Abstract
Right ventricular (RV) failure is one of the strongest predictors of mortality both in the presence of left ventricular decompensation and in the context of pulmonary vascular disease. Despite this, there is a limited understanding of the biochemical and mechanical characteristics of the pressure-overloaded RV at the level of the cardiac myocyte. To better understand this, we studied ventricular muscle obtained from neonatal calves that were subjected to hypobaric atmospheric conditions, which result in profound pulmonary hypertension. We found that RV pressure overload resulted in significant changes in the phosphorylation of key contractile proteins. Total phosphorylation of troponin I was decreased with pressure overload, predominantly reflecting changes at the putative PKA site at Ser22/23. Similarly, both troponin T and myosin light chain 2 showed a significant decline in phosphorylation. Desmin was unchanged, and myosin-binding protein C (MyBP-C) phosphorylation was apparently increased. However, the apparent increase in MyBP-C phosphorylation was not due to phosphorylation but rather to an increase in MyBP-C total protein. Importantly, these findings were seen in all regions of the RV and were paralleled by reduced Ca2+ sensitivity with preserved maximal Ca2+ saturated developed force normalized to cross-sectional area in isolated skinned right ventricular myocyte fragments. No changes in total force or cooperativity were seen. Taken together, these results suggest that RV failure is mechanistically unique from left ventricular failure.
Keywords: myofilament, heart failure, protein, phosphorylation
the right ventricle (RV) of the heart is anatomically and functionally distinct from the left ventricle (LV) (47). It has thinner walls and is crescentic rather than circular, and contraction occurs via longitudinal shortening (7, 15) rather than via the lateral torsion and shortening seen in the LV. Adrenergic receptor density is more robust in the RV and is heterogeneous, with a higher receptor density in the apex relative to the base (4). These distinct anatomic properties suggest that the cellular mechanisms defining the mechanical response of the RV to a pathological load may also be distinct. The need to identify the cellular mechanisms of RV contraction is underscored by the fact that RV function is a strong and independent predictor of adverse clinical outcomes, not only in conditions of RV overload but also in the presence of intercurrent LV dysfunction (12, 23, 28).
To approach this question, we studied sarcomeric protein biochemistry and cardiac myocyte mechanics in a unique large animal model, the neonatal calf subjected to hypobaric hypoxia (11, 16). This model has a number of features that make it particularly suitable to address the question of whether RV dysfunction parallels LV dysfunction (biochemically and mechanically): first, the hearts from these animals are of sufficient size so that the question of regional heterogeneity can be easily addressed, and, second, the cow heart, as opposed to rodents, is biochemically quite similar to that of the human, allowing straightforward extrapolation to clinically relevant circumstances. For example, adult cow heart myofilaments are >90% β-myosin (like humans), whereas rodent hearts express mainly α-myosin. Since the myosin isoform is a primary determinant of power output and shortening velocity (14), this is an important factor when examining myofilament mechanics. Finally, brief (∼2 wk) exposure to hypobaric conditions results in the development of rapid, definable pulmonary hypertension, which leads to RV pressure overload and, ultimately, to RV hypertrophy and dilation.
Since sarcomeric protein biochemistry has been postulated to be a major regulator of the mechanical behavior of cardiac tissue (34, 35), we hypothesized that there would be regional heterogeneity of sarcomeric protein isoform expression and/or post-translational modification that would characterize the physiology of the RV. We also hypothesized that the functional demands on the RV during failure would result in unique adaptations of contractility driven by sarcomeric protein phosphorylation.
We have established the isoform expression levels of α/β myosin, troponin T (TnT), and troponin I (TnI). While the fetal isoforms of myosin heavy chain (α-MHC) and TnI (slow TnI) are still partially present in the 2-wk calf model, there is no influence of RV pressure overload on the expression of these isoforms. Furthermore, we found no evidence for apex-to-base variation in mechanical function or sarcomeric protein expression or phosphorylation in the calf RV: the sarcomeric responses and biochemical modifications were consistent throughout the RV in control and experimental animals. However, we did establish that RV pressure overload in the calf resulted in a marked decrease in the Ca2+ sensitivity of contraction without a significant change in maximal force generation and that this was associated with a reduction in the overall phosphorylation of several contractile proteins [TnT, TnI, and myosin light chain (MLC) 2] with an increase in protein phosphatase (PP)1 content. Examination of length-dependent activation, a principle contributor to the Frank-Starling effect in cardiac muscle, showed no change with failure.
