Abstract
Diastolic heart failure is a major cause of mortality in the elderly population. It is often preceded by diastolic dysfunction, which is characterized by impaired active relaxation and increased stiffness. We tested the hypothesis that senescence-prone (SAMP8) mice would develop diastolic dysfunction compared with senescence-resistant controls (SAMR1). Pulsed-wave Doppler imaging of the ratio of blood flow velocity through the mitral valve during early (E) vs. late (A) diastole was reduced from 1.3 ± 0.03 in SAMR1 mice to 1.2 ± 0.03 in SAMP8 mice (P < 0.05). Tissue Doppler imaging of the early (E') and late (A') diastolic mitral annulus velocities found E' reduced from 25.7 ± 0.9 mm/s in SAMR1 to 21.1 ± 0.8 mm/s in SAMP8 mice and E'/A' similarly reduced from 1.1 ± 0.02 to 0.8 ± 0.03 in SAMR1 vs. SAMP8 mice, respectively (P < 0.05). Invasive hemodynamics revealed an increased slope of the end-diastolic pressure-volume relationship (0.5 ± 0.05 vs. 0.8 ± 0.14; P < 0.05), indicating increased left ventricular chamber stiffness. There were no differences in systolic function or mean arterial pressure; however, diastolic dysfunction was accompanied by increased fibrosis in the hearts of SAMP8 mice. In SAMR1 vs. SAMP8 mice, interstitial collagen area increased from 0.3 ± 0.04 to 0.8 ± 0.09% and perivascular collagen area increased from 1.0 ± 0.11 to 1.6 ± 0.14%. Transforming growth factor-β and connective tissue growth factor gene expression were increased in the hearts of SAMP8 mice (P < 0.05 for all data). In summary, SAMP8 mice show increased fibrosis and diastolic dysfunction similar to those seen in humans with aging and may represent a suitable model for future mechanistic studies.
Keywords: heart failure, transforming growth factor, aging
heart failure is a major and growing public health concern in the United States; there are an estimated 5 million people living with this disease, and another 550,000 patients will be diagnosed yearly (6). Approximately one-half of all heart failure patients in the United States suffer from diastolic heart failure, and it is a major cause of mortality in the elderly population (35). Diastolic heart failure describes a group of patients whose clinical manifestation of congestive heart failure is characterized by normal left ventricular diastolic volume, a normal ejection fraction, delayed active relaxation, and increased passive stiffness of the left ventricle (34). Diastolic dysfunction precedes diastolic heart failure and is often clinically silent; it is characterized by abnormal ventricular distensibility, relaxation, and filling (1). Both diastolic dysfunction and diastolic heart failure are most common in the elderly population. In a study that examined the prevalence of this form of heart failure, 50% of patients over the age of 70 showed evidence of diastolic heart failure. Studies (35) indicate that the most important determinants for development of diastolic dysfunction and diastolic heart failure are age and hypertension. Although the exact molecular mechanisms behind diastolic dysfunction are poorly understood, fibrosis is thought to contribute to its progression. Increased activity of cytokines and accumulation of extracellular matrix proteins are key features of most fibrotic diseases (16). Transforming growth factor-β (TGF-β), a profibrotic cytokine, works synergistically with connective tissue growth factor (CTGF) to promote fibroblast proliferation and deposition of collagen and fibronectin (18).
While well-established murine models exist to study such common cardiovascular diseases as hypertension, atherosclerosis, and congestive heart failure, a model of isolated age-related diastolic dysfunction has yet to be established and characterized. Previous studies have established that the ratio of early to late mitral filling velocity is decreased in aged wild-type mice (27) and that caloric restriction improves diastolic function in aged mice (28), but there has not been a model of pure spontaneous diastolic dysfunction or a model that has established the connection between age-related diastolic dysfunction and fibrosis.
Since advancing age is such an important risk factor for the development of this pathophysiological condition, we used the senescence-accelerated mouse model (SAM), a murine model of spontaneous senescence that displays many common geriatric disorders in the human population (13, 29–31). This model was derived from the AKR/J strain of mice by continuous sister-brother mating selecting for a tendency toward either accelerated or normal senescence; breeders were retrospectively chosen based on the degree of senescence at 8 mo as determined by life span and clinical signs of aging (30). The SAM model is comprised of the senescence-prone (SAMP) and control senescence-resistant (SAMR) strains. Accelerated senescence refers to the tendency of SAMP mice to experience a rapid progression of senescence after reaching maturity and a shorter life span by ∼40% compared with the SAMR series (29). Although some variability exists with respect to life span of these mice, the median life span of SAMR1 mice has been reported to be between 12 and 21 mo of age, and the median life span of SAMP8 mice has been reported as 10 to 17 mo of age (12). While these mice display a number of pathological changes at autopsy after their natural death, including pneumonia, abscess, colitis, amyloidosis, contracted kidney, neoplasms, and a number of other abnormalities, the most likely causes of death in SAMP8 mice are lymphoid neoplasms and contracted kidney (31). Of all the SAM strains, SAMR1 and SAMP8 strains are the best-studied strains with respect to cardiovascular disease and oxidative stress. Using these strains, we demonstrated that aging results in diastolic dysfunction and that diastolic dysfunction is accompanied by increased cardiac fibrosis and increased profibrotic cytokines.
METHODS
Animal maintenance.
SAMR1 and SAMP8 mice were purchased from Harlan (Indianapolis, IN). All experiments were carried out using 3-mo-old and 6-mo-old male mice. All experiments were approved by the Atlanta Veterans Affairs Institutional Animal Care and Use Committee (Protocol Approval No. V018–03). The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Assessment of cardiac dimensions and diastolic function using echocardiography.
