Abstract
Endothelin (ET)-1-mediated vasoconstrictor tone contributes to the development and progression of several adiposity-related conditions, including hypertension and atherosclerotic vascular disease. The aims of the present study were to determine 1) whether endogenous ET-1 vasoconstrictor activity is elevated in overweight and obese adults, and, if so, 2) whether increased ET-1-mediated vasoconstriction contributes to the adiposity-related impairment in endothelium-dependent vasodilation. Seventy-nine adults were studied: 34 normal weight [body mass index (BMI) < 25 kg/m2], 22 overweight (BMI ≥ 25 and < 30 kg/m2), and 23 obese (BMI ≥ 30 kg/m2). Forearm blood flow (FBF) responses to intra-arterial infusion of ET-1 (5 pmol/min for 20 min) and selective ET-1 receptor blockade (BQ-123, 100 nmol/min for 60 min) were determined. In a subset of the study population, FBF responses to ACh (4.0, 8.0, and 16.0 μg·100 ml tissue−1·min−1) were measured in the absence and presence of selective ET-1 receptor blockade. The vasoconstrictor response to ET-1 was significantly blunted in overweight and obese adults (∼70%) compared with normal weight adults. Selective ET-1 receptor blockade elicited a significant vasodilator response (∼20%) in overweight and obese adults but did not alter FBF in normal weight adults. Coinfusion of BQ-123 did not affect FBF responses to ACh in normal weight adults but resulted in an ∼20% increase (P < 0.05) in ACh-induced vasodilation in overweight and obese adults. These results demonstrate that overweight and obesity are associated with enhanced ET-1-mediated vasoconstriction that contributes to endothelial vasodilator dysfunction and may play a role in the increased prevalence of hypertension with increased adiposity.
Keywords: endothelial function, vascular function, vasoconstriction, hypertension
overweight and obesity are associated with impaired endothelium-dependent vasodilation, a hallmark characteristic of endothelial dysfunction that heightens the risk of coronary artery disease, hypertension, and atherothrombotic events (36, 37, 49). Forearm blood flow (FBF) responses to a variety of endothelial vasodilator agonists have been shown to be markedly impaired in overweight and obese adults compared with their normal weight peers (49). The mechanisms underlying the endothelial vasodilator dysfunction associated with excess adiposity are not completely understood. Interestingly, the contribution of nitric oxide (NO) to endothelium-dependent vasodilation is well preserved in overweight and obese adults, suggesting that other factors, besides NO, underlie the adiposity-related impairment in endothelial vasodilator function (49).
Endothelin (ET)-1 is the most potent vasoconstrictor peptide released by the endothelium and is recognized to play a key role in the regulation of vascular tone and the etiology of atherosclerotic vascular disease (32, 44). Enhanced ET-1 system activity has been linked to the development and progression of a number of adiposity-related cardiovascular pathologies, including hypertension, type 2 diabetes, coronary artery disease, and chronic heart failure (7, 22, 30, 46, 48). Mather and colleagues (30) have shown that ET-1 vasoconstrictor tone is elevated in obese adults with type 2 diabetes and metabolic syndrome. It is currently unknown if ET-1-mediated vasoconstrictor tone is elevated in overweight and obese adults independent of other cardiovascular risk factors.
Accordingly, the aims of the present study were to determine 1) whether endogenous ET-1 vasoconstrictor activity is elevated in overweight and obese adults, and, if so, 2) whether increased ET-1-mediated vasoconstriction contributes to the adiposity-related impairment in endothelium-dependent vasodilation in this population. To address these aims, we performed two complementary experiments. First, FBF responses to exogenous ET-1 and selective ETA receptor antagonism were determined in normal weight, overweight, and obese adults that were free of cardiometabolic risk factors. Second, in a separate group of normal weight, overweight, and obese adults, ACh-mediated endothelium-dependent vasodilation was assessed in the absence and presence of ETA receptor blockade. We hypothesized that ET-1 vasoconstrictor activity is greater in overweight and obese adults compared with normal weight adults and that the elevation in ET-1-mediated vasoconstriction contributes to impaired endothelium-dependent vasodilation in overweight and obese adults.
METHODS
Subjects
Seventy-nine middle-aged and older adults (age range: 43–66 yr) were studied: 34 normal weight [body mass index (BMI) < 25 kg/m2, 16 men and 18 women], 22 overweight (BMI ≥ 25 and < 30 kg/m2, 10 men and 12 women), and 23 obese (BMI ≥ 30 kg/m2, 17 men and 6 women). All subjects were sedentary and had not participated in a regular aerobic exercise program for at least 1 yr before the start of the study. Subjects were excluded from the study if they presented a history or evidence of hepatic, renal, or hematological disease; peripheral vascular disease; stroke; diabetes (fasting plasma glucose > 7.0 mmol/l) (3); dyslipoproteinemia (total cholesterol ≥ 6.2 mmol/l, triglycerides ≥ 3.5 mmol/l) (12); and hypertension (arterial blood pressure ≥ 140/90 mmHg) (11). All subjects were screened for clinical evidence of cardiovascular disease by medical history, physical examination, fasting blood chemistries, and electrocardiograms and blood pressure at rest and during incremental exercise performed to exhaustion. None of the subjects smoked or were taking medications, including vitamins. All of the women were at least 1 yr postmenopausal and had never taken or had discontinued use of hormone replacement therapy at least 1 yr before the start of the study. Before participation, all of the subjects had the research study and its potential risks and benefits explained fully before providing written informed consent. This study was approved by the Institutional Review Board of the University of Colorado (Boulder, CO).
