Abstract
S-nitrosation of thiols in key proteins in cell signaling pathways is thought to be an important contributor to nitric oxide (NO)-dependent control of vascular (patho)physiology. Multiple metabolic enzymes are targets of both NO and S-nitrosation, including those involved in glycolysis and oxidative phosphorylation. Thus it is important to understand how these metabolic pathways are integrated by NO-dependent mechanisms. Here, we compared the effects of NO and S-nitrosation on both glycolysis and oxidative phosphorylation in bovine aortic endothelial cells using extracellular flux technology to determine common and unique points of regulation. The compound S-nitroso-l-cysteine (l-CysNO) was used to initiate intracellular S-nitrosation since it is transported into cells and results in stable S-nitrosation in vitro. Its effects were compared with the NO donor DetaNONOate (DetaNO). DetaNO treatment caused only a decrease in the reserve respiratory capacity; however, l-CysNO impaired both this parameter and basal respiration in a concentration-dependent manner. In addition, DetaNO stimulated extracellular acidification rate (ECAR), a surrogate marker of glycolysis, whereas l-CysNO stimulated ECAR at low concentrations and inhibited it at higher concentrations. Moreover, a temporal relationship between NO- and S-nitrosation-mediated effects on metabolism was identified, whereby NO caused a rapid impairment in mitochondrial function, which was eventually overwhelmed by S-nitrosation-dependent processes. Taken together, these results suggest that severe pharmacological nitrosative stress may differentially regulate metabolic pathways through both intracellular S-nitrosation and NO-dependent mechanisms. Moreover, these data provide insight into the role of NO and related compounds in vascular (patho)physiology.
Keywords: mitochondria, glycolysis, S-nitrosation, S-nitroso-cysteine
understanding the fundamental action of nitric oxide (NO) within its target cells is important to many aspects of vascular (patho)physiology. Although significant evidence suggests that the interaction of free NO with heme-containing proteins is a fundamental mechanism of NO action in vascular cells, there is a large body of literature that implicates the post-translational S-nitrosation of protein cysteine residues (thiols) downstream of NO as an important element of NO-dependent signaling. NO is an essential component of vascular function in vivo because of its ability to regulate vessel tone through binding of NO to the heme group of soluble guanylyl cyclase (sGC) (10, 19, 44). In addition, S-nitrosation of multiple protein targets in the endothelium regulates cell viability, platelet aggregation, and NO synthase activity (26). Understanding how these two mechanistically distinct pathways interact has been a major challenge to the field.
Of particular interest are the recent observations that NO-mediated and S-nitrosation-dependent effects have therapeutic value in the context of ischemia-reperfusion injury. Complex I of the mitochondrial electron transport chain has been identified as a target of S-nitrosation (7), and modification limits electron flux through the chain to minimize oxidative damage during reperfusion (30). In addition, infusion of a mitochondria-targeted S-nitrosothiol was shown to be protective through modulation of mitochondrial respiration when administered during reperfusion (36). In this case, the beneficial effects were attributed to both direct protein S-nitrosation and NO-dependent mechanisms. We have also demonstrated that treatment of rat hearts with the intracellular S-nitrosating agent S-nitroso-l-cysteine (l-CysNO) significantly improved functional recovery after ischemia-reperfusion injury (21). Moreover, the biological activity of NO and S-nitrosothiols is not limited to only protection against ischemia-reperfusion injury. S-nitrosothiol formation after inhaled NO administration has recently been proposed as a mechanism of NO delivery to the alveolar epithelium (3), and others have suggested that NO metabolites such as S-nitrosothiols, rather than free NO, mediate some of the beneficial vascular effects of inhaled NO (15).
Pharmacological intervention with S-nitrosothiols exposes cells and tissues to nitrosative stresses that are orders of magnitude greater than endogenous S-nitrosation from NO production. For example, induction of inducible NO synthase in murine macrophages generates ∼20 pmol/mg protein of intracellular S-nitrosothiols (48), whereas titration of cells with S-nitrosocysteine, or other membrane permeable S-nitrosothiols, can generate up to nanomoles per milligram of protein levels of intracellular S-nitrosothiols (5, 36, 48). This pharmacological nitrosative stress may affect cells in many ways, and understanding the similarities and differences between this and endogenous NO-dependent effects is important in understanding the scope and context of endogenous NO-dependent signaling as well as defining pharmacological mechanisms of action.
Our previous studies indicate that NO and S-nitrosating agents have differential effects on diverse cell functions including regulation of glycolysis and activation of the apoptosis pathway (4, 5). For example, S-nitrosation, but not free NO, inhibits caspase-3 activity in endothelial cells (5), whereas both S-nitrosation and NO inhibit the glycolytic enzyme GAPDH through different mechanisms (irreversible cofactor binding vs. reversible thiol modification, respectively) (4). We have recently shown, using activation of sGC as a biomarker, that S-nitrosothiols can be metabolized in the intracellular environment to liberate free NO (39), indicating further that the effects of protein S-nitrosation and the direct effects of NO on cellular processes may be difficult to disentangle.
NO has been shown to regulate several metabolic pathways including glycolysis and mitochondrial oxidative phosphorylation. NO binding to the heme group of Complex IV of the mitochondrial electron transport chain is well established (9). In addition, both stimulation and inhibition of glycolytic flux by NO have been reported under different physiological conditions (12, 14), and both mitochondrial and glycolytic enzymes are known to be targets of S-nitrosation (4, 7). Recent evidence suggests that modulation of metabolic pathways in endothelial cells regulates cellular responses to stress (12); therefore, a more complete understanding of the differential regulation of metabolic pathways by NO and S-nitrosation is required.
Here, we attempt to disentangle the effects of NO and S-nitrosation on glycolysis and oxidative phosphorylation using extracellular flux technology. To this end, we examined the effects of the slow-releasing NO donor, DetaNONOate (DetaNO), and the cell-transportable S-nitrosothiol, l-CysNO, on metabolic parameters in bovine aortic endothelial cells (BAEC). In agreement with previous work (12), DetaNO stimulates the extracellular acidification rate (ECAR) and inhibits the reserve respiratory capacity without impacting basal mitochondrial respiration. In contrast, l-CysNO treatment causes a biphasic ECAR response whereby low concentrations stimulate ECAR, whereas high concentrations inhibit this parameter and impair both basal respiration and the reserve capacity. In addition, we show that stimulation of ECAR by l-CysNO occurs as a result of S-nitrosothiol metabolism to free NO. Inhibition of basal respiration and the reserve capacity by l-CysNO is partially reversible with a NO scavenger at an early time point, but irreversible at a later time point. These data demonstrate that NO and S-nitrosation differentially regulate metabolic pathways and that l-CysNO-dependent mitochondrial dysfunction is temporally regulated. This regulation occurs through rapid inhibition of basal respiration from the release of NO from l-CysNO, which is eventually superseded by S-nitrosation-dependent pathways.