Echocardiography studies were completed as described previously (25). Briefly, mice were anesthetized with 4% isoflurane, hair was removed from the thorax, and they were maintained under light anesthesia (1–1.5% isoflurane) at ∼37°C and demonstrated a physiological heart rate >500 beats/min during the procedure. Two-dimensional and M-mode transthoracic echocardiography modalities were used to assess wall motion, chamber dimensions, and wall thickness and to calculate fractional shortening. Pulsed-wave Doppler echocardiography was used to measure early (E) and late (A) blood flow velocities through the mitral valve. Tissue Doppler imaging was used to measure the early (E') and late (A') velocity of the mitral annulus. For each measured data point, at least three beats were averaged per measurement, at least three measurements were taken per animal, and beats were taken at end expiration. Studies were reviewed by two different investigators, one of which was blinded. Interobserver variability was based on six studies and was 9.7 ± 0.8%. Intraobserver variability was based on four studies and was 7.4 ± 0.9%. Measurements were made using a VisualSonics Vevo 770TM in vivo micro-imaging system equipped with a RMV-707B cardiovascular scanhead (Toronto, ON). Different groups of mice were used for studies carried out at 3, 6, and 12 mo of age.
Assessment of cardiac function using invasive hemodynamics.
Invasive hemodynamic studies were performed as described previously using a closed-chest procedure (26). Mice were anesthetized with 1–2% isoflurane. An initial intraperitoneal bolus injection of 0.3 ml normal saline was given, and the body temperature was maintained at 37–38°C during the procedure. After a single dose of pancuronium (0.12 mg/kg iv) through the left jugular vein, the mechanical ventilation (MA1 55–7059; Harvard Apparatus, Holliston, MA) was started via tracheoectomy with a rate of 118–133 breaths per minute and a tidal volume of 0.19–0.28 ml. A pressure-volume catheter (SPR-839; Millar Instruments, Houston, TX) was inserted into the right common carotid artery and advanced into the left ventricle. Inferior vena cava occlusion was performed via a midline abdominal incision. Volume and parallel conductance calibration were performed as previously described (32). The end-diastolic pressure volume points were fitted using the following linear function: LVEDP = EDPVR × LVEDV + intercept, where LVEDP and LVEDV are left venticular end-diastolic pressure and volume and EDPVR is end-diastolic pressure-volume relationship (for both groups combined: median: r2 = 0.97 ± 0.04; range: 0.84 to 0.99). The group of mice used for invasive hemodynamic studies was separate from the group used for echocardiography; however, all mice were 6 mo old at the time of study.
Sarcomere length shortening measurements.
The mechanical properties of the cardiomyocytes were assessed using an IonOptix Myocam System (Ionoptix, Milton, MA). Unloaded cardiomyocytes were placed on a glass slide and allowed to adhere for 10 min. Cardiomyocytes were then imaged with an inverted microscope and perfused with a normal Tyrode's solution containing 1.2 mmol/l CaCl2 at 37°C by temperature controller and heater (mTC-II; Ionoptix, Milton, MA). Cardiomyocytes were paced at 1.0 Hz for a 4-ms duration, and sarcomere shortening and relengthening were assessed using the following indexes: peak fractional shortening, time to 90% peak shortening, and τ, the relaxation time constant (a0 + a1et/τ, t = time). Cardiomyocytes (47–59) from five to six different mice were used to measure sarcomere shortening. A separate group of 6-mo-old male mice was used for sacrcomere length shortening measurements.
Blood pressure data acquisition.
Blood pressure was measured as described previously (15). Anesthesia was induced using 4% isoflurane and maintained at 1–1.5% isoflurane. A 2-cm ventral incision was made from the chin to the sternum, and the carotid artery was isolated by blunt dissection. A 25-guage bent needle was used to cannulate the artery with a sterile TA11PA-C10 transmitter (Data Sciences International, St. Paul, MN). The catheter connected to the transducer was advanced into the thoracic aorta and held in place with sutures, and the transmitter was positioned along the right flank, close to the hindlimb. Mice were allowed to recover for 1 wk before initiation of monitoring. Blood pressure measurements were telemetrically collected for 10 s each minute during a 24-h period at the baseline time point of 3 mo of age and then weekly up to 6 mo of age. The group of mice used for telemetry was separate from the groups used for echocardiography and invasive hemodynamics.
Collagen staining.
Hearts were fixed in 10% buffered formalin for 24 h and embedded in paraffin, and 5-μm transverse sections were cut using a microtome. Tissue sections were dewaxed, rehydrated, and then stained. For picrosirius red staining, sections were stained in a 0.1% solution of sirius red in a saturated aqueous solution of picric acid for 1 h and then washed in acidified water, dehydrated with graded alcohols, and mounted on slides. Both bright-field and polarized light microscopy were used to image and photograph the slides. Slides were imaged using a using a Zeiss microscope and AxioVision 4.5 software. For slides stained with Masson's trichrome, ImagePro 6.2 software was used to calculate the percent area of collagen content.
Quantitative real-time PCR.
Total RNA was isolated from left ventricular heart tissue homogenates and was reverse transcribed using the SuperScript II kit (Invitrogen, San Diego, CA). cDNA was purified then amplified using gene-specific primers and a LightCycler real-time thermocycler (Roche Diagnostics, Indianapolis, IN). Transcripts were detected using SYBR Green I (Molecular Probes) and were normalized to 18S mRNA.
Western blot analysis.
Western blotting was performed as described previously (20). Left ventricular heart tissue homogenates were prepared in a buffer containing 20 mM Tris (pH 7.4), 2.5 mM EDTA, 1% Triton X-100, 1% deoxycholic acid, 0.1% SDS, 100 mM NaCl, 10 mM NaF, and 1 mM Na3VO4 and were quantified using the BCA protein assay according to the manufacturer's instructions (Thermo Scientific, Rockford, IL). Heart samples (40 μg protein per lane) were run on a 10% SDS-PAGE gel (Invitrogen) for 90 min at 150 V and then transferred to a PVDF membrane. Membranes were blocked for 30 min in 5% nonfat dry milk and probed with primary antibody (1:1,000) specific to TGF-β (Santa Cruz Biotechnology) or α-smooth muscle actin (α-SMA; Thermo Fisher Scientific, Fremont, CA) on a rocking platform overnight at 4°C. Membranes were washed, then incubated with horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch), and detected using the SuperSignal West Pico peroxide and luminol enhancer solution (Thermo Scientific). Membranes were imaged, photographed, and quantified using the Bio-Rad ChemiDoc system (Hercules, CA). Proteins of interest were normalized to cdk4 content.
