Abstract
A growing body of evidence indicates that a number of common complex diseases, including hypertension, heart failure, and obesity, are characterized by alterations in central neurocardiovascular regulation. However, our understanding of how changes within the central nervous system contribute to the development and progression of these and other diseases remains unclear. As with many areas of cardiovascular research, the mouse has emerged as a key species for investigations of neuroregulatory processes because of its amenability to highly specific genetic manipulations. In parallel with the development of increasingly sophisticated murine models has come the miniaturization and advancement in methodologies for in vivo assessment of neurocardiovascular end points in the mouse. The following brief review will focus on a number of key direct and indirect experimental approaches currently in use, including measurement of arterial blood pressure, assessment of cardiovascular autonomic control, and evaluation of arterial baroreflex function. The advantages and limitations of each methodology are highlighted to allow for a critical evaluation by the reader when considering these approaches.
Keywords: baroreflex, blood pressure, parasympathetic, sympathetic, transgenic mice
this article is part of a collection on Assessing Cardiovascular Function in Mice: New Developments and Methods. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
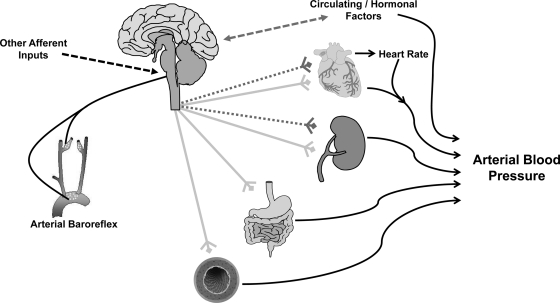
The central nervous system (CNS) plays an essential role in cardiovascular regulation: from the level of the peripheral vasculature, to modulation of cardiac function, to body fluid homeostasis (see Fig. 1). Intricate neural networks, along with reflex inputs, influence the activity of autonomic nerves innervating organ systems involved in cardiovascular regulation. Aside from direct efferent innervation of target organs, the CNS is also critically involved in the release and modulation of hormonal factors that influence cardiovascular control. CNS control of the cardiovascular system has become an area of intense investigation because of a growing body of evidence that a number of common complex diseases, including hypertension, heart failure, and obesity, are characterized by alterations in neurocardiovascular regulation (25, 54).
Fig. 1.
Schematic illustrating neural cardiovascular control. Sympathetic (light gray lines) and parasympathetic (dotted gray lines) autonomic efferent nerves arise from the central nervous system and influence arterial blood pressure via innervation of peripheral end-organ targets. The activity of efferent autonomic outflow is determined within central neural circuits, as well as modulated by a number of peripheral afferent inputs, in particular the arterial baroreflex. In addition, the central nervous system is intimately involved in the control of a number of hormonal factors that may influence cardiovascular regulation through bidirectional communication with central autonomic regions or by acting on peripheral targets. While neurocardiovascular regulation is a complex and integrated process, the mouse represents a critical model that allows for the dissection of the individual systems involved.
The mouse has emerged as a critical model in nearly all areas of biomedical research, and neural mechanisms of cardiovascular function and disease are no exception. The availability of well-defined genetic strains; advanced technologies for precise manipulations of the murine genome, including cell- and tissue-specific alterations; and increasingly sophisticated methods for integrative physiological analyses have converged to allow us to ask and answer questions of complex neuroregulatory processes in ways not possible before. The following brief review will focus on a number of key experimental approaches currently used for the in vivo assessment of neurocardio-regulatory control in the mouse, with an emphasis on arterial blood pressure (ABP) regulation. New developments, advantages, and limitations of these various methods for measurement of CNS effector mechanisms in murine models are presented. The field of neurocardiovascular control is a vast area of research, and therefore it would be impossible to cover and reference all of the work. To aid the reader, we have cited a number of reviews and representative articles when appropriate.
Neural Control of ABP
Because of the importance of the CNS in both short- and long-term control of ABP, many investigations have focused on the central mechanisms and brain regions involved in the neurogenic control of blood pressure. In this regard, several indirect and direct methodologies have been developed that allow for the measurement of ABP in murine models. For an extensive review, the reader is referred to previously published expert recommendations on the appropriate techniques for the measurement of ABP in experimental animals (45).
Indirect measurements of ABP.
In the mouse, a classic approach used for the measurement of ABP has been through indirect estimates obtained using tail-cuff plethysmography (23, 24, 45). ABP is measured as the cuff pressure at which blood flow in the tail is restored upon release of an occlusion cuff. While this method allows for a noninvasive, inexpensive, and high-throughput approach, the physiological validity of ABP obtained using tail cuff has been questioned (45). This is due, in part, to the necessary restraint and thermal stress (heating of the animal) imposed on the mouse during periods of measurement. While appropriate training and handling of the mice has been suggested to minimize the stress on the animal (43, 50), a number of studies have shown that even more than a week of “conditioning” fails to prevent the large increase in ABP and heart rate induced by restraint stress (27, 59). These external factors are particularly confounding for neurocardiovascular research in which important changes in ABP between groups and/or following an experimental intervention may be relatively subtle. For example, murine models allow for discrete genetic manipulations in specific neural regions (20, 84). Such highly targeted experimental approaches may result in small, albeit physiologically, significant changes in blood pressure, an effect that could potentially be masked because of the limitations of the tail-cuff technique. In addition, restraint stress alone influences the amount of neural outflow to the cardiovascular system, thus possibly confounding the interpretation of results when examining neural control of ABP. Furthermore, tail-cuff measurements normally only provide measures of systolic blood pressure during a limited number of cardiac cycles from the tail. Recent advances in the equipment available, including volume pressure recordings, allow for an estimation of diastolic blood pressure, although the accuracy of these measures remains a matter of debate (24). As such, indirect methods have been recommended primarily for use when quantifying frank systolic hypertension (45). In addition, tail-cuff methodologies are useful when screening large numbers of animals (22, 78), although the measurements should be confirmed with direct methods (45).
Direct measurements of ABP.
