Abstract
Acetylcholine evokes endothelium-dependent vasodilation subsequent to a rise in intracellular calcium. Despite widespread application in human and animal studies, calcium responses to intravascular ACh have not been visualized in vivo. Microiontophoresis of ACh in tissue adjacent to an arteriole activates abluminal muscarinic receptors on endothelial cells within a “local” region of diffusion, but it is unknown whether ACh released in such fashion gains access to the flow stream resulting in further actions downstream. To test this hypothesis and provide new insight into calcium signaling in vivo, we studied the cremaster muscle microcirculation of anesthetized male Cx40BAC-GCaMP2 transgenic mice (n = 22; 5–9 mo; 33 ± 1 g) expressing the fluorescent calcium sensor GCaMP2 selectively in arteriolar endothelial cells. Submaximal ACh stimuli were delivered using microiontophoresis (1-μm pipette tip, 500 nA). With stimulus duration <500 ms or with the micropipette positioned within one vessel diameter (∼30 μm) away from an arteriole, endothelial cell calcium fluorescence was restricted to the region of ACh diffusion (<200 μm). In contrast, with the micropipette tip positioned immediately adjacent to an arteriole or within its lumen, calcium fluorescence encompassed entire networks downstream. The velocity of downstream calcium signaling in response to ACh increased with centerline velocity of fluorescent tracer microbeads (r2 > 0.99; range: <1 mm/s to >10 mm/s). Diverting arteriolar blood flow into a side branch redirected downstream fluorescence responses to ACh; occluding flow abolished responses. Blocking luminal muscarinic receptors (intravascular glycopyrrolate; 6 μg/kg) inhibited downstream responses reversibly. Through visualizing the actions of a “local” ACh stimulus on endothelial cell calcium fluorescence in vivo, the present findings illustrate that transmural diffusion and convection of an agonist can activate entire networks of arteriolar endothelial cells concomitant with its delivery in the flow stream.
Keywords: blood flow, calcium signaling, cremaster muscle, endothelial cells, intravital microscopy, microcirculation
acetylcholine (ach) evokes endothelium-dependent vasodilation subsequent to a rise in intracellular calcium initiated by the activation of muscarinic receptors (5, 37). Despite widespread use of ACh infusion to study endothelium-dependent vasodilation in animals (7, 45) and humans (6, 35), calcium responses to intravascular ACh have not been visualized in vivo. With the use of microiontophoresis to deliver ACh from a micropipette onto an arteriole, imaging studies have revealed that the calcium response initiated by activation of muscarinic receptors on the abluminal surface of endothelial cells (32) spreads along the endothelium for several hundred micrometers (1, 13, 37). The ensuing spread of vasodilation entails rapid intercellular conduction of hyperpolarization (∼45 mm/s; Ref. 16) over distances that encompass several millimeters and multiple vessel branches, thereby lowering smooth muscle calcium to promote relaxation. This electromechanical response is complemented by the slower spread (∼100 μm/s) of calcium signaling along arteriolar endothelium (1, 37), which can release nitric oxide to promote vasodilation for several hundred micrometers in each direction (4, 12).
Whereas the spread of vasodilation initiated by ACh from a discrete location in the tissue is bidirectional along the arteriolar wall (4, 14, 33), remote responses to local delivery of an agonist from a micropipette have typically been recorded at defined distances upstream from the site of stimulation to avoid possible effects of convective transport of vasoactive agents in the stream of flowing blood (9, 33, 37). Despite such precautions, the ability of a vasoactive agent to cross the arteriolar wall and enter the flow stream to initiate signaling events throughout a microvascular network downstream from the site of delivery has not been resolved in previous studies. Indeed, several laboratories have routinely applied substances onto an arteriole from a micropipette and assumed that the agent was acting only at the “local” site of application. However, if present, transmural diffusion and intravascular convection would result in such “local” stimuli exerting direct actions over much greater regions of microvascular networks than has been recognized previously in vivo. A key goal of the present study was to determine whether such concerns are warranted.
The development of the Cx40BAC-GCaMP2 mouse, which expresses a genetically encoded calcium indicator molecule in precapillary endothelial cells under endogenous transcriptional regulation of connexin 40 (Cx40), enables visualization of calcium signaling in arteriolar endothelium without necessitating the use of indicator dyes (1, 37). Thus, in contrast to studying discrete segments following dye loading, responses throughout entire networks can be visualized in vivo. By studying the microcirculation in the cremaster muscle of these mice, we tested the hypothesis that a vasoactive stimulus released from a micropipette positioned in the tissue can gain access to the flow stream and thereby exert direct effects on the endothelium over much greater distances than have been previously documented. The present findings are the first to illustrate that ACh released abluminally can gain access to the vessel lumen. Moreover, when convected in the flow stream at a concentration sufficient to activate muscarinic receptors, entire networks of arteriolar endothelium respond nearly instantaneously to the delivery of ACh.
METHODS
Cx40BAC-GCaMP2 Mice
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Missouri. Experiments were performed on Tg(RP24–25504-GCaMP2)1Mik mice. Original breeder stocks (N3) were obtained (M. I. Kotlikoff; Cornell University, Ithaca, NY) with subsequent generations (N4 to N7) maintained on a C57BL/6J (stock no. 000664; Jackson Laboratories; Bar Harbor, ME) background in the animal facility at the University of Missouri. Briefly, a GCaMP2-IRES-GCaMP2-pA cassette replaced (via homologous recombination) six nucleotides at the initiation codon of the Cx40 locus contained within a bacterial artificial chromosome (BAC). The GCaMP2 construct was introduced as a random insertion transgene, effectively targeting expression to arteriolar endothelial cells without affecting endogenous Cx40 expression (37, 38). This strategy enables calcium fluorescence to be visualized in vivo without the use of indicator dyes or interference with constitutive mechanisms of blood flow control.
Mouse Cremaster Muscle Preparation
Adult male Cx40BAC-GCaMP2 mice (n = 22; 5–9 mo; 33 ± 1 g) were anesthetized with pentobarbital sodium (60 mg/kg ip injection). Hair in the scrotal region was removed by shaving. The anesthetized mouse was positioned in a supine position on a clear acrylic platform with its legs straddling a transparent silicone rubber pedestal (Sylgard 184; Dow Corning; Midland MI). Esophageal temperature was maintained at 37°C using a heating plate positioned under the mouse (2). Throughout surgery and experimental procedures, exposed tissue was superfused continuously (3–4 ml/min) with a bicarbonate-buffered physiological salt solution (34°C, pH 7.4) of the following composition (in mmol/l): 137 NaCl, 4.7 KCl, 1.2 MgS04, 2 CaCl2, and 18 NaHCO3 equilibrated with 5% CO2-95% N2. Reagents were obtained from Sigma-Aldrich (St. Louis, MO).
Surgical procedures were performed while viewing took place through a stereomicroscope as recently described (2). Briefly, an incision was made through the ventral surface of the left scrotal sac, the cremaster muscle was separated from surrounding skin and opened longitudinally, and an orchiectomy was performed. The cremaster muscle was cleared of connective tissue and spread radially on the surface of the Sylgard pedestal to create a flat sheet, and its edges were pinned to secure the preparation. Effluent superfusion solution was continuously removed by vacuum line to maintain a constant fluid layer over the muscle and thereby maintain a stable optical image. The completed preparation was transferred to the stage of an intravital microscope based on an Olympus MVX10 Stereo Zoom platform (Center Valley, PA). Preparations were equilibrated for ≥30 min before experimental procedures. As evidenced by the maintenance of vasomotor tone in arterioles and lack of leukocyte accumulation in venules, cremaster muscle preparations remain viable for 4–5 h. Criterion experiments were typically completed within 3–4 h, and then sodium nitroprusside (10 μmol/l) was added to the superfusate to obtain maximal arteriolar diameter (2). Anesthesia was maintained with hourly supplements (10–20% of the initial injection, intraperitoneal) to prevent withdrawal to toe or tail pinch. At the end of the day's procedures, the mouse was euthanized by injection of an overdose (intraperitoneal) of pentobarbital followed by cervical dislocation.
