Abstract
Tracking the fate and function of cells in vivo is paramount for the development of rational therapies for cardiac injury. Bioluminescence imaging (BLI) provides a means for monitoring physiological processes in real time, ranging from cell survival to gene expression to complex molecular processes. In mice and rats, BLI provides unmatched sensitivity because of the absence of endogenous luciferase expression in mammalian cells and the low background luminescence emanating from animals. In the field of stem cell therapy, BLI provides an unprecedented means to monitor the biology of these cells in vivo, giving researchers a greater understanding of their survival, migration, immunogenicity, and potential tumorigenicity in a living animal. In addition to longitudinal monitoring of cell survival, BLI is a useful tool for semiquantitative measurements of gene expression in vivo, allowing a better optimization of drug and gene therapies. Overall, this technology not only enables rapid, reproducible, and quantitative monitoring of physiological processes in vivo but also can measure the influences of therapeutic interventions on the outcome of cardiac injuries.
Keywords: bioluminescence imaging, heart, gene therapy, stem cell therapy, in vivo cell tracking
this article is part of a collection on Assessing Cardiovascular Function in Mice: New Developments and Methods. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
Cell-based therapies have rapidly emerged as a potential therapeutic approach for heart disease. After the initial work to characterize putative endothelial progenitor cells (1) and their potential to promote cardiac neovascularization and to attenuate ischemic injury, a decade of intense research has examined several novel approaches to promote cardiac repair in adult life. A variety of adult stem and progenitor cells from different sources have been examined for their potential to promote cardiac repair and regeneration: bone marrow-derived cells (55, 72), circulating and mobilized CD133+ and CD34+ stem and progenitor cells (36), mesenchymal stem cells (56, 65, 83), cardiac resident stem cells (43, 62), and skeletal myoblasts (54, 97). However, many questions such as the optimal type and number of progenitor cells to be administered, the route of administration, and the best time to administer cells after injury remain unresolved. This is particularly true in vivo where it is difficult to assess cellular activity in the heart in real time. Therefore, it would be helpful to have practical tools to accurately track cell location and their functional status over time in vivo.
Histopathological examination has been the main approach for ex vivo evaluation of the distribution, the engraftment, and the differentiation of injected cells. However, histopathology precludes the evaluation and monitoring of these parameters in real time and in vivo. Molecular and cellular imaging has provided various techniques for identifying and tracking transplanted cells in cardiovascular research (15). Magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET), and single photon emission computed tomography (SPECT) offer deep tissue penetration and high spatial resolution (64, 70, 94a). However, in small animal studies, these techniques are more costly and time consuming to implement compared with optical imaging. Among the optical imaging tools, bioluminescence imaging (BLI) is a promising technique that is especially useful in small animal models and does not require the use of radionuclides with their associated hazards. BLI is a high throughput technique that can provide unmatched sensitivity because of the absence of endogenous luciferase expression in mammalian cells and the low background luminescence emanating from animals. BLI can be very useful in evaluating the delivery efficiency of therapeutic genes and their expression levels in vivo. By the use of different cellular promoters to drive the expression of a luminescent reporter gene, BLI allows monitoring of the transplanted cells' differentiation status as well as their location and functional characteristics in vivo (39, 51, 72, 78, 94).
Overall, BLI of reporters cloned into promoter/enhancer sequences or engineered into fusion proteins has demonstrated the modality's ability to monitor fundamental processes such as transcriptional regulation, signal transduction cascades, protein-protein interactions, protein degradation, oncogenic transformation, cell trafficking, and targeted drug action under in vivo spatial registration. In this review, we will discuss recent advances and applications of BLI, specifically as they apply to cardiovascular research.
Principles of Bioluminescence
In nature, numerous luminous species exist in more than 700 genera, of which 80% are marine species (93). Luciferase enzymes have been cloned from both marine (e.g., Renilla luciferase) and terrestrial (e.g., firefly and click beetle luciferase) eukaryotic organisms and are commonly used as reporters for in vitro and in vivo studies. They emit long wavelengths of bioluminescence (>600 nm) in the red and near-infrared regions of the spectrum and are efficiently transmitted through mammalian tissues (20, 86). These wavelengths can avoid absorbing and scattering environment of mammalian tissues (69), thus can be efficiently detected outside a small animal's body using BLI.
BLI is based on the detection of light emitted by cells that express light-generating enzymes such as luciferase. In bioluminescent reactions, luciferase generates visible light through the oxidation of enzyme-specific substrates such as d-luciferin for terrestrial organisms (29, 30) and coelenterazine for marine organisms (31, 53). Luciferases from different organisms can be distinguished by their abbreviations: lux (bacterial), luc (firefly), and lcf (dinoflagellate). To track cells in vivo by BLI, the cells of interest need to be genetically modified to express luciferase. The animal recipient of the cells receives the luciferase substrate either intraperitoneally or intravenously and is placed in a light-tight dark box where luminescence is detected (Fig. 1). In a bioluminescent reaction, the generation of light depends on several factors. Firefly luciferase requires ATP, Mg2+, and oxygen to catalyze the oxidation of its substrate d-luciferin and generates CO2, AMP, inorganic pyrophosphate, oxyluciferin, and a yellow-green light at a wavelength that peaks at 562 nm (Fig. 2). By comparison, Renilla luciferase catalyzes the oxidative decarboxylation of coelenterazine in the presence of dissolved oxygen to yield oxyluciferin, CO2, and blue light that peaks at 480 nm (49). Bioluminescence generated by the luciferase reporter reaction is captured by a cooled charge-coupled device camera that can detect very low levels of visible light emitted from internal organs (19). Charge-coupled device camera-imaged bioluminescence can then be superimposed on photographic images of the mouse to detect quantitatively and repetitively the bioluminescent signal from a given location (95). Imaging can be conducted 10 to 15 min after intraperitoneal injection of d-luciferin (reporter probe), with relatively stable light emission levels for 30 to 60 min, depending on the experimental conditions. The sensitivity of detection depends on the wavelength of light emission, expression levels of the enzyme in the target cells, the location of the source of bioluminescence in the animal, the efficiency of the collection optics, and the sensitivity of the detector (95).
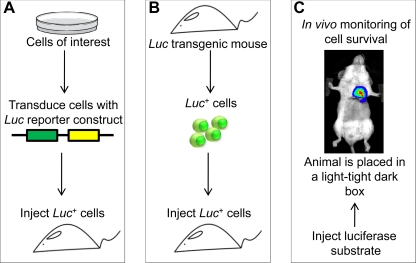
Fig. 1.