Statistical analysis.
Data are represented as the means ± SE and are compared using the Student's t-test when SAMR1 and SAMP8 mice are being compared for 6 mo time point studies. A two-way ANOVA is used when SAMR1 and SAMP8 mice are being compared for 3 and 6 mo studies. Bonferroni post hoc tests were used to determine significance of specific pair-wise comparisons. A P value <0.05 is considered statistically significant.
RESULTS
SAMP8 mice showed accelerated aging at 6 mo of age.
To determine if the SAMP8 mice have accelerated senescence, levels of p19ARF also known as ARF, a tumor suppressor protein encoded by the INK4a/ARF locus regulating the p53 pathway by stabilizing p53, were measured. Since senescence requires activation of the p53 pathway, elevated p19ARF is consistent with aging and accelerated senescence (24). p19ARF was increased in 6-mo-old SAMP8 mice compared with SAMR1 controls as measured by quantitative real-time PCR (Supplemental Fig. S1; Supplemental Material for this article is available online at the Am J Physiol Heart Circ Physiol website).
SAMP8 mice show diastolic dysfunction at 6 mo of age.
At 6 mo of age, SAMP8 mice displayed echocardiographic evidence of diastolic dysfunction compared with SAMR1 controls. With the use of conventional pulsed-wave Doppler echocardiography, the ratio of early to late mitral inflow velocity (E/A) was reduced in SAMP8 mice compared with SAMR1 controls (1.2 ± 0.03 vs. 1.3 ± 0.03; P < 0.05). Tissue Doppler imaging was used to measure the mitral valve annulus velocity. In SAMP8 mice, E' was reduced compared with SAMR1 controls (21.1 ± 0.8 vs. 25.7 ± 0.9 mm/s; P < 0.05). Likewise, the ratio of early to late tissue mitral annulus velocities (E'/A') was reduced in SAMP8 mice (0.8 ± 0.03 vs. 1.1 ± 0.02; P < 0.05; Table 1). The dependency of the phenotype on age was confirmed with echocardiographic studies performed at 3 mo of age that showed no differences in diastolic function (Table 1). Furthermore, we studied 12-mo-old SAMR1 and SAMP8 mice to more solidly establish that diastolic dysfunction in the model is age related. Similar to the findings at 6 mo of age, the E'/A' ratio was reduced in SAMP8 mice compared with SAMR1 controls (0.8 ± 0.03 vs. 1.0 ± 0.04; P < 0.05; Supplemental Table S1). In addition, when diastolic function was compared between both types of mice at 6 and 12 mo of age, it seemed that diastolic remained impaired in SAMP8 mice at 12 mo of age, and in SAMR1 mice at 12 mo of age, E'/A' decreased to a level comparable to SAMP8 mice at 6 mo of age. Therefore, it appears that with advancing age, SAMR1 mice begin to display evidence of diastolic dysfunction, further supporting our observation that diastolic dysfunction is related to aging.
Table 1.
Echocardiographic comparison of SAMR1 and SAMP8 mice at 3 and 6 mo of age
| SAMR1 at 3 mo (n = 7) | SAMP8 at 3 mo (n = 7) | SAMR1 at 6 mo (n = 8) | SAMP8 at 6 mo (n = 8) | |
|---|---|---|---|---|
| Body weight, g | 29.0 ± 0.3 | 30.7 ± 0.5 | 39.1 ± 1.1† | 42.3 ± 1.0*† |
| LV weight,mg | 82.0 ± 2.2 | 90.5 ± 2.3* | 110.4 ± 1.9† | 120.0 ± 2.2*† |
| LV/body weight | 2.8 ± 0.05 | 2.9 ± 0.4 | 2.8 ± 0.07 | 2.9 ± 0.08 |
| Heart rate, beats/min | 550 ± 25 | 550 ± 25 | 550 ± 25 | 550 ± 25 |
| LV dimensions | ||||
| LVID;s, mm | 2.9 ± 0.07 | 2.7 ± 0.1 | 2.6 ± 0.07 | 2.6 ± 0.07 |
| LVID;d, mm | 4.0 ± 0.05 | 4.0 ± 0.1 | 4.0 ± 0.06 | 4.0 ± 0.08 |
| LVESV, μl | 32.3 ± 1.8 | 28.1 ± 2.8 | 25.1 ± 1.7 | 24.8 ± 1.7 |
| LVEDV, μl | 71.6 ± 2.2 | 70.9 ± 4.7 | 69.7 ± 2.3 | 70.5 ± 3.6 |
| LVWT, mm | 0.8 ± 0.01 | 1.0 ± 0.03* | 0.7 ± 0.04 | 0.9 ± 0.03 |
| Systolic | ||||
| SV, μl | 39.3 ± 1.2 | 42.7 ± 2.7 | 44.5 ± 1.0 | 45.7 ± 2.2 |
| EF, % | 55.0 ± 1.6 | 60.5 ± 2.0 | 64.3 ± 1.5 | 65.0 ± 1.2 |
| FS, % | 28.3 ± 1.1 | 32.0 ± 1.4 | 34.7 ± 1.1 | 35.3 ± 0.8 |
| Diastolic | ||||
| E/A | 1.4 ± 0.03 | 1.4 ± 0.04 | 1.3 ± 0.03 | 1.2 ± 0.03*† |
| E', mm/s | 28.1 ± 1.03 | 30.8 ± 2.0 | 25.7 ± 0.9 | 21.1 ± 0.8† |
| A', mm/s | 20.7 ± 0.9 | 20.8 ± 1.7 | 23.3 ± 0.8 | 25.8 ± 1.1† |
| E'/A' | 1.4 ± 0.03 | 1.4 ± 0.04 | 1.1 ± 0.02† | 0.8 ± 0.03*† |
Values are means ± SE. SAMR and SAMP, senescence-resistant and senescence-prone mice; LV, left ventricular; LVID;s and LVID;d, LV internal diameter systolic and diastolic; LVESV and LVEDV, LV end-systolic and diastolic volume; LVWT, LV wall thickness; SV, stroke volume; EF, ejection fraction; FS, fractional shortening; E/A, ratio of early to late mitral inflow velocity; E'/A', ratio of diastolic mitral annulus velocities.
