Abstract
All four adenosine receptor subtypes have been shown to play a role in cardioprotection, and there is evidence that all four subtypes may be expressed in cardiomyocytes. There is also increasing evidence that optimal adenosine cardioprotection requires the activation of more than one receptor subtype. The purpose of this study was to determine whether adenosine A2A and/or A2B receptors modulate adenosine A1 receptor-mediated cardioprotection. Isolated perfused hearts of wild-type (WT), A2A knockout (KO), and A2BKO mice, perfused at constant pressure and constant heart rate, underwent 30 min of global ischemia and 60 min of reperfusion. The adenosine A1 receptor agonist N6-cyclohexyladenosine (CHA; 200 nM) was administrated 10 min before ischemia and for the first 10 min of reperfusion. Treatment with CHA significantly improved postischemic left ventricular developed pressure (74 ± 4% vs. 44 ± 4% of preischemic left ventricular developed pressure at 60 min of reperfusion) and reduced infarct size (30 ± 2% with CHA vs. 52 ± 5% in control) in WT hearts, effects that were blocked by the A1 antagonist 8-cyclopentyl-1,3-dipropylxanthine (100 nM). Treatments with the A2A receptor agonist CGS-21680 (200 nM) and the A2B agonist BAY 60-6583 (200 nM) did not exert any beneficial effects. Deletion of adenosine A2A or A2B receptor subtypes did not alter ischemia-reperfusion injury, but CHA failed to exert a cardioprotective effect in hearts of mice from either KO group. These findings indicate that both adenosine A2A and A2B receptors are required for adenosine A1 receptor-mediated cardioprotection, implicating a role for interactions among receptor subtypes.
Keywords: A2a adenosine receptor, A2b adenosine receptor, knockout mice
the beneficial effects of adenosine in the ischemic-reperfused myocardium have been recognized for >25 yr. Adenosine can exert protection when given either before ischemia or at the onset of reperfusion. Substantial evidence now exists that these cardioprotective effects are achieved by the activation of extracellular adenosine receptors. All four adenosine receptor subtypes (A1, A2A, A2B, and A3) are expressed in the heart, and there is evidence that all four receptors may be expressed in ventricular myocytes (4, 15, 26, 42).
There is substantial evidence that the activation of all four adenosine receptors is cardioprotective, although there do appear to be differences in the specific roles of each receptor subtype. Activation of A1 and A3 receptors before ischemia is cardioprotective (2, 19, 20, 24, 30, 33, 37, 38, 41), whereas A2A and A2B receptors appear to exert their effects during reperfusion (10, 13, 17, 18, 28, 34, 42, 43). Adenosine A3 receptor activation during reperfusion has been reported to be cardioprotective (8), but there are conflicting reports on whether A1 receptor activation during reperfusion is beneficial (3, 7, 30, 39, 40). Likewise, all four adenosine receptors have been reported to activate protein kinases that appear to play a critical role in adenosine-mediated cardioprotection (9, 17, 33, 35). The similar cardioprotective and signaling effects of all four adenosine receptors raise the question of whether there is redundancy in adenosine receptor cardioprotection or interactions among receptor subtypes (27, 32).
There is increasing evidence that the expression and/or activation of more than one adenosine receptor subtype is needed for optimal protection from ischemia-reperfusion injury. We (19, 33) have previously reported that antagonism of adenosine A2A and/or A2B receptors with ZM-241385 blocked adenosine A1 receptor agonist-mediated infarct size reduction in intact rats. Urmaliya et al. (39) reported that A1 receptor-mediated protection of H9c2(2-1) cells during simulated ischemia was blocked by both A2A and A2B receptor antagonists. The authors concluded that endogenous adenosine activation of A2A and A2B receptors played a cooperative role in A1 receptor protection. These same authors subsequently reported that reperfusion A1 receptor-mediated infarct size reduction in mice also required the activation of A2A and A2B receptors, based on the use of receptor antagonists and adenosine A2A receptor knockout (KO) mice (40), although A1 receptor-mediated preservation of left ventricular (LV) end-diastolic pressure (LVEDP) was maintained in A2AKO hearts. There have been no reports to date providing direct evidence of A2B receptors modulating A1 receptor cardioprotection.
The purpose of this study was to determine whether the cardioprotective effects of adenosine A1 receptor activation before ischemia and at the onset of reperfusion are modulated by A2A and/or A2B receptors. Experiments were conducted in isolated perfused hearts of wild-type (WT), A2AKO, and A2BKO mice.
MATERIALS AND METHODS
Animals.
All animals in this study were maintained and used in accordance with guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996) and the Institutional Animal Care and Use Committee of Wayne State University. Experiments were conducted using male mice (10–15 wk of age) on a C57BL/6 background. WT mice were purchased from Jackson Laboratories (Bar Harbor, ME). A2AKO and A2BKO male mice were bred from homozygous A2AKO and A2BKO breeders, which were generous gifts from Dr. Joel Linden (La Jolla Institute for Allergy and Immunology, La Jolla, CA) and Dr. Stephen Tilley (University of North Carolina, Chapel Hill, NC), respectively. Genotypes were validated by PCR. The characterization of these two KO mouse models has been previously described in detail (5, 11).
Isolated heart preparation.