Measurements
Body composition.
Body mass was measured to the nearest 0.1 kg using a medical beam balance (Detecto, Webb City, MO). Percent body fat was determined by dual-energy X-ray absorptiometry (Lunar Radiation, Madison, WI). BMI was calculated as weight (in kg) divided by height (in m2). Minimal waist circumference was measured according to published guidelines (28).
Metabolic measurements.
Fasting plasma lipid, lipoprotein, glucose, and insulin concentrations were determined using standard techniques as previously described (49). Insulin resistance was estimated using homeostasis model assessment (HOMA-IR) according to the following equation: fasting insulin (in mU/ml) × fasting glucose (in mmol/l)/22.5 (31).
Intra-Arterial Infusion Experiments
All experiments were performed between 7:00 and 10:00 AM after a 12-h overnight fast in a temperature-controlled room. Under strict aseptic conditions, a 5-cm, 20-gauge catheter was inserted into the brachial artery of the nondominant arm under local anesthesia (1% lidocaine). Heart rate and arterial blood pressure were continuously measured throughout the infusion protocol. FBF was measured in both the experimental (nondominant) and contralateral (dominant) forearm using strain-gauge venous occlusion plethysmography (D. E. Hokanson, Bellevue, WA), as previously described by our laboratory (50).
Experiment 1: FBF responses to exogenous ET-1 and ETA receptor blockade.
Thirty-nine of the seventy-nine subjects participated in experiment 1. FBF responses to exogenous ET-1 and selective ETA receptor blockade were determined in 15 normal weight (BMI: 22.6 ± 0.4 kg/m2, 7 men and 8 women), 13 overweight (BMI: 27.6 ± 0.3 kg/m2, 7 men and 6 women) and 11 obese (BMI: 32.4 ± 0.5 kg/m2, 8 men and 3 women) adults. To rule out the possibility of nonspecific differences to vasoconstrictor agents with overweight and obesity, vascular responses to norepinephrine were determined. Norepinephrine was infused at a rate of 260 pmol/min for 5 min, and FBF was measured during the last 3 min. After a 30-min rest period to allow FBF to return to baseline levels, ET-1 (Clinalfa) was infused at a rate of 5 pmol/min for 20 min, and FBF was measured during the last 3 min. After FBF was allowed to return to baseline (∼30 min), BQ-123 (Clinalfa), a selective ETA receptor antagonist, was infused at a rate of 100 nmol/min for 60 min. FBF was measured every 10 min throughout the infusion period. The selected dose of BQ-123 has been shown to completely inhibit the vasoconstrictor effect of ET-1 in the human forearm of healthy adults (9, 50).
Experiment 2: effect of ETA receptor blockade on endothelium-dependent vasodilation.
Forty of the seventy-nine subjects participated in experiment 2. None of the subjects in experiment 2 participated in experiment 1. FBF responses to ACh were measured in the absence and presence of BQ-123 in 19 normal weight (BMI: 23.0 ± 0.3 kg/m2, 9 men and 10 women), 9 overweight (BMI: 27.3 ± 0.4 kg/m2, 3 men and 6 women), and 12 obese (BMI: 32.6 ± 0.6 kg/m2, 9 men and 3 women) subjects. After the measurement of resting blood flow for 5 min, FBF was assessed in response to infusions of ACh (IOLAB Pharmaceuticals, Duluth, GA) at 4.0, 8.0, and 16.0 μg·100 ml tissue−1·min−1 and sodium nitroprusside (SNP; Nitropress, Abbott Laboratories) at 1.0, 2.0, and 4.0 μg·100 ml tissue−1·min−1. Each dose of ACh and SNP was infused for ∼5 min, and sufficient time (∼20 min) was allowed for FBF to return to resting levels between each vasoactive agent. To avoid an order effect, the sequence of drug administration was randomized. After the initial infusion of ACh and SNP, BQ-123 was infused in an identical manner to experiment 1. After 60 min, the infusion of BQ-123 was continued at the same dose, and FBF was reassessed during coadministration of ACh as performed earlier.
Statistical Analysis
Differences in subject baseline characteristics and the magnitude of change in FBF to norepinephrine and ET-1 were determined by between-groups ANOVA. Group differences in FBF responses to BQ-123, ACh, and SNP were determined by repeated-measures ANOVA. Relations between variables of interest were assessed by linear and stepwise regression analysis. There were no significant sex interactions; therefore, the data were pooled and presented together. All data are expressed as means ± SE. Statistical significance was set a priori at P < 0.05.