For clarity in this paper, ‘NO’ refers only to the free radical molecule NO, and therefore ‘NO-dependent’ refers to processes that are dependent on the formation of NO. ‘NO-independent’ refers to processes that do not require NO formation, such as S-nitrosation of protein thiols by the direct transfer of the nitroso functional group from an S-nitrosothiol to a protein thiol.
MATERIALS AND METHODS
Materials.
All chemicals were of analytical grade and purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. DetaNO; 2-(4-carboxyphenyl)-4,5-dihydro-4,4,5,5-tetramethyl-1H-imidazolyl-1-oxy-3-oxide, monopotassium salt (CPTIO); and 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) were purchased from Cayman Chemical (Ann Arbor, MI). CysNO was synthesized by incubating cysteine with acidified nitrite as previously described (48).
Cell culture.
BAEC were obtained from Lonza (Walkersville, MD) and cultured routinely as described previously (4). BAEC were maintained in high glucose (25 mmol/l) DMEM media supplemented with 10% FBS (Invitrogen, Carlsbad, CA). Cultures were incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air. For all experiments except HPLC, cells were seeded at 40,000 cells/well in specialized Seahorse Bioscience microplates 24 h before assays. For HPLC experiments, cells in six-well cluster plates were used. Cells were used between passages 4 and 10 for all experiments and at ∼95% confluence at the beginning of each assay.
Extracellular flux technology.
To measure mitochondrial function in intact BAEC, a Seahorse Bioscience XF24 Extracellular Flux Analyzer was used (13, 16, 32, 45). The mitochondrial function assay employed in the present study used a sequential injection of oligomycin, carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP), and antimycin A to define a number of mitochondrial parameters such as basal oxygen consumption rate (OCR), ATP-linked OCR, proton leak, maximal respiratory capacity, reserve respiratory capacity, and nonmitochondrial oxygen consumption. ECAR, a surrogate marker of glycolysis, was measured concomitantly. All assays were conducted using a seeding density of 40,000 cells/well in XF running media [Dulbecco's phosphate-buffered saline with Ca++ and Mg++ supplemented with 5.56 mmol/l glucose, 1 mmol/l sodium pyruvate, and 100 μmol/l diethylene triamine pentaacetic acid (DTPA)], and the AKOS algorithm was used. Cells were switched to XF running media 1 h before the beginning of the assay, and incubation in XF running media did not cause changes in cell morphology or viability. Results represented as percentage of baseline are normalized to the third basal OCR or ECAR measurement in each experiment. Across all experimental plates, basal OCR was 21.24 ± 7.37 pmol O2·min−1·μg protein−1 (means ± SD; n = 277) and ECAR was 0.63 ± 0.28 mpH·min−1·μg protein−1 (means ± SD; n = 200).
S-nitrosothiol measurements.
S-nitrososthiols were detected using triiodide-dependent ozone-based chemiluminescence as previously described (38, 41, 47). Samples were pretreated with 10% (vol/vol) sulfanilamide (100 mmol/l in 2 N HCl) to remove nitrite, and HgCl2 (5 mmol/l) was used to verify the presence of S-nitrosothiols. A standard curve was generated using S-nitroso-glutathione.
Adenine nucleotide extraction.
Perchloric acid precipitation was used to extract adenine nucleotides as described previously (35). Solvent A [75 μl; 0.1 mol/l potassium phosphate, 4 mmol/l tetrabutylammonium bisulfate (pH 6.0), and water 64:36] was added to supernatants, and they were filtered and used immediately for HPLC analysis. Protein pellets were resuspended, and protein concentration was determined by the method of Bradford.
HPLC analysis of adenine nucleotides.
HPLC analysis of adenine nucleotides based on a previously described method (49) was performed on Kromasil C-18 column using solvent A and solvent B [0.1 mol/l potassium phosphate, 4 mmol/l tetrabutylammonium bisulfate (pH 6.0), and methanol 64:36] with a flow rate of 1 ml/min. The chromatographic conditions are detailed in the Supplemental Material. ATP and ADP peaks were measured for each sample, compared with the standard, and expressed in nanomoles per milligram of protein.
Statistical analysis.
Results are reported as means ± SE for n ≥ 3, as indicated in the figure legends. Statistical significance was evaluated by Student's t-test. The minimum level of significance was set at P < 0.05.
RESULTS
Effect of nitrosative stress on cellular bioenergetics.
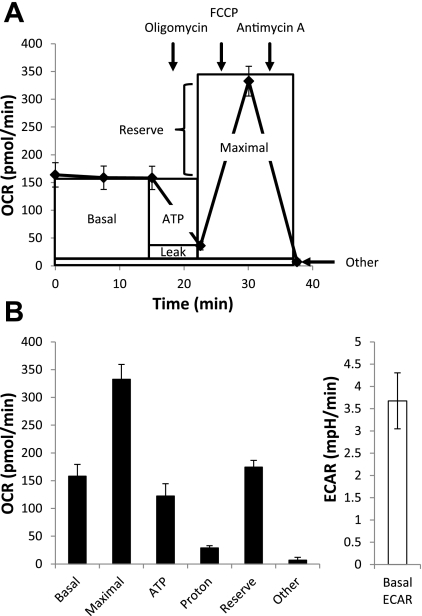
Extracellular flux technology was used to assess a number of mitochondrial function parameters as previously described (12). A schematic of the mitochondrial function assay is shown in Fig. 1A. After baseline is established, sequential injection of oligomycin, FCCP, and antimycin A with subsequent measurement of OCR allows for the determination of six mitochondrial function parameters in BAEC (Fig. 1B). Concomitant measurement of basal ECAR yields this additional metabolic parameter.
Fig. 1.
Assessment of bioenergetics using extracellular flux technology. A schematic representation of a mitochondrial function assay is shown (A). After baseline oxygen consumption rate (OCR) is established, sequential injection of oligomycin, carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP), and antimycin A allows for the determination of the mitochondrial function parameters basal OCR, ATP-linked OCR (ATP), proton leak (Leak), maximal OCR, reserve capacity (Reserve), and oxygen consumption, which occurs independent of Complex IV (Other). Mitochondrial function parameters and basal extracellular acidification rate (ECAR) determined in bovine aortic endothelial cells (BAEC) are shown (B). Values represent means ± SE; n = 3 to 4.
