Abstract
The involvement of reactive oxygen species (ROS) in regulating vascular function both in normal vessels and as part of an adaptive response during disease has been intensively studied. From the recognition that ROS serve as important signaling molecules has emerged multiple lines of evidence that there is a functional connectivity between intracellular sites of ROS production. This cross talk has been termed ROS-induced ROS release (RIRR) and is supported by a variety of observations showing that RIRR is a common mechanism for ROS amplification and regional ROS generation. The compartmentalization of ROS production within a cell is critical to its signaling function and is facilitated by microlocalization of specific scavengers. This review will provide descriptions and examples of important mechanisms of RIRR.
Keywords: reactive oxygen species, mitochondria, endothelial dysfunction
aerobic organisms continuously produce reactive oxygen species (ROS), redox active molecules with unpaired electrons derived from molecular oxygen. Cellular levels of ROS are determined by the steady-state balance between rates of formation and decomposition by endogenous enzymatic antioxidant systems and by small molecules that function as antioxidants (Table 1).
Table 1.
Intracellular sources of ROS and cellular defenses modulating oxidative stress
| Possible Defenses from Oxidative Stress |
|||
|---|---|---|---|
| Increased Activity of Cellular Enzymatic Sources of ROS | Extracellular or Noncellular Sources of Oxidative Stress | Enzymatic antioxidants | Small molecules |
| NADPH oxidase | Cigarette smoke | Cytosolic superoxide dismutase, containing copper and zinc (Cu,ZnSOD) and Manganese (Mn2+ dependent) SOD, catalase, glutathione system (glutathione, glutathione peroxidase, and glutathione reductase), peroxiredoxins, thioredoxin reductase, heme oxygenase, and biliverdin reductase | Salicylic acid; carotenoids; vitamins C, E, and A; α-tocopherol; ascorbic acid; uric acid; thioredoxins; coenzyme Q; melatonin; flavonoids; and polyphenols |
| Mitochondrial electron transport | Radiation (UV, gamma) | ||
| Nitric oxide synthase | Neutrophil activation | ||
| Xanthine oxidase | Ischemia-reperfusion | ||
| Arachidonic acid metabolism (lipoxygenase and cyclooxygenase) | Hyperoxia | ||
| Electrical current | |||
| Elevated glucose levels, lipid peroxidation, | |||
| Metal-catalyzed oxidation | |||
| Catecholamine autoxidation | |||
| Neuromelanin | |||
ROS, reactive oxygen species.
Cellular Enzymatic Sources of ROS and Stimuli for ROS Production
ROS derived from the reduction of molecular oxygen include superoxide (O2·−), hydrogen peroxide (H2O2), hydroxyl radical (OH·−), and peroxynitrite (ONOO−). O2·− is converted to H2O2 spontaneously or more than a thousand times faster by an enzymatic process involving one of three isoforms of superoxide dismutase (SOD) (15). H2O2 can undergo one electron reduction to the highly evanescent and reactive OH·− in the presence of reducing metal ions or, through two-electron reduction, can be converted to water by peroxisomal catalase. Intracellular ROS originate from multiple sites, including the mitochondrial electron transport chain, cytochrome P-450 oxygenase, xanthine oxidase (XO), lipoxygenase, cyclooxygenase, and uncoupled nitric oxide (NO) synthase (NOS) (7). NADPH oxidase, a prominent source of ROS in vascular tissue, has several isoforms localized to different sites within the cell. NADPH oxidase that contains the NOX2 catalytic subunit can be plasmalemmal bound and produce O2·− extracellularly or within the cytosol (1, 12, 14). The extracellularly generated O2·− can reenter the cell through anion-selective chloride channel-3 channels (16) or by conversion to H2O2 via extracellular SOD. The NOX4 containing oxidase is located in endosomes (28, 39), focal adhesions, and nuclei (19) and generates O2·− intracellularly. Other members of the NOX family include NOX1, which can be found in various subcellular localizations such as nuclei (5) and caveolae (19), NOX3 and NOX5, which both have been shown to colocalize with the plasma membrane (42, 50). Thus subcellular localization of NADPH oxidase allows for stereospecific release of O2·−, which is spontaneously or catalytically (SOD) converted to H2O2, the primary signaling ROS. As an uncharged molecule, H2O2 can traverse cell membranes, is rapidly inactivated by endogenous catalase and peroxiredoxins, and can reversibly alter enzyme function through oxidative modification of susceptible residues, including arginine, cysteine, histidine, and others (24, 52). These properties strongly support a signaling role for intermediate doses of H2O2. Signaling dose ranges for H2O2 were established in human and animal models and vary from 1 μM to 10 mM (21, 32, 55). Interestingly, in rat coronary arterioles, sensitivity to H2O2 is increased with aging (21).