RESULTS
Selected subject characteristics are shown in Table 1. By design, body mass and body composition values were greater (P < 0.05) in the overweight and obese groups compared with the normal weight group. All subjects were normotensive, normolipidemic, and normoglycemic; overweight and obese subjects demonstrated higher (P < 0.05) resting systolic and diastolic blood pressure, plasma triglycerides, and insulin concentrations compared with normal weight subjects. FBF in the noninfused arm and mean arterial blood pressure remained constant throughout the infusion protocol and did not differ significantly between groups (data not shown).
Table 1.
Selected subject characteristics
| Parameter | Normal Weight | Overweight | Obese |
|---|---|---|---|
| No. of subjects | 34 | 22 | 23 |
| Sex, men/women | 16/18 | 10/12 | 17/6 |
| Age, yr | 55 ± 1 | 56 ± 1 | 57 ± 1 |
| Body mass, kg | 67.3 ± 1.6 | 78.9 ± 2.2* | 98.1 ± 1.9*† |
| Body mass index, kg/m2 | 22.9 ± 0.2 | 27.6 ± 0.3* | 32.5 ± 0.4*† |
| Body fat, % | 28.5 ± 1.7 | 36.9 ± 1.8* | 38.3 ± 1.6* |
| Waist circumference, cm | 79.4 ± 1.5 | 91.1 ± 1.7* | 105.2 ± 1.7*† |
| Systolic blood pressure, mmHg | 118 ± 2 | 125 ± 2* | 126 ± 2* |
| Diastolic blood pressure, mmHg | 73 ± 1 | 79 ± 1* | 79 ± 1* |
| Total cholesterol, mmol/l | 5.2 ± 0.1 | 5.3 ± 0.1 | 5.3 ± 0.1 |
| LDL-cholesterol, mmol/l | 3.2 ± 0.1 | 3.4 ± 0.1 | 3.4 ± 0.1 |
| HDL-cholesterol, mmol/l | 1.5 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.1 |
| Triglycerides, mmol/l | 1.1 ± 0.1 | 1.4 ± 0.1* | 1.3 ± 0.1* |
| Glucose, mmol/l | 5.0 ± 0.1 | 5.2 ± 0.1 | 5.1 ± 0.1 |
| Insulin, pmol/l | 37.0 ± 3.3 | 54.7 ± 7.1 | 71.1 ± 8.4* |
| Homeostasis model assessment | 1.2 ± 0.1 | 1.8 ± 0.2 | 2.4 ± 0.3* |
Values are means ± SE.
P < 0.05 vs. normal weight adults;
P < overweight adults.
Experiment 1: FBF Responses to Exogenous ET-1 and ETA Receptor Blockade
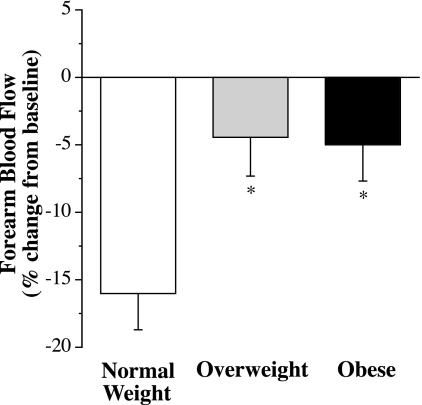
The vasoconstrictor response to norepinephrine was not significantly different among groups (data not shown). FBF was reduced by ∼30% in all three groups. In contrast, the vasoconstrictor response to ET-1 was significantly lower (P = 0.009) in overweight and obese adults compared with normal weight adults. Resting FBF was reduced by ∼5% in overweight (from 4.1 ± 0.3 to 3.9 ± 0.3 ml·100 ml tissue−1·min−1) and obese (from 4.1 ± 0.2 to 3.9 ± 0.3 ml·100 ml tissue−1·min−1) adults compared with ∼15% in normal weight (from 3.6 ± 0.2 to 2.9 ± 0.2 ml·100 ml tissue−1·min−1) adults (Fig. 1). The vasoconstrictor response to ET-1 was not significantly different between overweight and obese adults. In the overall study population, the magnitude of vasoconstriction in response to ET-1 was inversely related to the following (all P < 0.05): BMI (r = −0.43, P = 0.006), waist circumference (r = −0.39, P = 0.018), diastolic blood pressure (r = −0.39, P = 0.018), systolic blood pressure (r = −0.38, P = 0.019), serum triglyceride concentrations (r = −0.35, P = 0.035), and body mass (r = −0.33, P = 0.036). Stepwise regression analysis revealed that BMI was the primary determinant of the FBF response to ET-1, accounting for ∼20% of the variability.
Fig. 1.
Forearm blood flow (FBF) responses to endothelin (ET)-1 (5 pmol/min for 20 min) in normal weight, overweight, and obese adults. Values are means ± SE. *P < 0.05 vs. normal weight adults.