Diagram illustrating steps required for bioluminescence imaging (BLI). A: therapeutic cells are transduced with a luciferase (Luc) reporter construct and injected into an animal. B: alternatively, luciferase-expressing cells can be isolated from a luciferase transgenic animal and injected to the experimental subject. C: after systemic substrate injection, a charge-coupled device camera can be used to localize the luciferase photon signals in vivo. Pseudo-colored images that represent signal intensity are overlaid with grayscale reference images of the animal to facilitate localization of the signal. Cell survival, expansion, and homing, as well as the expression of therapeutic genes, can be monitored using this strategy.
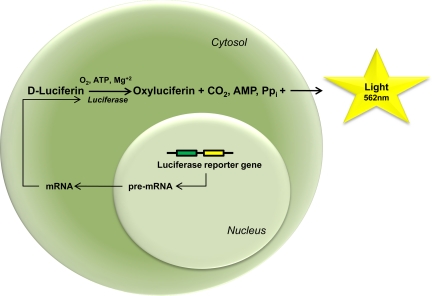
Fig. 2.
Representation of the bioluminescence reaction. A cell expressing the firefly luciferase reporter gene produces the luciferase enzyme that in the presence of oxygen, ATP, and Mg2+ catalyzes d-luciferin into oxyluciferin, CO2, inorganic pyrophosphate (PPi), and light. Light can then be detected, collected, and quantified by a charge-coupled device camera.
Reporter genes commonly used for BLI.
To track cells in vivo, reporter genes and reporter probes must be able to reveal cellular and molecular processes throughout an entire study period, be highly sensitive to small changes in cell function and distribution over time, and must not alter the labeled biological process itself. A variety of reporter genes have been introduced and validated for different imaging modalities, including various luciferases for BLI (5, 67, 74). Luciferase (Luc) is the only one that produces light without requiring an external excitation source, and they offer inherently low background signals because animal tissues do not emit significant amounts of light. Since the first report of the cDNA encoding Luc in 1985 (23), DNA sequences have been optimized so that the Luc gene can be expressed at high levels and its product localized in the cytoplasm of the cells. For all reporter systems, the intensity of light is proportional to the amount of luciferase expressed in each individual cell or the number of cells in which a gene has been transferred.
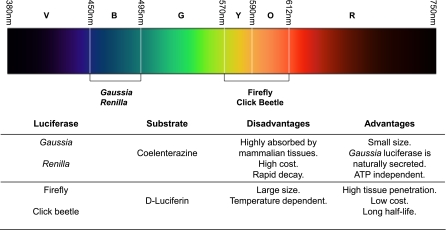
Of the many available luciferase enzymes, only a subset have been developed and used as reporter genes. Luciferase from the North American firefly Photinus pyralis is the most common choice, but other luciferases such as the sea pansy Renilla reniformis, the click beetle Pyrophorus plagiophalamus, and the copepod Gaussia princeps have also been investigated (48, 79). Gaussia and Renilla luciferase enzymes emit in the blue/green region of the UV visible spectrum, where light is strongly absorbed and scattered by tissues. Consequently, the imaging performance suffers from poor sensitivity and spatial resolution. On the contrary, Photinus pyralis and click beetle luciferase emit ∼60% of their light at >600 nm, which enables great tissue penetration. Photinus pyralis has emission at 620 nm when collected at 37°C, making it among the longest emitting luciferases at mammalian body temperatures and the most sensitive for in vivo applications (100).
Bacterial luciferases (Lux) such as from Photorhabdus luminescens emit blue light that has been used for BLI of bacterial infections. They are unique in that their lux operon cassette codes for the luciferase and also for enzymes that produce the substrate required for luminescence reaction, thereby eliminating the need for exogenous substrate. Transferring this operon to mammalian cells would be advantageous for in vivo imaging; however, limited research has been done to determine whether this is possible (16). Mutations in luciferases can change the wavelength of the luminescence emitted. A mutation of a single amino acid has been shown to cause red-shifted luminescence and improve in vivo performance. Recently, a red-shifted mutant of luciferase from Photinus pyralis was created and described to have an emission maximum of 612 nm at pH 7.0, a narrow emission bandwidth, and to be thermostable (with a half-life 8.8 h at 37°C vs. 0.26 h for the wild-type luciferase). This Photinus pyrallis mutant, called Ppy RE-TS reporter, has been successfully used in small animals to visualize cancer progr ession and shown to have superior in vivo imaging performance compared with the wild-type photinus pyralis luciferase (6, 7).
The choice of reporter gene reporter probe pair to be used should ultimately be based on the specific biological process to be monitored, the duration and intensity of the signal needed, and the tissue to be imaged (21, 100) (Fig. 3). As longer wavelengths of light penetrate mammalian tissues with less absorbance, luciferase from Photinus pyralis (>600 nm) is more readily detected (17). Moreover, its substrate d-luciferin remains in circulation longer than other substrates because it is poorly catalyzed by mammalian tissues (101). However, Photinus pyralis luciferase has the disadvantage of having a longer coding sequence (1,653 bp) compared with marine luciferases such as Renilla and Gaussia. Renilla and Gaussia luciferases have coding sequences of 936 and 558 bp, respectively, making them more suitable for studies that require compact transgene sequences, such as gene transfer and gene expression studies. Another advantage of Renilla and Gaussia luciferases over Photinus pyralis is the fact that they do not require ATP as a cofactor during bioluminescent reaction and thus can be used for imaging cells independent of their metabolic state (5, 66). One particular characteristic of Gaussia luciferase is that it is naturally secreted (96) and therefore can be used as a reporter for quantitative assessment of cells in vivo by measuring its concentration in blood (61). However, Renilla and Gaussia luciferases produce shorter wavelengths of light (peaking at 480 nm), which are not transmitted through tissues as effectively as Photinus pyralis (100). Overall, the utility of Renilla and Gaussia for in vivo BLI can benefit from improvements in sensitivity (46–48).
Fig. 3.
Emission wavelengths for the most commonly used reporter luciferase enzymes in BLI and their advantages and disadvantages. V, violet; B, blue; Y, yellow; O, orange; R, red.
Codon-optimized humanized Gaussia (2, 79) has improved its sensitivity in mammalian cells, but the pharmacokinetics of its reporter probe coelenterazine are somewhat limiting in vivo. Coelenterazine is prone to quick inactivation, including degradation through autoxidation. This substrate is also costly with low solubility. Furthermore, coelenterazine binds to serum proteins, is cleared rapidly from the bloodstream (101), and decays rapidly with time (5). Therefore, when imaging in vivo with coelenterazine, the signal needs to be acquired immediately after substrate administration. On the other hand, this short half-life can be advantageous in cases in which sequential imaging of two luciferases, such as Gaussia princeps and Photinus pyralis, is required to monitor two biological processes in tandem in the same animal (5).