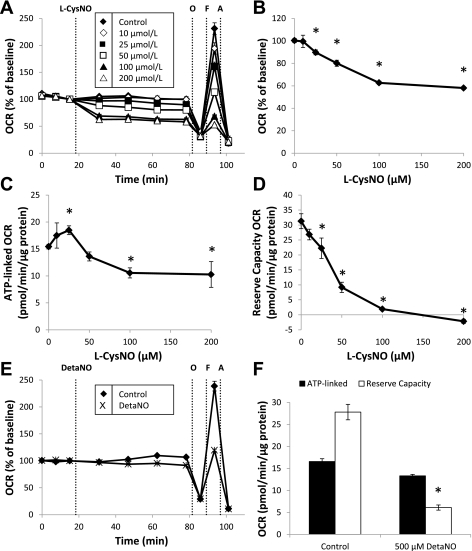
Using this mitochondrial function assay, we assessed the effect of both the transnitrosating agent, l-CysNO, and NO donor, DetaNO, on mitochondrial function in BAEC. DetaNO has a half-life of 20 h at 37°C (25) and produces NO at levels similar to those observed in inflammation (1, 34). After baseline OCR was established, increasing concentrations of l-CysNO (10–200 μmol/l) were injected directly into assay wells. OCR was monitored every 15 min for 1 h before assessment of mitochondrial function parameters (Fig. 2A). l-CysNO caused a concentration-dependent decrease in basal OCR after 1 h (Fig. 2B), and both ATP-linked respiration and reserve capacity were also impacted in a similar manner (Fig. 2, C and D); however, the reserve capacity was the parameter most sensitive to inhibition by l-CysNO. In contrast, treatment with DetaNO (500 μmol/l) under the same experimental conditions resulted in no change in basal OCR (Fig. 2, E and F). Consistent with previous reports (12), DetaNO did, however, decrease the reserve capacity by ∼78% (Fig. 2F).
Fig. 2.
Effect of S-nitroso-cysteine (CysNO) and DetaNONOate (DetaNO) on mitochondrial function in BAEC. After baseline was established, OCR was monitored upon addition of the compound S-nitroso-l-cysteine (l-CysNO; 10–200 μmol/l) for 1 h before sequential injection of oligomycin (O; 1 μg/ml), FCCP (F; 1 μmol/l), and antimycin A (A; 10 μmol/l). Time-resolved data are shown in A. Basal OCR after 1 h (B), ATP-linked OCR (C), and reserve capacity (D) are shown. OCR after DetaNO (500 μmol/l) addition was also monitored in the same manner. Basal OCR after 1 h and reserve capacity (E and F) are shown. OCRs were normalized to total protein per well after completion of assay. Values represent means ± SE; n = 3 to 4. *P < 0.05 compared with control.
Effect of CysNO uptake on bioenergetic function.
We have previously demonstrated that l-CysNO is actively transported into cells through amino acid transport system L (L-AT), and intracellular nitrosation by l-CysNO can be attenuated by high concentrations of the competitive substrate of transport, l-leucine (48). The D isomer of CysNO (d-CysNO) is transported with significantly reduced efficiency by L-AT, but has the same nitrosothiol moiety; thus it can be used as a control for intracellular versus extracellular effects of the nitrosothiol (39). As anticipated, l-CysNO treatment caused a concentration-dependent increase in intracellular S-nitrosothiol content, whereas d-CysNO treatment resulted in significantly lower levels as reported previously (Supplemental Fig. S1) (48). DetaNO treatment resulted in very low S-nitrosothiol formation (0.036 ± 0.013 nmol/mg protein) when compared with l-CysNO.
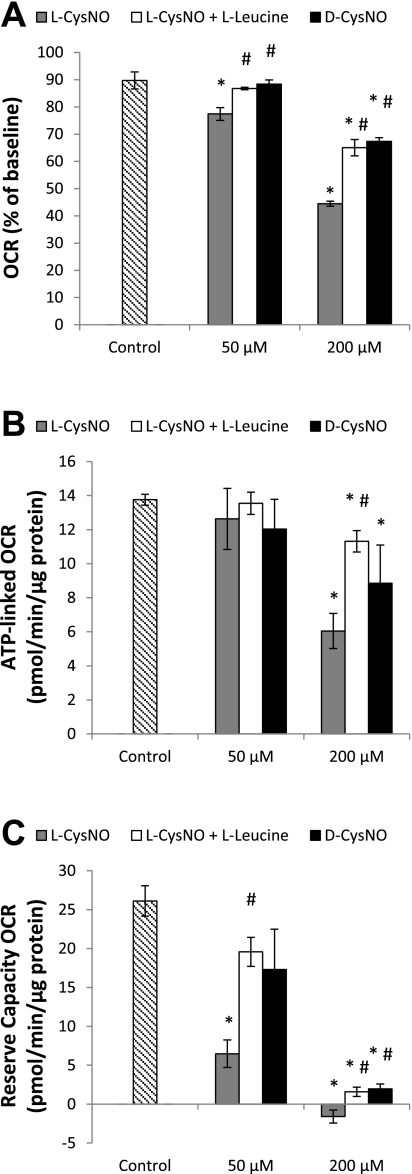
We next examined the effect of l-leucine with l-CysNO and d-CysNO to determine whether the impairment of mitochondrial function observed in Fig. 2 occurs as a result of intracellular interactions of the nitrosothiol. Although l-leucine itself had no effect on basal respiration after 1 h (control: 89.7 ± 3.1% of baseline; l-leucine: 91.8 ± 3.8% of baseline), it partially attenuated l-CysNO-dependent inhibition of basal respiration (Fig. 3A). Moreover, d-CysNO, which is not readily taken up by the cell, also inhibited basal OCR, although only to the same extent as the l-CysNO/l-leucine combination (Fig. 3A). This same trend was also observed when the effects of l-leucine with l-CysNO and d-CysNO on ATP-linked OCR and reserve capacity were examined (Fig. 3, B and C). In addition, concentration-dependent effects of d-CysNO (10–200 μmol/l) on mitochondrial function parameters were examined and demonstrate d-CysNO is less potent at every concentration than l-CysNO (Supplemental Fig. S2). This suggests that l-CysNO regulates mitochondrial function through an L-AT transport-dependent process.
Fig. 3.
Effect of CysNO uptake on mitochondrial function in BAEC. OCR was monitored for 1 h after the addition of l-CysNO, l-CysNO ± l-leucine (8 mmol/l), or the D isomer of CysNO (d-CysNO). Sequential injection of oligomycin (1 μg/ml), FCCP (1 μmol/l), and antimycin A (10 μmol/l) was used to determine mitochondrial function parameters. Basal OCR after 1 h (A), ATP-linked OCR (B), and reserve capacity (C) are shown. OCRs were normalized to total protein per well after completion of assay. Values represent means ± SE; n = 3 to 4. *P < 0.05 compared with control; #P < 0.05 compared with matched CysNO concentration.