Micropipettes and Microiontophoresis
Micropipettes were prepared from borosilicate glass capillary tubes (GC120F-10; Warner Instruments; Hamden, CT) using a horizontal pipette puller (model P-97; Sutter Instruments; Novato, CA). For microiontophoresis, tip internal diameters were ∼1 μm and micropipettes were backfilled with acetylcholine chloride (1 mol/l; Sigma-Aldrich) dissolved in ultrapure deionized (18.2 MΩ) water. For microocclusion, a micropipette tip was melted to form a blunt end (outer diameter: ∼25 μm) using a microforge. Each micropipette was secured in a holder in a three-axis micromanipulator (DT3–100; Siskiyou Design Instruments; Grants Pass, OR) mounted onto the acrylic platform.
The stimulus micropipette containing ACh was connected to a constant current source (Model 260 Iontophoresis Programmer; World Precision Instruments; Sarasota, FL) via a silver wire (diameter: 250 μm). A second silver wire secured at the perimeter of the cremaster preparation served as the reference electrode. A stimulus micropipette was positioned with its tip located adjacent to the abluminal surface of an arteriole (resting diameter: 30–40 μm) located in the central region of the cremaster muscle preparation. The onset and duration of ejection current were controlled by a timer that provided a coincident reference signal (LED flash). Preliminary studies defined the criterion stimulus for these experiments as 500-nA ejection current with a pulse duration of 500 ms. The amount of ACh thereby released from the micropipette evokes vasodilation (10–15 μm) at the site of stimulation similar to that obtained during superfusion of the tissue with 1 μmol/l ACh and is slightly less than the maximal diameter obtained with 10 μmol/l sodium nitroprusside (2). Retain current (∼200 nA) was determined experimentally as that required to prevent vasodilation or a fluorescence response when the tip of the ACh micropipette was positioned adjacent to the arteriolar wall. Controls in which NaCl (1 mol/l) was used for microiontophoresis in a fashion identical to that used for ACh had no effect on arteriolar fluorescence or diameter (n = 4). Thus responses reported here are attributable to the actions of ACh independent of current ejection.
Image Acquisition and Analysis
Images were acquired using an intensified charge-coupled device camera (Mega-10; Stanford Photonics; Palo Alto, CA) using Piper Control Software (Stanford Photonics) on a personal computer (1). For fluorescence imaging, GCaMP2 was excited at 472/30 nm using a mercury lamp (X-Cite Series 120PC; EXFO, Vanier, Canada) with emission recorded at 520/35 nm. Total magnification on the digital monitor was ×149. Fluorescence recorded under resting conditions is referred to as “Fo” (taken as the mean of 10 baseline data points), and fluorescence during a calcium response (from baseline through recovery) is referred to as “F.” Fluorescence data are presented as F/Fo or in arbitrary fluorescence units.
Image stacks were acquired in tagged image file format at 15 or 120 frames per second (fps) and were analyzed off-line using SparkAn software (kindly provided by A. Bonev and M. T. Nelson, University of Vermont). Scan lines were placed normal to the axis of the arteriole at designated sites and the software output the following parameters: peak response amplitude = peak F − Fo, rise time = time from response onset (F > Fo) until peak F, and response time = rise time + recovery time to 50% of peak F. Area under the response curve was calculated by the trapezoid rule using a custom program in Igor Pro (Version 6.4; WaveMetrics, Lake Oswego, OR). Arteriolar diameter was measured by tracking the outer edge of the fluorescent endothelial cell layer (1).
All analyses were performed on raw image stacks. For data presentation, background fluorescence was reduced and images were pseudocolored to more prominently display calcium response characteristics. One arteriolar network (either an unbranched or branched vessel) was examined per animal. Summary data represent at least five animals per group and are presented as means ± SE with n indicating the number of independent observations. Data were analyzed using linear regression and ANOVA with Prism software (Version 5; GraphPad, La Jolla, CA) or SigmaStat (Version 3.5; Systat Software, San Jose, CA). Differences were considered statistically significant with P < 0.05.
Experimental Protocols to Evaluate the Nature of Calcium Fluorescence Responses
Stimulus proximity and intensity.
With the stimulus micropipette tip adjacent to the arteriolar wall, ACh was delivered as a 100-, 250-, 500-, or 1,000-ms pulse (randomized across experiments) with ejection current maintained at 500 nA. The vessel recovered for 3 min between successive stimuli. In separate experiments, the ACh micropipette was positioned either directly adjacent to the arteriole (as above), in the tissue located 30 μm (∼1 vessel diameter) away from the arteriole, or within the arteriolar lumen and a constant stimulus (500 nA, 500 ms) was delivered. Scan lines positioned perpendicular to the vessel axis at designated sites along the arteriole were used to record evoked calcium responses.
Role of blood flow.
The role of blood flow in endothelial cell calcium responses to ACh was evaluated using microocclusion with blunted micropipettes positioned upstream of the stimulation site (Supplemental Movie S3; Supplemental Material for this article is available online at the Am J Physiol Heart Circ Physiol website.). A fluorescence response initiated by ACh was recorded at 15 fps under control conditions, and then the microocclusion micropipette was lowered gently onto the arteriole until flow ceased as confirmed visually by stationary red blood cells in the vessel lumen. The ACh stimulus was repeated in the absence of blood flow and the ensuing response recorded. The occluder was removed, and ACh was delivered a final time during spontaneous reperfusion. To estimate blood flow velocity, fluorescent microbeads (0.5-μm diameter; Polysciences; Warrington, PA; ∼1.4 × 105 particles) were injected retroorbitally. While the charge-coupled device camera focused on the widest aspect of the arteriolar lumen, images were acquired at 15 and 120 fps to record beads at low (<1 mm/s) and high (∼1–10 mm/s) velocities, respectively (Supplemental Movie S4). Centerline bead velocity was determined with SparkAn software by placing two line scans, each perpendicular to the axis of an arteriole, and dividing their separation distance (∼300–600 μm) by the time it took the leading edge of the calcium fluorescence response or of the fluorescent microbead in the flow centerline to initiate a response (defined when F increased by 20% above Fo) at respective sites.
Role for luminal muscarinic receptors.
After downstream activation of endothelial cells was confirmed by calcium fluorescence responses to abluminal delivery of ACh under control conditions, the muscarinic receptor antagonist glycopyrrolate (American Regent, Shirley, NY) was introduced into the vascular compartment via retroorbital injection (6 μg/kg). Glycopyrrolate was allowed to equilibrate for 3–5 min, and responses to ACh were evaluated. The hydrophilic nature of glycopyrrolate (a quaternary ammonium compound) facilitated its retention within the vascular compartment to test the role of luminal muscarinic receptors in downstream fluorescence responses. In contrast, the commonly used muscarinic receptor antagonist atropine (41) is an alkaloid with relatively poor solubility in water and more likely to cross the vascular wall, thereby affecting both luminal and abluminal receptors. This strategy is consistent with the use of the antagonists scopolamine (lipophilic) and methscopolamine (hydrophilic) to define respective populations of muscarinic receptors in arterioles of the hamster cheek pouch (32). The preparation recovered for 60 min, and the responses to ACh were recorded a final time.