In contrast to indirect measurements, direct recordings of ABP, using fluid-filled catheters or implantable radiotelemeters, allow the dynamic intricacies of neural control of blood pressure to be examined. The variability of ABP between cardiac cycles and throughout a 24-h period may be quite large, particularly in nocturnal animals that are more active at night (such as mice). Importantly, small alterations in the inherent variability of ABP can have important physiological and pathophysiological consequences, such as ABP-related effects on organ function (e.g., target organ damage) (64). In this regard, individual neural genes and/or CNS loci may be involved in the control of different aspects of blood pressure regulation (e.g., systolic vs. diastolic, diurnal rhythms, etc.), an effect that cannot be evaluated using indirect ABP measurement techniques. It is therefore not surprising that the ability to directly measure ABP for extended periods of time has begun to provide novel insight into neurohormonal cardiovascular regulation.
One methodology to obtain direct recordings of ABP is via the insertion of a fluid-filled catheter into a major artery. While a variety of vessels has been used in larger animal models, the femoral artery has been the common vessel employed in the mouse (30, 59). The proximal portion of the fluid-filled catheter is introduced into the artery, whereas the distal portion is exteriorized and connected to a calibrated pressure transducer, in line with an amplifier and recording device. Importantly, for long-term continuous recordings of ABP, it is necessary for the exteriorized portion of the system to be connected to a tether/swivel device that allows stress-free movement of the mouse. Following recovery from surgical procedures, direct recordings using fluid-filled catheters are accurate and allow for the continuous measurement of ABP over time. The major disadvantage of this technique is that maintaining a functional arterial catheter for an extended period of time is challenging and meticulous, labor-intensive care is necessary to ensure the patency and accuracy of the catheter. As such, the majority of studies in mice using fluid-filled catheters have been generally limited to a recording time of ∼1 to 2 wk (45), although patent arterial catheters for up to 5 wk have been reported (59).
Within recent years, the advent of miniature wireless radiotelemetric technology has provided an alternative approach for direct recordings of ABP (11, 12, 42, 61). Implantable radiotelemeters allow for high-fidelity measurements of ABP to be obtained over weeks to months, without the need for tethering of arterial catheters or restraining of the mouse. The common approach involves the placement of a gel-filled, pressure-sensitive catheter into the thoracic aorta via the common carotid artery (11, 12). Subsequently, the attached radiotransmitter body is placed in a subcutaneous “pocket” along the flank. The small size of the radiotelemeter (∼1 g) is minimally stressful to an adult mouse (20–30 g), and the carotid artery catheterization approach provides highly reliable short- and long-term measurements of ABP. However, a consideration with this placement of the telemeter is the potential of inducing cerebral ischemia due to ligation of the carotid artery. While C57/BL6 mice with carotid placed radiotelemeters are behaviorally indistinguishable from unoperated control mice (11), other murine strains are particularly susceptible to cerebral ischemia induced by carotid occlusion (4, 18), and therefore this should be considered during the study design.
The abdominal aorta has been used as an alternative catheterization site for radiotelemetry (42, 61), although this technique typically requires larger than average mice (>30 g) and is associated with a higher mortality rate (11, 12). Our laboratory and others have begun to employ the femoral artery, with advancement of the catheter tip to the lower aorta, as an alternative to the carotid and/or abdominal aorta implantation. We have found that comparable ABP recordings can be obtained using the femoral or carotid artery approach. In addition, these recordings can be maintained for an extended period of time, and we have successfully kept recordings for over 30 days in mice as small as 18 g. While the obstruction of blood flow to the extremity is a concern with this approach and further miniaturization of the catheter will prove to be beneficial, proper surgical training and expertise appear to minimize these effects.
The major disadvantage with radiotelemetry is the high cost associated with the initial purchase of the devices, hardware and software, as well as the continued expense for refurbishment of the radiotransmitter battery and catheter (41). However, the added benefits of the data that can be obtained may outweigh the expense of radiotelemetry. In addition to providing accurate long-term continuous evaluation of ABP, radiotelemetric measurements also allow for simultaneous recordings of locomotor and biopotential activity (19, 26, 82). In this regard, the ability to record electrical activity in a conscious mouse, such as an electrocardiogram, represents a significant advantage over anesthetized preparations in which the well-known effects of anesthesia on the autonomic control of the heart have been extensively documented (85, 86). In addition, behavioral activity should be considered. Indeed, several studies performing experimental interventions (i.e., genetic manipulation) to evaluate ABP and heart rate control have come to different conclusions when locomotor activity was taken into consideration (36, 72). Furthermore, it appears that newer transmitters will soon become available, which will also allow for the concurrent recording of body temperature (www.datasci.com). Simultaneous recordings of blood pressure, cardiac electrical activity, locomotor activity, and body temperature will likely prove to be beneficial to not only investigate the role of the CNS on individual systems but also allow for integration of the central neural factors on multiple systems at the same time. An overview of the advantages and disadvantages of the methods for ABP measurement in the mouse is highlighted in Table 1.
Table 1.
Experimental approaches to investigate neural control of arterial blood pressure
| Pros | Cons/Caveats | |
|---|---|---|
| Indirect methods | ||
| Tail cuff |
|
|
| Direct methods | ||
| Fluid-filled catheters |
|
|
| Implantable radiotelemetry |
|
|
Central Neural Control of the Autonomic Nervous System
Direct innervation of target organs by autonomic parasympathetic and sympathetic nerves is the major mechanism by which the CNS affects cardiovascular regulation. Given that a number of pathophysiological states are characterized by alterations in autonomic function (25, 54), a major focus of neurocardio-regulatory research has focused on the central mechanisms involved in the control of the branches of the autonomic nervous system. A number of approaches are currently used for the examination of the autonomic nervous system in the mouse, each with their benefits and limitations (see Table 2).
Table 2.
Experimental approaches to investigate neural control of the autonomic nervous system
| Pros | Cons/Caveats | |
|---|---|---|
| Indirect methods | ||
| Biochemical measurements |
|
|
| Pharmacological blockade |
|
|
| Frequency/time domain analysis |
|
|
| Direct methods | ||
| Efferent nerve recordings |
|
|
Indirect methods for evaluating the autonomic nervous system.
The most commonly employed investigations of parasympathetic and sympathetic control in the mouse have relied on indirect measurements of autonomic neural activity. In this regard, the methodologies used can be classified into biochemical assays, pharmacological approaches, or mathematical indices, including frequency and time-domain assessments.
PLASMA AND URINE BIOCHEMICAL MEASUREMENTS.