In summary, the development of reporter gene variants with better emission spectra, brightness, and stability can ultimately improve sensitivity and the overall performance of luciferases in BLI imaging studies. Moreover, cloning new luciferases from different organisms, such as those from Luciola italica and Cratomorphus distinctus, can certainly improve current applications and make novel ones possible (8, 88).
Vector-mediated expression of reporter genes.
A fundamental requirement for BLI is the expression of a reporter gene (e.g., luciferase) by the cells or tissues to be imaged, which requires the introduction of genetically encoded imaging reporters into cells cultured in vitro (50). Alternatively, luciferase-expressing cells can be obtained from luciferase transgenic mice (Fig. 1). This can be achieved by using a reporter vector that incorporates a luciferase gene driven by a promoter that allows luciferase to be constitutively expressed by all cells in the animal's body (11, 83).
Delivery of bioluminescent reporter genes to cells has been achieved through several means, but lentiviral-based gene transfer has been the method of choice because of its effective gene delivery and high expression levels of transgenes in mammalian cells in culture as well as in vivo (22). Lentiviruses have the capability to deliver target genes to both dividing and nondividing cells and are capable of inserting genetic information into the host genome, ensuring prolonged gene expression with a more limited host immune response (80). The safety of the lentiviral vectors has been further improved with the generation of self-inactivating vectors and the use of minimal packaging systems. The efficiency of gene expression has been improved by the introduction of a relatively strong internal promoter such as cytomegalovirus. This promoter drives the expression of the reporter gene and guarantees that the reporter expression is always “on” under all conditions, in all tissue types. Moreover, lentiviral vectors can be used to simultaneously induce the expression of multiple genes in a cell. Coupling the expression of a gene with a luciferase reporter gene provides a simple yet effective mechanism for studying the regulation of gene expression and monitoring it by BLI. This provides exciting opportunities for transcriptional targeting, double reporter labeling for BLI monitoring, as well as gene therapy. For example, by linking the expression of luciferase to the cardiac-specific promoter myosin light chain 2v, it is possible to monitor cells undergoing cardiac differentiation over time via the detection of reporter gene expression by BLI (27, 35).
Applications of BLI in Cardiovascular Research
Because physiological processes are dynamic in time and space, end-point assays do not always provide a comprehensive understanding of biology in vivo. In cardiovascular research and many other scientific areas, BLI has been used for noninvasive visualization of a variety of biological processes in real time. In this section, we will review the many applications of BLI in cardiovascular research.
Monitoring expression of therapeutic genes in the heart.
Gene therapy is a rapidly evolving field in cardiovascular medicine. This technology allows for the correction of functional gene loss and enables the expression of a therapeutic gene in a target tissue (52). Nevertheless, gene therapy studies continue to be plagued by suboptimal delivery of genes, poor survival of cells carrying the therapeutic gene(s), and the inability to evaluate the levels of gene expression in vivo (28). Thus the information gathered from BLI studies in small animals can be used to design solid clinical trials that will help improve gene therapy for cardiac repair. In cardiovascular research, BLI has been used to address a variety of questions that range from determining the effects of transgene expression (e.g., BCL2) on cardiomyoblast survival and cardiac repair (40) to testing the efficiency of anti-inflammatory drugs on the expression of specific genes (e.g., inducible nitric oxide synthase) (99). Additionally, BLI has been used to optimize vector systems (33) and treatment regimens (71) for delivery of therapeutic genes (e.g., hypoxia-inducible factor-1α) to the heart.
The applications of BLI in research are constantly expanding with the development of new therapeutic modalities. For instance, BLI enabled tracking the activity of a short hairpin RNA plasmid in knocking down inhibitory factors of angiogenic genes in the heart (32). The advances in reporter gene technology and dual reporter labeling have enabled a simultaneous monitoring of distinct physiological events via BLI. Dual-reporter labeling can address questions such as how the expression of a gene affects the survival or differentiation of a specific cell type or how it alters the expression of another gene. However, challenges in designing dual-reporter systems, such as finding adequate means for separating the two bioluminescent signals while retaining sensitivity, remain (60, 87).
Monitoring survival and homing of therapeutic cells.
Stem cell-based therapies are expected to have an enormous impact in the treatment and cure of various diseases and disorders in the future. These cells, given the right conditions, have the ability to differentiate into the constituent cells of various organs. When stem cells are used in therapies, it would be ideal to track their migration or differentiation process in vivo. The therapeutic use of bone marrow stem and progenitor cells initially was popular and has been evaluated furthest in the clinic setting. More recently, circulating stem and progenitor cells, resident cardiac stem cells, and mesenchymal stem cells have also been used in translational studies for clinical applications (15). Although small animal studies have provided promising results, significant questions regarding the biology of transplanted cells and their ability to promote cardiac repair remain (92) (Table 1).
Table 1.
Cells types that have been investigated for their potential to promote cardiac repair using BLI
| Cell Type | Information Gained from BLI | References |
|---|---|---|
| Bone marrow mononuclear cells | Comparison of different cell types for treatment of MI | 83 |
| Timing of cell delivery on acute vs. chronic MI | 76 | |
| Systemic homing to injured heart | 72 | |
| Adipose tissue-derived stem cells (ASCs) | Long-term survival of cells in injured heart | 3 |
| Homing and survival of fresh versus cultured cells to injured heart | 4 | |
| Mesenchymal stem cells (MSCs) | Comparison of ASCs vs. MSCs for treatment of MI | 82 |
| Growth factor-treated MSCs for treatment of MI | 24 | |
| Human CD34+ cells | Cell fate in the heart using a MI model | 89 |
| Rat cardiomyoblasts | Improvement of engraftment and survival with collagen matrix | 38, 39 |
| Embryonic stem cells | Cell survival, proliferation, and migration | 9, 42, 77 |
| Skeletal myoblasts | Cell fate in MI model | 83 |
| ESC-derived endothelial cells (ESC-EC) | Cell fate in MI model | 44, 45 |
| Effects of nicotine on the therapeutic effects ESC-ECs | 98 | |
| Resident cardiac stem cells | Cell fate and function in MI model | 43 |
BLI, bioluminescence imaging; MI, myocardial infarction.