RESULTS
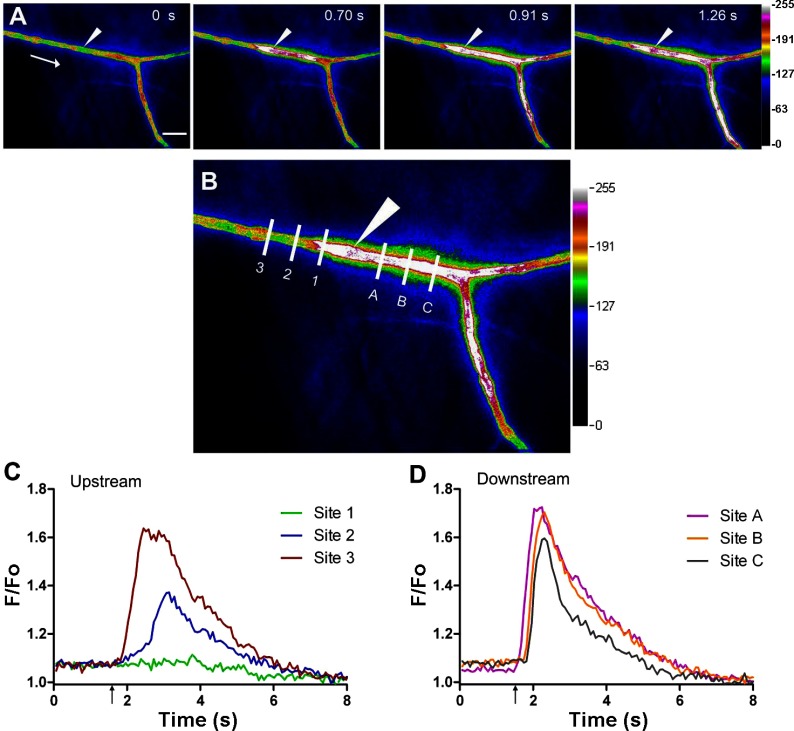
When the ACh stimulus was delivered from a micropipette positioned several micrometers from the arteriole, endothelial cell calcium fluorescence spread with similar velocity (∼100 μm/s) in both directions over several hundred micrometers (Supplemental Movie S1) as recently reported (1, 37). However, with the micropipette tip positioned immediately adjacent to the arteriolar wall, the fluorescence response in the downstream direction was faster and extended beyond the field of view (Fig. 1; Supplemental Movie S2). In approximately half of these experiments, it was necessary to manipulate the tip of the micropipette adjacent to the arteriolar wall to observe the downstream fluorescence response, which has not previously been reported. Respective signaling events were distinguished by their fluorescence kinetics: upstream from the stimulus the fluorescence response decayed within ∼200 μm (Fig. 1, B and C, sites 1–3). In contrast, downstream responses exhibited negligible decay over the same distance (Fig. 1, B and C, sites A-C). These different patterns of endothelial cell calcium fluorescence responses led to protocols designed to manipulate the proximity and intensity of ACh stimuli and the flow of blood through the arteriolar lumen and thereby determine whether the activation of the endothelium via transmural diffusion and convection of the agonist could extend throughout entire arteriolar networks.
Fig. 1.
Upstream vs. downstream calcium fluorescence responses along arterioles. A: endothelial cell calcium signaling events in arterioles of the mouse cremaster muscle in response to a brief pulse (500 ms, 500 nA) of ACh delivered from a micropipette (white arrowhead). Pseudocolored (arbitrary units; low→high = black→white) 4-panel image sequence shows a calcium event in the direction of blood flow (arrow in panel 1) in addition to the slower calcium response upstream (the latter component is masked in the downstream direction by the downstream calcium fluorescence response). Elapsed time from stimulus delivery in seconds (s) indicated in top right corner of panels 1–4. B: defined distances (65-μm intervals) at sites upstream (sites 1–3) and downstream (sites A-C) from the ACh stimulus micropipette where line scan recordings of fluorescence were obtained (image corresponds to 0.91 s in A). C and D: plot of fluorescence (F/Fo; analyses performed on original grayscale images) vs. time from sites marked in B with ACh stimulus delivered at ↑. Fluorescence responses upstream decay rapidly with distance from the site of stimulation (sites 1–3), while downstream responses show little decay with distance (sites A-C). Scale bar in A = 100 μm and applies to panels 1–4. (Images correspond to network in Supplemental Movie S2.)
Stimulus Proximity and Intensity
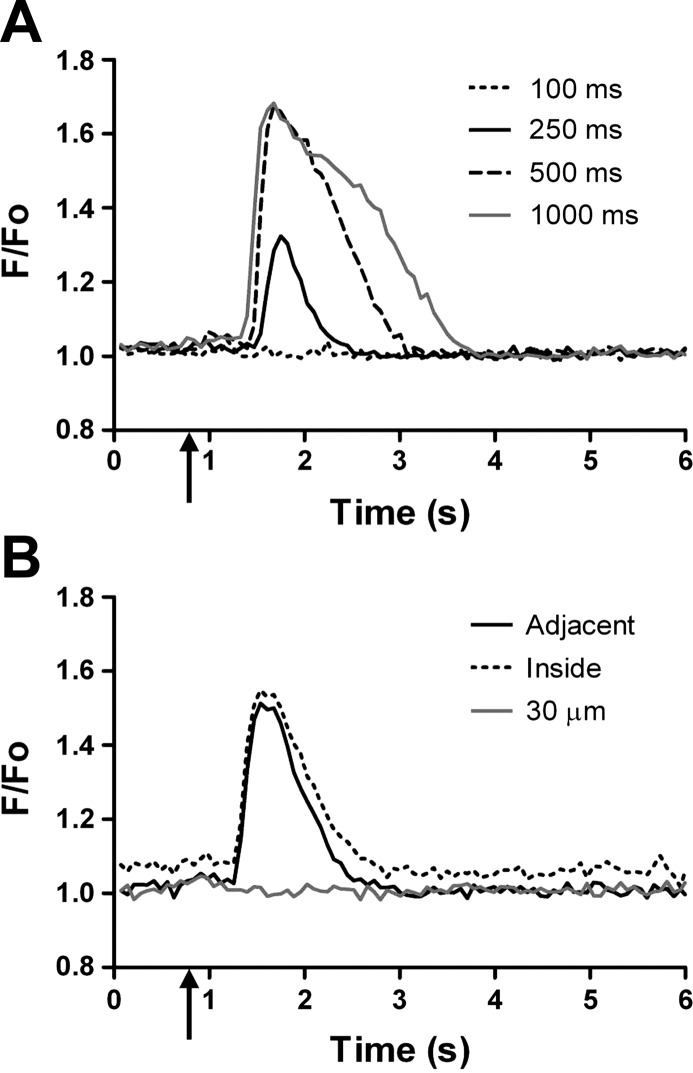
With constant ejection current (500 nA) and the ACh micropipette tip positioned adjacent to the arteriolar wall, the amplitude, rise time, response time, and area under the calcium fluorescence response curve increased with pulse duration from 100 to 1,000 ms (Fig. 2A; Table 1, stimulus duration). With no change in amplitude, response times increased significantly with pulse durations longer than 500 ms but were inconsistent with pulse durations <500 ms. Thus 500 ms was used for criterion measurements in subsequent experiments. These characteristics of fluorescence responses were similar when the ACh micropipette tip was positioned within the arteriolar lumen. In contrast, no response was observed when the micropipette tip was positioned in the tissue parenchyma ∼1 vessel diameter (30 μm) away from the arteriolar wall (Fig. 2B; Table 1, stiumulus proximity). This behavior suggested that ACh released from an appropriate site adjacent to the arteriole can diffuse across the vessel wall and enter the flow stream.
Fig. 2.