The activity of the sympathetic nervous system can be estimated from urinary or plasma measurements of norepinephrine, epinephrine, and other metabolites of the norepinephrine synthesis pathway (21, 29, 40, 48). While beneficial, such approaches only provide a “global” estimate of sympathetic function. Another drawback is that circulating norepinephrine levels are determined by secretion, tissue clearance, and reuptake processes, all of which influence the measured plasma concentration (21, 40). In addition, urinary catecholamine excretion is intimately linked to kidney function and thus is not reliable in situations in which renal function is altered (40).
Given the above limitations, alternative approaches using radiotracer techniques have been used, so far predominately in humans, for the measurement of norepinephrine kinetics. Briefly, the infusion of radiolabeled norepinephrine, combined with venous sampling, allows for an estimate of the spillover of norepinephrine into the plasma. While this technique can be applied to estimate “whole body” kinetics, the evaluation of specific regional beds may also be performed via local arterial and venous cannulation, along with measurements of blood flow to calculate region-specific norepinephrine release. Details of this technique have been extensively reviewed (21). Interestingly, the translation of this methodology to the rat has recently occurred (35, 37). While a few examinations of norepinephrine turnover in the heart and brown adipose tissue have been reported (38, 39, 80), application in a murine model awaits further development.
PHARMACOLOGICAL APPROACHES.
The increase in heart rate following muscarinic blockade of the heart, such as with methylatropine, can be used as an estimate of the tonic level of cardiac parasympathetic activity. In contrast, the decrease in heart rate following blockade of β-adrenergic receptors (e.g., propanolol) allows for the estimation of tonic cardiac sympathetic control (16, 30). In line with this, sympathetic control of the vasculature may be evaluated after the removal of α-adrenergic receptor restraint or ganglionic blockade, with pharmacological agents such as phentolamine or hexamethonium, respectively (29, 30, 48). Importantly, acute infusions of pharmacological agents can be performed in conscious mice, eliminating the need and the confounding effect of anesthesia. However, it is important to realize that alterations in the receptor density (adrenergic or muscarinic), as well as inherent vascular properties (i.e., vascular hypertrophy), will also influence the hemodynamic changes following pharmacological manipulation. As such, pharmacologically induced changes in heart rate and ABP primarily reflect the tonic level of autonomic control of the cardiovascular system but may not always mirror efferent parasympathetic or sympathetic neural activity.
FREQUENCY DOMAIN MEASUREMENTS.
Regular oscillations in ABP and heart rate occur at various frequencies because of the influence of central autonomic rhythms, as well as local vascular mechanisms, respiratory patterns, and circulating factors (53, 64, 65). With the use of sophisticated mathematical partitioning, individual rhythms in cardiovascular parameters can be determined. The underlying theory is that the timing of cardiovascular responses to individual physiological mechanisms differs. For example, cardiac vagal control affects the variability of heart rate at higher frequencies (HFs) than the sympathetic nervous system. A detailed methodology on the acquisition and frequency domain analysis of cardiovascular parameters in mice has been previously published (7). It is important to note that to obtain measurements of cardiovascular variability, continuous recordings of ABP and heart rate are required, with radiotelemetry being the most common technique applied.
In this regard, power spectral analysis methods of heart rate variability have revealed that parasympathetic modulation of the mouse heart occurs at HFs (2.5–5.0 Hz); the variability within this frequency range is reduced by muscarinic receptor blockade with atropine (5, 32). Importantly, the heart rate variability within the HF range primarily results from respiratory-driven, vagal-mediated variations in pulse interval (i.e., respiratory sinus arrythmia) (75). For this reason, some investigators center the HF range around the respiratory rate of each individual animal, instead of using a fixed range (74, 75). In contrast, the sympathetic nervous system influences low-frequency (LF: 0.4–1.5 Hz) heart rate oscillations, although atropine significantly reduces the power within this range, indicating a parasympathetic contribution to this frequency band as well (5, 32). As such, the LF-to-HF ratio is often reported as a marker of “sympathovagal balance.” Although this approach is common, because of the influence of vagal modulation on all frequency bands, changes in this ratio (e.g., group comparisons) can be difficult to interpret and the individual frequency spectrums should also be considered (5, 16, 32).
Comparable spectral analysis approaches have been used to evaluate the autonomic influence on ABP variability. Using systemic α1-adrenergic blockade with prazosin and elegant statistical approaches, Baudrie and colleagues (5) determined that sympathetic modulation of the mouse peripheral vasculature likely occurs in the LF spectrum at 0.15–0.6 Hz. The HF component of ABP variability is encompassed by respiratory influences, and the physiological factors contributing to this band remain unclear but probably reflect nonautonomic influences on ABP control (31, 73). Interestingly, a large number of studies in the mouse present the LF-to-HF ratio for ABP, whereas reports in other species have commonly focused on only the LF domain.
At the present time, spectral analysis of ABP represents the primary means for obtaining estimates of long-term sympathetic control of the peripheral vasculature in a conscious mouse. However, although it is easily performed, a number of caveats to the technique should be considered. Sympathetic neural outflow to individual vascular beds may occur differentially (see Direct methods for evaluating the autonomic nervous system). By only considering ABP, spectral analysis cannot take into account this complex heterogeneity of sympathetic activity. In line with this, differences may exist in the frequency responsiveness between vascular beds (31, 76). Perhaps an even more important consideration of spectral analysis is whether the measurements obtained truly reflect “sympathetic drive” or “sympathetic nerve activity.” Reports obtained in rats have demonstrated that while directly measured sympathetic nerve activity was higher in spontaneously hypertensive rats compared with Wistar-Kyoto controls, the LF blood pressure variability spectrum was similar between strains (77). These results are in line with other reports of a weak relationship between sympathetic nerve activity and LF blood pressure variability (13, 66, 83). As such, several investigators have concluded that while LF blood pressure variability may reflect the contribution of sympathetic nerve activity on an acute basis (α-blockade), it does not appear to reflect the prevailing level of sympathetic nerve activity per se (66, 77). In the mouse, a comprehensive evaluation of the usefulness of ABP variability to reflect sympathetic nerve activity in healthy and sympathetically overactive models is yet to be determined; however, it is greatly needed to evaluate the utility of the technique.
TIME DOMAIN MEASUREMENTS.