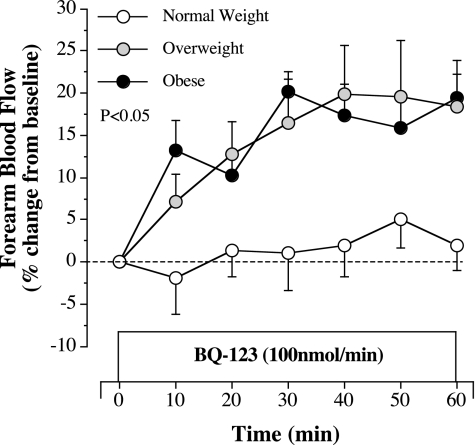
FBF responses to selective ETA receptor blockade with BQ-123 were markedly different (P = 0.041) between groups. In normal weight adults, resting FBF was not significantly altered by BQ-123, whereas a significant vasodilator response (∼20%) was observed in both the overweight and obese groups (Fig. 2). Of note, FBF responses to BQ-123 were not significantly different between the overweight and obese groups. The peak FBF response to BQ-123 was significantly related to body mass (r = 0.54, P = 0.001), waist circumference (r = 0.52, P = 0.001), systolic blood pressure (r = 0.51, P = 0.001), BMI (r = 0.50, P = 0.001), diastolic blood pressure (r = 0.49, P = 0.004), and waist-to-hip ratio (r = 0.39, P = 0.017) in the study population. Stepwise regression analysis revealed that body mass (R2 = 0.29) was the primary determinant of the FBF response to BQ-123.
Fig. 2.
FBF responses to BQ-123 (100 nmol/min), a selective ETA receptor antagonist, in normal weight, overweight, and obese adults included in experiment 1. Values are means ± SE. The P value refers to the difference in the FBF response to ETA blockade in the overweight versus normal weight group and in the obese versus normal weight group.
Experiment 2: Effect of ETA Receptor Blockade on Endothelium-Dependent Vasodilation
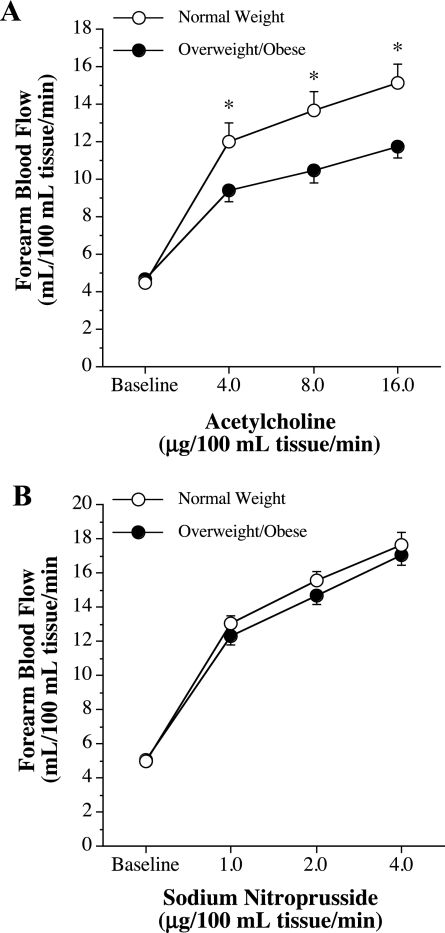
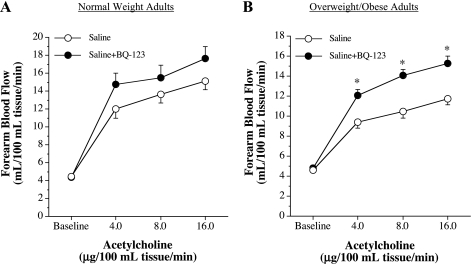
Since there were no significant differences in the FBF responses to BQ-123 and ET-1 between overweight and obese adults, these groups were combined and are presented as overweight/obese group. FBF responses to ACh were blunted (∼30%, P = 0.031) in the overweight/obese (from 4.6 ± 0.2 to 11.7 ± 0.6 ml·100 ml tissue−1·min−1) group compared with the normal weight (from 4.5 ± 0.2 to 15.2 ± 0.9 ml·100 ml tissue−1·min−1) group (Fig. 3). FBF responses to SNP were not significantly different between the groups (Fig. 3).
Fig. 3.
FBF responses to ACh (A) and sodium nitroprusside (B) in normal weight and overweight/obese adults. Values are means ± SE. *P < 0.05 vs. normal weight adults.