BLI is an important tool that can answer some of these pressing questions by uncovering the dynamics of cell expansion, migration, and survival upon transplantation into the heart (90, 102). Several studies have indicated that cardiomyocytes purified from embryonic stem cells (ESCs) can benefit cardiac function following myocardial ischemia (10, 12, 13, 41, 59) with a low risk of tumor formation. However, one of the central issues in cell therapy is the lack of long-term survival of these therapeutic cells in vivo. BLI has shown that more than 90% of transplanted adult stem cells die within the first 3 wk of delivery (43, 45, 83). This could be the reason for the short duration of improvement of cardiac function observed in multiple studies focused on cell therapy for cardiac repair. Thus the problem of donor cell death continues to be troublesome and may limit the overall efficacy of stem cell-based therapy, making further studies to improve cell fate monitoring via imaging technologies essential.
Failure of long-term survival of therapeutic cells has been demonstrated for a variety of cell types (43, 45, 83) (Fig. 4). BLI has demonstrated the poor survival of bone marrow mononuclear cells (72), cardiac resident stem cells (43), mesenchymal stem cells (83), adipose stromal cells (82), ESC-derived cardiac cells (10), and ESC-derived endothelial cells (45) by 8 wk after cell injection. Conversely, the tumorigenic potential of ESCs can also be uncovered by BLI. Lee et al. (42) demonstrated that a minimum of 100,000 human ESCs are necessary to form teratoma in the heart. Overall, BLI studies have provided significant insights into the biology of stem cells in vivo and how issues such as poor survival and potential tumorigenicity must be overcome before translation into the clinic.
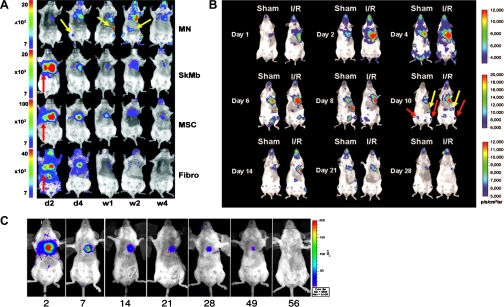
Fig. 4.
Monitoring survival and homing of cells via BLI. A: skeletal myoblasts (SkMb), bone marrow-derived mononuclear cells (MN), mesenchymal stem cells (MSC), and fibroblasts (Fibro) were injected intramyocardially after myocardial infarction. All cell types demonstrated a decrease in bioluminescence signal intensity starting at 4 wk postinjection. Yellow arrows indicate homing of MN to femur, spleen, and liver. Red arrows indicate cells retained in the heart and lungs. Values in y-axis are in photons·s−1·cm−2·sr−1. Abbreviations in x-axis: d, day; w, week. Reprinted with permission (83). B: BLI demonstrating preferential homing of bone marrow mononuclear cells to the ischemic myocardium in a model of ischemic reperfusion injury (I/R). Bioluminescence was also detected in the spleen and bone marrow (yellow and red arrows, respectively). A progressive decrease in bioluminescence signal was observed starting at day 14 postinjection. Reprinted with permission (72). C: cardiac resident stem cells were injected in the heart following myocardial infarction. Robust bioluminescence activity was detected on day 2 but mostly disappeared by 8 wk postinjection. Values in x-axis represent days post-cell injection. Reprinted with permission (43).
Monitoring immune rejection of heart transplant and cell grafts.
Allogeneic heart transplantation is the most commonly used therapy for end-stage heart disease. Transplant loss due to immune rejection remains a significant problem in clinical heart transplantation despite current immunosuppressive therapies (73). Acute rejection of heart transplants is an immune response mediated by the coordinated infiltration and function of host alloantigen-specific T cells in the allograft (57). In this context, BLI has allowed monitoring of cardiac allograft over time and revealed its rejection by day 12 after transplantation. A decrease in the intensity of bioluminescence signals was detected as early as day 4, suggesting acute graft rejection (78). The correlation of bioluminescence intensity to other measures of heart function such as beating score, fractional shortening, and lymphocyte infiltration has provided additional means to assess function and to determine the possible mechanisms responsible for rejection.
Acute rejection can also be a major issue in human ESC-based therapy (25, 84). BLI has provided significant information for the development of therapeutic strategies to prevent the rejection of stem cells. An immunosuppressant cocktail consisting of tacrolimus and sirolimus has been shown by BLI to mitigate the rejection of human ESCs (77). Recently, Pearl et al. (63) used BLI to demonstrate that a cocktail of costimulatory blockade agents (anti-CD40 ligand, cytotoxic T-lymphocyte antigen 4-immunoglobulin, and anti-lymphocyte function-associated antigen-1) induced long-term allogeneic and xenogeneic human ESCs and induced pluripotent stem cell engraftment. Overall, these studies elucidate the importance of in vivo imaging for the development of therapies that may one day enable stem cell therapy to become feasible.
Limitations
BLI has been successfully used to obtain semiquantitative measurements of biological processes because of a strong correlation between the number of cells and the bioluminescence signal detected both in vitro (75) and in vivo (68). However, a simple quantification of light emission may not provide a true representation of biological processes. This is because the firefly luciferase reaction is a complex interaction of a variety of molecules (e.g., ATP, Mg2+, oxygen, and luciferin) and because the intensity of the bioluminescence signal depends on multiple factors. In particular, the number of metabolically active luciferase-transfected cells, the concentration of luciferin, ATP and oxygen levels, the spectral emission of bioluminescence probes, and the depth and optical properties of tissues are known to alter the intensity of bioluminescence signal (69). Another issue that should be considered during quantitative BLI is the limited and wavelength-dependent transmission of light through animal tissues. Light sources closer to the surface of the animal appear brighter compared with deeper sources because of tissue attenuation properties (91). It is estimated that for every centimeter of depth, there is a 10-fold decrease in bioluminescence signal intensity (18). Mathematical models can be used to predict in vivo imaging signal levels and spatial resolution as a function of depth and to help define the requirements for imaging instrumentation. However, the overall low spatial resolution (3–5 mm range) and limited tissue penetrance restrict the use of BLI to small animal studies (69).
Changes in tissue oxygenation can also alter bioluminescence signal. In rat gliosarcoma for instance, bioluminescence signal has been shown to decrease by ∼50% at 0.2% oxygen (58). Thus, for reliable BLI measurements, it is important to understand the effects of local niche in which the luciferase-expressing cells of interest reside. This is especially the case in cardiovascular studies involving hypoxia (e.g., myocardial infarction). Similarly, BLI quantification has to be carefully interpreted in studies that involve surgical procedures. Changes in tissue thickness because of the presence of inflammation, edema, sutures, and animal growth can alter light absorption and scattering as well as the bioluminescence signal.