P < 0.05 when comparison is made between SAMR1 and SAMP8 mice of the same age;
P < 0.05 when comparison is made between the same type of mice at 3 and 6 mo of age.
To confirm our echocardiographic observations, we used invasive hemodynamics, and as expected, SAMP8 mice displayed hemodynamic evidence of diastolic dysfunction compared with SAMR1 controls. Compared with SAMR1 mice at 6 mo of age, SAMP8 mice had an increased end-diastolic pressure (5.6 ± 0.9 vs. 3.4 ± 0.3 mmHg; P < 0.05). Additionally, transient occlusion of the inferior vena cava was used to generate a family of pressure-volume loops at varying volumes. The left ventricular EDPVR is represented as the slope of the best-fitting line connecting the end-diastolic pressure-volume points, and it reflects the passive diastolic properties of the left ventricle (7). Consistent with our data demonstrating diastolic dysfunction, the slope of the EDPVR is increased in SAMP8 mice compared with SAMR1 controls (0.8 ± 0.1 vs. 0.5 ± 0.05 mmHg/μl; P < 0.05; Table 2). We did not observe any differences in dP/dtmin, the maximal slope of ventricular pressure decline during diastole, or in τGlantz, τWeiss, or t1/2, three different ways of assessing LV pressure decay, however, suggesting that the pressure-volume relation, and hence passive diastole, but not active relaxation were affected in the model. We also measured arterial elastance (Ea) and the ventricular-vascular coupling ratio (Ea/Es) to determine whether abnormal ventricular-vascular coupling could account for the changes in cardiac function we observed. There were no differences between SAMP8 and SAMR1 mice in Ea (8.8 ± 1.1 vs. 7.0 ± 0.5) or Ea/Es (1.2 ± 0.2 vs. 1.3 ± 0.2), respectively, suggesting changes are not the result of altered ventricular-vascular coupling.
Table 2.
Invasive hemodynamic comparison of SAMR1 and SAMP8 mice at 6 mo of age
| SAMR1 (n = 11) | SAMP8 (n = 8) | |
|---|---|---|
| Baseline HR, beats/min | 603.1 ± 12.05 | 583.4 ± 11.41 |
| LVESP, mmHg | 85.82 ± 3.37 | 79.50 ± 4.00 |
| LVEDP, mmHg | 3.41 ± 0.28 | 5.59 ± 0.93* |
| dP/dtmax, mmHg/s | 8,093 ± 721.2 | 7534 ± 787.7 |
| dP/dtmin, mmHg/s | −9,138 ± 831.8 | −9,089 ± 1,055 |
| dP/dtEDV, mmHg/s | 461.7 ± 68.00 | 624.5 ± 102.5 |
| Ea, mmHg/μl | 6.95 ± 0.45 | 8.79 ± 1.10 |
| Ea/Es | 1.30 ± 0.16 | 1.23 ± 0.18 |
| τGlantz, ms | 8.50 ± 0.59 | 8.66 ± 0.66 |
| τWeiss, ms | 5.09 ± 0.26 | 5.73 ± 0.43 |
| t1/2 | 4.10 ± 0.21 | 4.20 ± 0.30 |
| EDPVR, mmHg/μl | 0.49 ± 0.05 | 0.79 ± 0.14* |
| ESPVR, mmHg/μl | 5.90 ± 0.59 | 7.87 ± 1.00 |
| PRSW | 72.11 ± 8.39 | 65.40 ± 11.47 |
Values are means ± SE. HR, heart rate; LVESP and LVEDP, left ventricular end-systolic and diastolic pressure; dP/dtmax, dP/dtmin, and dP/dtEDV, maximal, minimal, and end-diastolic volume change of ventricular pressure over time; Ea, arterial elastance; Ea/Es, ventricular-vascular coupling ratio; τGlantz, τWeiss, or t1/2, three different ways of assessing LV pressure decay; EDPVR and ESPVR, end-diastolic and systolic pressure-volume relationship; PRSW, preload recruitable stroke work.
P < 0.05.
Conventional M-mode echocardiography was used to measure cardiac dimensions. There were no differences in left ventricular dimensions between SAMR1 and SAMP8 mice during either systole or diastole. Furthermore, the stroke volume (44.5 ± 1.0 vs. 45.7 ± 2.2 μl), the ejection fraction (64.3 ± 1.5 vs. 65.0 ± 1.2%), and the percent fractional shortening (34.7 ± 1.1 vs. 35.3 ± 0.8%) were unchanged between SAMP8 and SAMR1 mice, respectively, suggesting that changes in diastolic function could not be explained by changes in systolic function (Table 1).
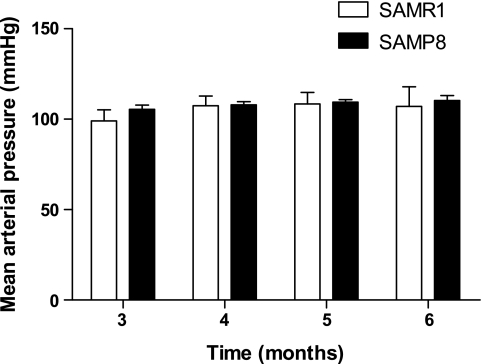
Since hypertension is an established risk factor for the development of diastolic dysfunction (35, 36), we monitored mean arterial pressure by telemetry in SAMR1 and SAMP8 mice from 3 through 6 mo of age. There were no differences in mean arterial pressure between the two groups of mice at any time point, and there was no change in pressure over the 3 mo course of measurement (Fig. 1). Therefore, changes in diastolic function observed were independent of blood pressure changes.