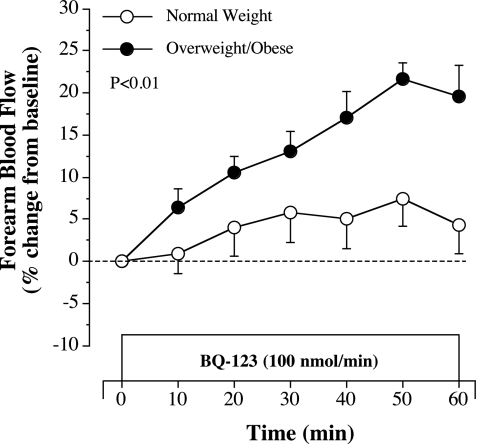
FBF responses to selective ETA receptor blockade with BQ-123 were similar to those observed in experiment 1. Indeed, resting FBF increased ∼20% in response to BQ-123 in the overweight/obese group but was largely unchanged in the normal weight group (Fig. 4). Coinfusion of BQ-123 with ACh did not significantly affect ACh-mediated vasodilation in normal weight subjects (Fig. 5). However, FBF responses to ACh increased significantly (∼30%, P = 0.001) in overweight/obese adults with BQ-123 (Fig. 5). In fact, coinfusion of BQ-123 abolished the adiposity-related difference in ACh-mediated vasodilation between the groups. FBF responses to ACh in the presence of BQ-123 were not significantly different between normal weight (from 4.4 ± 0.3 to 17.7 ± 1.3 ml·100 ml tissue−1·min−1) and the overweight/obese (from 4.7 ± 0.2 to 15.3 ± 0.7 ml·100 ml tissue−1·min−1) adults.
Fig. 4.
FBF responses to BQ-123 (100 nmol/min), a selective ETA receptor antagonist, in normal weight and overweight/obese adults included in experiment 2. Values are means ± SE. The P value refers to the difference in the FBF response to ETA blockade between the groups.
Fig. 5.
FBF responses to ACh in the absence and presence of ETA receptor blockade with BQ-123 in normal weight (A) and overweight/obese adults (B). Values are means ± SE. *P < 0.05 vs. saline.
DISCUSSION
The key findings of the present study are as follows: 1) overweight and obese adults demonstrated a blunted forearm vasoconstrictor response to exogenous ET-1 compared with normal weight adults, 2) selective ETA receptor blockade elicited a significant forearm vasodilator response in overweight and obese adults but not normal weight adults, and 3) selective ETA receptor blockade increased ACh-mediated endothelium-dependent vasodilation in overweight and obese adults to levels similar to that of normal weight adults. Collectively, these findings indicate that ET-1-mediated vasoconstrictor tone is elevated with overweight and obesity and contributes to the adiposity-related impairment in endothelium-dependent vasodilation.
Overweight and obesity are characterized by a marked impairment in endothelium-dependent vasodilation, which is thought to underlie the heightened risk of cardiovascular disease associated with increased adiposity (37, 49). Previous studies (1, 36, 45, 49) have demonstrated a reduction in endothelium-dependent vasodilation (∼40%) in the peripheral and coronary circulation of overweight and obese adults that is not limited to muscarinic receptor agonists. The results of the present study significantly extend these findings by demonstrating that overweight and obesity, independent of traditional cardiovascular risk factors, are associated with elevated ET-1-mediated vasoconstriction. Indeed, overweight and obese adults demonstrated a greater vasodilator response to selective ETA receptor blockade than their normal weight counterparts, indicative of enhanced ETA receptor-mediated ET-1 vasoconstrictor tone. In line with augmented ETA receptor activation, the vasoconstrictor response to exogenous ET-1 was lower in overweight and obese adults, suggesting that ET-1 bioavailability is elevated with increased adiposity (15). A functional consequence of the increase in ET-1 vasoconstrictor tone with overweight and obesity appears to be reduced endothelial vasodilator function. A seminal finding of the present study is that blockade of the ETA receptor restored ACh-mediated endothelium-dependent vasodilation in overweight and obese adults. Thus, ET-1-mediated vasoconstriction is a key component of adiposity-related vascular dysfunction and a viable target for therapeutic interventions aimed at improving vascular health and reducing risk in overweight and obese adults.
The mechanisms underlying increased ET-1 system activation with adiposity are unclear. Overweight and obesity are typically associated with hyperinsulinemia (13), and insulin has been shown to stimulate ET-1 production (14, 21). In the present study, overweight and obese adults had significantly higher plasma insulin concentrations and HOMA-IR values (obese group only) compared with the normal weight group; however, neither plasma insulin concentrations nor HOMA-IR were associated with FBF responses to either exogenous ET-1 or BQ-123, arguing against an insulin-related effect. Our findings with respect to insulin concentrations are consistent with those of Lteif et al. (29), who recently reported that hyperinsulinemia fails to augment ET-1 action in vivo. Other factors that may contribute to the adiposity-related elevation in ET-1 system activity include inflammation and oxidative stress. Overweight and obesity are conditions characterized by increased oxidative and inflammatory burden (16, 26), and several oxidative and inflammatory mediators associated with adiposity (such as superoxide anion, C-reactive protein, and TNF-α) can upregulate the synthesis and release of ET-1 from endothelial cells (19, 35, 39, 51). It is also possible that elevations in the sympathetic nervous system or renin-angiotensin system activity are involved in the elevation of ET-1 vasoconstrictor tone with excess adiposity. Obesity is associated with activation of these systems (2), and both epinephrine and angiotensin II can stimulate ET-1 production (38, 40). Future studies are needed to delineate the mechanisms underlying this aspect of adiposity-related vascular dysfunction.