It has recently been demonstrated that these diverse anatomic and chemical ROS-generating systems interact to facilitate an integrated redox modulation of vascular tissue. These interactions can result in enhanced elimination or amplification of the cellular ROS signal. The latter response, termed ROS-induced ROS release (RIRR) (69), is the subject of this review.
Concentration-Dependent Effects of ROS
ROS are implicated in the etiology of aging, angiogenesis, apoptosis, and a myriad of diseases including atherosclerosis, hypertension, hypercholesterolemia, obesity, cancer, diabetes mellitus, and neurodegenerative disorders [see reviews (6, 41, 53)]. Most pathology is the result of excessive ROS formation that promotes inflammation in surrounding tissues and accelerates cell death or senescence. In these cases, levels of ROS can reach high concentrations, often exceeding 500 μM for H2O2 in sites of inflammation or injury (17). O2·− concentrations are tightly controlled by the cytosolic Cu,Zn-SOD, which can rapidly lower O2·− levels from the nanomolar to picomolar concentration range (62). Interestingly, excessively low levels of ROS can also invoke pathological changes by interrupting the physiological role of oxidants in proliferation, vasodilation, and host defense and may promote carcinogenesis (33, 60). The presence of a “physiological window” of ROS concentrations could explain some of the negative results from clinical trials where large doses of exogenously administered antioxidants failed to improve cardiovascular outcomes [see review (20)]. There appears to be a physiological range of concentrations where intermediate levels of ROS can function as critical signaling molecules and mediate cellular growth, protein phosphorylation, and cell migration (47, 59). This dose dependency should be considered when addressing the pathophysiological relevance of ROS.
Decomposition of ROS: Role of Antioxidants
The cellular response to oxidative stress involves the elimination of, protection against, and repair of damage caused by ROS. Scavenger antioxidant enzymes including SOD, peroxiredoxin, and catalase are responsible for the direct elimination of ROS, whereas systems that reconstitute antioxidants [e.g., glutathione (GSH) and GSH peroxidase (GSH-Px)] can indirectly reduce ROS. The protective role of vitamins C and E in modulating ROS levels in athletes following maximal exercise is an example of such an indirect effect. These vitamins may upregulate GSH-Px levels, thereby ameliorating exercise-induced oxidative damage (43).
Protection from oxidative damage can be achieved by a variety of mechanisms. The driving force for oxidant generation can be reduced. Production of ROS has been shown to correlate positively with changes in mitochondrial membrane potential, such that even a small increase in membrane potential results in a rapid elevation of O2·− levels (23). Therefore, mild mitochondrial depolarization can result in more efficient electron transfer and less uncoupling, thus less diversion to oxygen to form O2·− (23). However, this effect is not universal, since mild depolarization has also been shown to have the opposite effect (45). Molecules sensitive to oxidative stress can be surrounded by decoys that are preferentially oxidized, thereby protecting key cellular molecules (e.g., GSH, α-tocopherol, bilirubin, ascorbate, urate, albumin plasmogens, and cytosolic amino acids) (15). Direct physical quenching of O2·− or other free radicals (e.g., by carotenoids) is another biologically deployed mechanism (15). Each of these processes contributes to the local and global cellular redox balance, which has implications for cell signaling, cell growth, and cell death.
Functional Effect of ROS and RIRR
Recent investigations indicate that ROS participate in regulating vascular function both in normal vessels (36) and as part of an adaptive response during disease (57, 66). The concentration and localization of oxidant-generating and -quenching enzymes indicate high stereospecificity and compartmentalization within the cell. Given the ubiquitous nature of ROS and their involvement in signaling transduction and cell injury, their accumulation must be tightly controlled both spatially and temporally by systems capable of regulating regional ROS production. RIRR is an example of such a control, by allowing ROS formed in one region to activate specific sites in another region of the cell. While not comprehensive, the sections that follow highlight several distinct mechanisms of RIRR that have been described in a variety of cellular systems. They are grouped based on whether they represent direct or complex forms of RIRR.
Direct RIRR
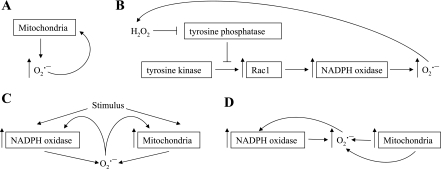
Mitochondria are important sources of ROS production and a common target for the damaging effect of oxidative stress. Using mitochondria from freshly isolated adult rat cardiac myocytes, Zorov et al. (69) demonstrated that inhibition of complex III with antimycin at the inward electron transfer site diverted electrons toward the inner-membrane space leading to O2·− formation (Fig. 1A). In single mitochondria ROS production (H2O2 detected by dichlorofluorescein fluorescence) occurred in two distinct phases: the initial or “trigger phase,” followed by a delayed amplified release of ROS, which could be attenuated with the complex I inhibitor, rotenone (70).