Conclusions and Future Directions
Many different organisms, ranging from bacteria and fungi to fireflies and fish, are endowed with the ability to emit light. The discovery of new luminescence reporters and the use of genetically modified reporters may further strengthen reporter gene expression in mammalian cells, thus improving the sensitivity and expanding the applications of this imaging modality (7, 14). Additionally, alternative methods for transduction of reporter gene may reduce the risk of anomalous and inappropriate transcription of unwanted sequences in mammalian cells (37).
BLI allows real-time monitoring of survival and homing of various therapeutic cells that are currently being investigated to promote cardiac repair. By the use of cardiac-specific promoters linked to reporter genes, BLI can be used to monitor cell differentiation in vivo. It also provides excellent opportunities to evaluate strategies designed to improve the survival of therapeutic cells or transplanted hearts. The ability to monitor physiological processes in vivo in real time is extremely beneficial to evaluate the success or failure of the various therapies designed to promote cardiac repair. As most basic cardiovascular research is performed in rodent models, BLI can efficiently reveal problems and provide insight into solutions for validating and optimizing novel therapies for the treatment of heart disease. Overall, this technology can furnish great insights that can drive the development of better clinical trials in cardiovascular medicine.
GRANTS
This work was supported in part by National Institutes of Health Grants HL-093172, HL-099117, and EB-009689 (to J. C. Wu) and by the International Society for Heart and Lung Transplantation (to P. E. de Almeida).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Because of space limitations, we were unable to cite all the important papers relevant to bioluminescence imaging and cardiovascular research. We apologize to investigators not mentioned here who have made significant contributions to this field.
REFERENCES
- 1. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Badr CE, Hewett JW, Breakefield XO, Tannous BA. A highly sensitive assay for monitoring the secretory pathway and ER stress. PLoS One 2: e571, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bai X, Yan Y, Coleman M, Wu G, Rabinovich B, Seidensticker M, Alt E. Tracking long-term survival of intramyocardially delivered human adipose tissue-derived stem cells using bioluminescence imaging. Mol Imaging Biol 13: 633–645, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Bai X, Yan Y, Song YH, Seidensticker M, Rabinovich B, Metzele R, Bankson JA, Vykoukal D, Alt E. Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. Eur Heart J 31: 489–501, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Bhaumik S, Gambhir SS. Optical imaging of Renilla luciferase reporter gene expression in living mice. Proc Natl Acad Sci USA 99: 377–382, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Branchini BR, Ablamsky DM, Davis AL, Southworth TL, Butler B, Fan F, Jathoul AP, Pule MA. Red-emitting luciferases for bioluminescence reporter and imaging applications. Anal Biochem 396: 290–297, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Branchini BR, Ablamsky DM, Murtiashaw MH, Uzasci L, Fraga H, Southworth TL. Thermostable red and green light-producing firefly luciferase mutants for bioluminescent reporter applications. Anal Biochem 361: 253–262, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Branchini BR, Southworth TL, DeAngelis JP, Roda A, Michelini E. Luciferase from the Italian firefly Luciola italica: molecular cloning and expression. Comp Biochem Physiol B Biochem Mol Biol 145: 159–167, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Cao F, Lin S, Xie X, Ray P, Patel M, Zhang X, Drukker M, Dylla SJ, Connolly AJ, Chen X, Weissman IL, Gambhir SS, Wu JC. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation 113: 1005–1014, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cao F, Wagner RA, Wilson KD, Xie X, Fu JD, Drukker M, Lee A, Li RA, Gambhir SS, Weissman IL, Robbins RC, Wu JC. Transcriptional and functional profiling of human embryonic stem cell-derived cardiomyocytes. PLoS One 3: e3474, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao YA, Bachmann MH, Beilhack A, Yang Y, Tanaka M, Swijnenburg RJ, Reeves R, Taylor-Edwards C, Schulz S, Doyle TC, Fathman CG, Robbins RC, Herzenberg LA, Negrin RS, Contag CH. Molecular imaging using labeled donor tissues reveals patterns of engraftment, rejection, and survival in transplantation. Transplantation 80: 134–139, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol 50: 1884–1893, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Habib IH, Gepstein L, Levenberg S. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res 100: 263–272, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Caysa H, Jacob R, Muther N, Branchini B, Messerle M, Soling A. A redshifted codon-optimized firefly luciferase is a sensitive reporter for bioluminescence imaging. Photochem Photobiol Sci 8: 52–56, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Chen IY, Wu JC. Cardiovascular molecular imaging: focus on clinical translation. Circulation 123: 425–443, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Close DM, Patterson SS, Ripp S, Baek SJ, Sanseverino J, Sayler GS. Autonomous bioluminescent expression of the bacterial luciferase gene cassette (lux) in a mammalian cell line. PLoS One 5: e12441, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Contag CH. In vivo pathology: seeing with molecular specificity and cellular resolution in the living body. Annu Rev Pathol 2: 277–305, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Contag CH, Contag PR, Mullins JI, Spilman SD, Stevenson DK, Benaron DA. Photonic detection of bacterial pathogens in living hosts. Mol Microbiol 18: 593–603, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Contag CH, Jenkins D, Contag FR, Negrin RS. Use of reporter genes for optical measurements of neoplastic disease in vivo. Neoplasia 2: 41–52, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Contag PR, Olomu IN, Stevenson DK, Contag CH. Bioluminescent indicators in living mammals. Nat Med 4: 245–247, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Cui K, Xu X, Zhao H, Wong ST. A quantitative study of factors affecting in vivo bioluminescence imaging. Luminescence 23: 292–295, 2008 [DOI] [PubMed] [Google Scholar]