Effect of stimulus duration and proximity on downstream calcium fluorescence. Representative tracings of calcium fluorescence recorded 500 μm downstream from the ACh stimulus pipette. ACh stimuli delivered at ↑. A: with constant ejection current (500 nA) and the stimulus micropipette positioned immediately adjacent to the arteriolar wall, the downstream fluorescence response increased with stimulus duration (summary data for stimulus duration in Table 1). B: with a constant ACh stimulus (500 nA, 500 ms), the downstream response occurred only when the micropipette tip was immediately adjacent to the arteriolar wall or within the vessel lumen. Moving the stimulus micropipette 30 μm (∼1 vessel diameter) away from the arteriole abolished downstream fluorescent responses (summary data for stimulus proximity in Table 1).
Table 1.
Dependence of downstream calcium fluorescence response upon stimulus duration and location
| Amplitude | Rise Time, s | Response Time, s | Area | |
|---|---|---|---|---|
| Stimulus duration | ||||
| Duration, ms | ||||
| 100 | 0.00 ± 0.00* | 0.00 ± 0.00* | 0.00 ± 0.00* | 0.3 ± 0.16‡ |
| 250 | 1.00 ± 0.18* | 0.15 ± 0.02* | 0.29 ± 0.06* | 3.6 ± 0.85‡ |
| 500 | 1.65 ± 0.04† | 0.27 ± 0.03† | 0.59 ± 0.06* | 9.1 ± 0.91* |
| 1000 | 1.68 ± 0.04† | 0.31 ± 0.05† | 1.09 ± 0.09* | 14.8 ± 1.65* |
| Stimulus proximity | ||||
| Location | ||||
| Adjacent | 1.60 ± 0.06 | 0.22 ± 0.02 | 0.64 ± 0.07 | 8.4 ± 1.20 |
| Inside | 1.65 ± 0.07 | 0.21 ± 0.02 | 0.76 ± 0.09 | 10.5 ± 1.81 |
| 30 μm | 0.00 ± 0.00§ | 0.00 ± 0.00§ | 0.00 ± 0.00§ | 0.1 ± 0.11§ |
Summary data are means ± SE. Respective data sets were obtained from the same vessels (n = 8). Calcium fluorescence responses were initiated by an ACh stimulus, and measurements were taken 500 μm downstream of the stimulus site. For stimulus duration, the ACh micropipette was positioned immediately adjacent to the arteriolar wall and ejection current was held constant at 500 nA. As the duration of the ACh stimulus was varied, fluorescence increased with pulse duration. Amplitude, rise time, decay, and area under the curve were calculated as described in methods. For stimulus location, the ACh stimulus was held constant (500 nA, 500-ms pulse duration) and the location of the ACh micropipette was varied. Downstream calcium fluorescence responses were observed only when the tip of the micropipette was immediately adjacent to the arteriole or within its lumen. Values for amplitude and area are arbitrary fluorescence units. For stimulus duration:
significantly different from other pulse durations (P < 0.05);
significantly different from 100 and 250 ms (P < 0.05);
significantly different from 500 and 1000 ms (P < 0.05). For stimulus location:
significantly different from other proximities (P < 0.05).
Role of Blood Flow
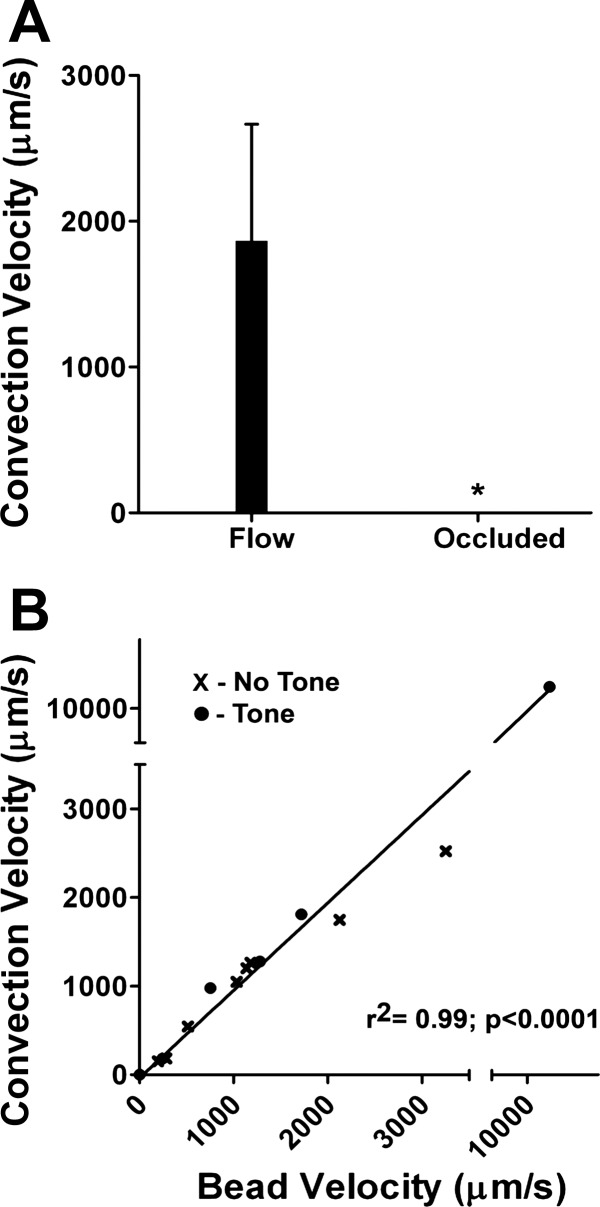
Because calcium fluorescence along the endothelium exhibited negligible decay downstream when the ACh stimulus was delivered immediately adjacent to the arteriolar wall, we tested the hypothesis that blood flow (i.e., convection) contributed to these responses. When blood flow was eliminated by occluding the arteriole, fluorescence responses in the downstream direction were no longer different from those recorded in the upstream direction and all responses were constrained to a few hundred micrometers (Supplemental Movie S3). In turn, fluorescence responses further downstream (e.g., in daughter branches; Supplemental Movie S2) recovered promptly following removal of the occlusion micropipette. During spontaneous flow, the velocities of downstream fluorescence responses were highly variable (note SE in Fig. 3A). With fluorescent microbeads in the circulation, the velocity of downstream calcium fluorescence responses varied directly with that of microbeads in the centerline flow through a velocity range encompassing 0 to >10,000 μm/s and this relationship was manifest irrespective of vasomotor tone (Fig. 3B; Supplemental Movie S4). As a positive control for the dependence on convection, diversion of blood flow into a side branch (using microocclusion) concomitantly diverted the calcium fluorescence response to ACh (n = 5, data not shown). However, before ACh microiontophoresis, neither microocclusion with flow cessation nor flow diversion into a side branch had any effect on endothelial cell calcium fluorescence. These data indicate that the downstream fluorescence response reflects convection of ACh in the arteriolar flow stream following transmural diffusion from its site of release and not the effect of changing flow on the endothelium.
Fig. 3.
Downstream calcium fluorescence responses are related to arteriolar blood flow. A: velocity of the downstream calcium response during luminal blood flow and during arteriolar occlusion. Velocity of the downstream calcium response varied across arterioles (n = 13) and was abolished when blood flow was eliminated with microocclusion (n = 6). *P < 0.05, significantly different from flow. B: blood flow velocity (estimated from fluorescent microbeads in flow stream) related linearly to the velocity of the downstream calcium response in the presence (●) and absence (x) of vasomotor tone (n = 14).