Fig. 1.
A–D: sequence of events illustrating direct reactive oxygen species (ROS)-induced ROS release (RIRR). O2·−, superoxide; H2O2, hydrogen peroxide.
Another example of feed-forward RIRR occurs through ROS amplification in smooth muscle NADPH oxidase. Exogenous exposure of vascular cells to H2O2 stimulates NADPH oxidase, resulting in O2·− production, which is converted to H2O2. The produced H2O2 can further stimulate O2·− production from NADPH oxidase, therefore initiating a self-promoting cycle (29). This form of RIRR signaling has been implicated in apoptosis of nonphagocytic cells (27). The mechanism responsible for this amplification of ROS might include the inhibition of tyrosine phosphatases by H2O2, allowing Rac1 assembly with NADPH oxidase subunits into an active complex. These two examples describe a spatially localized but temporally sustained form of RIRR (Fig. 1B).
Redox communication between mitochondria and NADPH oxidase is one of the best-studied forms of RIRR and is involved in a variety of physiological signaling pathways.
Mitochondria initiate ROS production by NADPH oxidase.
Lee et al. (26) demonstrated that complete serum withdrawal in human 293T cells rapidly elevates mitochondrial ROS production. ROS generated by mitochondria stimulated phosphoinositide 3-kinase followed by translocation of Rac1, allowing its interaction with NOX1. The dynamics of ROS generation in this model is such that initial ROS production from mitochondria is relatively transient, but the evoked activation of NOX1 is more prolonged (26). The importance of this study is twofold: 1) it demonstrates how a transient ROS signal (from mitochondria) can be converted into a more sustained ROS release (from NADPH oxidase), and 2) it also describes a pathway by which ROS generated at one subcellular site triggers ROS production in a different site through signal transduction (Fig. 1C).
NADPH oxidase triggers ROS production from mitochondria.
In converse fashion, ROS generation from mitochondria can be triggered from ROS produced by NADPH oxidase. Hawkins et al. (16) demonstrated in pulmonary microvascular endothelial cells that the activation of NADPH oxidase produces extracellular O2·−. The O2·− produced can then be transported intracellularly through a chloride channel-3, where it triggers rapid Ca2+ mobilization followed by mitochondrial O2·− production. These findings not only demonstrate a link between two enzymatic sources of ROS but also establish a role for O2·− as a signaling molecule involved in RIRR where extracellular ROS stimulate intracellular ROS production, resulting in endothelial dysfunction and cellular apoptosis (Fig. 1C) (16).
Interactions between NADPH oxidase and mitochondria represent one of the more commonly observed examples of RIRR (8, 22, 61). Studying the cardioprotective effects of angiotensin II (ANG II), Kimura et al. identified a novel signaling pathway by which ANG-II stimulation of ANG-I receptors activates NADPH oxidase in the heart by a protein kinase C-mediated mechanism (22, 31). Although the direct contribution of ROS produced by NADPH oxidase via this mechanism does not induce preconditioning, the NADPH oxidase-derived ROS are essential for the activation of mitochondrial ATP-sensitive K+ (KATP) channels, which elicit preconditioning through an increase in mitochondrial ROS release (22). The findings of this study reveal how ROS generated in one region of the cell (membrane) orchestrate ROS production in a separate compartment (mitochondria) to effect a physiological response (22).
As mentioned, O2·− from mitochondria can activate mitochondrial KATP channels to produce a substantial (over 50%) reduction in the mitochondrial membrane potential, which leads to excess ROS generation. It can also combine with NO to generate ONOO−. Both O2·− and ONOO− oxidize electron transport chain components, uncoupling respiration and further augmenting ROS generation (8). This mitochondrial ROS production activates NADPH oxidase, creating a positive feedback loop that substantially increases ROS and reduces NO bioavailability, thereby contributing to vascular pathophysiology (8). Thus a reciprocal activation of RIRR between mitochondria and NADPH oxidase can produce a feed-forward acceleration of vascular ROS generation (Fig. 1D).
Nitrate tolerance may also occur through RIRR between mitochondria and NADPH oxidase as suggested by Wenzel et al. (61). Chronic nitrate therapy results in mitochondrial oxidative stress, perhaps by inhibiting mitochondrial SOD activity (MnSOD). Mitochondria to NADPH oxidase redox signaling may increase cytosolic O2·−, thereby quenching NO. This concept is supported by the observation that nitrate tolerance can be attenuated either by blocking mitochondrial permeability transition pore or by treatment with rotenone, an inhibitor of mitochondrial complex I (61), and raises the therapeutic possibility of using mitochondria-targeted antioxidants as a treatment for nitrate tolerance by interrupting the RIRR tolerance mechanism.