- 22. De A, Yaghoubi SS, Gambhir SS. Applications of lentiviral vectors in noninvasive molecular imaging. Methods Mol Biol 433: 177–202, 2008 [DOI] [PubMed] [Google Scholar]
- 23. de Wet JR, Wood KV, Helinski DR, DeLuca M. Cloning of firefly luciferase cDNA and the expression of active luciferase in Escherichia coli. Proc Natl Acad Sci USA 82: 7870–7873, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deuse T, Peter C, Fedak PW, Doyle T, Reichenspurner H, Zimmermann WH, Eschenhagen T, Stein W, Wu JC, Robbins RC, Schrepfer S. Hepatocyte growth factor or vascular endothelial growth factor gene transfer maximizes mesenchymal stem cell-based myocardial salvage after acute myocardial infarction. Circulation 120: S247–S254, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Drukker M, Benvenisty N. The immunogenicity of human embryonic stem-derived cells. Trends Biotechnol 22: 136–141, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Gritti A, Frolichsthal-Schoeller P, Galli R, Parati EA, Cova L, Pagano SF, Bjornson CR, Vescovi AL. Epidermal and fibroblast growth factors behave as mitogenic regulators for a single multipotent stem cell-like population from the subventricular region of the adult mouse forebrain. J Neurosci 19: 3287–3297, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gruber PJ, Li Z, Li H, Worrad D, Huang B, Abdullah I, Wang W, El-Deiry W, Ferrari VA, Zhou R. In vivo imaging of MLC2v-luciferase, a cardiac-specific reporter gene expression in mice. Acad Radiol 11: 1022–1028, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gupta R, Tongers J, Losordo DW. Human studies of angiogenic gene therapy. Circ Res 105: 724–736, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hastings JW, Gibson QH. The role of oxygen in the photoexcited luminescence of bacterial luciferase. J Biol Chem 242: 720–726, 1967 [PubMed] [Google Scholar]
- 30. Hastings JW, McElroy WD, Coulombre J. The effect of oxygen upon the immobilization reaction in firefly luminescence. J Cell Physiol 42: 137–150, 1953 [DOI] [PubMed] [Google Scholar]
- 31. Hastings JW, Wilson T. Bioluminescence and chemiluminescence. Photochem Photobiol 23: 461–473, 1976 [DOI] [PubMed] [Google Scholar]
- 32. Huang M, Chan DA, Jia F, Xie X, Li Z, Hoyt G, Robbins RC, Chen X, Giaccia AJ, Wu JC. Short hairpin RNA interference therapy for ischemic heart disease. Circulation 118: S226–S233, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang M, Chen Z, Hu S, Jia F, Li Z, Hoyt G, Robbins RC, Kay MA, Wu JC. Novel minicircle vector for gene therapy in murine myocardial infarction. Circulation 120: S230–S237, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kammili RK, Taylor DG, Xia J, Osuala K, Thompson K, Menick DR, Ebert SN. Generation of novel reporter stem cells and their application for molecular imaging of cardiac-differentiated stem cells in vivo. Stem Cells Dev 19: 1437–1448, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, Kearney M, Zak V, Asahara T, Losordo DW. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation 114: 2163–2169, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Keravala A, Calos MP. Site-specific chromosomal integration mediated by phiC31 integrase. Methods Mol Biol 435: 165–173, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Kutschka I, Chen IY, Kofidis T, Arai T, von Degenfeld G, Sheikh AY, Hendry SL, Pearl J, Hoyt G, Sista R, Yang PC, Blau HM, Gambhir SS, Robbins RC. Collagen matrices enhance survival of transplanted cardiomyoblasts and contribute to functional improvement of ischemic rat hearts. Circulation 114: I167–I173, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Kutschka I, Chen IY, Kofidis T, von Degenfeld G, Sheikh AY, Hendry SL, Hoyt G, Pearl J, Blau HM, Gambhir SS, Robbins RC. In vivo optical bioluminescence imaging of collagen-supported cardiac cell grafts. J Heart Lung Transplant 26: 273–280, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Kutschka I, Kofidis T, Chen IY, von Degenfeld G, Zwierzchoniewska M, Hoyt G, Arai T, Lebl DR, Hendry SL, Sheikh AY, Cooke DT, Connolly A, Blau HM, Gambhir SS, Robbins RC. Adenoviral human BCL-2 transgene expression attenuates early donor cell death after cardiomyoblast transplantation into ischemic rat hearts. Circulation 114: I174–I180, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol 25: 1015–1024, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Lee AS, Tang C, Cao F, Xie X, van der Bogt K, Hwang A, Connolly AJ, Robbins RC, Wu JC. Effects of cell number on teratoma formation by human embryonic stem cells. Cell Cycle 8: 2608–2612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li Z, Lee A, Huang M, Chun H, Chung J, Chu P, Hoyt G, Yang P, Rosenberg J, Robbins RC, Wu JC. Imaging survival and function of transplanted cardiac resident stem cells. J Am Coll Cardiol 53: 1229–1240, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li Z, Wilson KD, Smith B, Kraft DL, Jia F, Huang M, Xie X, Robbins RC, Gambhir SS, Weissman IL, Wu JC. Functional and transcriptional characterization of human embryonic stem cell-derived endothelial cells for treatment of myocardial infarction. PLoS One 4: e8443, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li Z, Wu JC, Sheikh AY, Kraft D, Cao F, Xie X, Patel M, Gambhir SS, Robbins RC, Cooke JP. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation 116: I46–I54, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Loening AM, Dragulescu-Andrasi A, Gambhir SS. A red-shifted Renilla luciferase for transient reporter-gene expression. Nat Methods 7: 5–6, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Loening AM, Fenn TD, Wu AM, Gambhir SS. Consensus guided mutagenesis of Renilla luciferase yields enhanced stability and light output. Protein Eng Des Sel 19: 391–400, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Loening AM, Wu AM, Gambhir SS. Red-shifted Renilla reniformis luciferase variants for imaging in living subjects. Nat Methods 4: 641–643, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Lorenz WW, Mccann RO, Longiaru M, Cormier MJ. Isolation and Expression of a Cdna-Encoding Renilla-Reniformis Luciferase. Proc Natl Acad Sci USA 88: 4438–4442, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Luker GD, Pica CM, Song J, Luker KE, Piwnica-Worms D. Imaging 26S proteasome activity and inhibition in living mice. Nat Med 9: 969–973, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Ma L, Xiang Z, Sherrill TP, Wang L, Blackwell TS, Williams P, Chong A, Chari R, Yin DP. Bioluminescence imaging visualizes activation of nuclear factor-kappaB in mouse cardiac transplantation. Transplantation 85: 903–910, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Markkanen JE, Rissanen TT, Kivela A, Yla-Herttuala S. Growth factor-induced therapeutic angiogenesis and arteriogenesis in the heart—gene therapy. Cardiovasc Res 65: 656–664, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Mccapra F, Hart R. The origins of marine bioluminescence. Nature 286: 660–661, 1980 [Google Scholar]