Parasympathetic heart rate control can also be evaluated from mathematical measurements obtained in the time domain. Such measures are calculated based on the time interval between successive cardiac cycles. A number of indices can be considered, including the square root of the mean of the sum of the squares of differences, the standard deviation of differences between beat-to-beat or NN intervals, the standard deviation of NN intervals, and the heart rate variability triangular index. A detailed description of these parameters has been provided by the Task Force of the European Society of Cardiology (1), and normal values have been established for the mouse (47, 81).
Direct methods for evaluating the autonomic nervous system.
A very powerful approach to examine the neural signals arising from the CNS is via the recording of efferent parasympathetic and sympathetic nerve activity. The placement of a bipolar electrode around an intact nerve, followed by amplification of the neural signal, allows for the direct measurement of postganglionic autonomic nervous system activity (28, 54). Efferent parasympathetic nerve activity can be directly recorded from nerve fibers of the cardiac vagal or cervical vagus nerve branches (16, 34, 44). Whereas this technique has been applied in rats and larger animals, to the best of our knowledge, no published reports of direct efferent parasympathetic nerve recordings have been reported in the mouse. This is likely due to the technical difficulty associated with recording vagal efferent nerve activity (16).
In contrast, a growing number of laboratories have begun to directly record sympathetic nerve activity in murine models. In relation to cardiovascular regulation, recordings from the lumbar (70, 71), renal (49, 51, 56), and splanchnic (17) sympathetic nerves have been the primary nerves evaluated to date. It is likely that with the progression of the technique, other regional outputs, such as the cardiac nerves, will be examined, as reported in other animal models. The measured efferent sympathetic output is due to a complex integration of neural pathways, hormonal mediators, and a plethora of afferent reflex inputs, in particular the arterial baroreflex. Because of the multifaceted interplay of all of these factors, efferent nerve activity to specific organs is highly differentially regulated (54, 62). For example, anatomical tracer studies have revealed a distinct topography of CNS circuits, with separate groups of neurons associated with organ-specific sympathetic pathways (68, 69, 79). Importantly, whole nerve recordings represent the summation of the ongoing activity of hundreds to thousands of unmyelinated postganglionic sympathetic nerve fibers. While multiunit recordings are the common approach in most animal models, the potential also exists for the recording of single-unit firing properties from individual nerve fibers (54). However, we are unaware of any reports of single-unit recordings in the mouse.
So far, published reports of direct recordings of sympathetic nerve activity in mice have been restricted to anesthetized preparations. The well-known depression of the cardiovascular system during anesthesia is a clear limitation of this strategy (85, 86). Furthermore, measurements have been typically limited to one discrete time point. While such approaches lend important insight into neural mechanisms, they do not allow for dissection of the time course and/or evaluation of differential sympathetic responses during the development of disease or following an experimental perturbation. For example, chronic recordings in rats have recently demonstrated distinct regional sympathetic responses during the development of angiotensin II/salt-sensitive hypertension (63). Over 24 days of recording, renal sympathetic nerve activity transiently decreased, whereas lumbar sympathetic nerve activity remained unchanged, leaving the authors to conclude that sympathetic nerve activity to another vascular region, such as the splanchnic bed, was the preferential driver in this form of hypertension (although not directly measured). In line with this, Malpas and colleagues have developed telemetric instrumentation for chronic recordings of sympathetic nerve activity in larger rodents and animals (9, 55, 60), with beta testing of other manufactured systems currently in progress. Given the power of genetic manipulation in murine models, there is a clear need for further miniaturization of equipment, as well as development of telemetry systems for chronic nerve recordings in the mouse. Although challenging, the ability to record sympathetic nerve activity in the conscious mouse will likely become feasible in the future.
The Arterial Baroreflex
Although a number of afferent reflex pathways are involved in the modulation of the autonomic nervous system because of its importance in blood pressure homeostasis, the arterial baroreflex has received a considerable amount of attention. Afferent neural signals from baroreceptors located within the carotid sinus and aortic arch are relayed to brain stem regions crucial for the control of efferent autonomic outflow. Through these central pathways, reflex-mediated changes in autonomic outflow function to regulate ABP on a beat-to-beat, as well as potentially a long-term, basis. A number of methodologies have been applied in the mouse to examine baroreflex regulation, including an examination of the overall reflex function and investigation of the afferent and efferent neural arcs.
Indirect assessment of arterial baroreflex function.
The ability to obtain continuous recordings of ABP and heart rate with direct recordings (i.e., fluid-filled catheters or radiotelemetry) allows for the estimation of “spontaneous” measures of cardiovagal baroreflex sensitivity (i.e., gain). Computer-based analysis of random fluctuations in ABP and heart rate can be applied in the time and frequency domain to calculate arterial baroreflex gain (46). In the time domain, the most commonly employed approach has been the sequence technique (6). This method involves an identification of three or more consecutive beats of ABP that are positively correlated with pulse interval. For example, progressive falls in blood pressure may be paralleled by falls in pulse interval, due in part to baroreflex-mediated regulation. Linear regression is applied to calculate the slope of all individual ABP pulse-interval relationships, and the average of all “sequences” during a recording period is taken as cardiovagal baroreflex sensitivity. Baroreflex estimates obtained using the sequence technique can also be separately examined for sequences in which ABP is increasing versus decreasing. This is beneficial, given the well-characterized hysteresis in cardiac baroreflex control; baroreflex sensitivity to falls in pressure are greater compared with rises in pressure (33). An alternative to the sequence method is the measurement of baroreflex gain using frequency-based power spectral analysis methods. In this regard, the calculation of the LF transfer function gain between ABP and heart rate has been used as a measure of cardiac baroreflex sensitivity (46). Importantly, muscarinic blockade with atropine has been shown to significantly reduce the sensitivity measures obtained using these indirect methods, whereas β-blockade has no effect (46). These findings suggest a vagal predominance in baroreflex control of the heart in conscious mice.