Adenine nucleotide levels after CysNO exposure.
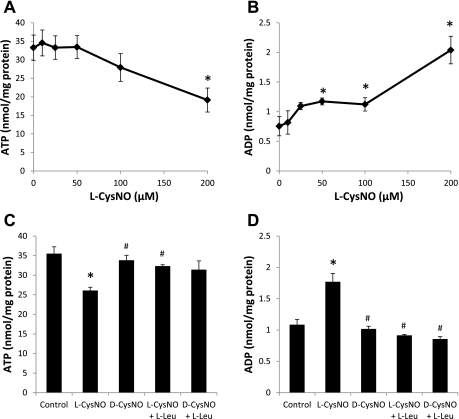
Because treatment with both CysNO and DetaNO resulted in changes in mitochondrial function, we next determined the impact of l-CysNO on ATP levels. Exposure to l-CysNO (10–200 μmol/l) for 1 h resulted in a concentration-dependent decrease in cellular ATP levels (Fig. 4A). This decrease was accompanied by an increase in ADP levels (Fig. 4B); however, all ATP loss could not be accounted for by the increase in ADP levels, suggesting further degradation of adenine nucleotides may occur. l-leucine (8 mmol/l) blocked l-CysNO-dependent ATP depletion and ADP accumulation, and d-CysNO (200 μmol/l; 1 h) did not have an effect on either ATP or ADP levels (Fig. 4, C and D). In sum, ATP depletion occurs under conditions of high intracellular S-nitrosothiol levels and requires CysNO transport into cells. It is important to note that DetaNO (500 μmol/l) exposure did not deplete ATP (control, 22.1 ± 1.7 nmol/mg protein; DetaNO, 25.7 ± 0.6 nmol/mg protein).
Fig. 4.
Adenine nucleotide levels after CysNO exposure. BAEC were treated with increasing concentrations of l-CysNO (10–200 μmol/l) in XF running media for 1 h, and cell lysates were analyzed for adenine nucleotide levels (A and B). ATP (C) and ADP (D) were also measured from cells treated with l-CysNO (200 μmol/l) or d-CysNO (200 μmol/l) ± l-leucine (l-Leu; 8 mmol/l) for 1 h. Nucleotide levels were normalized to total protein. Values represent means ± SE; n = 3. *P < 0.05 compared with control; #P < 0.05 compared with matched CysNO concentration.
Regulation of mitochondrial function by NO-dependent effects of CysNO.
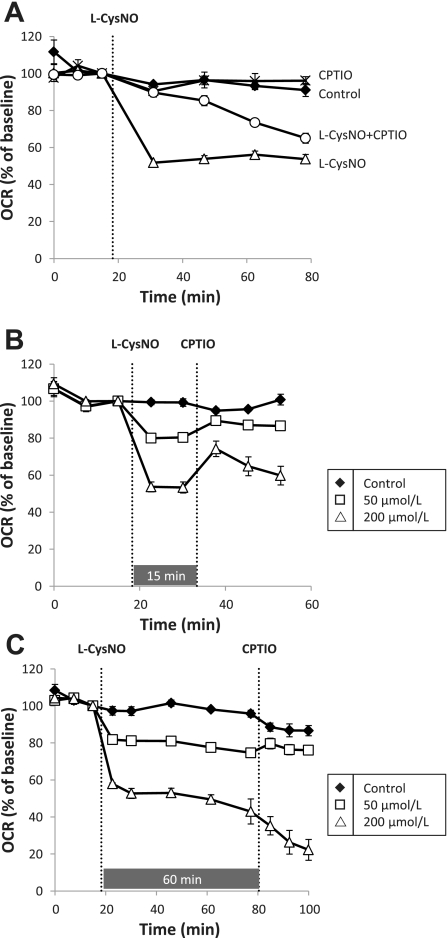
We have previously observed the activation of sGC from intracellular CysNO through a DPI-inhibitable mechanism (39), suggesting that CysNO can be metabolized to generate NO. It has previously been shown, and we confirm here, that NO regulates mitochondrial function. It is for these reasons we sought to determine whether CysNO-induced mitochondrial inhibition of basal oxygen consumption occurs through a mechanism involving free NO. To address this, we examined the effect of the NO scavenger CPTIO (24) on CysNO-induced mitochondrial dysfunction. First, BAEC were prepared for extracellular flux analysis and CPTIO (100 μmol/l) was added to the XF running media 1 h before assay and was found to have no effect on OCR. After baseline was established, l-CysNO (200 μmol/l) was injected, and OCR was monitored over 1 h. The presence of CPTIO caused a time-dependent lag in l-CysNO-induced inhibition of basal OCR (Fig. 5A), consistent with a role for free NO in the regulation of mitochondrial function by CysNO.
Fig. 5.
2-(4-Carboxyphenyl)-4,5-dihydro-4,4,5,5-tetramethyl-1H-imidazolyl-1-oxy-3-oxide, monopotassium salt (CPTIO) reversibility of CysNO-dependent impairment of basal mitochondrial function. BAEC were treated with or without CPTIO in the running media before measurement of OCR. After baseline was established, l-CysNO (200 μmol/l) was injected, and OCR was monitored for 1 h (A). The effect of CPTIO on OCR after treatment with l-CysNO (50 or 200 μmol/l) is shown in B (15 min after l-CysNO) and C (60 min after l-CysNO). OCRs were normalized to total protein per well after completion of assay. Values represent means ± SE; n = 3 to 4.
In the next series of experiments the reversibility of the l-CysNO (50 or 200 μmol/l)-dependent inhibition of OCR examined was by adding CPTIO after l-CysNO. When CPTIO was injected after 15 min, there was reversibility of l-CysNO-dependent inhibition of basal OCR at both concentrations (Fig. 5B). However, if CPTIO was added 60 min after l-CysNO treatment, inhibition of basal OCR was no longer reversible with CPTIO (Fig. 5C). Using the same protocol the reversibility of the cell impermeable d-CysNO was tested at 15 and 60 min and in this case reversibility was evident at both time points (Supplemental Fig. S3).