Fig. 1.
Mean arterial pressure was unchanged in senescence-resistant controls (SAMR1) and senescence-prone (SAMP8) mice from 3 to 6 mo of age (n = 5; P = NS).
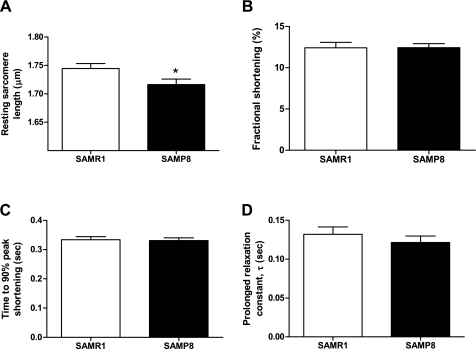
Since diastolic dysfunction could result from impairment in the relaxation of cardiac myocytes, we examined sarcomere length shortening and relengthening. Relaxation was measured in freshly isolated ventricular cardiomyocytes at 1.0-Hz stimulation and 37°C. The baseline sarcomere length of cardiac myocytes was modestly shorter in SAMP8 mice compared with SAMR1 mice (1.72 ± 0.01 vs. 1.78 ± 0.01 μm; P < 0.05; Fig. 2A). Nevertheless, there were no significant differences in fractional shortening (12.42 ± 0.67 vs. 12.43 ± 0.48%), time to 90% peak shortening (0.33 ± 0.01 vs. 0.33 ± 0.01 s), and τ the relaxation time constant (0.13 ± 0.01 vs. 0.12 ± 0.01) between SAMR1 and SAMP8 mice, respectively (Fig. 2, B–D). This suggests that diastolic dysfunction observed in this model was not the result of changes in cardiomyocyte function.
Fig. 2.
Functional analysis of isolated cardiomyocytes. A: mean of diastolic sarcomere length was significantly shorter in cardiomyocytes from SAMP8 compared with SAMR1 at 6 mo of age (n = 53, 59; *P < 0.05). B: fractional shortening of isolated cardiomyocytes paced at 1.0 Hz at 37°C represented as the peak shortening divided by the baseline sarcomere length (n = 53, 59; P = NS). C: time to 90% peak contraction in isolated cardiomyocytes (n = 47, 51; P = NS). D: isolated cardiomyocytes from SAMP8 mice have a prolonged relaxation constant (τ) compared with SAMP1 mice (n = 53, 59; P = NS).
Since the SAM model develops other pathologies in addition to those related to cardiac function and because cardiac and renal function are often interrelated, we examined the metabolic profiles of SAM mice at 6 mo of age. Plasma blood urea nitrate was 15.9 ± 0.5 in 6-mo-old SAMR1 mice and was only mildly elevated to 17.8 ± 0.4 in SAMP8 mice. Plasma creatinine was unchanged in SAMR1 vs. SAMP8 mice (0.21 ± 0.01 vs. 0.20 ± 0.0). Finally, the diastolic dysfunction observed did not progress fully to diastolic heart failure; we observed no difference between lung weights between SAMR1 and SAMP8 mice at 6 mo of age (data not shown).
SAMP8 mice demonstrated increased cardiac fibrosis.
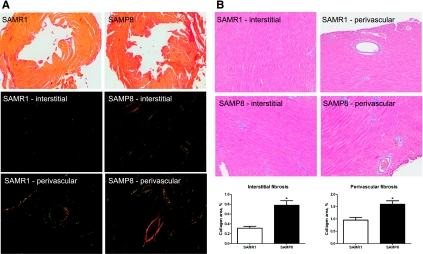
Since diastolic dysfunction in the SAM model is not secondary to hypertension, we sought to investigate other causes and mechanisms of the age-associated diastolic dysfunction. Since diastolic dysfunction has been found to be correlated with stiffening of the left ventricle and diminished distensibility of the heart muscle in a population of patients with the disorder (4), we next sought to examine the fibrotic response in our model. Collagens represent an important component of fibrotic tissue, so myocardial collagen content was examined using two different histological methods. When transverse myocardial tissue sections were stained with sirius red, greater collagen accumulation in whole hearts of SAMP8 mice was evident due to increased red staining of the tissue using brightfield microscopy (Fig. 3A, top). Tissue sections were also imaged using polarized light, where large collagen fibers appear yellow or orange, and thinner fibers appear green (14). In both interstitial and perivascular regions of the myocardium, SAMP8 mice showed increased collagen deposition compared with SAMR1 controls. The accumulation of large collagen fibers was particularly markedly increased in the perivascular regions of hearts from SAMP8 mice (Fig. 3A, bottom). To confirm and quantify these findings, Masson's trichrome staining was used. Again, greater collagen accumulation was observed in the interstitial and perivascular regions of the myocardium in SAMP8 mice. In the interstitial and perivascular tissue, the percentage of tissue comprised of collagen was greater in SAMP8 mice compared with SAMR1 controls [0.8 ± 0.1 vs. 0.3 ± 0.04 (P < 0.05) and 1.6 ± 0.1 vs. 1.0 ± 0.1 (P < 0.05), respectively; Fig. 3B].
Fig. 3.
SAMP8 mice show greater collagen deposition and hence increased cardiac fibrosis in both the interstitial areas and perivascular areas compared with SAMR1 controls at 6 mo of age. A: picrosirius red staining to evaluate collagen deposition. With the use of bright-field microscopy, SAMP8 mice show a more intense red stain than SAMR1 mice, indicating greater collagen accumulation. When polarized light is used, larger collagen fibers appear as bright yellow or orange, and thinner fibers are green. Both modalities show increased collagen accumulation in SAMP8 mice at 6 mo of age. B: Masson's trichrome staining to evaluate collagen deposition (n = 4; *P < 0.01).