Indirect measures of arterial baroreflex control are cost effective and inherently straightforward, especially if mice are already instrumented for direct ABP and heart rate recordings. The strength of these experimental approaches is provided in their ability to assess the arterial baroreflex without the need for additional external manipulation. Moreover, with extended recordings of ABP, they can provide insight into potential diurnal variations and or activity-dependent influences on arterial baroreflex control (58). A caveat to the indirect baroreflex techniques is the assumption that spontaneous oscillations in blood pressure cause oscillations in pulse interval, because of baroreflex-mediated mechanisms. While baroreceptor denervation significantly reduces the sensitivity estimates obtained with these indices, it does not completely eliminate the parallel fluctuations in ABP and pulse interval (57). As such, because of the spontaneous nature of the techniques, a number of baroreflex-independent effects on heart rate control, such as respiration or circulating hormonal influences, may contribute to the calculations. These external influences likely contribute to the highly variable gains previously reported for these measurements, with between-day coefficients of variation in the same animal approaching 50% (46). Furthermore, it is important to recognize that transfer function and sequence method estimates only reflect baroreflex sensitivity around the prevailing heart rate and ABP (i.e., operating point of the entire baroreflex curve) (6). Indeed, spontaneous changes in ABP are typically <10 mmHg in mice (46). The arterial baroreflex stimulus-response curve is sigmoidal in nature, with a maximal sensitivity at the midpoint of the curve and a lesser gain at the threshold and saturation portions of the curve. Therefore, experimental manipulations in which a decreased sensitivity is noted may be due to the fact that the operating point is simply located on a flatter portion of the baroreflex curve, while the maximal sensitivity of the curve remains unchanged (67). Lastly, spontaneous measures only provide insight into cardiovagal baroreflex control (6, 16). Importantly, cardiac baroreflex function does not always parallel arterial baroreflex control of the peripheral vasculature (i.e., sympathetic nerve activity) (14, 51). For example, in a number of hypertensive models, cardiovagal baroreflex sensitivity is reduced, whereas reductions in sympathetic arterial baroreflex gain have not been conclusively shown (2).
Direct assessment of arterial baroreflex function.
Although spontaneous baroreflex examinations are beneficial, an inherent limitation is the assumption that changes in ABP lead to reciprocal changes in heart rate. Manipulations in which the input to the system (i.e., ABP) is stressed across a wide range of pressures allow for direct baroreflex-mediated changes in vagal and sympathetic control to be examined. Vasodilator (e.g., nitroprusside) and vasoconstrictor (e.g., phenylephrine) drugs can be administered via implanted intravenous catheters to elicit baroreflex-mediated responses. The slope of the relationship between ABP (commonly systolic ABP) and pulse interval provides a measure of cardiovagal baroreflex sensitivity (8, 10, 16). In addition, this technique has also been applied to examine the sympathetic baroreflex by examining the relationship between ABP (commonly diastolic or mean ABP) and the reciprocal changes in efferent sympathetic nerve activity (49, 52). Whereas a number of direct examinations of heart rate control in conscious mice have been reported, examinations of the sympathetic arc of the baroreflex in the mouse have been limited to a few laboratories to date.
With the infusion of pharmacological agents, the full extent of arterial baroreflex engagement can be examined. In this regard, the entire sigmoid response curve may be evaluated, including calculations of the maximal gain, as well as the response range of the baroreflex (8, 51). Importantly, because of the beat-to-beat nature of baroreflex responses, the study drugs should be administered in a rapid manner to eliminate the potential for reflex compensatory influences on the measured responses (87). However, to establish “average” responses, it is common that an infusion of study drugs be performed multiple times within the same animal. This may be a confounding factor when considering this technique, given that the common vasoactive agents used, nitroprusside and phenylephrine, also exert direct actions on autonomic centers within the CNS (15). Moreover, if the study design requires multiple assessments of arterial baroreflex control, such as during the development of disease, maintaining a patent venous catheter for extended periods of time in a mouse can be challenging.
Pharmacological measurements of arterial baroreflex sensitivity consider the entire arterial baroreflex arc, from the input into the system (ABP) to the ensuing end-organ response. Thus the derived gains comprehensively reflect arterial baroreflex afferent input, to central processing, to the resultant efferent output (3, 14). Each of these points in the baroreflex arc work in concert to determine the integrated baroreflex response. In this regard, an alteration in one portion of the baroreflex network may be compensated for by other areas, and therefore it may be necessary to investigate the individual pieces of the reflex pathway independently. Chapleau and colleagues (51) have illustrated elegant methodologies to individually investigate the afferent, central, and efferent components of the arterial baroreflex in mice. For example, direct recordings of aortic depressor nerve activity during pharmacologically induced changes in ABP allow for the investigation of afferent baroreflex input into the CNS. In addition, direct stimulation of the aortic depressor nerve bypasses the afferent pathway and provides a method to evaluate the central and efferent components. Lastly, direct stimulation of vagal or sympathetic efferent nerves, while simultaneously recording the end-organ response (i.e., heart rate or blood flow/pressure), serves to investigate the efferent arm of the baroreflex. Although these methods have not been extensively applied to mouse models, they represent a strong approach to dissect out the complexity of arterial baroreflex control. A summary of the major pros, cons, and caveats for the investigation of the arterial baroreflex in mouse models are presented in Table 3.
Table 3.