- 54. Menasche P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, Lake S, Chatellier G, Solomon S, Desnos M, Hagege AA. The myoblast autologous grafting in ischemic cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation 117: 1189–1200, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Meyer GP, Wollert KC, Lotz J, Pirr J, Rager U, Lippolt P, Hahn A, Fichtner S, Schaefer A, Arseniev L, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up from the randomized-controlled BOOST trial. Eur Heart J 30: 2978–2984, 2009 [DOI] [PubMed] [Google Scholar]
- 56. Mias C, Lairez O, Trouche E, Roncalli J, Calise D, Seguelas MH, Ordener C, Piercecchi-Marti MD, Auge N, Salvayre AN, Bourin P, Parini A, Cussac D. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells 27: 2734–2743, 2009 [DOI] [PubMed] [Google Scholar]
- 57. Miura M, Morita K, Kobayashi H, Hamilton TA, Burdick MD, Strieter RM, Fairchild RL. Monokine induced by IFN-gamma is a dominant factor directing T cells into murine cardiac allografts during acute rejection. J Immunol 167: 3494–3504, 2001 [DOI] [PubMed] [Google Scholar]
- 58. Moriyama EH, Niedre MJ, Jarvi MT, Mocanu JD, Moriyama Y, Subarsky P, Li B, Lilge LD, Wilson BC. The influence of hypoxia on bioluminescence in luciferase-transfected gliosarcoma tumor cells in vitro. Photochem Photobiol Sci 7: 675–680, 2008 [DOI] [PubMed] [Google Scholar]
- 59. Mummery CL, van Laake LW, Passier R, den Ouden K, Schreurs C, Monshouwer-Kloots J, Ward-van Oostwaard D, van Echteld CJ, Doevendans PA. Improvement of mouse cardiac function by hESC-derived cardiomyocytes correlates with vascularity but not graft size. Stem Cell Res 3: 106–112, 2009 [DOI] [PubMed] [Google Scholar]
- 60. Na IK, Markley JC, Tsai JJ, Yim NL, Beattie BJ, Klose AD, Holland AM, Ghosh A, Rao UK, Stephan MT, Serganova I, Santos EB, Brentjens RJ, Blasberg RG, Sadelain M, van den Brink MR. Concurrent visualization of trafficking, expansion, and activation of T lymphocytes and T-cell precursors in vivo. Blood 116: e18–e25, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Niers JM, Kerami M, Pike L, Lewandrowski G, Tannous BA. Multimodal in vivo imaging and blood monitoring of intrinsic and extrinsic apoptosis. Mol Ther 19: 1090–1096, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA 100: 12313–12318, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pearl JI, Lee AS, Leveson-Gower DB, Sun N, Ghosh Z, Lan F, Ransohoff J, Negrin RS, Davis MM, Wu JC. Short-term immunosuppression promotes engraftment of embryonic and induced pluripotent stem cells. Cell Stem Cell 8: 309–317, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pennell DJ. Cardiovascular magnetic resonance. Circulation 121: 692–705, 2010 [DOI] [PubMed] [Google Scholar]
- 65. Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci USA 106: 14022–14027, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Raty JK, Liimatainen T, Unelma Kaikkonen M, Grohn O, Airenne KJ, Yla-Herttuala S. Non-invasive imaging in gene therapy. Mol Ther 15: 1579–1586, 2007 [DOI] [PubMed] [Google Scholar]
- 67. Ray P, Bauer E, Iyer M, Barrio JR, Satyamurthy N, Phelps ME, Herschman HR, Gambhir SS. Monitoring gene therapy with reporter gene imaging. Semin Nucl Med 31: 312–320, 2001 [DOI] [PubMed] [Google Scholar]
- 68. Rehemtulla A, Stegman LD, Cardozo SJ, Gupta S, Hall DE, Contag CH, Ross BD. Rapid and quantitative assessment of cancer treatment response using in vivo bioluminescence imaging. Neoplasia 2: 491–495, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rice BW, Cable MD, Nelson MB. In vivo imaging of light-emitting probes. J Biomed Opt 6: 432–440, 2001 [DOI] [PubMed] [Google Scholar]
- 70. Rubinshtein R, Halon DA, Gaspar T, Jaffe R, Karkabi B, Flugelman MY, Kogan A, Shapira R, Peled N, Lewis BS. Usefulness of 64-slice cardiac computed tomographic angiography for diagnosing acute coronary syndromes and predicting clinical outcome in emergency department patients with chest pain of uncertain origin. Circulation 115: 1762–1768, 2007 [DOI] [PubMed] [Google Scholar]
- 71. Sato T, Ramsubir S, Higuchi K, Yanagisawa T, Medin JA. Vascular endothelial growth factor broadens lentivector distribution in the heart after neonatal injection. J Cardiol 54: 245–254, 2009 [DOI] [PubMed] [Google Scholar]
- 72. Sheikh AY, Lin SA, Cao F, Cao Y, van der Bogt KE, Chu P, Chang CP, Contag CH, Robbins RC, Wu JC. Molecular imaging of bone marrow mononuclear cell homing and engraftment in ischemic myocardium. Stem Cells 25: 2677–2684, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stehlik J, Edwards LB, Kucheryavaya AY, Aurora P, Christie JD, Kirk R, Dobbels F, Rahmel AO, Hertz MI. The registry of the international society for heart and lung transplantation: twenty-seventh official adult heart transplant report—2010. J Heart Lung Transplant 29: 1089–1103, 2010 [DOI] [PubMed] [Google Scholar]
- 74. Sun X, Annala AJ, Yaghoubi SS, Barrio JR, Nguyen KN, Toyokuni T, Satyamurthy N, Namavari M, Phelps ME, Herschman HR, Gambhir SS. Quantitative imaging of gene induction in living animals. Gene Ther 8: 1572–1579, 2001 [DOI] [PubMed] [Google Scholar]
- 75. Sweeney TJ, Mailander V, Tucker AA, Olomu AB, Zhang W, Cao Y, Negrin RS, Contag CH. Visualizing the kinetics of tumor-cell clearance in living animals. Proc Natl Acad Sci USA 96: 12044–12049, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Swijnenburg RJ, Govaert JA, van der Bogt KE, Pearl JI, Huang M, Stein W, Hoyt G, Vogel H, Contag CH, Robbins RC, Wu JC. Timing of bone marrow cell delivery has minimal effects on cell viability and cardiac recovery after myocardial infarction. Circ Cardiovasc Imag 3: 77–85, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Swijnenburg RJ, Schrepfer S, Govaert JA, Cao F, Ransohoff K, Sheikh AY, Haddad M, Connolly AJ, Davis MM, Robbins RC, Wu JC. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc Natl Acad Sci USA 105: 12991–12996, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tanaka M, Swijnenburg RJ, Gunawan F, Cao YA, Yang Y, Caffarelli AD, de Bruin JL, Contag CH, Robbins RC. In vivo visualization of cardiac allograft rejection and trafficking passenger leukocytes using bioluminescence imaging. Circulation 112: I105–I110, 2005 [DOI] [PubMed] [Google Scholar]