Hearts were excised from anesthetized (pentobarbital sodium, 60 mg/kg) and heparinized (500 units) mice, placed in ice-cold saline, and mounted on a perfusion apparatus in ≤90 s. The aorta was cannulated on a blunt 20-gauge needle, and perfusion was initiated at a constant pressure of 70 mmHg. Hearts were perfused with modified Krebs-Henseleit buffer (KHB), which contained (in mM) 116 NaCl, 11 glucose, 25 NaHCO3, 4.7 KCl, 1.2 MgCl2, 1.2 KH2PO4, 2.0 CaCl2, 0.05 EDTA, and 2 pyruvate. The fluid was bubbled with a mix of 95% O2 and 5% CO2 at 37°C to maintain a pH of 7.4 and was filtered through an in-line 0.45-μm filter.
A fluid-filled balloon was introduced into the LV through an incision in the atrial appendage to record ventricular function. The balloon was inflated to yield a LVEDP of 5–8 mmHg during the stabilization period. Temperature was maintained at 37°C with constant-temperature reservoirs and by partially submerging the heart into a water-jacketed chamber filled with KHB. Ventricular function (LV pressure, +dP/dt, and −dP/dt), heart rate, coronary flow (CF), and coronary perfusion pressure were continuously monitored and recorded throughout the experiments. All data were collected and analyzed using PowerLab data-acquisition systems and LabChart Software for Windows (AD Instruments, Colorado Springs, CO). Hearts were paced at 420 beats/min via pacing wires inserted into the right ventricle.
Experimental protocols.
All hearts underwent 30 min of global ischemia followed by 60 min of reperfusion.
Hearts were allowed 15–20 min of stabilization after instrumentation before the experimental protocols were initiated. Mice were divided into six primary groups: 1) WT control (n = 8), 2) WT + N6-cyclohexyladenosine (CHA; n = 8), 3) A2AKO control (n = 5), 4) A2AKO + CHA (n = 7), 5) A2BKO control (n = 5), and 6) A2BKO + CHA (n = 6).
Five additional groups (n = 4–5 mice/group) of WT mice were studied to confirm the A1 receptor cardioprotective effect of CHA and to determine the effects of A2A and A2B receptor activation and blockade. These groups were as follows: 1) WT + the adenosine A1 antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX; 100 nM) + CHA (200 nM), 2) WT + the A2A adenosine receptor-selective agonist CGS-21680 (200 nM), 3) WT + the A2B adenosine receptor-selective agonist BAY 60-6583 (200 nM); 4) CHA + 7-(2-phenylethyl)2-(2-furyl)pyrazolo[4,3e]1,2,4-triazolo[1,5c]pyrimidine (SCH-58261; 100 nM), and 5) CHA + N-(4-cyanophenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)phenoxy]-acetamide (MRS-1754; 100 nM).
The three adenosine receptor agonists were administered for 10 min before ischemia and for the first 10 min of reperfusion. Treatment with the A1 receptor antagonist DPCPX was initiated 5 min before CHA and then administered concurrently with the agonist. In the final two groups, SCH-58261 and MRS-1754 were administered for the first 10 min of reperfusion in the presence of CHA to determine whether A1 agonist protection requires A2A or A2B receptor activation during this period. The selectivity of these various agonists and antagonists for their respective receptors has been previously established (1, 12, 16, 21, 29, 44).
Drugs.
CHA, DPCPX, and CGS-21680 were obtained from Sigma-Aldrich (St. Louis, MO). SCH-58261 and MRS-1754 were obtained from Tocris-Cookson (Ellisville, MO). The A2B receptor agonist BAY 60-6583 was a kind gift from Dr. Thomas Krahn (Bayer Healthcare, Wuppertal, Germany). All of these agents were made as concentrated stock solutions (10 mM) in DMSO and then diluted in distilled H2O to 2 mM. The diluted solutions were added directly to the KHB immediately before use.
Determination of myocardial necrosis.
At the end of the reperfusion period, hearts were removed from the cannula, the aorta and atria were removed, and hearts were weighed. Hearts were sliced into five pieces parallel to the long axis and immersed in 1% triphenyltetrazolium chloride buffer (37°C) for 15 min and then in 10% neutral-buffered formalin for 10 min to enhance the contrast between triphenyltetrazolium chloride-negative and -positive staining. After removal of the right ventricle, heart sections were then photographed with a digital camera, and infarct size was quantified using SigmaScan Pro 4.0 software (Jandel Scientific, San Rafael, CA). Infarct size was expressed as a percentage of the total LV.
Statistical analysis.
Data are expressed as means ± SE. Baseline hemodynamic parameters among the groups were analyzed by one-way ANOVA. Differences between baseline and CHA treatments were analyzed by two-way ANOVA with repeated measures. Differences in postischemic function and infarct size among the experimental groups were analyzed by two-way ANOVA followed by a Bonferroni post hoc test. Differences were considered statistically significant at P < 0.05.
RESULTS
Baseline hemodynamics and the effects of the A1 agonist CHA before ischemia are shown in Table 1. There were no significant differences in any baseline parameter in WT, A2AKO, and A2BKO hearts. CHA significantly increased all four hemodynamic parameters [LV developed pressure (LVDP), +dP/dt, −dP/dt, and CF] in WT and A2BKO mice (P < 0.05). In A2AKO mice, A1 receptor agonist treatment increased −dP/dt (P < 0.05) but had no effect on LVDP, +dP/dt, or CF.