Experimental approaches to investigate arterial baroreflex function
| Pros | Cons/Caveats | |
|---|---|---|
| Indirect methods | ||
| Frequency/time domain analysis |
|
|
| Direct methods | ||
| Pharmacological approaches |
|
|
| Analysis of afferent/central/efferent components |
|
|
Summary
Neural control of the cardiovascular system is a complex and integrated process, and neurocardiovascular dysregulation is clearly implicated in a number of pathophysiological states. However, the precise CNS mechanisms underlying the development and progression of neurocardiovascular diseases remain poorly understood. Because of the ease of genetic manipulation, the mouse has become a powerful model for investigating complex neuroregulatory principles. As presented in this review, a number of indirect and direct methodologies exist for the in vivo examination of neural effector mechanisms in murine models, each with their associated benefits and pitfalls. We have highlighted areas in which the advancement in neurocardiovascular assessment methodologies will be particularly important as the field continues to progress and new questions are posed using murine model systems.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-63887, HL-84624, and HL-96571 (to R. L. Davisson). C. N. Young is supported by an American Physiological Society Postdoctoral Fellowship and by the Cornell University Genomics Scholars Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Anonymous Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93: 1043–1065, 1996 [PubMed] [Google Scholar]
- 2. Abboud FM. The sympathetic system in hypertension. State-of-the-art review. Hypertension 4: 208–225, 1982 [PubMed] [Google Scholar]
- 3. Abboud FM, Heistad DD, Mark AL, Schmid PG. Reflex control of the peripheral circulation. Prog Cardiovasc Dis 18: 371–403, 1976 [DOI] [PubMed] [Google Scholar]
- 4. Barone FC, Knudsen DJ, Nelson AH, Feuerstein GZ, Willette RN. Mouse strain differences in susceptibility to cerebral ischemia are related to cerebral vascular anatomy. J Cereb Blood Flow Metab 13: 683–692, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Baudrie V, Laude D, Elghozi JL. Optimal frequency ranges for extracting information on cardiovascular autonomic control from the blood pressure and pulse interval spectrograms in mice. Am J Physiol Regul Integr Comp Physiol 292: R904–R912, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Bertinieri G, di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. A new approach to analysis of the arterial baroreflex. J Hypertens 3: S79–S81, 1985 [PubMed] [Google Scholar]
- 7. Bhatia V, Rarick KR, Stauss HM. Effect of the data sampling rate on accuracy of indices for heart rate and blood pressure variability and baroreflex function in resting rats and mice. Physiol Meas 31: 1185–1201, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Bissonnette JM, Knopp SJ, Maylie J, Thong T. Autonomic cardiovascular control in methyl-CpG-binding protein 2 (Mecp2) deficient mice. Auton Neurosci 136: 82–89, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Booth LC, Bennet L, Barrett CJ, Guild SJ, Wassink G, Gunn AJ, Malpas SC. Cardiac-related rhythms in sympathetic nerve activity in preterm fetal sheep. Am J Physiol Regul Integr Comp Physiol 293: R185–R190, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Braga VA, Burmeister MA, Sharma RV, Davisson RL. Cardiovascular responses to peripheral chemoreflex activation and comparison of different methods to evaluate baroreflex gain in conscious mice using telemetry. Am J Physiol Regul Integr Comp Physiol 295: R1168–R1174, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Butz GM, Davisson RL. Long-term telemetric measurement of cardiovascular parameters in awake mice: a physiological genomics tool. Physiol Genomics 5: 89–97, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Carlson SH, Wyss JM. Long-term telemetric recording of arterial pressure and heart rate in mice fed basal and high NaCl diets. Hypertension 35: E1–E5, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Cerutti C, Barres C, Paultre C. Baroreflex modulation of blood pressure and heart rate variabilities in rats: assessment by spectral analysis. Am J Physiol Heart Circ Physiol 266: H1993–H2000, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Chapleau MW, Abboud FM. Neuro-cardiovascular regulation: from molecules to man. Ann NY Acad Sci 940: xiii–xxii, 2001 [PubMed] [Google Scholar]
- 15. Chapleau MW, Hajduczok G, Sharma RV, Wachtel RE, Cunningham JT, Sullivan MJ, Abboud FM. Mechanisms of baroreceptor activation. Clin Exp Hypertens 17: 1–13, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Chapleau MW, Sabharwal R. Methods of assessing vagus nerve activity and reflexes. Heart Fail Rev 16: 109–127, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen D, Bassi JK, Walther T, Thomas WG, Allen AM. Expression of angiotensin type 1A receptors in C1 neurons restores the sympathoexcitation to angiotensin in the rostral ventrolateral medulla of angiotensin type 1A knockout mice. Hypertension 56: 143–150, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Comi AM, Trescher WH, Abi-Raad R, Johnston MV, Wilson MA. Impact of age and strain on ischemic brain injury and seizures after carotid ligation in immature mice. Int J Dev Neurosci 27: 271–277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Costoli T, Sgoifo A, Stilli D, Flugge G, Adriani W, Laviola G, Fuchs E, Pedrazzini T, Musso E. Behavioural, neural and cardiovascular adaptations in mice lacking the NPY Y1 receptor. Neurosci Biobehav Rev 29: 113–123, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Davisson RL. Physiological genomic analysis of the brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol 285: R498–R511, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Esler M, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiol Rev 70: 963–985, 1990 [DOI] [PubMed] [Google Scholar]
- 22. Feng M, Deerhake ME, Keating R, Thaisz J, Xu L, Tsaih SW, Smith R, Ishige T, Sugiyama F, Churchill GA, DiPetrillo K. Genetic analysis of blood pressure in 8 mouse intercross populations. Hypertension 54: 802–809, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Feng M, DiPetrillo K. Non-invasive blood pressure measurement in mice. Methods Mol Biol 573: 45–55, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Feng M, Whitesall S, Zhang Y, Beibel M, D'Alecy L, DiPetrillo K. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens 21: 1288–1291, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Fisher JP, Young CN, Fadel PJ. Central sympathetic overactivity: maladies and mechanisms. Auton Neurosci 148: 5–15, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goecke JC, Awad H, Lawson JC, Boivin GP. Evaluating postoperative analgesics in mice using telemetry. Comp Med 55: 37–44, 2005 [PubMed] [Google Scholar]