Complex RIRR
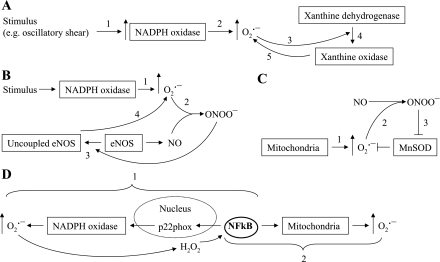
NADPH oxidase regulates the conversion of xanthine dehydrogenase to the O2·−-generating enzyme, XO.
Xanthine oxidoreductase is a critical enzyme in the metabolic pathway of purine degradation, catalyzing the oxidation of hypoxanthine to xanthine and xanthine to uric acid. It exhibits either dehydrogenase (XDH) or oxidase (XO) activities, depending on its redox state. The enzyme normally exists as XDH, which transfers electrons to NAD+. However, XDH can undergo redox modulation to XO, which reduces oxygen to O2·− and H2O2 (15). McNally et al. (37) determined that excess ROS generation in cultured endothelial cells during oscillatory shear stress is dependent on XO (37). NADPH oxidase is known to induce proteolytic cleavage of XDH to XO. Oxypurinol, a specific blocker of XO, or cells lacking NADPH oxidase activity both show reduced O2·− production in vascular areas of oscillatory shear (Fig. 2A) (37).
Fig. 2.
A–D: sequence of events illustrating complex RIRR. ONOO−, peroxynitrite; NO, nitric oxide; eNOS, endothelial NO synthase.
NADPH oxidase-derived ROS uncouples endothelial NOS, thereby increasing ROS production.
Another interenzymatic form of RIRR links NADPH oxidase and NOS. Landmesser et al. (25), using a rodent model of salt-induced hypertension, showed that O2·− generated from NADPH oxidase reacts with the product of NOS, NO, forming ONOO−, which oxidizes the critical NOS cofactor, tetrahydrobiopterin, to dihydrobiopterin and biopterin. This induces a conformational change in the NOS enzyme subunits that uncouple the oxidase and reductase domains, allowing a diversion of electrons directly to oxygen. This process converts endothelial NOS from a NO-forming (and O2·− scavenging) enzyme to one that generates O2·−. The resulting reduction in NO bioavailability impairs endothelium-dependent vasorelaxation (Fig. 2B).
ROS-generating enzymes can overwhelm or otherwise diminish the efficacy of antioxidant defenses, leading to increased ROS production.
An imbalance between ROS production and ROS decomposition constitutes an important form of RIRR. Using a well-established model of oxidative stress, global ischemia, and reperfusion, Brown et al. (4) demonstrated that oxidation of GSH to the disulfide form (GSSG) reduced the GSH-to-GSSG ratio (4). This resulted in an accumulation of ROS in the mitochondria of isolated cardiomyocytes from guinea pigs and oscillations in mitochondrial membrane potential that activated sarcolemmal KATP channels leading to arrhythmias (4). This study used diamide, a thiol-selective oxidant that promotes protein S-glutathiolation and chemically oxidizes GSH without directly producing ROS. Reduced GSH plays an important role in the detoxification of H2O2 via GSH-Px. Decreasing GSH-to-GSSG ratio with diamide leads to an accumulation of H2O2 and a collapse of the mitochondrial membrane potential, followed by increased ROS production (4). The loss of membrane potential and increase in GSSG was prevented with a ligand of the mitochondrial benzodiazepine receptor 4′-chlorodiazepam, resulting in the attenuation of associated arrhythmias (3, 4). High specificity of diamide for oxidation of internal GSH to GSSG has been confirmed by a recent study reporting similar biological responses in the diamide and H2O2 data sets (18). The effect of diamide can be inhibited by exogenous catalase (67). However, the pharmacological properties of diamide include cell stiffening [see review (11)], making this a complex, probably nonphysiological stimulus.
Valdez et al. (58) studied intramitochondrial pathways of ONOO− disposition and found that supplementation of submitochondrial particles with relatively low (1–3 μM) concentrations of ONOO− elicit mitochondrial O2·− formation, whereas the addition of relatively high ONOO− concentrations (200 μM) result in protein nitration. Previously, it has been reported that ONOO− inactivates MnSOD, therefore eliminating the decomposition of O2·−. The excess O2·− reacts with mitochondrial NO and generates more ONOO−, followed by an increased nitration and oxidation of mitochondrial proteins (38). The damaging effects of ONOO− can be attenuated by the addition of reductants, including ascorbic acid, ubiquinol, uric acid, and GSH (Fig. 2C) (58).