The noted increase in endothelium-dependent vasodilation with ETA receptor blockade in the overweight/obese adults is intriguing. A logical explanation for this response is that blockade of the ETA receptor improved endothelium-dependent vasodilation by directly, or indirectly, increasing the bioavailability of NO. It has been shown that ETA-mediated ET-1 activity can reduce NO bioavailability directly via the inhibition of endothelial NO synthase or indirectly through the production of superoxide anion and subsequent inactivation of NO (6, 18, 23). However, we (49) have previously demonstrated that the contribution of NO to ACh-mediated vasodilation is not diminished in overweight/obese adults compared normal weight adults, arguing against a NO-related mechanism. It is possible that blockade of the ETA receptor simply relieved the vasoconstricting influence of ET-1, allowing the vessel to dilate in response to ACh without opposition.
From a public health perspective, ET-1 vasoconstrictor tone is involved in the regulation of blood pressure and pathogenesis of human hypertension (40). For example, systemic infusion of ET-1, or its precursor big ET-1, increases blood pressure and vascular resistance in humans (25, 41), whereas blood pressure and vascular resistance decrease after the systemic blockade of ET-1 receptors (20, 42). Moreover, chronic ET-1 receptor blockade results in a reduction in blood pressure in patients with clinical hypertension (27, 33). Excess adiposity and weight gain are also associated with high blood pressure (10, 43). Data from the Framingham Offspring Study indicated that ∼70% of hypertension in men and women can be attributed to obesity (17). A potential role for ET-1 in the pathogenesis of obesity-related hypertension has been suggested (5, 24), supported by data showing an association between ET-1 gene polymorphisms and blood pressure levels in overweight and obese adults (4, 47). Although none of the subjects in the present study were hypertensive, we observed a significant relation between blood pressure and FBF responses to exogenous ET-1 (r = −0.39) and ETA receptor antagonism (r = 0.50), supporting a putative role of ET-1 system activation in adiposity-related elevations in blood pressure. Conversely, blood pressure in the prehypertensive range, although not considered to be clinically abnormal, may be a factor in the group differences observed in the present study.
There are three experimental considerations regarding this study that should be mentioned. First, given our cross-sectional study design, we cannot discount the possibility that genetic and/or lifestyle behaviors may have influenced our results. To minimize the influence of lifestyle behaviors, we studied subjects who were nonsmokers, not currently taking any medication that could influence endothelial vasomotor function, and did not differ in habitual physical activity. In addition, in an effort to isolate the primary influence of adiposity, we studied overweight and obese adults, independent of traditional cardiovascular risk factors that often accompany increased adiposity and are associated with increased ET-1 system activity, such as hypertension (8) and type 2 diabetes (7, 30, 34). Although enhanced ET-1-mediated vasoconstriction has previously been demonstrated in obese adults with these comorbidities (8, 30), this is the first study, to our knowledge, to demonstrate elevated ET-1 vasoconstrictor tone in overweight/obese adults without concomitant risk factors. It should be noted that Cardillo and colleagues (8) previously reported a modest, nonsignificant (∼5–10%) increase in FBF to BQ-123 in normotensive obese adults, a finding that appears to contradict those from the present study. The reason for this discrepancy is unclear, although several factors may be involved, such as differences in physical and metabolic characteristics or the racial makeup of the subject population included in each study. Our study population included primarily Caucasian adults, whereas almost 40% of the adults studied by Cardillo and colleagues (8) were African-American. Second, due to limited drug availability, we were unable to administer the selective ETB receptor antagonist BQ-788 and therefore cannot comment on the influence of overweight and obesity on the vascular actions of the ETB receptor. Finally, we did not measure circulating plasma levels of ET-1 in the present study. ET-1 produced by the endothelium is predominantly (>80%) released abluminally toward the vascular smooth muscle (52); consequently, the pathophysiological significance of circulating ET-1 levels is unclear, and values reported are often inconsistent and misleading (40). Circulating plasma concentrations of the peptide do not necessarily reflect local vascular production but rather variable spillover into, and clearance from, the bloodstream (9). Intra-arterial infusion of exogenous ET-1 and ET-1 receptor antagonists offers a more direct biological assessment of ET-1 system activity in vivo (8, 9, 50).
In conclusion, the results of the present study demonstrate that both overweight and obesity are associated with enhanced ET-1-mediated vasoconstrictor tone. In addition, elevated ET-1-mediated vasoconstrictor tone contributes to diminished endothelium-dependent vasodilation observed with overweight and obesity. Given the link between ET-1 system activity and blood pressure, adiposity-related elevations in ET-1-mediated vasoconstrictor tone may contribute to the increased risk of hypertension as well as atherosclerotic vascular disease in overweight and obese adults.
GRANTS
This work was supported by National Institutes of Health Grants HL-077450, HL-076434, and MOI-RR-00051.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank all of the subjects who participated in the study as well as Yoli Casas for administrative assistance.