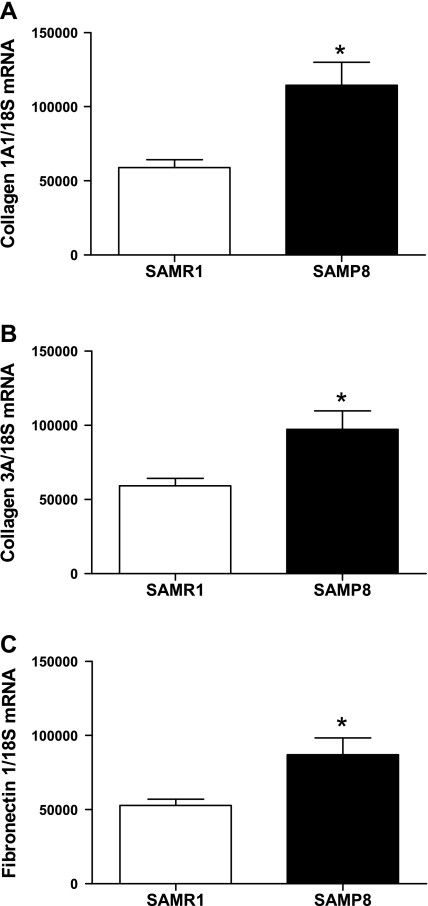
To further examine which collagens and other associated proteins might be increased in the myocardium of senescence-accelerated mice, quantitative real-time PCR was used to measure myocardial gene expression of collagen 1A1, collagen 3A, and fibronectin. In SAMP8 mice compared with SAMR1 controls, collagen 1A1, which is the main component of scar tissue, and collagen 3A, commonly associated with collagen 1A1, were increased (Fig. 4, A and B). Fibronectin is an extracellular matrix protein that can bind to collagen, and expression of fibronectin 1 was increased in SAMP8 mice (Fig. 4C).
Fig. 4.
Expression of extracellular matrix proteins. SAMP8 mice show increased gene expression of collagens 1A1 (A) and 3A (B) and as well as fibronectin 1 (C) compared with SAMR1 controls at 6 mo of age (n = 7; *P < 0.05).
Cardiac fibrosis observed in senescence-accelerated mice was associated with increased expression of profibrotic cytokines.
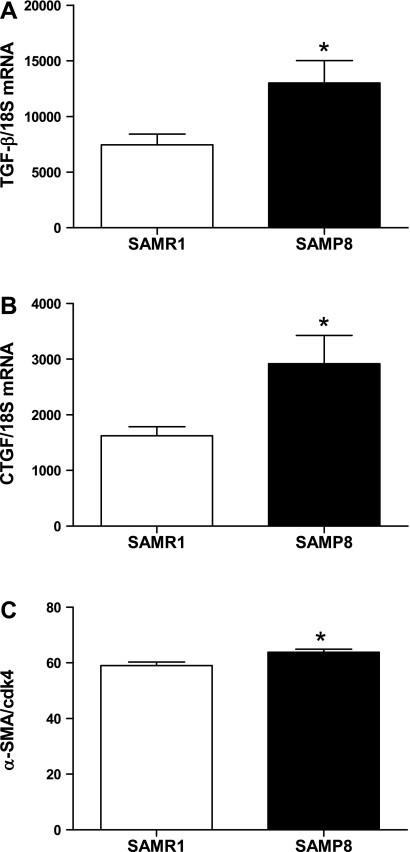
Since cardiac fibrosis is present in this model of age-related diastolic dysfunction, we sought to investigate the signaling pathways that might contribute to the fibrotic response. TGF-β is a potent profibrotic cytokine that influences the development of cardiac fibrosis by promoting cellular events such as increased collagen synthesis and decreased protease expression (17, 18). CTGF is induced by TGF-β and acts synergistically with TGF-β to promote deposition of extracellular matrix proteins (8, 16, 18). These profibrotic cytokines are capable of converting fibroblasts into myofibroblasts, which express α-SMA and synthesize collagen, promoting the fibrotic process (17). Cardiac gene expression of TGF-β and CTGF was increased in SAMP8 mice compared with SAMR1 controls (Fig. 5, A and B). Consistent with increased collagen disposition, α-SMA protein expression was increased in SAMP8 mice compared with controls, suggesting a conversion of fibroblasts into myofibroblasts (Fig. 5C).
Fig. 5.
Expression of profibrotic cytokines. A: transforming growth factor-β (TGF-β). B: connective tissue growth factor (CTGF). C: α-smooth muscle actin (α-SMA). SAMP8 mice show increased gene expression of TGF-β compared with SAMR1 controls at 6 mo of age (n = 7; *P < 0.05).
DISCUSSION
Diastolic heart failure is increasing in prevalence (23). It carries significant morbidity and mortality, and treatment strategies are nonspecific because of a poor mechanistic understanding of the disease (22). Diastolic heart failure is characterized by abnormal relaxation of the left ventricle (1). Since diastolic dysfunction is more common in the elderly population, we hypothesized that the SAM model would show diastolic dysfunction. In the present study, we show that SAMP8 mice have diastolic dysfunction in the absence of alterations in systolic function using two different modalities, echocardiography and invasive hemodynamics, which we have previously found to be well correlated (25). Additionally, we demonstrate that diastolic dysfunction can occur in the absence of an increase in blood pressure, perhaps explaining the lack of efficacy of antihypertensive medications in human clinical trials (10, 19, 33). Since renal function often impacts both blood pressure and cardiac function, we examined plasma metabolic profiles of SAM mice as well and found only minor differences in blood urea nitrogen between SAMR1 and SAMP8 mice. Taken in combination with the lack of change in blood pressure, we do not believe that renal abnormalities contribute to diastolic dysfunction in this aging model. Another potential pathophysiologic variable that could contribute to abnormal diastolic function is altered ventricular-vascular coupling. Nevertheless, when we measured arterial elastance and the ratio of ventricular-vascular coupling using invasive hemodynamics, we found no differences between the two groups of mice, suggesting that the diastolic dysfunction we have observed cannot be explained by increased vascular stiffness or abnormalities in the interaction between the heart and the systemic vasculature. Another variable that could contribute to abnormal diastolic function is impaired cardiac myocyte relaxation. We measured the baseline sarcomere length of cardiac myocytes, fractional shortening, time to 90% peak shortening, and the relaxation time constant τ to assess myocyte function. There were no differences in time to 90% peak shortening or τ between SAMR1 and SAMP8 mice, which suggests myocyte relaxation is not impaired and is not responsible for diastolic dysfunction. Taken together, we believe we have demonstrated that the senescence-accelerated mouse develops isolated age-related diastolic dysfunction by 6 mo of age and could thus be a useful model for continued studies.