ROS-induced activation of transcription factors that stimulate production of ROS-producing enzymes.
ROS-induced signaling includes nuclear transcription, which can serve as a mechanism of RIRR. NF-κB signals an increase in the redox state and regulates the expression of genes involved in inflammation and oxidative stress (65, 68). In 1991, Shreck et al. (49) were the first to demonstrate that H2O2-treatment of Jurkat T cells results in a rapid activation of NF-κB. ROS induction of NF-κB occurs by the activation of inhibitory κB (IκB) kinases and IκB phosphorylation and degradation (10, 48). In hypertensive Dahl salt-sensitive rats, ANG-II stimulation of ROS resulted in the activation of NF-κB signaling pathway (68). Manea et al. (34) demonstrated the involvement of NF-κB in the regulation of NADPH oxidase subunit p22phox in human aortic smooth muscle cells. The p22phox subunit is essential for the activation of NADPH oxidase enzymes and is associated with an increased production of O2·− in human coronary arteries in the presence of atherosclerosis (51). The transcriptional regulation of p22phox occurs via NF-κB cis-acting elements located in the promoter of p22phox gene (Fig. 2D). Therefore, NF-κB modulates the formation of NADPH oxidase subunits, leading to an increase in production of O2·− and H2O2. Since H2O2 is involved in the activation of NF-κB (49), the ROS generated by NADPH oxidase will upregulate NF-κB, which in turn increases NADPH oxidase activity, creating a positive feedback mechanism for ROS production (Fig. 2D) (34).
NF-κB may also act nontranscriptionally to elicit ROS production. Recent findings of Mariappan et al. (35), using an obese mouse model of type II diabetes (db/db), suggest that NF-κB enters into the mitochondria of db/db mice, generating excess mitochondrial O2·− by a nontranscriptional mechanism with decreased ATP production and complex III activity. They also found that NF-κB gene and protein expressions are elevated in db/db animals and demonstrated a potential therapeutic effect of NF-κB blockers in reducing mitochondrial oxidative damage and protecting against cardiac dysfunction (Fig. 2D) (35). However, we must note that because of the complexity of NF-κB signaling and lack of selective inhibitors, the NF-κB signaling pathway is still not completely understood. Studies examining the effect of ROS on NF-κB signaling in diverse cells and tissues reveal no consistent redox responses (46), suggesting that redox effects might be stimulus and cell type specific.
A recent study revealed a feed-forward interaction between TNF-α and NF-κB via the IKK-β pathway and established a link between the activation of IKK-β and oxidative stress, resulting in endothelial dysfunction in type 2 diabetes (65). The increased production of TNF-α was associated with NF-κB activation, which in turn promoted TNF-α expression and elevated O2·− production by NADPH oxidase, resulting in NO scavenging, ONOO− formation, and endothelial dysfunction in diabetic mice (13).
In cultured rat aortic smooth muscle cells, H2O2 stimulated the expression of Ets-1, a well-known mediator of vascular inflammation and remodeling (2). Prior incubation of the cells with polyethylene glycol catalase blocked Ets-1 expression (30). Using a model of carotid artery balloon injury, Feng et al. (9) and others demonstrated that nuclear Ets-1 expression is increased in adventitial and perivascular regions in injured arteries and identified downstream targets of Ets-1, including monocyte-specific chemokyne monocyte chemoattractant protein-1 and adhesion molecules (9). In the study conducted on human aortic smooth muscle cells, Ets-1 has been shown to regulate the expression of NADPH oxidase subunit p47phox, therefore promoting vascular remodeling and ROS generation in response to ANG II (44). This effect was blocked by dominant-negative Ets-1 peptide, suggesting a role of Ets-1 as a critical transcriptional mediator of ROS production (44). The results of these studies allow us to propose a potential pathway in the setting of vascular inflammation where H2O2-stimulated Ets-1 expression results in the activation of NADPH oxidase and ROS generation.
ROS in inflammation: ROS-induced injury begets further ROS formation.