REFERENCES
- 1. Al Suwaidi J, Higano ST, Holmes DR, Jr, Lennon R, Lerman A. Obesity is independently associated with coronary endothelial dysfunction in patients with normal or mildly diseased coronary arteries. J Am Coll Cardiol 37: 1523–1528, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Alonso-Galicia M, Brands MW, Zappe DH, Hall JE. Hypertension in obese Zucker rats. Role of angiotensin II and adrenergic activity. Hypertension 28: 1047–1054, 1996 [DOI] [PubMed] [Google Scholar]
- 3. American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 32, Suppl 1: S62–S67, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Asai T, Ohkubo T, Katsuya T, Higaki J, Fu Y, Fukuda M, Hozawa A, Matsubara M, Kitaoka H, Tsuji I, Araki T, Satoh H, Hisamichi S, Imai Y, Ogihara T. Endothelin-1 gene variant associates with blood pressure in obese Japanese subjects: the Ohasama Study. Hypertension 38: 1321–1324, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Barton M, Carmona R, Morawietz H, d'Uscio LV, Goettsch W, Hillen H, Haudenschild CC, Krieger JE, Munter K, Lattmann T, Luscher TF, Shaw S. Obesity is associated with tissue-specific activation of renal angiotensin-converting enzyme in vivo: evidence for a regulatory role of endothelin. Hypertension 35: 329–336, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Callera GE, Touyz RM, Teixeira SA, Muscara MN, Carvalho MH, Fortes ZB, Nigro D, Schiffrin EL, Tostes RC. ETA receptor blockade decreases vascular superoxide generation in DOCA-salt hypertension. Hypertension 42: 811–817, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Cardillo C, Campia U, Bryant MB, Panza JA. Increased activity of endogenous endothelin in patients with type II diabetes mellitus. Circulation 106: 1783–1787, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Cardillo C, Campia U, Iantorno M, Panza JA. Enhanced vascular activity of endogenous endothelin-1 in obese hypertensive patients. Hypertension 43: 36–40, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Cardillo C, Kilcoyne CM, Waclawiw M, Cannon RO, 3rd, Panza JA. Role of endothelin in the increased vascular tone of patients with essential hypertension. Hypertension 33: 753–758, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Cassano PA, Segal MR, Vokonas PS, Weiss ST. Body fat distribution, blood pressure, and hypertension. A prospective cohort study of men in the normative aging study. Ann Epidemiol 1: 33–48, 1990 [DOI] [PubMed] [Google Scholar]
- 11. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289: 2560–2572, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Fedder DO, Koro CE, L'Italien GJ. New National Cholesterol Education Program III guidelines for primary prevention lipid-lowering drug therapy: projected impact on the size, sex, and age distribution of the treatment-eligible population. Circulation 105: 152–156, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR). J Clin Invest 100: 1166–1173, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferri C, Pittoni V, Piccoli A, Laurenti O, Cassone MR, Bellini C, Properzi G, Valesini G, De Mattia G, Santucci A. Insulin stimulates endothelin-1 secretion from human endothelial cells and modulates its circulating levels in vivo. J Clin Endocrinol Metab 80: 829–835, 1995 [DOI] [PubMed] [Google Scholar]
- 15. Ferro CJ, Haynes WG, Hand MF, Webb DJ. Forearm vasoconstriction to endothelin-1 is impaired, but constriction to sarafotoxin 6c and vasodilatation to BQ-123 unaltered, in patients with essential hypertension. Clin Sci (Lond) 103, Suppl 48: 53S–58S, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Festa A, D'Agostino R, Jr, Williams K, Karter AJ, Mayer-Davis EJ, Tracy RP, Haffner SM. The relation of body fat mass and distribution to markers of chronic inflammation. Int J Obes Relat Metab Disord 25: 1407–1415, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Garrison RJ, Kannel WB, Stokes J, 3rd, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prevent Med 16: 235–251, 1987 [DOI] [PubMed] [Google Scholar]
- 18. Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 320: 454–456, 1986 [DOI] [PubMed] [Google Scholar]
- 19. Haug C, Schmid-Kotsas A, Zorn U, Schuett S, Gross HJ, Gruenert A, Bachem MG. Endothelin-1 synthesis and endothelin B receptor expression in human coronary artery smooth muscle cells and monocyte-derived macrophages is up-regulated by low density lipoproteins. J Mol Cell Cardiol 33: 1701–1712, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Haynes WG, Ferro CJ, O'Kane KP, Somerville D, Lomax CC, Webb DJ. Systemic endothelin receptor blockade decreases peripheral vascular resistance and blood pressure in humans. Circulation 93: 1860–1870, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Hu RM, Levin ER, Pedram A, Frank HJ. Insulin stimulates production and secretion of endothelin from bovine endothelial cells. Diabetes 42: 351–358, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Iglarz M, Clozel M. At the heart of tissue: endothelin system and end-organ damage. Clin Sci (Lond) 119: 453–463, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Iglarz M, Clozel M. Mechanisms of ET-1-induced endothelial dysfunction. J Cardiovasc Pharmacol 50: 621–628, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Jonk AM, Houben AJ, de Jongh RT, Serne EH, Schaper NC, Stehouwer CD. Microvascular dysfunction in obesity: a potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Physiology (Bethesda) 22: 252–260, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Kaasjager KA, Koomans HA, Rabelink TJ. Endothelin-1-induced vasopressor responses in essential hypertension. Hypertension 30: 15–21, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol 23: 434–439, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Krum H, Viskoper RJ, Lacourciere Y, Budde M, Charlon V. The effect of an endothelin-receptor antagonist, bosentan, on blood pressure in patients with essential hypertension. Bosentan Hypertension Investigators. N Engl J Med 338: 784–790, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Lohman TG, Roche AF, Mortorelli R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics, 1988 [Google Scholar]