- 79. Tannous BA, Kim DE, Fernandez JL, Weissleder R, Breakefield XO. Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol Ther 11: 435–443, 2005 [DOI] [PubMed] [Google Scholar]
- 80. Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet 4: 346–358, 2003 [DOI] [PubMed] [Google Scholar]
- 81. Tromberg BJ, Shah N, Lanning R, Cerussi A, Espinoza J, Pham T, Svaasand L, Butler J. Non-invasive in vivo characterization of breast tumors using photon migration spectroscopy. Neoplasia 2: 26–40, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. van der Bogt KE, Schrepfer S, Yu J, Sheikh AY, Hoyt G, Govaert JA, Velotta JB, Contag CH, Robbins RC, Wu JC. Comparison of transplantation of adipose tissue- and bone marrow-derived mesenchymal stem cells in the infarcted heart. Transplantation 87: 642–652, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. van der Bogt KE, Sheikh AY, Schrepfer S, Hoyt G, Cao F, Ransohoff KJ, Swijnenburg RJ, Pearl J, Lee A, Fischbein M, Contag CH, Robbins RC, Wu JC. Comparison of different adult stem cell types for treatment of myocardial ischemia. Circulation 118: S121–S129, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. van der Bogt KE, Swijnenburg RJ, Cao F, Wu JC. Molecular imaging of human embryonic stem cells: keeping an eye on differentiation, tumorigenicity and immunogenicity. Cell Cycle 5: 2748–2752, 2006 [DOI] [PubMed] [Google Scholar]
- 86. Verkhusha VV, Otsuna H, Awasaki T, Oda H, Tsukita S, Ito K. An enhanced mutant of red fluorescent protein DsRed for double labeling and developmental timer of neural fiber bundle formation. J Biol Chem 276: 29621–29624, 2001 [DOI] [PubMed] [Google Scholar]
- 87. Vilalta M, Jorgensen C, Degano IR, Chernajovsky Y, Gould D, Noel D, Andrades JA, Becerra J, Rubio N, Blanco J. Dual luciferase labelling for non-invasive bioluminescence imaging of mesenchymal stromal cell chondrogenic differentiation in demineralized bone matrix scaffolds. Biomaterials 30: 4986–4995, 2009 [DOI] [PubMed] [Google Scholar]
- 88. Viviani VR, Arnoldi FG, Brochetto-Braga M, Ohmiya Y. Cloning and characterization of the cDNA for the Brazilian Cratomorphus distinctus larval firefly luciferase: similarities with European Lampyris noctiluca and Asiatic Pyrocoelia luciferases. Comp Biochem Physiol B Biochem Mol Biol 139: 151–156, 2004 [DOI] [PubMed] [Google Scholar]
- 89. Wang J, Zhang S, Rabinovich B, Bidaut L, Soghomonyan S, Alauddin MM, Bankson JA, Shpall E, Willerson JT, Gelovani JG, Yeh ET. Human CD34+ cells in experimental myocardial infarction: long-term survival, sustained functional improvement, and mechanism of action. Circ Res 106: 1904–1911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang XL, Rosol M, Ge SD, Peterson D, McNamara G, Pollack H, Kohn DB, Nelson MD, Crooks GM. Dynamic tracking of human hematopoietic stem cell engraftment using in vivo bioluminescence imaging. Blood 102: 3478–3482, 2003 [DOI] [PubMed] [Google Scholar]
- 91. Weissleder R. A clearer vision for in vivo imaging. Nat Biotechnol 19: 316–317, 2001 [DOI] [PubMed] [Google Scholar]
- 92. Welt FG, Losordo DW. Cell therapy for acute myocardial infarction: curb your enthusiasm? Circulation 113: 1272–1274, 2006 [DOI] [PubMed] [Google Scholar]
- 93. Widder EA. Bioluminescence in the ocean: origins of biological, chemical, and ecological diversity. Science 328: 704–708, 2010 [DOI] [PubMed] [Google Scholar]
- 94. Wu JC, Chen IY, Sundaresan G, Min JJ, De A, Qiao JH, Fishbein MC, Gambhir SS. Molecular imaging of cardiac cell transplantation in living animals using optical bioluminescence and positron emission tomography. Circulation 108: 1302–1305, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94a. Wu JC, Inubushi M, Sundaresan G, Schelbert HR, Gambhir SS. Positron emission tomography imaging of cardiac reporter gene expression in living rats. Circulation 106: 180–183, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wu JC, Sundaresan G, Iyer M, Gambhir SS. Noninvasive optical imaging of firefly luciferase reporter gene expression in skeletal muscles of living mice. Mol Ther 4: 297–306, 2001 [DOI] [PubMed] [Google Scholar]
- 96. Wurdinger T, Badr C, Pike L, de Kleine R, Weissleder R, Breakefield XO, Tannous BA. A secreted luciferase for ex vivo monitoring of in vivo processes. Nat Methods 5: 171–173, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ye L, Haider H, Tan R, Toh W, Law PK, Tan W, Su L, Zhang W, Ge R, Zhang Y, Lim Y, Sim EK. Transplantation of nanoparticle transfected skeletal myoblasts overexpressing vascular endothelial growth factor-165 for cardiac repair. Circulation 116: I113–I120, 2007 [DOI] [PubMed] [Google Scholar]
- 98. Yu J, Huang NF, Wilson KD, Velotta JB, Huang M, Li Z, Lee A, Robbins RC, Cooke JP, Wu JC. nAChRs mediate human embryonic stem cell-derived endothelial cells: proliferation, apoptosis, and angiogenesis. PLoS One 4: e7040, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhang N, Weber A, Li B, Lyons R, Contag PR, Purchio AF, West DB. An inducible nitric oxide synthase-luciferase reporter system for in vivo testing of anti-inflammatory compounds in transgenic mice. J Immunol 170: 6307–6319, 2003 [DOI] [PubMed] [Google Scholar]
- 100. Zhao H, Doyle TC, Coquoz O, Kalish F, Rice BW, Contag CH. Emission spectra of bioluminescent reporters and interaction with mammalian tissue determine the sensitivity of detection in vivo. J Biomed Opt 10: 41210, 2005 [DOI] [PubMed] [Google Scholar]
- 101. Zhao H, Doyle TC, Wong RJ, Cao Y, Stevenson DK, Piwnica-Worms D, Contag CH. Characterization of coelenterazine analogs for measurements of Renilla luciferase activity in live cells and living animals. Mol Imaging 3: 43–54, 2004 [DOI] [PubMed] [Google Scholar]
- 102. Zhou R, Acton PD, Ferrari VA. Imaging stem cells implanted in infarcted myocardium. J Am Coll Cardiol 48: 2094–2106, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]