Increased ROS levels are a ubiquitous consequence of chronic inflammation. The role of proinflammatory cytokines in activating NADPH oxidase is well described [see review (56)]. In microvascular endothelial cells, ROS produced by NADPH oxidase increase the expression of inducible NOS (iNOS) (63). In a murine model of abdominal aortic aneurisms (AAAs), both NADPH oxidase and iNOS activity are mechanistically linked to inflammation and aortic wall degeneration (64). In human patients with AAAs, an increased expression of NADPH oxidase and elevated O2·− levels were detected not only in the regions of inflammatory cell infiltration but also within the vessel wall responsible for degradation of the extracellular matrix [stimulation of matrix metalloproteinase-2 (MMP-2)] and apoptosis of smooth muscle cell (64). The likely culprit mediating these responses in AAAs is ONOO−, produced from the interaction between NO (from iNOS) and O2·− (from NADPH oxidase) (40, 64). The direct infusion of ONOO− has similar proteolytic effects on extracellular matrix proteins (54). This sequence of events is consistent with the RIRR pathway, whereby proinflammatory cytokines activate NADPH oxidase, resulting in increased O2·− production. This together with NO generates ONOO−, which in turn activates MMP-2, resulting in the remodeling of the extracellular matrix and the progression of AAAs.
Conclusions
Enhanced oxidative stress does not always emanate from a single cellular source but may represent a complex interplay among prooxidant enzymes, antioxidants, nuclear transcription, and disruption of normal electron transfer in key regulatory enzymes. The complexity of this system affords several potential advantages to the cell: 1) amplification of ROS generation enhances the efficiency of the process, 2) interenzymatic stimulation allows stereospecific intracellular targeting of ROS generation where small amounts of ROS from one site can activate larger amounts from a separate intracellular site, 3) one ROS can stimulate the production of another distinct ROS within the same cell, and 4) better temporal control of ROS generation in response to diverse stimuli can be achieved. Future studies of the molecular mechanisms of RIRR should suggest treatments that allow a tighter control of ROS production, targeting of pathological sources of ROS, and preserving physiological ROS signaling.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-080704 and HL-094971.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Bonello MR, Bobryshev YV, Khachigian LM. Peroxide-inducible Ets-1 mediates platelet-derived growth factor receptor-alpha gene transcription in vascular smooth muscle cells. Am J Pathol 167: 1149–1159, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown DA, Aon MA, Akar FG, Liu T, Sorarrain N, O'Rourke B. Effects of 4′-chlorodiazepam on cellular excitation-contraction coupling and ischaemia-reperfusion injury in rabbit heart. Cardiovasc Res 79: 141–149, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown DA, Aon MA, Frasier CR, Sloan RC, Maloney AH, Anderson EJ, O'Rourke B. Cardiac arrhythmias induced by glutathione oxidation can be inhibited by preventing mitochondrial depolarization. J Mol Cell Cardiol 48: 673–679, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chamulitrat W, Schmidt R, Tomakidi P, Stremmel W, Chunglok W, Kawahara T, Rokutan K. Association of gp91phox homolog Nox1 with anchorage-independent growth and MAP kinase-activation of transformed human keratinocytes. Oncogene 22: 6045–6053, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Csordas G, Hajnoczky G. SR/ER-mitochondrial local communication: calcium and ROS. Biochim Biophys Acta 1787: 1352–1362, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension 49: 717–727, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102: 488–496, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Feng W, Xing D, Hua P, Zhang Y, Chen YF, Oparil S, Jaimes EA. The transcription factor ETS-1 mediates proinflammatory responses and neointima formation in carotid artery endoluminal vascular injury. Hypertension 55: 1381–1388, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Flohe L, Brigelius-Flohe R, Saliou C, Traber MG, Packer L. Redox regulation of NF-kappa B activation. Free Radic Biol Med 22: 1115–1126, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Forsyth AM, Wan J, Ristenpart WD, Stone HA. The dynamic behavior of chemically “stiffened” red blood cells in microchannel flows. Microvasc Res 80: 37–43, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Frey RS, Ushio-Fukai M, Malik AB. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal 11: 791–810, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gao X, Belmadani S, Picchi A, Xu X, Potter BJ, Tewari-Singh N, Capobianco S, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation 115: 245–254, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Gongora MC, Qin Z, Laude K, Kim HW, McCann L, Folz JR, Dikalov S, Fukai T, Harrison DG. Role of extracellular superoxide dismutase in hypertension. Hypertension 48: 473–481, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. New York: Oxford University Press, 2006 [Google Scholar]