- 27. Gross V, Luft FC. Exercising restraint in measuring blood pressure in conscious mice. Hypertension 41: 879–881, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Guild SJ, Barrett CJ, McBryde FD, Van Vliet BN, Head GA, Burke SL, Malpas SC. Quantifying sympathetic nerve activity: problems, pitfalls and the need for standardization. Exp Physiol 95: 41–50, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Infanger DW, Cao X, Butler SD, Burmeister MA, Zhou Y, Stupinski JA, Sharma RV, Davisson RL. Silencing nox4 in the paraventricular nucleus improves myocardial infarction-induced cardiac dysfunction by attenuating sympathoexcitation and periinfarct apoptosis. Circ Res 106: 1763–1774, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Janssen BJ, Leenders PJ, Smits JF. Short-term and long-term blood pressure and heart rate variability in the mouse. Am J Physiol Regul Integr Comp Physiol 278: R215–R225, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Julien C, Malpas SC, Stauss HM. Sympathetic modulation of blood pressure variability. J Hypertens 19: 1707–1712, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Just A, Faulhaber J, Ehmke H. Autonomic cardiovascular control in conscious mice. Am J Physiol Regul Integr Comp Physiol 279: R2214–R2221, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Katona PG, Barnett GO. Central origin of asymmetry in the carotid sinus reflex. Ann NY Acad Sci 156: 779–786, 1969 [DOI] [PubMed] [Google Scholar]
- 34. Katona PG, Poitras JW, Barnett GO, Terry BS. Cardiac vagal efferent activity and heart period in the carotid sinus reflex. Am J Physiol 218: 1030–1037, 1970 [DOI] [PubMed] [Google Scholar]
- 35. Keeton TK, Biediger AM. The measurement of norepinephrine clearance and spillover rate into plasma in conscious spontaneously hypertensive rats. Naunyn Schmiedebergs Arch Pharmacol 338: 350–360, 1988 [DOI] [PubMed] [Google Scholar]
- 36. Kim SM, Huang Y, Qin Y, Mizel D, Schnermann J, Briggs JP. Persistence of circadian variation in arterial blood pressure in α1/β2-adrenergic receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol 294: R1427–R1434, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. King AJ, Novotny M, Swain GM, Fink GD. Whole body norepinephrine kinetics in ANG II-salt hypertension in the rat. Am J Physiol Regul Integr Comp Physiol 294: R1262–R1267, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Knehans AW, Romsos DR. Norepinephrine turnover in obese (ob/ob) mice: effects of age, fasting, and acute cold. Am J Physiol Endocrinol Metab 244: E567–E574, 1983 [DOI] [PubMed] [Google Scholar]
- 39. Knehans AW, Romsos DR. Reduced norepinephrine turnover in brown adipose tissue of ob/ob mice. Am J Physiol Endocrinol Metab 242: E253–E261, 1982 [DOI] [PubMed] [Google Scholar]
- 40. Kopin IJ. Catecholamine metabolism: basic aspects and clinical significance. Pharmacol Rev 37: 333–364, 1985 [PubMed] [Google Scholar]
- 41. Kramer K, Kinter L, Brockway BP, Voss HP, Remie R, Van Zutphen BL. The use of radiotelemetry in small laboratory animals: recent advances. Contemp Top Lab Anim Sci 40: 8–16, 2001 [PubMed] [Google Scholar]
- 42. Kramer K, Voss HP, Grimbergen JA, Mills PA, Huetteman D, Zwiers L, Brockway B. Telemetric monitoring of blood pressure in freely moving mice: a preliminary study. Lab Anim 34: 272–280, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension 25: 1111–1115, 1995 [DOI] [PubMed] [Google Scholar]
- 44. Kunze DL. Reflex discharge patterns of cardiac vagal efferent fibres. J Physiol 222: 1–15, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE. and Subcommittee of Professional and Public Education of the American Heart Association Recommendations for blood pressure measurement in humans and experimental animals. Part 2: Blood pressure measurement in experimental animals: a statement for professionals from the subcommittee of professional and public education of the American Heart Association council on high blood pressure research. Hypertension 45: 299–310, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Laude D, Baudrie V, Elghozi JL. Applicability of recent methods used to estimate spontaneous baroreflex sensitivity to resting mice. Am J Physiol Regul Integr Comp Physiol 294: R142–R150, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Laude D, Baudrie V, Elghozi JL. Effects of atropine on the time and frequency domain estimates of blood pressure and heart rate variability in mice. Clin Exp Pharmacol Physiol 35: 454–457, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Lindley TE, Doobay MF, Sharma RV, Davisson RL. Superoxide is involved in the central nervous system activation and sympathoexcitation of myocardial infarction-induced heart failure. Circ Res 94: 402–409, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Ling GY, Cao WH, Onodera M, Ju KH, Kurihara H, Kurihara Y, Yazaki Y, Kumada M, Fukuda Y, Kuwaki T. Renal sympathetic nerve activity in mice: comparison between mice and rats and between normal and endothelin-1 deficient mice. Brain Res 808: 238–249, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Lorenz JN. A practical guide to evaluating cardiovascular, renal, and pulmonary function in mice. Am J Physiol Regul Integr Comp Physiol 282: R1565–R1582, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Ma X, Abboud FM, Chapleau MW. Analysis of afferent, central, and efferent components of the baroreceptor reflex in mice. Am J Physiol Regul Integr Comp Physiol 283: R1033–R1040, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Ma X, Sigmund CD, Hingtgen SD, Tian X, Davisson RL, Abboud FM, Chapleau MW. Ganglionic action of angiotensin contributes to sympathetic activity in renin-angiotensinogen transgenic mice. Hypertension 43: 312–316, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation 84: 482–492, 1991 [DOI] [PubMed] [Google Scholar]
- 54. Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev 90: 513–557, 2010 [DOI] [PubMed] [Google Scholar]
