulzer M, Frohlich J, Ignaszewski A. The prognostic importance of endothelial dysfunction and carotid atheroma burden in patients with coronary artery disease. J Am Coll Cardiol 42: 1037–1043, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Clancy R, Varenika B, Huang W, Ballou L, Attur M, Amin AR, Abramson SB. Nitric oxide synthase/COX cross-talk: nitric oxide activates COX-1 but inhibits COX-2-derived prostaglandin production. J Immunol 165: 1582–1587, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Corretti MC, Plotnick GD, Vogel RA. Technical aspects of evaluating brachial artery vasodilatation using high-frequency ultrasound. Am J Physiol Heart Circ Physiol 268: H1397–H1404, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Dietz NM. Gender and nitric oxide-mediated vasodilation in humans. Lupus 8: 402–408, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments alpha-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol 287: H2576–H2584, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Doshi SN, Naka KK, Payne N, Jones CJ, Ashton M, Lewis MJ, Goodfellow J. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci (Lond) 101: 629–635, 2001 [PubMed] [Google Scholar]
- 13. Engelke KA, Halliwill JR, Proctor DN, Dietz NM, Joyner MJ. Contribution of nitric oxide and prostaglandins to reactive hyperemia in human forearm. J Appl Physiol 81: 1807–1814, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol 568: 1057–1065, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferrer M, Encabo A, Conde MV, Marin J, Balfagon G. Heterogeneity of endothelium-dependent mechanisms in different rabbit arteries. J Vasc Res 32: 339–346, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Fischer D, Landmesser U, Spiekermann S, Hilfiker-Kleiner D, Hospely M, Muller M, Busse R, Fleming I, Drexler H. Cytochrome P450 2C9 is involved in flow-dependent vasodilation of peripheral conduit arteries in healthy subjects and in patients with chronic heart failure. Eur J Heart Fail 9: 770–775, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Freitas MR, Schott C, Corriu C, Sassard J, Stoclet JC, Andriantsitohaina R. Heterogeneity of endothelium-dependent vasorelaxation in conductance and resistance arteries from Lyon normotensive and hypertensive rats. J Hypertens 21: 1505–1512, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Gates PE, Boucher ML, Silver AE, Monahan KD, Seals DR. Impaired flow-mediated dilation with age is not explained by l-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J Appl Physiol 102: 63–71, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Ghiadoni L, Versari D, Magagna A, Kardasz I, Plantinga Y, Giannarelli C, Taddei S, Salvetti A. Ramipril dose-dependently increases nitric oxide availability in the radial artery of essential hypertension patients. J Hypertens 25: 361–366, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Gimbrone MA, Jr, Alexander RW. Angiotensin II stimulation of prostaglandin production in cultured human vascular endothelium. Science 189: 219–220, 1975 [DOI] [PubMed] [Google Scholar]
- 21. Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol 41: 1769–1775, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension 57: 363–369, 2011 [DOI] [PubMed] [Google Scholar]
- 23. Gryglewski RJ, mbinska-Kiec A, Korbut R. A possible role of thromboxane A2 (TXA2) and prostacyclin (PGI2) in circulation. Acta Biol Med Ger 37: 715–723, 1978 [PubMed] [Google Scholar]
- 24. Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heiss C, Kleinbongard P, Dejam A, Perre S, Schroeter H, Sies H, Kelm M. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J Am Coll Cardiol 46: 1276–1283, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Huang A, Sun D, Koller A, Kaley G. Gender difference in flow-induced dilation and regulation of shear stress: role of estrogen and nitric oxide. Am J Physiol Regul Integr Comp Physiol 275: R1571–R1577, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Ishibashi Y, Shimada T, Sakane T, Takahashi N, Sugamori T, Ohhata S, Inoue S, Katoh H, Sano K, Murakami Y, Hashimoto M. Contribution of endogenous nitric oxide to basal vasomotor tone of peripheral vessels and plasma B-type natriuretic peptide levels in patients with congestive heart failure. J Am Coll Cardiol 36: 1605–1611, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Jiang B, Seddon M, Fok H, Donald A, Chowienczyk P. Flow-mediated dilation of the radial artery is offset by flow-induced reduction in transmural pressure. Hypertension 57: 1145–50, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation 91: 1314–1319, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Katz SD, Krum H. Acetylcholine-mediated vasodilation in the forearm circulation of patients with heart failure: indirect evidence for the role of endothelium-derived hyperpolarizing factor. Am J Cardiol 87: 1089–1092, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol 36: 1233–1238, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Kooijman M, Thijssen DH, de Groot PC, Bleeker MW, van Kuppevelt HJ, Green DJ, Rongen GA, Smits P, Hopman MT. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J Physiol 586: 1137–1145, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Levenson J, Pessana F, Gariepy J, Armentano R, Simon A. Gender differences in wall shear-mediated brachial artery vasoconstriction and vasodilation. J Am Coll Cardiol 38: 1668–1674, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Li RC, Cindrova-Davies T, Skepper JN, Sellers LA. Prostacyclin induces apoptosis of vascular smooth muscle cells by a cAMP-mediated inhibition of extracellular signal-regulated kinase activity and can counteract the mitogenic activity of endothelin-1 or basic fibroblast growth factor. Circ Res 94: 759–767, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Liuni A, Luca MC, Lisi M, Dragoni S, di SG, Mariani JA, Uxa A, Gori T, Parker JD. Observations of time-based measures of flow-mediated dilation of forearm conduit arteries: implications for the accurate assessment of endothelial function. Am J Physiol Heart Circ Physiol 299: H939–H945, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Maes M, Scharpe S, Cooreman W, Wauters A, Neels H, Verkerk R, De MF, D'Hondt P, Peeters D, Cosyns P. Components of biological, including seasonal, variation in hematological measurements and plasma fibrinogen concentrations in normal humans. Experientia 51: 141–149, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Mayahi L, Mason L, Bleasdale-Barr K, Donald A, Trender-Gerhard I, Sweeney MG, Davis MB, Wood N, Mathias CJ, Watson L, Pellerin D, Heales S, Deanfield JE, Bhatia K, Murray-Rust J, Hingorani AD. Endothelial, sympathetic, and cardiac function in inherited (6R)-l-erythro-5,6,7,8-tetrahydro-l-biopterin deficiency. Circ Cardiovasc Genet 3: 513–522, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Meyer MF, Lieps D, Schatz H, Pfohl M. Impaired flow-mediated vasodilation in type 2 diabetes: lack of relation to microvascular dysfunction. Microvasc Res 76: 61–65, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Moncada S, Gryglewski R, Bunting S, Vane JR. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature 263: 663–665, 1976 [DOI] [PubMed] [Google Scholar]
- 40. Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res 88: 145–151, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Pak KJ, Geary GG, Duckles SP, Krause DN. Male-female differences in the relative contribution of endothelial vasodilators released by rat tail artery. Life Sci 71: 1633–1642, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Proctor DN. Sex-specific influence of aging on exercising leg blood flow. J Appl Physiol 104: 655–664, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Perregaux D, Chaudhuri A, Mohanty P, Bukhari L, Wilson MF, Sung BH, Dandona P. Effect of gender differences and estrogen replacement therapy on vascular reactivity. Metabolism 48: 227–232, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Pyke K, Green DJ, Weisbrod C, Best M, Dembo L, O'Driscoll G, Tschakovsky M. Nitric oxide is not obligatory for radial artery flow-mediated dilation following release of 5 or 10 min distal occlusion. Am J Physiol Heart Circ Physiol 298: H119–H126, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol 568: 357–369, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Regensteiner JG, Popylisen S, Bauer TA, Lindenfeld J, Gill E, Smith S, Oliver-Pickett CK, Reusch JE, Weil JV. Oral l-arginine and vitamins E and C improve endothelial function in women with type 2 diabetes. Vasc Med 8: 169–175, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Ribeiro ML, Cella M, Farina M, Franchi A. Crosstalk between nitric oxide synthase and cyclooxygenase metabolites in the estrogenized rat uterus. Prostaglandins Leukot Essent Fatty Acids 68: 285–290, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol 51: 997–1002, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Saunders NR, Dinenno FA, Pyke KE, Rogers AM, Tschakovsky ME. Impact of combined NO and PG blockade on rapid vasodilation in a forearm mild-to-moderate exercise transition in humans. Am J Physiol Heart Circ Physiol 288: H214–H220, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol 579: 227–236, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Scotland RS, Madhani M, Chauhan S, Moncada S, Andresen J, Nilsson H, Hobbs AJ, Ahluwalia A. Investigation of vascular responses in endothelial nitric oxide synthase/cyclooxygenase-1 double-knockout mice: key role for endothelium-derived hyperpolarizing factor in the regulation of blood pressure in vivo. Circulation 111: 796–803, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Seddon M, Melikian N, Dworakowski R, Shabeeh H, Jiang B, Byrne J, Casadei B, Chowienczyk P, Shah AM. Effects of neuronal nitric oxide synthase on human coronary artery diameter and blood flow in vivo. Circulation 119: 2656–2662, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Taddei S, Versari D, Cipriano A, Ghiadoni L, Galetta F, Franzoni F, Magagna A, Virdis A, Salvetti A. Identification of a cytochrome P450 2C9-derived endothelium-derived hyperpolarizing factor in essential hypertensive patients. J Am Coll Cardiol 48: 508–515, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Cyclooxygenase inhibition restores nitric oxide activity in essential hypertension. Hypertension 29: 274–279, 1997 [DOI] [PubMed] [Google Scholar]
- 55. Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Uno K, Iuchi Y, Fujii J, Sugata H, Iijima K, Kato K, Shimosegawa T, Yoshimura T. In vivo study on cross talk between inducible nitric-oxide synthase and cyclooxygenase in rat gastric mucosa: effect of cyclooxygenase activity on nitric oxide production. J Pharmacol Exp Ther 309: 995–1002, 2004 [DOI] [PubMed] [Google Scholar]
- 57. Vassalle C, Domenici C, Lubrano V, L'Abbate A. Interaction between nitric oxide and cyclooxygenase pathways in endothelial cells. J Vasc Res 40: 491–499, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Wray DW, Witman MA, Ives SJ, McDaniel J, Fjeldstad AS, Trinity JD, Conklin JD, Supiano MA, Richardson RS. Progressive handgrip exercise: evidence of nitric oxide-dependent vasodilation and blood flow regulation in humans. Am J Physiol Heart Circ Physiol 300: H1101–H1107, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wu Y, Huang A, Sun D, Falck JR, Koller A, Kaley G. Gender-specific compensation for the lack of NO in the mediation of flow-induced arteriolar dilation. Am J Physiol Heart Circ Physiol 280: H2456–H2461, 2001 [DOI] [PubMed] [Google Scholar]
- 60. Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation 115: 2390–2397, 2007 [DOI] [PubMed] [Google Scholar]