Table 1.
Baseline and preischemic hemodynamics in WT and KO hearts
| Left Ventricular Developed Pressure, mmHg | +dP/dt, mmHg/s | −dP/dt, mmHg/s | Coronary Flow, ml/min | |
|---|---|---|---|---|
| WT hearts | ||||
| Control | 106 ± 4 | 4,359 ± 380 | −2,708 ± 177 | 2.20 ± 0.08 |
| Pre-CHA | 107 ± 3 | 4,856 ± 151 | −2,822 ± 69 | 2.38 ± 0.08 |
| CHA | 113 ± 3* | 5,144 ± 102* | −3,141 ± 64* | 3.20 ± 0.15* |
| A2A receptor KO hearts | ||||
| Control | 100 ± 3 | 4,235 ± 223 | −2,528 ± 97 | 2.15 ± 0.06 |
| Pre-CHA | 98 ± 4 | 3,879 ± 209 | −2,310 ± 109 | 2.29 ± 0.14 |
| CHA | 101 ± 4 | 4,013 ± 190 | −2,446 ± 106* | 2.35 ± 0.15 |
| A2B receptor KO hearts | ||||
| Control | 102 ± 3 | 4,308 ± 230 | −2,637 ± 86 | 2.46 ± 0.07 |
| Pre-CHA | 107 ± 3 | 4,453 ± 250 | −2,700 ± 118 | 2.45 ± 0.06 |
| CHA | 111 ± 3* | 4,687 ± 281* | −2,918 ± 116* | 3.38 ± 0.08* |
Values are means ± SE. WT, wild type; KO, knockout; CHA, N6-cyclohexyladenosine. Data for control hearts in all three groups represent preischemic values. Baseline (pre-CHA) and 10-min CHA values are shown for all treatment groups.
P < 0.05 vs. the respective pre-CHA value.
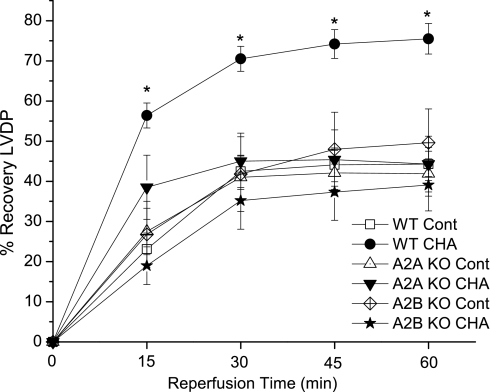
The postischemic recovery of LVDP is shown in Fig. 1. Treatment with the A1 agonist CHA significantly improved the recovery of LVDP in WT hearts at all reperfusion time points. At 30 and 60 min of reperfusion, hearts treated with CHA recovered 69 ± 3% and 74 ± 4%, respectively, of preischemic LVDP values compared with 43 ± 4% and 44 ± 4% recoveries in control WT hearts. Control A2AKO and A2BKO hearts recovered similar to WT control hearts. The cardioprotective effects of CHA were completely blocked in A2AKO and A2BKO hearts, as LVDP recovered 46 ± 7% in A2AKO mice and 39 ± 7% in A2BKO mice at 60 min of reperfusion.
Fig. 1.
Effects of the adenosine A1 receptor agonist N6-cyclohexyladenosine (CHA) on the recovery of left ventricular (LV) developed pressure (LVDP). CHA was administrated 10 min before ischemia and for the first 10 min of reperfusion. Recovery of LVDP is expressed as a percentage of the baseline value before the ischemic period in control (Cont) groups or before agonist administration in the CHA groups. WT, wild type; KO, knockout. Data are means ± SE. *P < 0.05 vs. the WT control group.
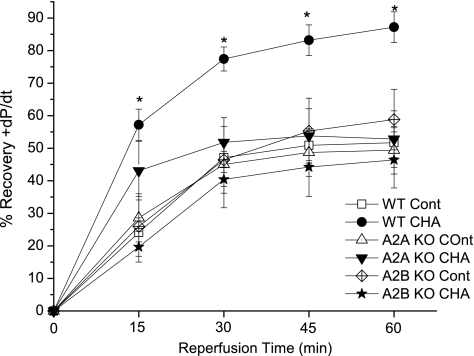
Figure 2 shows the effects of A1 agonist treatment on the recovery of +dP/dt. In WT hearts, CHA increased the postischemic recovery of +dP/dt at both 30 min (74 ± 4% vs. 47 ± 5%, P < 0.05) and 60 min (85 ± 5% vs. 52 ± 5%) of reperfusion. Deletion of adenosine A2A and A2B receptors did not alter the recovery of +dP/dt compared with WT hearts, but it prevented A1 agonist improvement in this contractile parameter. Similarly, the improvement in postischemic −dP/dt recovery with A1 agonist treatment was prevented in A2AKO and A2BKO hearts (data not shown).
Fig. 2.
Adenosine A1 receptor agonist effects on recovery of +dP/dt in WT, A2AKO, and A2BKO hearts. CHA was administrated 10 min before ischemia and during the initial 10 min of reperfusion. Recovery of +dP/dt is expressed as a percentage of the baseline value before the ischemic period in control groups or before drug administration in treatment groups. Data are means ± SE. *P < 0.05 vs. the WT control group.