- 29. Lteif AA, Fulford AD, Considine RV, Gelfand I, Baron AD, Mather KJ. Hyperinsulinemia fails to augment ET-1 action in the skeletal muscle vascular bed in vivo in humans. Am J Physiol Endocrinol Metab 295: E1510–E1517, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mather KJ, Mirzamohammadi B, Lteif A, Steinberg HO, Baron AD. Endothelin contributes to basal vascular tone and endothelial dysfunction in human obesity and type 2 diabetes. Diabetes 51: 3517–3523, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 32. Miyauchi T, Masaki T. Pathophysiology of endothelin in the cardiovascular system. Annu Rev Physiol 61: 391–415, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Nakov R, Pfarr E, Eberle S. Darusentan: an effective endothelinA receptor antagonist for treatment of hypertension. Am J Hypertens 15: 583–589, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Nugent AG, McGurk C, Hayes JR, Johnston GD. Impaired vasoconstriction to endothelin 1 in patients with NIDDM. Diabetes 45: 105–107, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Patel JN, Jager A, Schalkwijk C, Corder R, Douthwaite JA, Yudkin JS, Coppack SW, Stehouwer CD. Effects of tumour necrosis factor-alpha in the human forearm: blood flow and endothelin-1 release. Clin Sci (Lond) 103: 409–415, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Perticone F, Ceravolo R, Candigliota M, Ventura G, Iacopino S, Sinopoli F, Mattioli PL. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: protective effect of vitamin C. Diabetes 50: 159–165, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Poirier P, Eckel RH. Obesity and cardiovascular disease. Curr Atheroscler Rep 4: 448–453, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Pollock DM. Endothelin, angiotensin, and oxidative stress in hypertension. Hypertension 45: 477–480, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Pollock DM, Pollock JS. Endothelin and oxidative stress in the vascular system. Curr Vasc Pharmacol 3: 365–367, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Schiffrin EL. Vascular endothelin in hypertension. Vasc Pharmacol 43: 19–29, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Sorensen SS, Madsen JK, Pedersen EB. Systemic and renal effect of intravenous infusion of endothelin-1 in healthy human volunteers. Am J Physiol Renal Fluid Electrolyte Physiol 266: F411–F418, 1994 [DOI] [PubMed] [Google Scholar]
- 42. Spratt JC, Goddard J, Patel N, Strachan FE, Rankin AJ, Webb DJ. Systemic ETA receptor antagonism with BQ-123 blocks ET-1 induced forearm vasoconstriction and decreases peripheral vascular resistance in healthy men. Br J Pharmacol 134: 648–654, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stamler R, Stamler J, Riedlinger WF, Algera G, Roberts RH. Weight and blood pressure. Findings in hypertension screening of 1 million Americans. JAMA 240: 1607–1610, 1978 [DOI] [PubMed] [Google Scholar]
- 44. Stauffer BL, Westby CM, DeSouza CA. Endothelin-1, aging and hypertension. Curr Opin Cardiol 23: 350–355, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest 97: 2601–2610, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tesauro M, Schinzari F, Rovella V, Di Daniele N, Lauro D, Mores N, Veneziani A, Cardillo C. Ghrelin restores the endothelin 1/nitric oxide balance in patients with obesity-related metabolic syndrome. Hypertension 54: 995–1000, 2009 [DOI] [PubMed] [Google Scholar]
- 47. Tiret L, Poirier O, Hallet V, McDonagh TA, Morrison C, McMurray JJ, Dargie HJ, Arveiler D, Ruidavets JB, Luc G, Evans A, Cambien F. The Lys198Asn polymorphism in the endothelin-1 gene is associated with blood pressure in overweight people. Hypertension 33: 1169–1174, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Touyz RM, Schiffrin EL. Role of endothelin in human hypertension. Can J Physiol Pharmacol 81: 533–541, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Van Guilder GP, Stauffer BL, Greiner JJ, Desouza CA. Impaired endothelium-dependent vasodilation in overweight and obese adult humans is not limited to muscarinic receptor agonists. Am J Physiol Heart Circ Physiol 294: H1685–H1692, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension 50: 403–409, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Verma S, Li SH, Badiwala MV, Weisel RD, Fedak PW, Li RK, Dhillon B, Mickle DA. Endothelin antagonism and interleukin-6 inhibition attenuate the proatherogenic effects of C-reactive protein. Circulation 105: 1890–1896, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Wagner OF, Christ G, Wojta J, Vierhapper H, Parzer S, Nowotny PJ, Schneider B, Waldhausl W, Binder BR. Polar secretion of endothelin-1 by cultured endothelial cells. J Biol Chem 267: 16066–16068, 1992 [PubMed] [Google Scholar]