While it is recognized that diastolic dysfunction, which is often clinically silent, can progress to diastolic heart failure in humans, we did not observe progression to overt heart failure in the mice by 6 mo of age. At 6 mo of age, we observed no difference in lung weight between SAMR1 and SAMP8 mice, indicating the absence of pulmonary edema secondary to heart failure. Nevertheless, we believe that the development of diastolic dysfunction is significant in that it represents a point in the development of the disease where underlying mechanisms of pathology could be studied to investigate possible interventions before heart failure has occurred.
Since we sought to demonstrate that diastolic dysfunction is an age-dependent pathophysiological development in this model, we also evaluated the cardiac function of SAMR1 and SAMP8 mice at 12 mo of age. While there were no differences cardiac structure or systolic function between SAMR1 and SAMP8 mice, we again found evidence of diastolic dysfunction in SAMP8 mice at 12 mo of age, suggesting diastolic impairment first appeared by 6 mo of age and persisted to a similar degree through 12 mo of age. Furthermore, we observed a modest decrease in the diastolic function of SAMR1 mice at 12 mo of age compared with 3 or 6 mo of age, suggesting that as the control mice age further, they begin to show decreases in diastolic function as well. Taken with the absence of diastolic dysfunction at 3 mo of age, these data suggest that the diastolic dysfunction we have observed in the SAM model is age related.
One proposed cause of diastolic dysfunction is fibrosis, and, consistent with this, we found increased collagen deposition in the hearts of senescence-prone mice. This fibrotic response and collagen deposition were accompanied by an increase in several profibrotic cytokines. As expected, the increases in CTGF and TGF-β paralleled the increase in the myofibroblast phenotype. Many forms of tissue fibrosis are associated with increased signaling through the TGF-β pathway (16, 17). In this pathway, increased TGF-β upregulates CTGF, and these two cytokines act synergistically to increase Smad signaling in the nucleus, which ultimately leads to conversion of fibroblasts into myofibroblasts, which secrete additional extracellular matrix proteins (8). We surmise that, in this model, some aspect unique to the aging process triggers an increase in TGF-β, which then upregulates CTGF, and these two cytokines promote increased phosphorylation of Smad2/3, which ultimately leads to increased synthesis of extracellular matrix proteins such as collagen and fibronectin. It is also possible that there are changes in the expression and/or activity of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases, which contribute to the fibrotic response; however, these changes likely occur secondary to increased TGF-β signaling.
While models exist for the study of hypertension-related diastolic dysfunction (3, 21, 25), diabetic-associated diastolic dysfunction (2, 9, 11), and the relationship between age-dependent collagen alterations and diastolic dysfunction (5), there has not thus far been a spontaneous senescence model that recapitulates diastolic dysfunction as it naturally occurs in the elderly population. In this model, diastolic dysfunction develops between 3 and 6 mo of age in SAMP8 mice but not in SAMR1 controls. With respect to aging research, this is an early time point in the life span of these animals, and the physiologic abnormality manifests over a relatively short period of time. From an experimental standpoint, this is advantageous because it allows for a more rapid study of mechanism of disease. Furthermore, multiple models of the same disease state may ultimately provide a more comprehensive understanding of pathophysiologic mechanisms.
The senescence-accelerated mouse model is likely to prove a useful model for the study of age-related diastolic dysfunction. Furthermore, this model has many features similar to those seen in humans. In summary, we have characterized a model demonstrating isolated diastolic dysfunction associated with accelerated aging. Diastolic dysfunction is accompanied by fibrosis that arises in conjunction with an increase in profibrotic cytokines. This model may give new insight into the mechanisms of diastolic dysfunction and lead to specific pharmacological strategies to prevent or treat this pathology.
GRANTS
Support for this study was provided by National Institutes of Health Grants R01-HL-070892 (to R. L. Sutliff); R01-HL-085558, R01-HL-073753, and P01-HL-058000 (to S. C. Dudley, Jr.); and T32-ES-012870 (to A. L. Reed).
DISCLOSURES
S. C. Dudley, Jr., and R. L. Sutliff submitted a provisional patent on the use of antifibrotic therapy for treatment of diastolic dysfunction.
ACKNOWLEDGMENTS
Current address of E. R. Walp: Children's Hospital of Philadelphia, 3615 Civic Center Blvd., Abramson Research Building (ARC) 514G, Philadelphia, PA 19104.