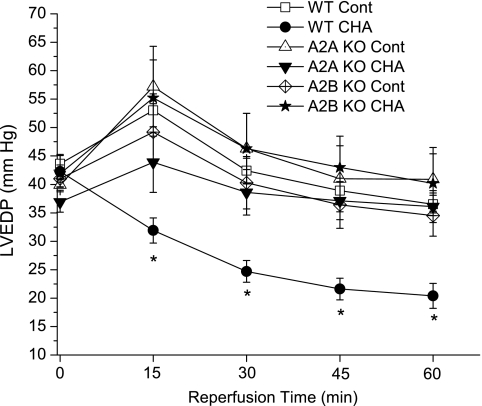
Reperfusion LVEDP results are shown in Fig. 3. There were no differences in end-ischemic diastolic pressures among the groups. CHA significantly decreased LVEDP in WT hearts throughout reperfusion; after 60 min of reperfusion, LVEDP values were reduced from 36 ± 2 mmHg in control hearts to 20 ± 2 mmHg in CHA-treated hearts. Deletion of A2A and A2B receptors alone exerted no effects on postischemic LVEDP, but, as with the aforementioned contractile parameters, these hearts exhibited no improvement in reperfusion LVEDP.
Fig. 3.
LV end-diastolic pressures (LVEDP) at the end of ischemia and during reperfusion. CHA was administrated 10 min before ischemia and during the initial 10 min of reperfusion. Data are means ± SE. *P < 0.05 vs. the WT control group.
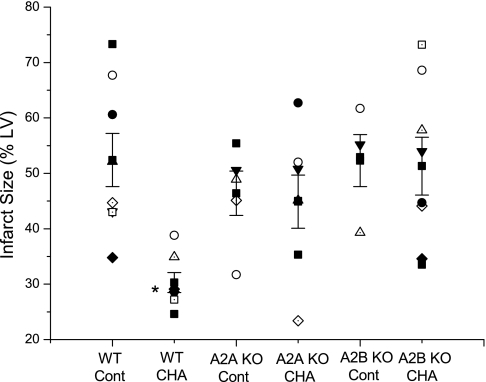
Myocardial infarct size results are shown in Fig. 4. The A1 agonist CHA significantly decreased infarct size in WT mice from 52 ± 5% to 30 ± 2%. Infarct sizes in control A2AKO (46 ± 4%) and A2BKO (52 ± 5%) hearts were similar to control WT hearts. Treatment with CHA exerted no beneficial effect on infarct size in either KO heart.
Fig. 4.
Effects of deletion of adenosine A2A and A2B receptors on A1 agonist-induced infarct size reduction. CHA was administrated 10 min before ischemia and during the initial 10 min of reperfusion. Infarct size is expressed as a percentage of the total area of the LV. Individual infarct sizes as well as means ± SE for each group are shown. *P < 0.05 vs. the WT control group.
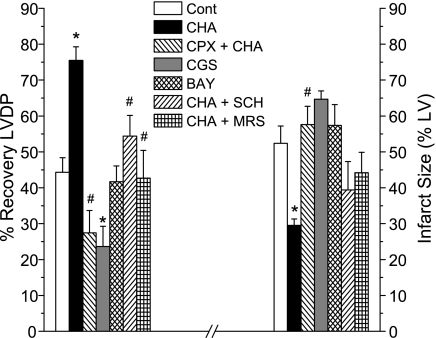
Additional experiments in WT hearts were conducted to verify that the effects of CHA were mediated via A1 receptor activation and to determine whether A2A and A2B receptor activation and blockade modulated ischemia-reperfusion injury. As shown in Fig. 5, hearts treated with the A1 receptor antagonist DPCPX + CHA exhibited 27 ± 6% recovery of LVDP after 60 min of reperfusion and an infarct size of 58 ± 5% (P < 0.05 vs. CHA). Hearts treated with the A2A agonist CGS-21680 and the A2B agonist BAY 60-6583 using the same concentration and treatment protocol as used with CHA did not show evidence of cardioprotection. These hearts recovered only 24 ± 6% and 42 ± 4% of preischemic LVDP and had infarct sizes of 65 ± 1% and 57 ± 6%, respectively. Although brief activation of A2A and A2B receptors was not protective, the results of additional experiments indicated that A2A (SCH-58261) and A2B (MR-S1754) receptor antagonists administered only during the reperfusion phase of CHA treatment significantly reduced the beneficial effects of this A1 agonist. The recovery of LVDP in hearts treated with SCH-58261 and MRS-1754 was comparable to that seen in control WT hearts and was statistically different (P < 0.05) than that observed in CHA-treated hearts. Similar findings in all of these groups on reperfusion LVEDP and +dP/dt were also observed (data not shown). Antagonism of A1 receptors with DPCPX throughout the CHA treatment protocol blocked the A1 infarct-reducing effect, and neither CGS-21680 nor BAY 60-6583 mimicked the beneficial effects of CHA. Hearts coinfused with SCH-58261 and MRS-1754 during reperfusion CHA treatment exhibited infarct sizes similar to control hearts, although these values were not statistically different from CHA infarct sizes.
Fig. 5.