- 16. Hawkins BJ, Madesh M, Kirkpatrick CJ, Fisher AB. Superoxide flux in endothelial cells via the chloride channel-3 mediates intracellular signaling. Mol Biol Cell 18: 2002–2012, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hecquet CM, Ahmmed GU, Vogel SM, Malik AB. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ Res 102: 347–355, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Held JM, Danielson SR, Behring JB, Atsriku C, Britton DJ, Puckett RL, Schilling B, Campisi J, Benz CC, Gibson BW. Targeted quantitation of site-specific cysteine oxidation in endogenous proteins using a differential alkylation and multiple reaction monitoring mass spectrometry approach. Mol Cell Proteomics 9: 1400–1410, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 24: 677–683, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol 4: 5, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kang LS, Reyes RA, Muller-Delp JM. Aging impairs flow-induced dilation in coronary arterioles: role of NO and H2O2. Am J Physiol Heart Circ Physiol 297: H1087–H1095, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Suzuki T, Maeta H, Abe Y. Role of NAD(P)H oxidase- and mitochondria-derived reactive oxygen species in cardioprotection of ischemic reperfusion injury by angiotensin II. Hypertension 45: 860–866, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 416: 15–18, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Kuo LY, Hwang GY, Yang SL, Hua YW, Chen W, Lin LL. Inactivation of Bacillus stearothermophilus leucine aminopeptidase II by hydrogen peroxide and site-directed mutagenesis of methionine residues on the enzyme. Protein J 23: 295–302, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee SB, Bae IH, Bae YS, Um HD. Link between mitochondria and NADPH oxidase 1 isozyme for the sustained production of reactive oxygen species and cell death. J Biol Chem 281: 36228–36235, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Li PF, Maasch C, Haller H, Dietz R, von HR. Requirement for protein kinase C in reactive oxygen species-induced apoptosis of vascular smooth muscle cells. Circulation 100: 967–973, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Li Q, Zhang Y, Marden JJ, Banfi B, Engelhardt JF. Endosomal NADPH oxidase regulates c-Src activation following hypoxia/reoxygenation injury. Biochem J 411: 531–541, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li WG, Miller FJ, Jr, Zhang HJ, Spitz DR, Oberley LW, Weintraub NL. H2O2-induced O2 production by a non-phagocytic NAD(P)H oxidase causes oxidant injury. J Biol Chem 276: 29251–29256, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu MY, Eyries M, Zhang C, Santiago FS, Khachigian LM. Inducible platelet-derived growth factor D-chain expression by angiotensin II and hydrogen peroxide involves transcriptional regulation by Ets-1 and Sp1. Blood 107: 2322–2329, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Liu Y, Tsuchida A, Cohen MV, Downey JM. Pretreatment with angiotensin II activates protein kinase C and limits myocardial infarction in isolated rabbit hearts. J Mol Cell Cardiol 27: 883–892, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res 93: 573–580, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Lu F. Reactive oxygen species in cancer, too much or too little? Med Hypotheses 69: 1293–1298, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Manea A, Manea SA, Gafencu AV, Raicu M. Regulation of NADPH oxidase subunit p22(phox) by NF-kB in human aortic smooth muscle cells. Arch Physiol Biochem 113: 163–172, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Mariappan N, Elks CM, Sriramula S, Guggilam A, Liu Z, Borkhsenious O, Francis J. NF-kappaB-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes. Cardiovasc Res 85: 473–483, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest 106: 1521–1530, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol 285: H2290–H2297, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Millan-Crow LA, Crow JP, Thompson JA. Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry 37: 1613–1622, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Miller FJ, Jr, Filali M, Huss GJ, Stanic B, Chamseddine A, Barna TJ, Lamb FS. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ Res 101: 663–671, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Miller FJ, Jr, Sharp WJ, Fang X, Oberley LW, Oberley TD, Weintraub NL. Oxidative stress in human abdominal aortic aneurysms: a potential mediator of aneurysmal remodeling. Arterioscler Thromb Vasc Biol 22: 560–565, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Muller G, Morawietz H. NAD(P)H oxidase and endothelial dysfunction. Horm Metab Res 41: 152–158, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Nakano Y, Banfi B, Jesaitis AJ, Dinauer MC, Allen LA, Nauseef WM. Critical roles for p22phox in the structural maturation and subcellular targeting of Nox3. Biochem J 403: 97–108, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Naziroglu M, Kilinc F, Uguz AC, Celik O, Bal R, Butterworth PJ, Baydar ML. Oral vitamin C and E combination modulates blood lipid peroxidation and antioxidant vitamin levels in maximal exercising basketball players. Cell Biochem Funct 28: 300–305, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Ni W, Zhan Y, He