Role for Luminal Muscarinic Receptors
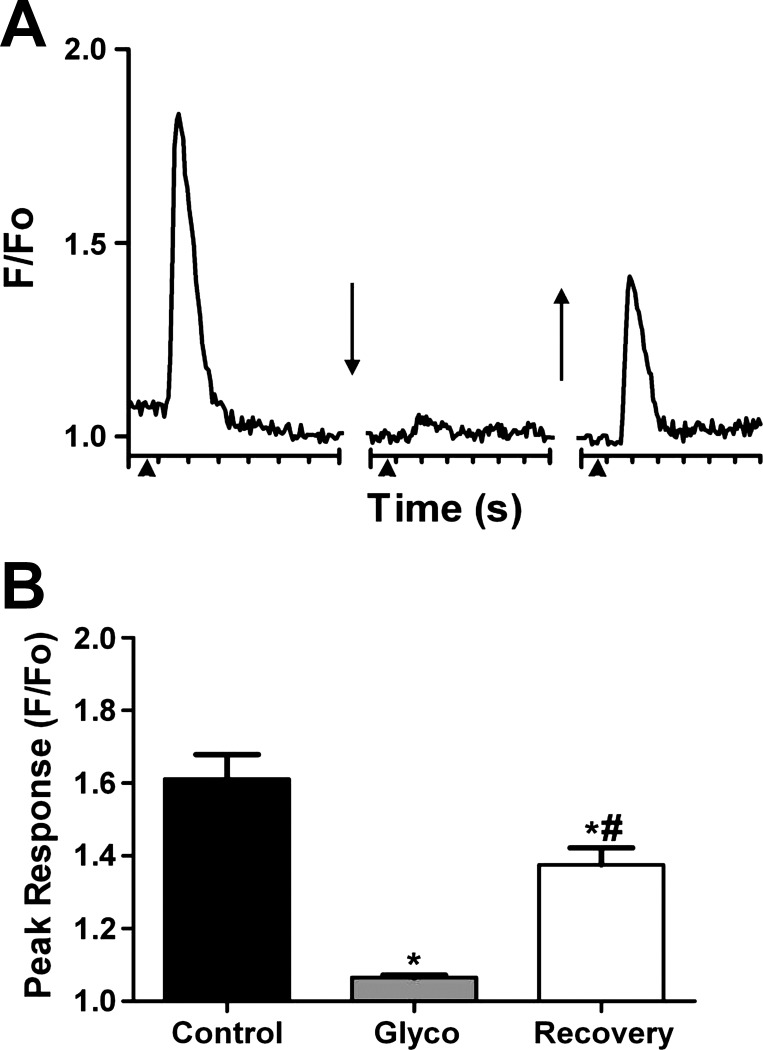
To test the hypothesis that the downstream calcium fluorescence response reflected activation of muscarinic receptors on the luminal surface of endothelial cells, the hydrophilic muscarinic receptor antagonist glycopyrrolate was introduced into the vascular compartment. As shown in Fig. 4, the downstream calcium fluorescence response was nearly abolished following glycopyrrolate treatment. After a recovery period of 60 min, downstream fluorescence responses had returned to ∼60% of control (n = 5).
Fig. 4.
Downstream calcium fluorescence entails activation of luminal muscarinic receptors. A: representative trace of fluorescence changes (F/Fo) vs. time recorded 500 μm downstream of the ACh stimulus. Each tick of x-axis = 1 s. First break (↓) indicates 3–5 min equilibration following retroorbital injection of glycopyrrolate into the bloodstream. Second break (↑) indicates 60-min recovery. Note robust calcium fluorescence response under control conditions, inhibition within several min of intraluminal muscarinic receptor inhibition, and response recovery after 60 min. B: summary data for glycopyrrolate treatment (Glyco) and recovery (n = 5). *P < 0.05, significantly different from control. #P < 0.05, significantly different from Glyco.
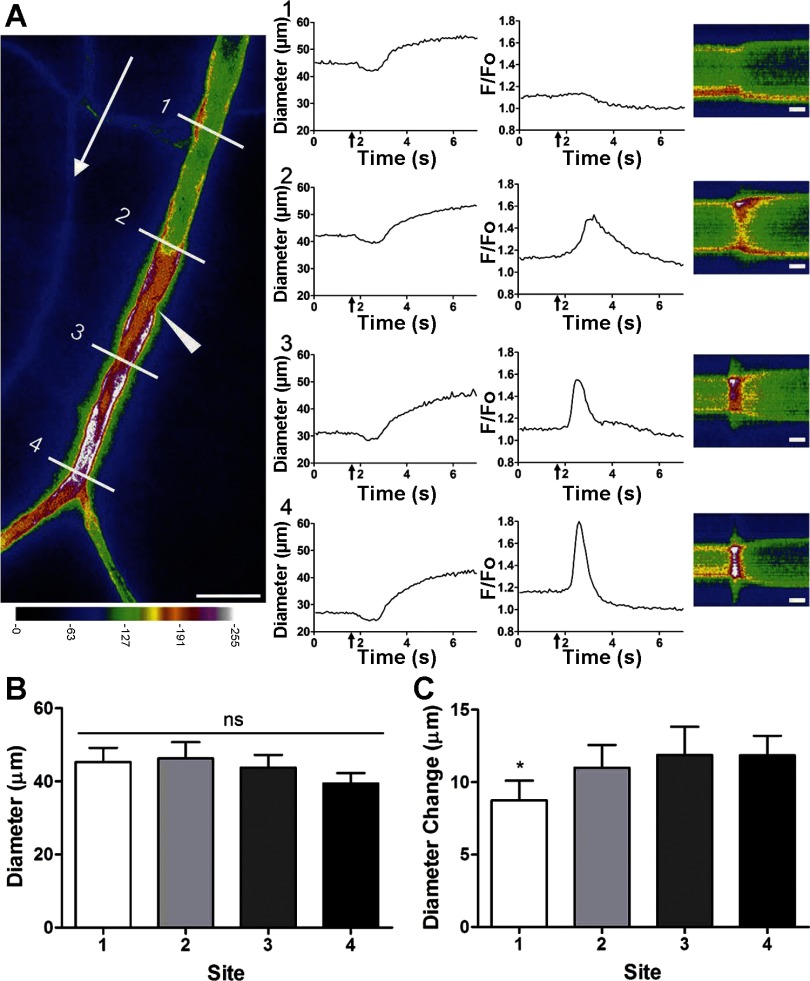
Previous studies suggest that ACh microiontophoresis evokes a rapid vasodilation at remote sites due to a conducted electrical signal (42, 44) that precedes a secondary slower dilation resulting from the spread of calcium fluorescence along the endothelium (12, 37, 40). However, previous studies have consistently focused their analyses on responses upstream from the site of stimulation. To determine if the vasomotor response along the arteriole varied with respect to differences in calcium signaling events, diameter and fluorescence responses were analyzed simultaneously at four defined sites within the field of view both upstream and downstream of the ACh stimulus. As illustrated in Fig. 5, there was no rise in fluorescence at the site furthest upstream (site 1), a relatively slow increase in fluorescence just upstream from the stimulus (site 2), a more rapid increase in fluorescence with a longer recovery lag just downstream from the stimulus (site 3), and an even faster fluorescence transient at the site furthest downstream (site 4). Thus, whereas the onset of vasodilation was indistinguishable across respective sites, the diameter change was greater (P < 0.05) at sites where fluorescence increased (Fig. 5C) and the fluorescence response was enhanced at downstream vs. upstream sites. In two of these experiments, a slight transient vasoconstriction preceded vasodilation (Fig. 5A, diameter panels 1–4).
Fig. 5.