Effects of adenosine agonists and antagonists in WT hearts. To confirm the A1 effects of CHA, the A1 antagonist 8-cyclopentyl-1,3-dipropylxanthine (CPX) was administered 5 min before and then concurrently with CHA. A2A and A2B agonists [CGS-21680 (CGS) and BAY 60-6583 (BAY), respectively] were administered at the same concentration and for the same duration as CHA. To determine the role of A2A and A2B receptors during reperfusion, antagonists [SCH-58261 (SCH) and MRS-1754 (MRS), respectively] were administered to CHA-treated hearts at the onset of reperfusion simultaneously with continued CHA treatment. The left axis shows 60-min reperfusion recovery of LVDP; the right axis indicates infarct size. Data are means ± SE. *P < 0.05 vs. the WT control group; #P < 0.05 vs. CHA.
DISCUSSION
The results of this study indicate that adenosine A1 receptor-induced cardioprotection in isolated perfused mouse hearts requires the expression and/or activation of additional adenosine receptor subtypes. Although neither deletion of A2A or A2B receptors nor brief activation of these receptors altered ischemia-reperfusion injury, KO of either receptor blocked the beneficial effects of adenosine A1 receptor stimulation on postischemic function and infarct size. The results of additional experiments in WT hearts indicated that pharmacological blockade of A2A or A2B receptors only during the reperfusion phase of CHA treatment completely blocked A1 agonist-induced functional protection and reduced the extent of A1 receptor-mediated infarct reduction. These observations indicate that maximal adenosine A1 receptor cardioprotection requires the input of more than one receptor subtype and support the hypothesis of interactions among adenosine receptor subtypes.
All four adenosine receptor subtypes are expressed in mammalian myocardium, and there is evidence that all four subtypes may be expressed in cardiac myocytes. In addition, all four adenosine receptors have been reported to reduce myocardial ischemia-reperfusion injury in multiple species (10, 27, 32). This raises the question of whether adenosine cardioprotection is modulated by interactions among adenosine receptor subtypes. Interactions among adenosine receptor subtypes have been reported in transfected cells and in the brain (14, 22, 23), and there is increasing evidence for adenosine receptor interactions in the heart (4, 19, 31, 40, 42). This is the first study to document that adenosine A1 receptor cardioprotection requires the activation of both A2A and A2B receptor subtypes.
Previous studies (19, 33, 39, 40) have provided evidence that adenosine A2 receptor subtypes modulate adenosine A1 receptor cardioprotection. We (19, 33) have reported that the adenosine receptor antagonist ZM-241385, which can block both A2A and A2B receptors, inhibited in vivo A1 receptor cardioprotection in rats. Urmaliya et al. (40) recently reported that the same antagonist blocked reperfusion A1 agonist cardioprotection in the isolated mouse heart. However, ZM-241385, which was developed as a potent and selective adenosine A2A receptor antagonist, has been shown to also have affinities of ≤100 nM for adenosine A2B receptors in multiple species, including the mouse (1). This indicates that alterations in adenosine receptor effects by ZM-241835 may be due to either A2A or A2B receptors.
The results of the present study indicate that neither deletion of A2A receptors nor administration of an A2A receptor agonist altered myocardial ischemia-reperfusion injury. However, A2A receptor deletion did block A1 receptor-induced cardioprotection. Our observations are consistent with the recent findings of Urmaliya et al. (40), who also reported that deletion of the A2A receptor blocked A1 cardioprotection in the isolated mouse heart. However, we administered CHA for 10 min before ischemia and 10 min at the onset of reperfusion, resulting in modulation of both ischemia and reperfusion injury, whereas in the previous report (40) A1 receptor stimulation was limited to only the first 15 min of reperfusion, thus modulating only reperfusion injury. We did not limit A1 agonist treatment to just the reperfusion period since there are reports (3, 37) showing that reperfusion A1 receptor stimulation does not exert beneficial effects. In contrast to Urmaliya et al. (40), we observed no preservation of reperfusion LVEDP in A2AKO hearts treated with CHA, and infarct sizes in our control hearts were ∼50%, in contrast to control infarcts in their study of only ∼30%. These differences could be due to the differences in the strains of mice, as we used C57Bl/6 mice and Urmaliya et al. (40) used CD-1 mice.
Much less is known about the role of A2B receptors in myocardial ischemia-reperfusion injury, although there are reports (17, 28, 42) showing that A2B receptor stimulation during reperfusion is cardioprotective. As stated above, our previous reports (19, 33) demonstrating that ZM-241385 blocked A1 cardioprotection could have been the result of antagonism of A2B receptors due to the high affinity of this antagonist for A2B receptors (1). Urmaliya et al. (40) recently reported that the beneficial effects of reperfusion treatment with an A1 agonist in isolated perfused mouse hearts was blocked by the A2B antagonist MRS-1754. Our present observations support these observations, as all of the beneficial effects of CHA were blocked in A2BKO hearts, although deletion of A2B receptors had no effect alone on ischemia-reperfusion injury, nor did brief treatment with the A2B agonist BAY 60-6583. Previous studies in A2BKO mice have yielded conflicting results, as Eckle et al. (6) reported that ischemic preconditioning in in vivo murine myocardium was blocked in A2B KO mice, but Maas et al. (25) observed no loss of ischemic preconditioning protection in A2BKO isolated mouse hearts. The present study is the first to report that A2B receptors are necessary for A1 cardioprotection.