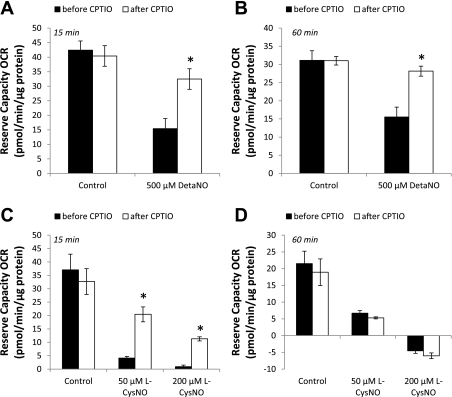
We also examined CPTIO-dependent reversibility of alterations in the reserve capacity after DetaNO and l-CysNO treatment. To do this, we used a modification of the mitochondrial function assay with injection of CPTIO after FCCP. The reserve capacity was then calculated before and after CPTIO injection. Because DetaNO treatment did not alter basal respiration, but only the reserve capacity, we first determined whether CPTIO reversed this effect of DetaNO. As expected, DetaNO caused a significant decrease in the reserve capacity after both 15 and 60 min treatment, and CPTIO reversed this effect (Fig. 6, A and B) resulting in the recovery of ∼80–90% of the reserve capacity. In contrast, the reserve capacity was only partially reversible with CPTIO after treatment with l-CysNO for 15 min and completely irreversible after 60 min (Fig. 6, C and D). These results are consistent with the fact that inhibition of basal OCR by l-CysNO seems to occur in part through the release of NO; however, these effects are overcome by events independent of free NO, which likely are related to protein thiol modification, at later time points.
Fig. 6.
CPTIO reversibility of DetaNO- and CysNO-dependent inhibition of the reserve capacity. The reserve capacity was measured before and after CPTIO (100 μmol/l) injection either 15 min or 60 min after the addition of DetaNO (500 μmol/l; A and B) or l-CysNO (50 or 200 μmol/l; C and D). OCRs were normalized to total protein per well after completion of assay. Values represent means ± SE; n = 3–5. *P < 0.05 compared with control.
Effect of CysNO uptake on glycolytic flux.
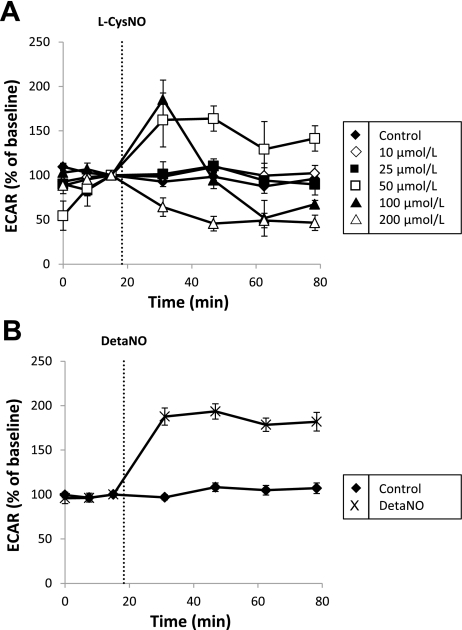
The effect of l-CysNO and DetaNO on ECAR, a surrogate marker of glycolytic flux (13), in BAEC was also examined. After baseline was established, increasing concentrations of l-CysNO (10–200 μmol/l) were injected into assay wells, and ECAR was monitored for 1 h. A biphasic response to l-CysNO was observed whereby low concentrations caused a transient stimulation of ECAR, whereas higher concentrations caused significant inhibition (Fig. 7A). In contrast, exposure to DetaNO (500 μmol/l) resulted in sustained stimulation of ECAR over the 1-h measurement period (Fig. 7B) as previously reported (12). d-CysNO treatment caused stimulation of ECAR without any inhibition observed even at high concentrations (e.g., 200 μmol/l; Supplemental Fig. S4A).
Fig. 7.
Effect of l-CysNO and DetaNO on ECAR in BAEC. After baseline was established, ECAR was monitored upon addition of l-CysNO (10–200 μmol/l) for 1 h (A). ECAR after DetaNO (500 μmol/l) addition was also monitored in the same manner (B). ECARs were normalized to total protein per well after completion of assay. Values represent means ± SE; n = 3–5.
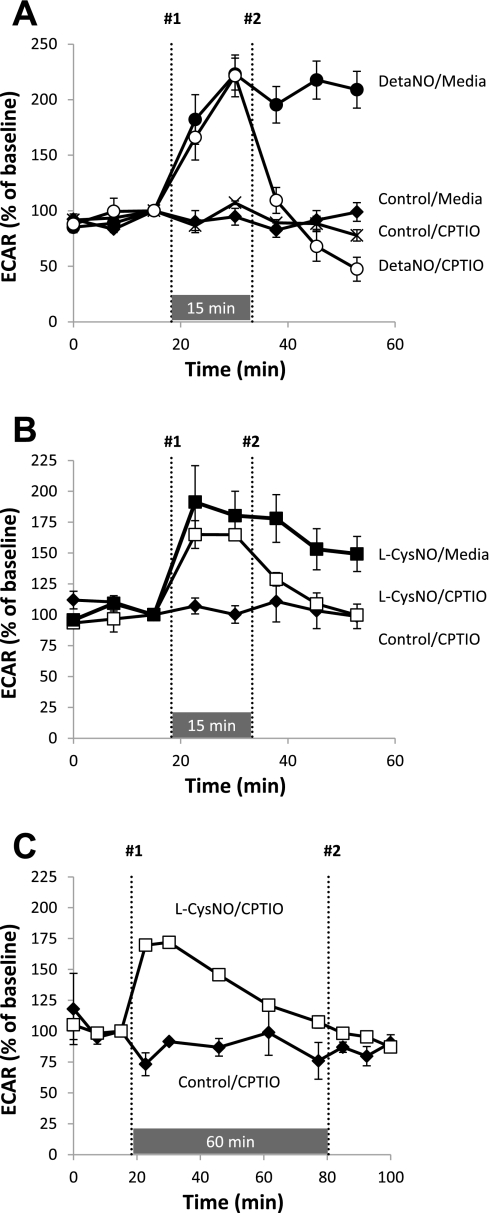
Because both DetaNO and low levels of l-CysNO caused an increase in ECAR, we again used CPTIO to identify free NO-dependent effects of l-CysNO. Figure 8A shows the CPTIO reversibility of DetaNO-induced stimulation of ECAR. Addition of CPTIO after DetaNO returned ECAR back to control levels. Moreover, treatment with l-CysNO (50 μmol/l) stimulated ECAR ∼75% over the basal rate, and CPTIO injection 15 min after l-CysNO treatment caused ECAR to decrease to levels similar to control (Fig. 8B). When CPTIO was added 60 min after l-CysNO treatment, no significant effect is observed due to the transient nature of stimulation of ECAR by l-CysNO (Fig. 8C). Similar experiments were conducted using d-CysNO (200 μmol/l), and stimulation of ECAR was reversible by CPTIO addition (Supplemental Fig. S4, B and C). Taken together, these results indicate that stimulation of ECAR by low concentrations of l-CysNO (e.g., 50 μmol/l) occurs through the release of NO from l-CysNO. At longer times and higher concentrations, this effect is reversed, and glycolysis appears to be inhibited.