Dilation along arterioles is enhanced by endothelial cell calcium signaling. A: image at left taken 1.5 s following ACh stimulus (500 ms, 500 nA; micropipette located at arrowhead) shows calcium fluorescence spreading over a limited distance upstream (to site 2 but not to site 1) while the fluorescence response increases more rapidly and without decrement in the direction of blood flow (white arrow) at sites 3 and 4. Scale bar = 100 μm. For panels 1–4, diameter and F/Fo traces encompass baseline through peak dilation, and ↑ indicates stimulus delivery. Distances for line scan lines for analysis of diameter and fluorescence: 1) 300 μm upstream, 2) 100 μm upstream, 3) 100 μm downstream, and 4) 300 μm downstream. To the right of panels 1–4 are pseudocolored line scans taken at respective sites over time (scale bars = 1 s); calcium changes at each site are indicated by increased F/Fo in red/white portion of the line scan. Diameter traces show vasodilation occurring simultaneously at all sites, while F/Fo responses vary according to proximity to the stimulus and direction of blood flow. At site 1 (furthest upstream), no calcium response occurred, whereas sites 2–4 exhibited robust calcium responses. B: baseline diameter was not significantly different between respective sites (ns). C: diameter change (peak-resting baseline) was significantly less at site 1 compared with sites 2–4 (*P < 0.05; n = 5). Maximal diameter during superfusion of 10 μM sodium nitroprusside was 51 ± 2 μm.
DISCUSSION
The present study has utilized a unique transgenic mouse expressing a genetically encoded calcium indicator protein (GCaMP2) in arteriolar endothelial cells. Localization of GCaMP2 to the arteriolar endothelium of the cremaster muscle is attributable to the constitutive pattern of Cx40 expression in these microvessels (10, 19, 27, 43). Thus using a bacterial artificial chromosome to direct GCaMP2 expression under endogenous transcriptional regulation of Cx40 enables visualization of calcium signaling throughout the endothelium of arteriolar networks in vivo (37, 38). Here we illustrate that when released from a micropipette immediately adjacent to an arteriole with sufficient stimulus intensity, ACh can diffuse across the vessel wall and enter the flow stream. This finding implies the need for appropriate controls when delivering substances to defined sites in microvascular networks to determine the spatial domain of their direct actions on the microvasculature. Moreover, in light of the widespread use of ACh as a vasodilator infused to evaluate endothelial cell function in animals and humans (6, 7, 35, 45), the present findings are the first to visualize endothelial cell activation by convective ACh delivery throughout resistance networks. We thereby illustrate that calcium responses along the endothelium are nearly instantaneous in response to ACh that is carried in the flow stream at a sufficient concentration to activate muscarinic receptors.
Calcium-Dependent and -Independent Endothelium-Mediated Vasodilation
Local delivery of agonists from a micropipette onto arterioles identified ACh as an agonist that was capable of initiating vasodilation over much greater distances that could be explained by diffusion (14). Ensuing studies utilized microocclusion to eliminate changes in flow (or transmural pressure) and thereby demonstrated that the conduction of vasodilation triggered by ACh could be explained by cell-to-cell coupling through gap junctions along the arteriolar wall (33). In the wake of these studies, Cx40 has been shown to be integral to conducted vasodilation (10, 19, 43). Intracellular recording has demonstrated that ACh triggers endothelial cell hyperpolarization through a rise intracellular calcium (5, 13) and the activation of KCa3.1 (IKCa) and KCa2.3 (SKCa) calcium-sensitive potassium channels (8, 12, 44), thereby generating an electrical signal that conducted rapidly along the endothelium and into surrounding smooth muscle cells through myoendothelial gap junctions (17, 18, 21, 27). In turn, hyperpolarization of the surrounding smooth muscle lowers intracellular calcium to promote relaxation and vasodilation along the vessel wall (3, 25). As shown previously (13, 37) and confirmed in the present study (Fig. 5), the rapid (electrical) conduction of vasodilation at remote sites occurs without corresponding changes in endothelial cell calcium. In the hamster cheek pouch, a second component of spreading vasodilation was attributed to the release of NO (4), implying an underlying calcium wave along the arteriolar wall (20). More recent studies (12) have revealed that, in response to ACh microiontophoresis, the local rise in intracellular calcium can spread along the endothelium for several hundred micrometers to release NO. Consistent with earlier findings (37, 40), our analysis of diameter changes at sites upstream and downstream from the ACh stimulus revealed an enhanced dilator response along arteriolar regions where calcium fluorescence increased (Fig. 5, sites 2–4). More importantly, by focusing on sites downstream from the stimulus, the present study illustrates for the first time that calcium fluorescence along the arteriolar endothelium is enhanced in the downstream vs. upstream direction and can be explained by the activation of luminal muscarinic receptors in this direction through the transmural diffusion and convection of ACh in the flow stream.
Dependence of Downstream Calcium Fluorescence Responses on Blood Flow, Stimulus Proximity, and Intensity
The consistency of vasomotor responses to ACh underlies its use as a preferred agonist to study conducted vasodilation (11, 14, 33). Whereas the vasomotor response spreads bidirectionally along arterioles (4, 14, 33), observation sites are typically located upstream from the site of stimulation. The present findings illustrate that ACh released near the arteriolar wall increases calcium fluorescence along the endothelium in a similar manner for several hundred micrometers in both directions (Supplemental Movie S1), attributable to the diffusion of ACh (1) activating muscarinic receptors on the abluminal surface of endothelial cells and the ensuing signaling events. However, when the micropipette tip is positioned immediately adjacent to the arteriolar wall, a robust calcium response often occurs downstream from the site of stimulation (Figs. 1 and 5) that can be explained by transmural diffusion of ACh and its convection in the flow stream to activate muscarinic receptors along the intimal surface of the arteriolar endothelium (Figs. 2 and 4). Consistent with the present findings in vivo, downstream calcium responses of cultured endothelial cells to fluid flow were attributed to the release of agonists (e.g., ATP and ACh) from cells upstream (28, 31, 34).
Previous studies (9, 33, 37, 44) of remote signaling in arteriolar networks have focused on events upstream from the stimulus to avoid possible contribution of the flow of blood to responses observed. In contrast, the use of Cx40BAC-GCaMP2 mice enables direct visualization of endothelial cell fluorescence along entire arteriolar networks in vivo and does so without necessitating the loading of indicator dyes. Thus the present experiments are the first to focus on events downstream from the local stimulus. The inability to elicit a downstream (convective) calcium fluorescence response at stimulus durations <500 ms (Fig. 2A) indicates that a threshold concentration is required to drive the diffusion of ACh across the arteriolar wall to activate muscarinic receptors within the vessel lumen. Above this threshold, the duration of the convective calcium fluorescence increased with stimulus duration (Fig. 2 and Table 1, stimulus duration). This relationship was investigated further by manipulating the site of ACh release relative to the arteriolar wall. Placement of the ACh micropipette within the arteriolar lumen consistently evoked similar fluorescence kinetics. In contrast, moving the micropipette tip 30 μm (∼1 vessel diameter) away from the arteriole abolished the calcium fluorescence response to ACh (Table 1, stimulus proximity). These data illustrate that the ACh stimulus must be delivered within a critical distance at an appropriate site to access the bloodstream by diffusing through the arteriolar wall.
Despite the great care taken when positioning a stimulus micropipette immediately adjacent to the arteriolar wall, additional repositioning of the micropipette tip was required for the downstream response to occur in approximately half of these experiments. This behavior suggests that subtle differences in connective tissue, adventitia, and/or ultrastructure of the arteriolar wall relative to where the micropipette tip is positioned can determine whether ACh (or other agents) released abluminally can enter the flow stream to activate endothelial cells. In the hamster cheek pouch, arterioles were nearly two orders of magnitude more sensitive when constricting to phenylephrine, vasopressin, and angiotensin II applied topically in the superfusion solution compared with their intraarterial (carotid) injection (26). This difference was attributed to the endothelium presenting an intramural barrier to the diffusion of intraluminal vasoconstrictors acting on surrounding smooth muscle. Our finding that endothelial cell fluorescence can be readily triggered by an abluminal stimulus without a convective fluorescence response supports this interpretation and confirms the presence of distinct populations of muscarinic receptors on luminal vs. abluminal surfaces of arteriolar endothelium (32). As illustrated here by the “threshold” ACh stimulus required to evoke the downstream convective response, the ability of vasoactive substances released by parenchymal cells or carried in venular blood (22, 39) to access the arteriolar lumen and stimulate downstream branches is likely to be affected in a similar manner.