Our observations that brief treatments with CGS-21680 and BAY 60-6583 exerted no beneficial effects indicate that CHA was not merely nonselectively activating A2A or A2B receptors. These findings may initially appear to contradict the numerous reports (10, 13, 17, 18, 28, 30, 34, 42, 43) showing that reperfusion infusions of A2A and A2B receptor agonists are cardioprotective. However, beneficial effects of A2A and A2B receptor agonists have been observed after prolonged (≥60 min) agonist infusions. In addition, the beneficial effects of reperfusion A2A agonists have been demonstrated primarily in in vivo preparations (13, 18, 28, 30, 43), with few such observations in isolated perfused hearts (34). There are two previous reports (6, 25) showing that treatment with the A2B receptor agonist BAY 60-6583 before ischemia reduces myocardial infarct size in intact mice and rats. However, in these studies, BAY 60-6583 was administered ≥30 min before myocardial ischemia. In the present study, reperfusion treatments with CGS-21680 and BAY 60-6583 were limited to only the first 10 min of reperfusion (in addition to the 10-min preischemic treatment) to directly compare the effects of A2A and A2B receptor activation with the same dose and timing that we used with the A1 agonist CHA.
Another interesting aspect of the present findings is that although A1 agonist treatment included both preischemic and early reperfusion components, including the A2A (SCH-58261) or A2B (MRS-1754) receptor antagonists only during the reperfusion phase significantly blunted A1 receptor agonist cardioprotection in WT hearts. Functional protection with CHA was completely lost with these two antagonists, whereas infarct size was similar to control WT hearts but not statistically different from that observed with CHA. These observations indicate that A2A and A2B receptor stimulation during reperfusion play a key role in A1 agonist-induced cardioprotection. We were surprised by this observation since we included 10 min of preischemic CHA treatment and hearts were exposed to this agonist throughout the 30-min ischemic period. Urmaliya et al. (40) arrived at similar conclusions when A1 agonist treatment was limited to only the first 15 min of reperfusion.
Our present findings using both genetic and pharmacological blockade of A2A and A2B receptors indicate that these two receptors are both necessary for optimal A1 receptor-mediated cardioprotection. These observations suggest that there may be functional and/or physical interactions among these receptor subtypes. Stimulation of A1, A2A, and A2B receptors in several tissues, including the heart, all activate similar protein kinase signaling pathways, which have been implicated in adenosine receptor cardioprotection (17, 27, 32, 33, 35). These results suggest that receptor cross-talk may be necessary to provide optimal subcellular signaling to induce adenosine A1 receptor-mediated cardioprotection.
There is increasing evidence for interactions among adenosine receptors in several tissues, including the heart. Lopes et al. (23) first reported evidence of A1 and A2A receptor functional interactions in rat hippocampal and cortical synaptosomes based on the observations that A2A receptor stimulation suppressed A1 receptor agonist binding. It has been reported that adenosine A2B receptor expression is increased in A2AKO mice (4, 5, 36). Norton et al. (31) concluded that the A2A receptor blunted the A1 receptor antiadrenergic effect in rat hearts, and we (4) have recently observed potentiation of the A1 antiadrenergic effect in A2AKO, but not A2BKO, mice. In that same study (4), BAY 60-6583, but not CGS-21680, produced dose-dependent increases in contractility. In the present study, we observed that preischemic infusion of CHA exerted a small, but statistically significant, increase in contractility in WT and A2BKO hearts but not in A2AKO hearts. The above observations suggest that A2A and A2B receptors differentially modulate A1 receptor effects on contractility but tht both receptors play an important role in A1 cardioprotection.
In summary, our present findings support the hypothesis of cross-talk between adenosine A1 and A2 receptor subtypes in the mouse myocardium. Both A2A and A2B receptors are necessary for A1 receptor-mediated myocardial protection, and these effects appear to manifest themselves primarily during reperfusion.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-066132 (to R. D. Lasley).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Dr. Joel Linden (La Jolla Institute for Allergy and Immunology, La Jolla, CA) and Dr. Stephen Tilley (University of North Carolina, Chapel Hill, NC) for the generous gifts of adenosine A2A and A2B receptor knockout breeders. The authors also acknowledge Dr. Thomas Krahn (Bayer Healthcare, Wuppertal, Germany) for providing BAY 60-6583.