H, Maynard E, Balschi JA, Oettgen P. Ets-1 is a critical transcriptional regulator of reactive oxygen species and p47(phox) gene expression in response to angiotensin II. Circ Res 101: 985–994, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Olsson M, Wilson M, Uller T, Mott B, Isaksson C, Healey M, Wanger T. Free radicals run in lizard families. Biol Lett 4: 186–188, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pantano C, Reynaert NL, van d V A, Janssen-Heininger YM. Redox-sensitive kinases of the nuclear factor-kappaB signaling pathway. Antioxid Redox Signal 8: 1791–1806, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE 2000: pe1, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med 175: 1181–1194, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J 10: 2247–2258, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Serrander L, Jaquet V, Bedard K, Plastre O, Hartley O, Arnaudeau S, Demaurex N, Schlegel W, Krause KH. NOX5 is expressed at the plasma membrane and generates superoxide in response to protein kinase C activation. Biochimie 89: 1159–1167, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of Nox family proteins in human atherosclerosis. Circulation 105: 1429–1435, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Stadtman ER, Berlett BS. Reactive oxygen-mediated protein oxidation in aging and disease. Chem Res Toxicol 10: 485–494, 1997 [DOI] [PubMed] [Google Scholar]
- 53. Storz P. Reactive oxygen species-mediated mitochondria-to-nucleus signaling: a key to aging and radical-caused diseases. Sci STKE 2006: re3, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Sung MM, Schulz CG, Wang W, Sawicki G, Bautista-Lopez NL, Schulz R. Matrix metalloproteinase-2 degrades the cytoskeletal protein alpha-actinin in peroxynitrite mediated myocardial injury. J Mol Cell Cardiol 43: 429–436, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Thengchaisri N, Kuo L. Hydrogen peroxide induces endothelium-dependent and -independent coronary arteriolar dilation: role of cyclooxygenase and potassium channels. Am J Physiol Heart Circ Physiol 285: H2255–H2263, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Touyz RM, Briones AM. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens Res 34: 5–14, 2011 [DOI] [PubMed] [Google Scholar]
- 57. Ungvari ZI, Bailey-Downs L, Gautam T, Jimenez R, Losonczy G, Zhang C, Ballabh P, Recchia FA, Wilkerson DC, Sonntag WE, Pearson KJ, de CR, Csiszar A. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol 300: H1133–H1140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Valdez LB, Alvarez S, Arnaiz SL, Schopfer F, Carreras MC, Poderoso JJ, Boveris A. Reactions of peroxynitrite in the mitochondrial matrix. Free Radic Biol Med 29: 349–356, 2000 [DOI] [PubMed] [Google Scholar]
- 59. Ventura A, Pelicci PG. Semaphorins: green light for redox signaling? Sci STKE 2002: pe44, 2002 [DOI] [PubMed] [Google Scholar]
- 60. Vincent A, Crozatier M. Neither too much nor too little: reactive oxygen species levels regulate Drosophila hematopoiesis. J Mol Cell Biol 2: 74–75, 2010 [DOI] [PubMed] [Google Scholar]
- 61. Wenzel P, Mollnau H, Oelze M, Schulz E, Wickramanayake JM, Muller J, Schuhmacher S, Hortmann M, Baldus S, Gori T, Brandes RP, Munzel T, Daiber A. First evidence for a crosstalk between mitochondrial and NADPH oxidase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antioxid Redox Signal 10: 1435–1447, 2008 [DOI] [PubMed] [Google Scholar]
- 62. Wolin MS, Gupte SA, Oeckler RA. Superoxide in the vascular system. J Vasc Res 39: 191–207, 2002 [DOI] [PubMed] [Google Scholar]
- 63. Wu F, Tyml K, Wilson JX. iNOS expression requires NADPH oxidase-dependent redox signaling in microvascular endothelial cells. J Cell Physiol 217: 207–214, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xiong W, MacTaggart J, Knispel R, Worth J, Zhu Z, Li Y, Sun Y, Baxter BT, Johanning J. Inhibition of reactive oxygen species attenuates aneurysm formation in a murine model. Atherosclerosis 202: 128–134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yang J, Park Y, Zhang H, Xu X, Laine GA, Dellsperger KC, Zhang C. Feed-forward signaling of TNF-α and NF-κB via IKK-β pathway contributes to insulin resistance and coronary arteriolar dysfunction in type 2 diabetic mice. Am J Physiol Heart Circ Physiol 296: H1850–H1858, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yang Y, Shi W, Cui N, Wu Z, Jiang C. Oxidative stress inhibits vascular KATP channels by S-glutathionylation. J Biol Chem 285: 38641–38648, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zago EB, Castilho RF, Vercesi AE. The redox state of endogenous pyridine nucleotides can determine both the degree of mitochondrial oxidative stress and the solute selectivity of the permeability transition pore. FEBS Lett 478: 29–33, 2000 [DOI] [PubMed] [Google Scholar]
- 68. Zhou MS, Schulman IH, Raij L. Vascular inflammation, insulin resistance, and endothelial dysfunction in salt-sensitive hypertension: role of nuclear factor kappa B activation. J Hypertens 28: 527–535, 2010 [DOI] [PubMed] [Google Scholar]
- 69. Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 192: 1001–1014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta 1757: 509–517, 2006 [DOI] [PubMed] [Google Scholar]