- 55. Malpas SC, Ramchandra R, Guild SJ, Budgett DM, Barrett CJ. Baroreflex mechanisms regulating mean level of SNA differ from those regulating the timing and entrainment of the sympathetic discharges in rabbits. Am J Physiol Regul Integr Comp Physiol 291: R400–R409, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Mark AL, Agassandian K, Morgan DA, Liu X, Cassell MD, Rahmouni K. Leptin signaling in the nucleus tractus solitarii increases sympathetic nerve activity to the kidney. Hypertension 53: 375–380, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Masuki S, Takeoka M, Taniguchi S, Nose H. Enhanced baroreflex sensitivity in free-moving calponin knockout mice. Am J Physiol Heart Circ Physiol 284: H939–H946, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Masuki S, Todo T, Nakano Y, Okamura H, Nose H. Reduced alpha-adrenoceptor responsiveness and enhanced baroreflex sensitivity in Cry-deficient mice lacking a biological clock. J Physiol 566: 213–224, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mattson DL. Long-term measurement of arterial blood pressure in conscious mice. Am J Physiol Regul Integr Comp Physiol 274: R564–R570, 1998 [DOI] [PubMed] [Google Scholar]
- 60. McBryde FD, Malpas SC, Guild SJ, Barrett CJ. A high-salt diet does not influence renal sympathetic nerve activity: a direct telemetric investigation. Am J Physiol Regul Integr Comp Physiol 297: R396–R402, 2009 [DOI] [PubMed] [Google Scholar]
- 61. Mills PA, Huetteman DA, Brockway BP, Zwiers LM, Gelsema AJ, Schwartz RS, Kramer K. A new method for measurement of blood pressure, heart rate, and activity in the mouse by radiotelemetry. J Appl Physiol 88: 1537–1544, 2000 [DOI] [PubMed] [Google Scholar]
- 62. Morrison SF. Differential control of sympathetic outflow. Am J Physiol Regul Integr Comp Physiol 281: R683–R698, 2001 [DOI] [PubMed] [Google Scholar]
- 63. Osborn JW, Fink GD. Region-specific changes in sympathetic nerve activity in angiotensin II-salt hypertension in the rat. Exp Physiol 95: 61–68, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Parati G, Faini A, Valentini M. Blood pressure variability: its measurement and significance in hypertension. Curr Hypertens Rep 8: 199–204, 2006 [DOI] [PubMed] [Google Scholar]
- 65. Parati G, Saul JP, Di Rienzo M, Mancia G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation. A critical appraisal. Hypertension 25: 1276–1286, 1995 [DOI] [PubMed] [Google Scholar]
- 66. Persson PB, Stauss H, Chung O, Wittmann U, Unger T. Spectrum analysis of sympathetic nerve activity and blood pressure in conscious rats. Am J Physiol Heart Circ Physiol 263: H1348–H1355, 1992 [DOI] [PubMed] [Google Scholar]
- 67. Potts JT, Shi XR, Raven PB. Carotid baroreflex responsiveness during dynamic exercise in humans. Am J Physiol Heart Circ Physiol 265: H1928–H1938, 1993 [DOI] [PubMed] [Google Scholar]
- 68. Pyner S, Coote JH. Rostroventrolateral medulla neurons preferentially project to target-specified sympathetic preganglionic neurons. Neuroscience 83: 617–631, 1998 [DOI] [PubMed] [Google Scholar]
- 69. Pyner S, Coote JH. Evidence that sympathetic preganglionic neurones are arranged in target-specific columns in the thoracic spinal cord of the rat. J Comp Neurol 342: 15–22, 1994 [DOI] [PubMed] [Google Scholar]
- 70. Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes 54: 2012–2018, 2005 [DOI] [PubMed] [Google Scholar]
- 71. Rahmouni K, Sigmund CD, Haynes WG, Mark AL. Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes 58: 536–542, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sheward WJ, Naylor E, Knowles-Barley S, Armstrong JD, Brooker GA, Seckl JR, Turek FW, Holmes MC, Zee PC, Harmar AJ. Circadian control of mouse heart rate and blood pressure by the suprachiasmatic nuclei: behavioral effects are more significant than direct outputs. PLoS One 5: e9783, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stauss HM. Identification of blood pressure control mechanisms by power spectral analysis. Clin Exp Pharmacol Physiol 34: 362–368, 2007 [DOI] [PubMed] [Google Scholar]
- 74. Stauss HM. Power spectral analysis in mice: what are the appropriate frequency bands? Am J Physiol Regul Integr Comp Physiol 292: R902–R903, 2007 [DOI] [PubMed] [Google Scholar]
- 75. Stauss HM. Heart rate variability. Am J Physiol Regul Integr Comp Physiol 285: R927–R931, 2003 [DOI] [PubMed] [Google Scholar]
- 76. Stauss HM, Kregel KC. Frequency response characteristic of sympathetic-mediated vasomotor waves in conscious rats. Am J Physiol Heart Circ Physiol 271: H1416–H1422, 1996 [DOI] [PubMed] [Google Scholar]
- 77. Stauss HM, Mrowka R, Nafz B, Patzak A, Unger T, Persson PB. Does low frequency power of arterial blood pressure reflect sympathetic tone? J Auton Nerv Syst 54: 145–154, 1995 [DOI] [PubMed] [Google Scholar]
- 78. Sugiyama F, Churchill GA, Li R, Libby LJ, Carver T, Yagami K, John SW, Paigen B. QTL associated with blood pressure, heart rate, and heart weight in CBA/CaJ and BALB/cJ mice. Physiol Genomics 10: 5–12, 2002 [DOI] [PubMed] [Google Scholar]
- 79. Sved AF, Cano G, Card JP. Neuroanatomical specificity of the circuits controlling sympathetic outflow to different targets. Clin Exp Pharmacol Physiol 28: 115–119, 2001 [DOI] [PubMed] [Google Scholar]
- 80. Takahashi A, Tabuchi M, Suzuki W, Iizuka S, Nagata M, Ikeya Y, Takeda S, Shimada T, Aburada M. Insulin resistance and low sympathetic nerve activity in the Tsumura Suzuki obese diabetic mouse: a new model of spontaneous type 2 diabetes mellitus and obesity. Metabolism 55: 1664–1669, 2006 [DOI] [PubMed] [Google Scholar]
- 81. Thireau J, Zhang BL, Poisson D, Babuty D. Heart rate variability in mice: a theoretical and practical guide. Exp Physiol 93: 83–94, 2008 [DOI] [PubMed] [Google Scholar]
- 82. Tovote P, Meyer M, Beck-Sickinger AG, von Horsten S, Ove Ogren S, Spiess J, Stiedl O. Central NPY receptor-mediated alteration of heart rate dynamics in mice during expression of fear conditioned to an auditory cue. Regul Pept 120: 205–214, 2004 [DOI] [PubMed] [Google Scholar]
- 83. Tsai ML, Tseng WT, Yen CT, Chen RF. The correlation of mean sympathetic activity with low-frequency blood pressure and sympathetic variability. Clin Exp Hypertens 31: 615–624, 2009 [DOI] [PubMed] [Google Scholar]
- 84. Vasquez EC, Meyrelles SS, Chapleau MW, Johnson AK. Approaches for gene delivery to the subfornical organ and magnocellular neurons. Clin Exp Pharmacol Physiol 28: 602–609, 2001 [DOI] [PubMed] [Google Scholar]
- 85. Vatner SF. Effects of anesthesia on cardiovascular control mechanisms. Environ Health Perspect 26: 193–206, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Vatner SF, Braunwald E. Cardiovascular control mechanisms in the conscious state. N Engl J Med 293: 970–976, 1975 [DOI] [PubMed] [Google Scholar]
- 87. Weinstock M, Rosin AJ. Relative contributions of vagal and cardiac sympathetic nerves to the reflex bradycardia induced by a pressor stimulus in the conscious rabbit: comparison of ‘steady state’ and ‘ramp’ methods. Clin Exp Pharmacol Physiol 11: 133–141, 1984 [DOI] [PubMed] [Google Scholar]