REFERENCES
- 1. Auchampach JA, Kreckler LM, Wan TC, Maas JE, van der Hoeven D, Gizewski E, Narayanan J, Maas GE. Characterization of the A2B adenosine receptor from mouse, rabbit, and dog. J Pharmacol Exp Ther 329: 2–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Auchampach JA, Rizvi A, Qiu Y, Tang XL, Maldonado C, Teschner S, Bolli R. Selective activation of A3 adenosine receptors with N6-(3-iodobenzyl)adenosine-5′-N-methyluronamide protects against myocardial stunning and infarction without hemodynamic changes in conscious rabbits. Circ Res 80: 800–809, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Baxter GF, Hale SL, Miki T, Kloner RA, Cohen MV, Downey JM, Yellon DM. Adenosine A1 agonist at reperfusion trial (AART): results of a three-center, blinded, randomized, controlled experimental infarct study. Cardiovasc Drugs Ther 14: 607–614, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Chandrasekera PC, McIntosh VJ, Cao FX, Lasley RD. Differential effects of adenosine A2a and A2b receptors on cardiac contractility. Am J Physiol Heart Circ Physiol 299: H2082–H2089, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA. A2A adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci 19: 9192–9200, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation 115: 1581–1590, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Finegan BA, Lopaschuk GD, Gandhi M, Clanachan AS. Inhibition of glycolysis and enhanced mechanical function of working rat hearts as a result of adenosine A1 receptor stimulation during reperfusion following ischaemia. Br J Pharmacol 118: 355–363, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ge ZD, van der Hoeven D, Maas JE, Wan TC, Auchampach JA. A3 adenosine receptor activation during reperfusion reduces infarct size through actions on bone marrow-derived cells. J Mol Cell Cardiol 49: 280–286, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Germack R, Griffin M, Dickenson JM. Activation of protein kinase B by adenosine A1 and A3 receptors in newborn rat cardiomyocytes. J Mol Cell Cardiol 37: 989–999, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Headrick JP, Lasley RD. Adenosine receptors and reperfusion injury of the heart. Handb Exp Pharmacol: 189–214, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hua X, Kovarova M, Chason KD, Nguyen M, Koller BH, Tilley SL. Enhanced mast cell activation in mice deficient in the A2b adenosine receptor. J Exp Med 204: 117–128, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ji X, Kim YC, Ahern DG, Linden J, Jacobson KA. [3H]MRS 1754, a selective antagonist radioligand for A2B adenosine receptors. Biochem Pharmacol 61: 657–663, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jordan JE, Zhao ZQ, Sato H, Taft S, Vinten-Johansen J. Adenosine A2 receptor activation attenuates reperfusion injury by inhibiting neutrophil accumulation, superoxide generation and coronary endothelial adherence. J Pharmacol Exp Ther 280: 301–309, 1997 [PubMed] [Google Scholar]
- 14. Karcz-Kubicha M, Quarta D, Hope BT, Antoniou K, Muller CE, Morales M, Schindler CW, Goldberg SR, Ferre S. Enabling role of adenosine A1 receptors in adenosine A2A receptor-mediated striatal expression of c-fos. Eur J Neurosci 18: 296–302, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Kilpatrick EL, Narayan P, Mentzer RM, Jr, Lasley RD. Cardiac myocyte adenosine A2a receptor activation fails to alter cAMP or contractility: role of receptor localization. Am J Physiol Heart Circ Physiol 282: H1035–H1040, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Klotz KN, Hessling J, Hegler J, Owman C, Kull B, Fredholm BB, Lohse MJ. Comparative pharmacology of human adenosine receptor subtypes–characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol 357: 1–9, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Kuno A, Solenkova NV, Solodushko V, Dost T, Liu Y, Yang XM, Cohen MV, Downey JM. Infarct limitation by a protein kinase G activator at reperfusion in rabbit hearts is dependent on sensitizing the heart to A2b agonists by protein kinase C. Am J Physiol Heart Circ Physiol 295: H1288–H1295, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lasley RD, Jahania MS, Mentzer RM., Jr Beneficial effects of adenosine A2a agonist CGS-21680 in infarcted and stunned porcine myocardium. Am J Physiol Heart Circ Physiol 280: H1660–H1666, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Lasley RD, Kristo G, Keith BJ, Mentzer RM., Jr The A2a/A2b receptor antagonist ZM-241385 blocks the cardioprotective effect of adenosine agonist pretreatment in in vivo rat myocardium. Am J Physiol Heart Circ Physiol 292: H426–H431, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Lasley RD, Mentzer RM., Jr Adenosine improves recovery of postischemic myocardial function via an adenosine A1 receptor mechanism. Am J Physiol Heart Circ Physiol 263: H1460–H1465, 1992 [DOI] [PubMed] [Google Scholar]
- 21. Lohse MJ, Klotz KN, Lindenborn-Fotinos J, Reddington M, Schwabe U, Olsson RA. 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX)–a selective high affinity antagonist radioligand for A1 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol 336: 204–210, 1987 [DOI] [PubMed] [Google Scholar]