An additional determinant of ACh efficacy is the prevailing levels of cholinesterase activity in blood and tissue (30, 36), and these were clearly exceeded when convective fluorescence responses occurred. In turn the inhibition of cholinesterase in the tissue would promote the ability of ACh to gain access to the vessel lumen, while inhibiting cholinesterase in the blood would augment its actions within the vessel lumen in a manner analogous to increasing stimulus duration (Fig. 2A). Such behavior is implied when ACh given intraarterially evokes increases in tissue blood flow in a concentration-dependent manner (7, 45). To further define the role of blood flow in downstream responses to ACh, we performed microocclusion experiments. Eliminating blood flow reversibly abolished the downstream calcium fluorescence responses (Fig. 3A) and thereby unmasked the downstream component of the slower, bidirectional spread of calcium fluorescence along the endothelium (1) (Supplemental Movie S3). Further, with the use of fluorescent microbeads as a tracer of centerline blood flow (Supplemental Movie S4), the velocity of downstream calcium signals was found to increase linearly with blood flow velocity (Fig. 3B). Inhibiting this response with glycopyrrolate confirmed that the actions of ACh were mediated by muscarinic receptors on the intimal surface of arteriolar endothelial cells (Fig. 4). Whereas flow-mediated arteriolar responses have been related to changes in luminal shear stress (23, 24), occluding or diverting blood flow in the present experiments had no effect on endothelial cell calcium fluorescence in the absence of an ACh stimulus, indicating that changes in flow (or luminal shear stress) per se did not activate calcium signaling in arteriolar endothelium.
Implications of Convection in Studying the Intact Microcirculation
The convection of molecules within the bloodstream to deliver substances (e.g., hormones) that initiate signaling events at remote sites in cells and tissues external to the vascular compartment is well established. In contrast, the present findings are the first to visually demonstrate the acute activation of entire networks of precapillary microvascular endothelium by a circulating molecule in vivo. Other agonists that gain access to the flow stream are likely to act in a complementary manner, e.g., histamine released from mast cells located near the vessel wall. Nevertheless, unmasking this response brings into question basic assumptions that have been associated with studying how signals travel along the arteriolar wall and draws attention to the details and implications of experimental design. For example, studies of conducted vasomotor responses at sites remote from the stimulus have applied agents at intermediate locations along arterioles in the attempt to manipulate the remote response (9, 15, 29, 33, 37, 44). However, if such agents can cross the arteriolar wall and enter the flow stream, the present data call into question how “local” such treatments may actually be. Further, the present findings demonstrate the importance of rigorously examining vascular responses downstream and upstream from the site of such experimental interventions to understand their full impact in the intact microcirculation. Collectively, both conducted and convective responses would serve to promote uniformity of vasomotor responses throughout resistance networks by coordinating changes in diameter among parent and daughter branches.
Summary and Conclusion
In humans and animals, the intraarterial delivery of ACh is used to evaluate the functional integrity of the endothelium, with changes in blood flow routinely measured upstream from the resistance microvessels that control tissue perfusion and dilate in response to ACh. Thus the correspondence between changes in tissue blood flow and endothelial cell activation in vivo is typically inferred from indirect measurements. In contrast, the correspondence between endothelial cell calcium responses and smooth muscle relaxation has been illustrated using isolated vessel preparations or perfused segments loaded with indicator dye. With the use of Cx40BAC-GCaMP2 transgenic mice that constitutively express the calcium-sensitive protein GCaMP2 throughout arteriolar endothelium, the present data are the first to visualize the activation of luminal muscarinic receptors of arteriolar endothelium resulting from calcium fluorescence concomitant with ACh delivery in the flow stream in vivo. When studying signaling events that govern blood flow control in the microcirculation, vasoactive agents are often delivered abluminally from micropipettes positioned near arterioles with the assumption that their actions are restricted to the site of delivery. In contrast, the present findings are the first to directly demonstrate that an agent released locally from a micropipette can activate entire networks of arteriolar endothelial cells through transmural diffusion and convection in the flow stream. In turn, these data suggest that additional caution and appropriate controls are essential when interpreting the actions of a “local” experimental or therapeutic intervention within the intact microcirculation.
GRANTS
This study was supported by National Institutes of Health Grants R37-HL-041026 (to S. S. Segal), F32-HL-097463 and T32-AR-048523 (to P. Bagher), and RO1-HL-072989 (to M. J. Davis).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Adrian Bonev, Dr. Joshua Scallan, and Samantha Bixby for valuable contributions.
REFERENCES
- 1. Bagher P, Davis MJ, Segal SS. Intravital macrozoom imaging and automated analysis of endothelial cell calcium signals coincident with arteriolar dilation in Cx40BAC-GCaMP2 transgenic mice. Microcirculation 18: 331–338, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bagher P, Duan D, Segal SS. Evidence for impaired neurovascular transmission in a murine model of Duchenne muscular dystrophy. J Appl Physiol 110: 601–610, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brekke JF, Jackson WF, Segal SS. Arteriolar smooth muscle Ca2+ dynamics during blood flow control in hamster cheek pouch. J Appl Physiol 101: 307–315, 2006. [DOI] [PubMed] [Google Scholar]
- 4. Budel S, Bartlett IS, Segal SS. Homocellular conduction along endothelium and smooth muscle of arterioles in hamster cheek pouch: unmasking an NO wave. Circ Res 93: 61–68, 2003. [DOI] [PubMed] [Google Scholar]
- 5. Busse R, Fichtner H, Luckhoff A, Kohlhardt M. Hyperpolarization and increased free calcium in acetylcholine-stimulated endothelial cells. Am J Physiol Heart Circ Physiol 255: H965–H969, 1988. [DOI] [PubMed] [Google Scholar]
- 6. Casey DP, Joyner MJ. NOS inhibition blunts and delays the compensatory dilation in hypoperfused contracting human muscles. J Appl Physiol 107: 1685–1692, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cox DA, Hintze TH, Vatner SF. Effects of acetylcholine on large and small coronary arteries in conscious dogs. J Pharmacol Exp Ther 225: 764–769, 1983. [PubMed] [Google Scholar]
- 8. Crane GJ, Gallagher N, Dora KA, Garland CJ. Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. J Physiol 553: 183–189, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Wit C, Esser N, Lehr HA, Bolz SS, Pohl U. Pentobarbital-sensitive EDHF comediates ACh-induced arteriolar dilation in the hamster microcirculation. Am J Physiol Heart Circ Physiol 276: H1527–H1534, 1999. [DOI] [PubMed] [Google Scholar]