REFERENCES
- 1. Aurigemma GP, Gaasch WH. Clinical practice. Diastolic heart failure. N Engl J Med 351: 1097–1105, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Basu R, Oudit GY, Wang X, Zhang L, Ussher JR, Lopaschuk GD, Kassiri Z. Type 1 diabetic cardiomyopathy in the Akita (Ins2WT/C96Y) mouse model is characterized by lipotoxicity and diastolic dysfunction with preserved systolic function. Am J Physiol Heart Circ Physiol 297: H2096–H2108, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Billet S, Bardin S, Verp S, Baudrie V, Michaud A, Conchon S, Muffat-Joly M, Escoubet B, Souil E, Hamard G, Bernstein KE, Gasc JM, Elghozi JL, Corvol P, Clauser E. Gain-of-function mutant of angiotensin II receptor, type 1A, causes hypertension and cardiovascular fibrosis in mice. J Clin Invest 117: 1914–1925, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borlaug BA, Kass DA. Mechanisms of diastolic dysfunction in heart failure. Trends Cardiovasc Med 16: 273–279, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Bonnema DD, Zile MR. Age-dependent alterations in fibrillar collagen content and myocardial diastolic function: role of SPARC in postsynthetic procollagen processing. Am J Physiol Heart Circ Physiol 298: H614–H622, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brutsaert DL. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev 83: 59–115, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol 289: H501–H512, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH. CTGF expression is induced by TGF- beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol 32: 1805–1819, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Chiu HC, Kovacs A, Blanton RM, Han X, Courtois M, Weinheimer CJ, Yamada KA, Brunet S, Xu H, Nerbonne JM, Welch MJ, Fettig NM, Sharp TL, Sambandam N, Olson KM, Ory DS, Schaffer JE. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res 96: 225–233, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J 27: 2338–2345, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Flagg TP, Cazorla O, Remedi MS, Haim TE, Tones MA, Bahinski A, Numann RE, Kovacs A, Schaffer JE, Nichols CG, Nerbonne JM. Ca2+-independent alterations in diastolic sarcomere length and relaxation kinetics in a mouse model of lipotoxic diabetic cardiomyopathy. Circ Res 104: 95–103, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Flood JF, Morley JE. Learning and memory in the SAMP8 mouse. Neurosci Biobehav Rev 22: 1–20, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Hosokawa M, Abe T, Higuchi K, Shimakawa K, Omori Y, Matsushita T, Kogishi K, Deguchi E, Kishimoto Y, Yasuoka K, Takeda T. Management and design of the maintenance of SAM mouse strains: an animal model for accelerated senescence and age-associated disorders. Exp Gerontol 32: 111–116, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 11: 447–455, 1979 [DOI] [PubMed] [Google Scholar]
- 15. Kleinhenz JM, Kleinhenz DJ, You S, Ritzenthaler JD, Hansen JM, Archer DR, Sutliff RL, Hart CM. Disruption of endothelial peroxisome proliferator-activated receptor-gamma reduces vascular nitric oxide production. Am J Physiol Heart Circ Physiol 297: H1647–H1654, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leask A. TGFbeta, cardiac fibroblasts, and the fibrotic response. Cardiovasc Res 74: 207–212, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-beta(1). Mol Genet Metab 71: 418–435, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Lim H, Zhu YZ. Role of transforming growth factor-beta in the progression of heart failure. Cell Mol Life Sci 63: 2584–2596, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 359: 2456–2467, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Nisbet RE, Graves AS, Kleinhenz DJ, Rupnow HL, Reed AL, Fan TH, Mitchell PO, Sutliff RL, Hart CM. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. Am J Respir Cell Mol Biol 40: 601–609, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ogata T, Miyauchi T, Sakai S, Takanashi M, Irukayama-Tomobe Y, Yamaguchi I. Myocardial fibrosis and diastolic dysfunction in deoxycorticosterone acetate-salt hypertensive rats is ameliorated by the peroxisome proliferator-activated receptor-alpha activator fenofibrate, partly by suppressing inflammatory responses associated with the nuclear factor-kappa-B pathway. J Am Coll Cardiol 43: 1481–1488, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355: 251–259, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Schocken DD, Benjamin EJ, Fonarow GC, Krumholz HM, Levy D, Mensah GA, Narula J, Shor ES, Young JB, Hong Y. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation 117: 2544–2565, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Sharpless NE. Ink4a/Arf links senescence and aging. Exp Gerontol 39: 1751–1759, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Silberman GA, Fan TH, Liu H, Jiao Z, Xiao HD, Lovelock JD, Boulden BM, Widder J, Fredd S, Bernstein KE, Wolska BM, Dikalov S, Harrison DG, Dudley SC., Jr Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation 121: 519–528, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Simpson D, Liu H, Fan TH, Nerem R, Dudley SC., Jr A tissue engineering approach to progenitor cell delivery results in significant cell engraftment and improved myocardial remodeling. Stem Cells 25: 2350–2357, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taffet GE, Hartley CJ, Wen X, Pham T, Michael LH, Entman ML. Noninvasive indexes of cardiac systolic and diastolic function in hyperthyroid and senescent mouse. Am J Physiol Heart Circ Physiol 270: H2204–H2209, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Taffet GE, Pham TT, Hartley CJ. The age-associated alterations in late diastolic function in mice are improved by caloric restriction. J Gerontol A Biol Sci Med Sci 52: B285–290, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Takeda T. Senescence-accelerated mouse (SAM): a biogerontological resource in aging research. Neurobiol Aging 20: 105–110, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Takeda T, Hosokawa M, Takeshita S, Irino M, Higuchi K, Matsushita T, Tomita Y, Yasuhira K, Hamamoto H, Shimizu K, Ishii M, Yamamuro T. A new murine model of accelerated senescence. Mech Ageing Dev 17: 183–194, 1981 [DOI] [PubMed] [Google Scholar]
- 31. Takeda T, Matsushita T, Kurozumi M, Takemura K, Higuchi K, Hosokawa M. Pathobiology of the senescence-accelerated mouse (SAM). Exp Gerontol 32: 117–127, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Yang B, Larson DF, Beischel J, Kelly R, Shi J, Watson RR. Validation of conductance catheter system for quantification of murine pressure-volume loops. J Invest Surg 14: 341–355, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 362: 777–781, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Zile MR, Baicu CF, Bonnema DD. Diastolic heart failure: definitions and terminology. Prog Cardiovasc Dis 47: 307–313, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation 105: 1387–1393, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part II: causal mechanisms and treatment. Circulation 105: 1503–1508, 2002 [DOI] [PubMed] [Google Scholar]