Fig. 8.
CPTIO reversibility of DetaNO- and CysNO-dependent effects on ECAR. After baseline was established, ECAR was monitored upon addition of DetaNO (500 μmol/l). CPTIO (100 μmol/l) was added 15 min after DetaNO (A). CPTIO (100 μmol/l) was also added 15 min (B) or 60 min (C) after the addition of l-CysNO (200 μmol/l). Compounds injected at point No. 1 and point No. 2 for each treatment group are denoted. ECARs were normalized to total protein per well after completion of assay. Values represent means ± SE; n = 3–5.
Finally, to determine whether stimulation of ECAR by DetaNO or low concentrations of l-CysNO (50 μmol/l) is regulated by canonical NO signaling through sGC, extracellular flux experiments were preformed in the presence of ODQ (5 μmol/l). ODQ had no effect on DetaNO or l-CysNO-dependent changes in ECAR or OCR (Supplemental Fig. S5), suggesting that sGC-mediated signaling is not involved.
DISCUSSION
In this study, we have compared the effects of NO and the S-nitrosothiol CysNO on integrated cellular metabolism using extracellular flux technology. It is well established that free NO regulates mitochondrial function at the level of cytochrome c oxidase by competing for the oxygen binding site of the enzyme (9). In addition, modulation of glycolysis by NO has been reported; however, contradicting evidence shows both stimulation and inhibition of this metabolic pathway by NO. A number of potential mechanisms have been invoked for how this regulation occurs. For example, direct inhibition of the glycolytic enzyme GAPDH by NO has been demonstrated in cells derived from different organs including endothelium (4) and hippocampal brain slices (22). In contrast, NO-mediated stabilization of hypoxia-inducible factor 1-α has been shown to stimulate glycolysis (14), and others have demonstrated the activation of nutrient-sensing pathways, such as AMP kinase, by NO (29), which are known to regulate glycolysis through phosphorylation cascades that increase glucose transport (40) and impact glycolytic enzyme activity (e.g., phosphofructokinase 2) (28).
Here, we show regulation of both glycolysis and mitochondrial function by free NO. Consistent with the previous report by Dranka et al. (12), treatment of BAEC with the NO donor DetaNO stimulated glycolysis (Fig. 7) and impaired the mitochondrial reserve capacity without impacting basal respiration (Fig. 2). Moreover, we show that both the stimulation of ECAR and impairment of the reserve capacity were readily reversible upon the addition of the free NO scavenger CPTIO after DetaNO administration (Figs. 6 and 8). These data suggest that both of these effects are due to the rapidly reversible binding of NO to a target, which is more consistent with NO/heme interactions than with protein S-nitrosation or other covalent thiol modifications. In addition, although the mechanism by which NO causes increased glycolytic flux remains unknown, because there is no change in basal respiration after exposure to NO, direct inhibition of Complex IV by NO does not seem to be the signal for stimulation of ECAR. Our results using the highly selective, irreversible inhibitor of sGC, ODQ, demonstrate that sGC/cGMP responses are also not involved (Supplemental Fig. S5). These results also suggest that significant inhibition of GAPDH activity is not a sensitive biological activity of free NO.
Although it is well known that multiple metabolic enzymes are targets of S-nitrosation (e.g., GAPDH and Complex I), and modification inhibits their activity (4, 7), the integrated metabolic response to intracellular S-nitrosation has not yet been defined. We used l-CysNO to initiate intracellular S-nitrosation and compared its effects with those of NO. In contrast with the effects observed with DetaNO treatment, l-CysNO caused a biphasic ECAR response (Fig. 7) whereby low concentrations of l-CysNO stimulated ECAR and high concentrations inhibited this parameter. In addition, we examined whether alterations in cellular metabolism caused by l-CysNO required transport of the nitrosating agent into cells. Inhibition of ECAR absolutely required l-CysNO uptake since the D isomer could not recapitulate this effect (Supplemental Fig. S4). Taken together, these data clearly demonstrate that high concentrations of l-CysNO inhibit glycolytic flux through intracellular S-nitrosation-dependent events, which are likely the result of inhibition of GAPDH as previously reported (4, 18) or other redox-sensitive glycolytic enzymes (e.g., aldolase) (31, 43).
Examination of the effect of l-CysNO on mitochondrial function parameters showed that l-CysNO inhibited basal respiration, ATP-linked OCR, and the reserve capacity in a concentration-dependent manner (Fig. 2) and that reserve capacity was most sensitively inhibited. The fact that the reserve capacity (also known as the spare respiratory capacity) is the most sensitive parameter to inhibition both by DetaNO and l-CysNO is particularly interesting given that it is thought that the reserve capacity is critical for maintaining cellular function in response to multiple stressors (8, 11, 23, 33, 46). In the context of cardiovascular pathologies, it has been observed that loss of the reserve capacity predicts cell death and/or organ dysfunction in cardiomyocytes (20), endothelial cells (12), and the failing heart in vivo (17). Loss of the reserve capacity in our model likely occurs both as the result of impaired glycolysis, which provides substrates to mitochondria to drive their activity (6), and through direct modification of redox-sensitive mitochondrial proteins such as Complex I (7). We also determined whether CysNO must be transported into cells to regulate mitochondrial function. Interestingly, the D isomer of CysNO, which is not efficiently transported into cells, has a partial effect on basal respiration, ATP-linked OCR, and reserve capacity compared with l-CysNO (Supplemental Fig. S3). This suggests that the regulation of mitochondrial function by l-CysNO occurs, at least in part, through mechanisms independent of its ability to initiate intracellular S-nitrosation.
Although CysNO treatment is an effective way to initiate S-nitrosation in cultured cells, we have previously shown that flavoprotein-dependent metabolism of CysNO activates sGC (39), implying that NO is released intracellularly from CysNO. To separate the free NO- versus S-nitrosation-dependent effects of CysNO on metabolism, we utilized CPTIO to scavenge NO released from CysNO. We demonstrated that stimulation of ECAR by CysNO occurs through the liberation of NO from CysNO since CPTIO completely reverses this effect (Fig. 8). This is further supported by the obersevation that d-CysNO, albeit at higher concentrations than l-CysNO, also stimulates ECAR, and this can be blocked with CPTIO (Supplemental Fig. S4). Here, we cannot determine whether CysNO-induced enhanced glycolytic flux is mediated through intracellular CysNO metabolism to NO, extracellular cell-mediated NO release, or through spontaneous decomposition of CysNO. However, all experiments were conducted in media supplemented with the metal chelator DTPA (100 μmol/l) to minimize metal-dependent CysNO decay. In previous studies, we have not observed extracellular NO-liberation from CysNO (39), but it is possible that the conditions required for the XF24 assay allow for some NO formation.