- 10. de Wit C, Roos F, Bolz SS, Kirchhoff S, Kruger O, Willecke K, Pohl U. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res 86: 649–655, 2000. [DOI] [PubMed] [Google Scholar]
- 11. Delashaw JB, Duling BR. Heterogeneity in conducted arteriolar vasomotor response is agonist dependent. Am J Physiol Heart Circ Physiol 260: H1276–H1282, 1991. [DOI] [PubMed] [Google Scholar]
- 12. Domeier TL, Segal SS. Electromechanical and pharmacomechanical signalling pathways for conducted vasodilatation along endothelium of hamster feed arteries. J Physiol 579: 175–186, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dora KA, Xia J, Duling BR. Endothelial cell signaling during conducted vasomotor responses. Am J Physiol Heart Circ Physiol 285: H119–H126, 2003. [DOI] [PubMed] [Google Scholar]
- 14. Duling BR, Berne RM. Propagated vasodilation in the microcirculation of the hamster cheek pouch. Circ Res 26: 163–170, 1970. [DOI] [PubMed] [Google Scholar]
- 15. Duza T, Sarelius IH. Conducted dilations initiated by purines in arterioles are endothelium dependent and require endothelial Ca2+. Am J Physiol Heart Circ Physiol 285: H26–H37, 2003. [DOI] [PubMed] [Google Scholar]
- 16. Emerson GG, Neild TO, Segal SS. Conduction of hyperpolarization along hamster feed arteries: augmentation by acetylcholine. Am J Physiol Heart Circ Physiol 283: H102–H109, 2002. [DOI] [PubMed] [Google Scholar]
- 17. Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ Res 87: 474–479, 2000. [DOI] [PubMed] [Google Scholar]
- 18. Emerson GG, Segal SS. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ Res 86: 94–100, 2000. [DOI] [PubMed] [Google Scholar]
- 19. Figueroa XF, Paul DL, Simon AM, Goodenough DA, Day KH, Damon DN, Duling BR. Central role of connexin40 in the propagation of electrically activated vasodilation in mouse cremasteric arterioles in vivo. Circ Res 92: 793–800, 2003. [DOI] [PubMed] [Google Scholar]
- 20. Fleming I. Bobbing along on the crest of a wave: NO ascends hamster cheek pouch arterioles. Circ Res 93: 9–11, 2003. [DOI] [PubMed] [Google Scholar]
- 21. Heberlein KR, Straub AC, Isakson BE. The myoendothelial junction: breaking through the matrix? Microcirculation 16: 307–322, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hester RL. Venular-arteriolar diffusion of adenosine in hamster cremaster microcirculation. Am J Physiol Heart Circ Physiol 258: H1918–H1924, 1990. [DOI] [PubMed] [Google Scholar]
- 23. Koller A, Kaley G. Endothelium regulates skeletal muscle microcirculation by a blood flow velocity-sensing mechanism. Am J Physiol Heart Circ Physiol 258: H916–H920, 1990. [DOI] [PubMed] [Google Scholar]
- 24. Koller A, Sun D, Kaley G. Role of shear stress and endothelial prostaglandins in flow- and viscosity-induced dilation of arterioles in vitro. Circ Res 72: 1276–1284, 1993. [DOI] [PubMed] [Google Scholar]
- 25. Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 21: 69–78, 2006. [DOI] [PubMed] [Google Scholar]
- 26. Lew MJ, Duling BR. Arteriolar reactivity in vivo is influenced by an intramural diffusion barrier. Am J Physiol Heart Circ Physiol 259: H574–H581, 1990. [DOI] [PubMed] [Google Scholar]
- 27. Looft-Wilson RC, Payne GW, Segal SS. Connexin expression and conducted vasodilation along arteriolar endothelium in mouse skeletal muscle. J Appl Physiol 97: 1152–1158, 2004. [DOI] [PubMed] [Google Scholar]
- 28. Milner P, Kirkpatrick KA, Ralevic V, Toothill V, Pearson J, Burnstock G. Endothelial cells cultured from human umbilical vein release ATP, substance P and acetylcholine in response to increased flow. Proc Biol Sci 241: 245–248, 1990. [DOI] [PubMed] [Google Scholar]
- 29. Murrant CL, Sarelius IH. Local and remote arteriolar dilations initiated by skeletal muscle contraction. Am J Physiol Heart Circ Physiol 279: H2285–H2294, 2000. [DOI] [PubMed] [Google Scholar]
- 30. Naik RS, Doctor BP, Saxena A. Comparison of methods used for the determination of cholinesterase activity in whole blood. Chem Biol Interact 175: 298–302, 2008. [DOI] [PubMed] [Google Scholar]
- 31. Nollert MU, McIntire LV. Convective mass transfer effects on the intracellular calcium response of endothelial cells. J Biomech Eng 114: 321–326, 1992. [DOI] [PubMed] [Google Scholar]
- 32. Rivers RJ, Duling BR. Arteriolar endothelial cell barrier separates two populations of muscarinic receptors. Am J Physiol Heart Circ Physiol 262: H1311–H1315, 1992. [DOI] [PubMed] [Google Scholar]
- 33. Segal SS, Duling BR. Flow control among microvessels coordinated by intercellular conduction. Science 234: 868–870, 1986. [DOI] [PubMed] [Google Scholar]
- 34. Shen J, Luscinskas FW, Gimbrone MA, Jr, Dewey CF., Jr Fluid flow modulates vascular endothelial cytosolic calcium responses to adenine nucleotides. Microcirculation 1: 67–78, 1994. [DOI] [PubMed] [Google Scholar]
- 35. Shoemaker JK, Halliwill JR, Hughson RL, Joyner MJ. Contributions of acetylcholine and nitric oxide to forearm blood flow at exercise onset and recovery. Am J Physiol Heart Circ Physiol 273: H2388–H2395, 1997. [DOI] [PubMed] [Google Scholar]
- 36. Stamler CJ, Arrieta DE, Basu N, Henderson JD, Wilson BW, Chan HM. Evaluation of a fluorescent method for measuring cholinesterase activity in mammalian blood and tissue. Bull Environ Contam Toxicol 77: 785–792, 2006. [DOI] [PubMed] [Google Scholar]
- 37. Tallini YN, Brekke JF, Shui B, Doran R, Hwang SM, Nakai J, Salama G, Segal SS, Kotlikoff MI. Propagated endothelial Ca2+ waves and arteriolar dilation in vivo: measurements in Cx40BAC GCaMP2 transgenic mice. Circ Res 101: 1300–1309, 2007. [DOI] [PubMed] [Google Scholar]
- 38. Tallini YN, Shui B, Greene KS, Deng KY, Doran R, Fisher PJ, Zipfel W, Kotlikoff MI. BAC transgenic mice express enhanced green fluorescent protein in central and peripheral cholinergic neurons. Physiol Genomics 27: 391–397, 2006. [DOI] [PubMed] [Google Scholar]
- 39. Tigno XT, Ley K, Pries AR, Gaehtgens P. Venulo-arteriolar communication and propagated response. A possible mechanism for local control of blood flow. Pflügers Arch 414: 450–456, 1989. [DOI] [PubMed] [Google Scholar]
- 40. Uhrenholt TR, Domeier TL, Segal SS. Propagation of calcium waves along endothelium of hamster feed arteries. Am J Physiol Heart Circ Physiol 292: H1634–H1640, 2007. [DOI] [PubMed] [Google Scholar]
- 41. Welsh DG, Segal SS. Coactivation of resistance vessels and muscle fibers with acetylcholine release from motor nerves. Am J Physiol Heart Circ Physiol 273: H156–H163, 1997. [DOI] [PubMed] [Google Scholar]
- 42. Welsh DG, Segal SS. Endothelial and smooth muscle cell conduction in arterioles controlling blood flow. Am J Physiol Heart Circ Physiol 274: H178–H186, 1998. [DOI] [PubMed] [Google Scholar]
- 43. Wolfle SE, Schmidt VJ, Hoepfl B, Gebert A, Alcolea S, Gros D, de Wit C. Connexin45 cannot replace the function of connexin40 in conducting endothelium-dependent dilations along arterioles. Circ Res 101: 1292–1299, 2007. [DOI] [PubMed] [Google Scholar]
- 44. Wolfle SE, Schmidt VJ, Hoyer J, Kohler R, de Wit C. Prominent role of KCa3.1 in endothelium-derived hyperpolarizing factor-type dilations and conducted responses in the microcirculation in vivo. Cardiovasc Res 82: 476–483, 2009. [DOI] [PubMed] [Google Scholar]
- 45. Zhao G, Xu X, Ochoa M, Shen W, Hintze TH. Interaction between prostacyclin and nitric oxide in the reflex control of the coronary circulation in conscious dogs. Cardiovasc Res 32: 940–948, 1996. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.