- 22. Lopes LV, Cunha RA, Kull B, Fredholm BB, Ribeiro JA. Adenosine A2A receptor facilitation of hippocampal synaptic transmission is dependent on tonic A1 receptor inhibition. Neuroscience 112: 319–329, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Lopes LV, Cunha RA, Ribeiro JA. Cross talk between A1 and A2A adenosine receptors in the hippocampus and cortex of young adult and old rats. J Neurophysiol 82: 3196–3203, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Louttit JB, Hunt AA, Maxwell MP, Drew GM. The time course of cardioprotection induced by GR79236, a selective adenosine A1-receptor agonist, in myocardial ischaemia-reperfusion injury in the pig. J Cardiovasc Pharmacol 33: 285–291, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Maas JE, Wan TC, Figler RA, Gross GJ, Auchampach JA. Evidence that the acute phase of ischemic preconditioning does not require signaling by the A2B adenosine receptor. J Mol Cell Cardiol 49: 886–893, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martens D, Lohse MJ, Schwabe U. [3H]-8-cyclopentyl-1,3-dipropylxanthine binding to A1 adenosine receptors of intact rat ventricular myocytes. Circ Res 63: 613–620, 1988 [DOI] [PubMed] [Google Scholar]
- 27. McIntosh VJ, Lasley RD. Adenosine receptor-mediated cardioprotection: are all 4 subtypes required or redundant? J Cardiovasc Pharmacol Ther; http://cpt.sagepub.com/content/early/2011/02/18/1074248410396877.long [DOI] [PubMed]
- 28. Methner C, Schmidt K, Cohen MV, Downey JM, Krieg T. Both A2a and A2b adenosine receptors at reperfusion are necessary to reduce infarct size in mouse hearts. Am J Physiol Heart Circ Physiol 299: H1262–H1264, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moos WH, Szotek DS, Bruns RF. N6-cycloalkyladenosines. Potent, A1-selective adenosine agonists. J Med Chem 28: 1383–1384, 1985 [DOI] [PubMed] [Google Scholar]
- 30. Norton ED, Jackson EK, Turner MB, Virmani R, Forman MB. The effects of intravenous infusions of selective adenosine A1-receptor and A2-receptor agonists on myocardial reperfusion injury. Am Heart J 123: 332–338, 1992 [DOI] [PubMed] [Google Scholar]
- 31. Norton GR, Woodiwiss AJ, McGinn RJ, Lorbar M, Chung ES, Honeyman TW, Fenton RA, Dobson JG, Jr, Meyer TE. Adenosine A1 receptor-mediated antiadrenergic effects are modulated by A2a receptor activation in rat heart. Am J Physiol Heart Circ Physiol 276: H341–H349, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Peart JN, Headrick JP. Adenosinergic cardioprotection: multiple receptors, multiple pathways. Pharmacol Ther 114: 208–221, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Reid EA, Kristo G, Yoshimura Y, Ballard-Croft C, Keith BJ, Mentzer RM, Jr, Lasley RD. In vivo adenosine receptor preconditioning reduces myocardial infarct size via subcellular ERK signaling. Am J Physiol Heart Circ Physiol 288: H2253–H2259, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Rork TH, Wallace KL, Kennedy DP, Marshall MA, Lankford AR, Linden J. Adenosine A2A receptor activation reduces infarct size in the isolated, perfused mouse heart by inhibiting resident cardiac mast cell degranulation. Am J Physiol Heart Circ Physiol 295: H1825–H1833, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schulte G, Fredholm BB. Human adenosine A1, A2A, A2B, and A3 receptors expressed in Chinese hamster ovary cells all mediate the phosphorylation of extracellular-regulated kinase 1/2. Mol Pharmacol 58: 477–482, 2000 [PubMed] [Google Scholar]
- 36. Teng B, Ledent C, Mustafa SJ. Up-regulation of A2B adenosine receptor in A2A adenosine receptor knockout mouse coronary artery. J Mol Cell Cardiol 44: 905–914, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thornton JD, Liu GS, Olsson RA, Downey JM. Intravenous pretreatment with A1-selective adenosine analogues protects the heart against infarction. Circulation 85: 659–665, 1992 [DOI] [PubMed] [Google Scholar]
- 38. Tracey WR, Magee W, Masamune H, Kennedy SP, Knight DR, Buchholz RA, Hill RJ. Selective adenosine A3 receptor stimulation reduces ischemic myocardial injury in the rabbit heart. Cardiovasc Res 33: 410–415, 1997 [DOI] [PubMed] [Google Scholar]
- 39. Urmaliya VB, Church JE, Coupar IM, Rose'Meyer RB, Pouton CW, White PJ. Cardioprotection induced by adenosine A1 receptor agonists in a cardiac cell ischemia model involves cooperative activation of adenosine A2A and A2B receptors by endogenous adenosine. J Cardiovasc Pharmacol 53: 424–433, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Urmaliya VB, Pouton CW, Ledent C, Short JL, White PJ. Cooperative cardioprotection through adenosine A1 and A2A receptor agonism in ischemia-reperfused isolated mouse heart. J Cardiovasc Pharmacol 56: 379–388, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Wan TC, Ge ZD, Tampo A, Mio Y, Bienengraeber MW, Tracey WR, Gross GJ, Kwok WM, Auchampach JA. The A3 adenosine receptor agonist CP-532,903 [N6-(2,5-dichlorobenzyl)-3′-aminoadenosine-5′-N-methylcarboxamide] protects against myocardial ischemia/reperfusion injury via the sarcolemmal ATP-sensitive potassium channel. J Pharmacol Exp Ther 324: 234–243, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xi J, McIntosh R, Shen X, Lee S, Chanoit G, Criswell H, Zvara DA, Xu Z. Adenosine A2A and A2B receptors work in concert to induce a strong protection against reperfusion injury in rat hearts. J Mol Cell Cardiol 47: 684–690, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang Z, Day YJ, Toufektsian MC, Xu Y, Ramos SI, Marshall MA, French BA, Linden J. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation 114: 2056–2064, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Zocchi C, Ongini E, Ferrara S, Baraldi PG, Dionisotti S. Binding of the radioligand [3H]-SCH 58261, a new non-xanthine A2A adenosine receptor antagonist, to rat striatal membranes. Br J Pharmacol 117: 1381–1386, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]