Our data suggest that the immediate effects of CysNO on mitochondrial function are mediated through the release of free NO, and in this way, l-CysNO may act as a cell-targeted NO releasing agent. The addition of CPTIO to assay media before the injection of l-CysNO resulted in a temporal lag in l-CysNO-dependent inhibition of basal respiration (Fig. 5A). Further examination of this effect using CPTIO to reverse inhibition of respiration revealed that NO plays a crucial role in inhibition of basal respiration at an early time point (e.g., 15 min); however, 60 min after administration of l-CysNO, CPTIO no longer reversed this effect (Fig. 5, B and C). CysNO-dependent inhibition of the reserve capacity was also partially reversible after 15 min and irreversible after 60 min (Fig. 6). These results highlight the fact that a temporal relationship exists between free NO- and S-nitrosation-mediated effects on mitochondrial function. NO caused an early, rapid response in mitochondrial function, which was eventually overtaken by a slower, S-nitrosothiol-driven process.
Finally, using ATP as a key energy currency output from cellular metabolism, we show that l-CysNO caused concentration-dependent depletion of ATP (Fig. 4). l-CysNO treatment also causes an increase in ADP levels. Furthermore, CysNO-dependent ATP depletion requires CysNO transport into cells (Fig. 4), and NO from DetaNO does not deplete ATP.
In summary, both CysNO and NO regulate integrated cellular metabolism in complex manners. NO stimulates glycolytic flux and inhibits the reserve capacity, whereas the effects of CysNO are more pervasive. CysNO causes a biphasic response in glycolytic flux and impairs basal respiration, ATP-linked OCR, and the reserve capacity. These data show differential sensitivity of metabolic pathways to regulation by S-nitrosation. Moreover, release of NO from CysNO accounts for the early, rapid inhibition of mitochondrial function. We find little evidence that the NO-dependent inhibition of respiration and stimulation of glycolysis occur via S-nitrosation, which implies that under physiological conditions, NO-mediated S-nitrosation is not a major mechanism by which glycolysis is regulated. The effects are more consistent with reversible binding to metal centers and are clearly different than the effects of S-nitrosation. The most important effect of pharmacological S-nitrosation appears to be the inhibition of glycolysis, likely at the level of GAPDH, which prevents the biosynthesis of mitochondrial substrates. Because the cells in this study were respiring on a combination of glucose and pyruvate, direct inhibition of mitochondrial electron transfer by S-nitrosation must also be invoked. Consequently, the pharmacological mechanism of vascular and cardioprotection by membrane transportable S-nitrosothiols may be quite different than those driven by NO. Because metabolic pathways are integrally related to cell health (2, 27, 37), and the reserve capacity has been shown to be linked to stress responses (12, 20, 42), the differential regulation of metabolism by endogenous NO and by endogenous or pharmacological S-nitrosation remains a key area to understanding the role of endothelial cell function in cardiovascular (patho)physiology.
GRANTS
This research was supported in part by the Redox Biology Program at the Medical College of Wisconsin and National Institutes of Health Grants R01-GM-55792 (to N. Hogg) and R01-ES-10167 (to V. M. Darley-Usmar).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Drs. B. Kalyanaraman and Brian P. Dranka for important support for these studies.
REFERENCES
- 1. Balbatun A, Louka FR, Malinski T. Dynamics of nitric oxide release in the cardiovascular system. Acta Biochim Pol 50: 61–68, 2003 [PubMed] [Google Scholar]
- 2. Ballinger SW. Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med 38: 1278–1295, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Brahmajothi MV, Mason SN, Whorton AR, McMahon TJ, Auten RL. Transport rather than diffusion-dependent route for nitric oxide gas activity in alveolar epithelium. Free Radic Biol Med 49: 294–300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Broniowska KA, Hogg N. Differential mechanisms of inhibition of glyceraldehyde-3-phosphate dehydrogenase by S-nitrosothiols and NO in cellular and cell-free conditions. Am J Physiol Heart Circ Physiol 299: H1212–H1219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Broniowska KA, Zhang Y, Hogg N. Requirement of transmembrane transport for S-nitrosocysteine-dependent modification of intracellular thiols. J Biol Chem 281: 33835–33841, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burwell LS, Nadtochiy SM, Brookes PS. Cardioprotection by metabolic shut-down and gradual wake-up. J Mol Cell Cardiol 46: 804–810, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J 394: 627–634, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choi SW, Gerencser AA, Lee DW, Rajagopalan S, Nicholls DG, Andersen JK, Brand MD. Intrinsic bioenergetic properties and stress sensitivity of dopaminergic synaptosomes. J Neurosci 31: 4524–4534, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cooper CE. Competitive, reversible, physiological? Inhibition of mitochondrial cytochrome oxidase by nitric oxide. IUBMB Life 55: 591–597, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Denninger JW, Marletta MA. Guanylate cyclase and the NO/cGMP signaling pathway. Biochim Biophys Acta 1411: 334–350, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Diers AR, Higdon AN, Ricart KC, Johnson MS, Agarwal A, Kalyanaraman B, Landar A, Darley-Usmar VM. Mitochondrial targeting of the electrophilic lipid 15-deoxy-Delta12,14-prostaglandin J2 increases apoptotic efficacy via redox cell signalling mechanisms. Biochem J 426: 31–41, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dranka BP, Hill BG, Darley-Usmar VM. Mitochondrial reserve capacity in endothelial cells: the impact of nitric oxide and reactive oxygen species. Free Radic Biol Med 48: 905–914, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferrick DA, Neilson A, Beeson C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov Today 13: 268–274, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Garedew A, Moncada S. Mitochondrial dysfunction and HIF1alpha stabilization in inflammation. J Cell Sci 121: 3468–3475, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Gaston B. Summary: systemic effects of inhaled nitric oxide. Proc Am Thorac Soc 3: 170–172, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Gerencser AA, Neilson A, Choi SW, Edman U, Yadava N, Oh RJ, Ferrick DA, Nicholls DG, Brand MD. Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal Chem 81: 6868–6878, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gong G, Liu J, Liang P, Guo T, Hu Q, Ochiai K, Hou M, Ye Y, Wu X, Mansoor A, From AH, Ugurbil K, Bache RJ, Zhang J. Oxidative capacity in failing hearts. Am J Physiol Heart Circ Physiol 285: H541–H548, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y, Takahashi M, Cheah JH, Tankou SK, Hester LD, Ferris CD, Hayward SD, Snyder SH, Sawa A. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol 7: 665–674, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6: 150–166, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Hill BG, Dranka BP, Zou L, Chatham JC, Darley-Usmar VM. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem J 424: 99–107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hogg N, Broniowska KA, Novalija J, Kettenhofen NJ, Novalija E. Role of S-nitrosothiol transport in the cardioprotective effects of S-nitrosocysteine in rat hearts. Free Radic Biol Med 43: 1086–1094, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Izumi Y, Zorumski CF. Neuroprotective effects of pyruvate following NMDA-mediated excitotoxic insults in hippocampal slices. Neurosci Lett 478: 131–135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jekabsons MB, Nicholls DG. In situ respiration and bioenergetic status of mitochondria in primary cerebellar granule neuronal cultures exposed continuously to glutamate. J Biol Chem 279: 32989–33000, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Joseph J, Kalyanaraman B, Hyde JS. Trapping of nitric oxide by nitronyl nitroxides: an electron spin resonance investigation. Biochem Biophys Res Commun 192: 926–934, 1993 [DOI] [PubMed] [Google Scholar]
- 25. Keefer LK, Nims RW, Davies KM, Wink DA. “NONOates” (1-substituted diazen-1-ium-1,2-diolates) as nitric oxide donors: convenient nitric oxide dosage forms. Methods Enzymol 268: 281–293, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res 106: 633–646, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res 100: 460–473, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den BG, Carling D, Hue L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol 10: 1247–1255, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Meares GP, Hughes KJ, Jaimes KF, Salvatori AS, Rhodes CJ, Corbett JA. AMP-activated protein kinase attenuates nitric oxide-induced beta-cell death. J Biol Chem 285: 3191–3200, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nadtochiy SM, Burwell LS, Brookes PS. Cardioprotection and mitochondrial S-nitrosation: effects of S-nitroso-2-mercaptopropionyl glycine (SNO-MPG) in cardiac ischemia-reperfusion injury. J Mol Cell Cardiol 42: 812–825, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Newman SF, Sultana R, Perluigi M, Coccia R, Cai J, Pierce WM, Klein JB, Turner DM, Butterfield DA. An increase in S-glutathionylated proteins in the Alzheimer's disease inferior parietal lobule, a proteomics approach. J Neurosci Res 85: 1506–1514, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Nicholls DG, Darley-Usmar VM, Wu M, Jensen PB, Rogers GW, Ferrick DA. Bioenergetic profile experiment using C2C12 myoblast cells. J Vis Exp 46: 2511, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oliva CR, Nozell SE, Diers A, McClugage SG, III, Sarkaria JN, Markert JM, Darley-Usmar VM, Bailey SM, Gillespie GY, Landar A, Griguer CE. Acquisition of temozolomide chemoresistance in gliomas leads to remodeling of mitochondrial electron transport chain. J Biol Chem 285: 39759–39767, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Orsi A, Beltran B, Clementi E, Hallen K, Feelisch M, Moncada S. Continuous exposure to high concentrations of nitric oxide leads to persistent inhibition of oxygen consumption by J774 cells as well as extraction of oxygen by the extracellular medium. Biochem J 346: 407–412, 2000 [PMC free article] [PubMed] [Google Scholar]
- 35. Perez J, Hill BG, Benavides GA, Dranka BP, Darley-Usmar VM. Role of cellular bioenergetics in smooth muscle cell proliferation induced by platelet-derived growth factor. Biochem J 428: 255–267, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prime TA, Blaikie FH, Evans C, Nadtochiy SM, James AM, Dahm CC, Vitturi DA, Patel RP, Hiley CR, Abakumova I, Requejo R, Chouchani ET, Hurd TR, Garvey JF, Taylor CT, Brookes PS, Smith RA, Murphy MP. A mitochondria-targeted S-nitrosothiol modulates respiration, nitrosates thiols, and protects against ischemia-reperfusion injury. Proc Natl Acad Sci USA 106: 10764–10769, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ramachandran A, Levonen AL, Brookes PS, Ceaser E, Shiva S, Barone MC, Darley-Usmar VM. Mitochondria, nitric oxide, and cardiovascular dysfunction. Free Radic Biol Med 33: 1465–1474, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Rassaf T, Bryan NS, Kelm M, Feelisch M. Concomitant presence of N-nitroso and S-nitroso proteins in human plasma. Free Radic Biol Med 33: 1590–1596, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Riego JA, Broniowska KA, Kettenhofen NJ, Hogg N. Activation and inhibition of soluble guanylyl cyclase by S-nitrosocysteine: involvement of amino acid transport system L. Free Radic Biol Med 47: 269–274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Russell RR, III, Bergeron R, Shulman GI, Young LH. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol Heart Circ Physiol 277: H643–H649, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Samouilov A, Zweier JL. Development of chemiluminescence-based methods for specific quantitation of nitrosylated thiols. Anal Biochem 258: 322–330, 1998 [DOI] [PubMed] [Google Scholar]
- 42. Sansbury BE, Jones SP, Riggs DW, Darley-Usmar VM, Hill BG. Bioenergetic function in cardiovascular cells: the importance of the reserve capacity and its biological regulation. Chem Biol Interact 191: 288–295, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shenton D, Grant CM. Protein S-thiolation targets glycolysis and protein synthesis in response to oxidative stress in the yeast Saccharomyces cerevisiae. Biochem J 374: 513–519, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Waldman SA, Murad F. Biochemical mechanisms underlying vascular smooth muscle relaxation: the guanylate cyclase-cyclic GMP system. J Cardiovasc Pharmacol 12, Suppl 5: S115–S118, 1988 [PubMed] [Google Scholar]
- 45. Wu M, Neilson A, Swift AL, Moran R, Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J, Chomicz S, Ferrick DA. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol 292: C125–C136, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Yadava N, Nicholls DG. Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. J Neurosci 27: 7310–7317, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang BK, Vivas EX, Reiter CD, Gladwin MT. Methodologies for the sensitive and specific measurement of S-nitrosothiols, iron-nitrosyls, and nitrite in biological samples. Free Radic Res 37: 1–10, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Zhang Y, Hogg N. The mechanism of transmembrane S-nitrosothiol transport. Proc Natl Acad Sci USA 101: 7891–7896, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zur NS, Eason R, Doney AS, Frenguelli BG. An ion-pair reversed-phase HPLC method for determination of fresh tissue adenine nucleotides avoiding freeze-thaw degradation of ATP. Anal Biochem 388: 108–114, 2009 [DOI] [PubMed] [Google Scholar]