MATERIALS AND METHODS
Induction of RV failure in calves.
Details of the animal model have been previously published (17, 21). Briefly, 1-day-old male Holstein calves (70–110 lb) were subjected to hypobaric hypoxia (barometric pressure: 445 mmHg) for 2 wk. Age-matched controls were kept at ambient altitude (barometric pressure: 640 mmHg). In response to hypobaric hypoxia, the RV both hypertrophies and dilates, anatomic and physiological features that can be well defined in vivo. The pulmonary vascular response shows elevations in pulmonary artery systolic pressures (>80 mmHg), which are associated with marked increases in stiffening of the proximal pulmonary arteries (38). In the present study, we studied seven animals that were exposed to hypoxia and subsequently developed severe elevations in pulmonary artery pressure and five age-matched controls. Standard veterinary care was used following institutional guidelines, and the procedures were approved by the Institutional Animal Care and Use Committee (Dept. of Physiology, School of Veterinary Medicine, Colorado State Univ., Fort Collins, CO). On the day of euthanization, hemodynamic experiments were carried out as previously described (21). After the hemodynamic experiments, the animal was euthanized by an overdose of pentobarbital sodium (160 mg/kg body wt) and exsanguinated, and the heart and lungs were excised. Biopsies (∼500 mg) were taken from the apex, mid, and basal portions of the RV free wall and from the free wall of the LV. The cardiac tissues were immediately placed in liquid nitrogen and subsequently stored at −70°C until analysis.
Gel electrophoresis.
Approximately 20-mg samples were homogenized in 8 M urea, 2.5 M thiourea, 4% CHAPS, and 2 mM EDTA buffer with DTT, tributylphosphine, and protease inhibitors. The protein concentration of each sample was measured using a modified protein assay (Bio-Rad). Proteins were separated on 12.5% SDS-PAGE gels and stained with phosphospecific Pro-Q Diamond Gel Stain (PQD; Invitrogen) to detect phosphorylation. Phosphoproteins were imaged using a Typhoon 9410 Gel Imager. After being imaged, the same gels were stained with Coomassie brilliant blue (CBB) to quantify total protein. Relative phosphorylation was determined by dividing the PQD signal of each protein by the total protein signal for MLC1 (48). Because myosin-binding protein C (MyBP-C) was not well separated from the MHC band using 12.5% electrophoresis, we further characterized MyBP-C phosphorylation using 7.5% SDS-PAGE gels followed by the same staining protocol. The MyBP-C phosphosignal (PQD) was divided by the total protein signal (CBB) for MyBP-C. Additionally, separation and quantification of α- and β-myosin were performed using 6% SDS-PAGE as previously published (48). All data shown are means ± SE unless otherwise stated.
Absolute MLC2 total protein and phosphorylation were further assessed by two-dimensional gel electrophoresis. Samples were separated in the first dimension by charge on isoelectric focusing tube gels (pH 4.5–5.4) and then by molecular weight in the second dimension on 15% polyacrylamide gels. Gels were stained with CBB.
Western blot analysis.
Samples separated on either 12.5% or 7.5% SDS-PAGE gels were transferred to nitrocellulose membranes and blocked for 1 h at room temperature with 5% nonfat dry milk in Tris-buffered saline containing 0.5% Tween. After being washed, membranes were incubated in primary antibody at 4°C overnight. Membranes were washed, incubated in secondary antibody for 1 h at room temperature, and washed again. The protein bands were visualized using a chemiluminescent substrate and autoradiography.
Myocyte contractility measurements.
Details of the myocyte isolation and experimentation protocols have been previously described in detail (19, 46). Briefly, myocytes were purified from the frozen RV samples by mechanical homogenization and subsequently permeabilized with 0.3% Triton X-100 in rigor buffer. These isolated skinned myocyte fragments were attached to a force transducer and motor and mechanical experiments were conducted. Length and width of the skinned myocyte fragment were measured when it was attached to the force transducer in relaxing solution, and a side view mirror was used to measure depth. To evaluate the Frank-Starling effect in RV myocytes under control and failing conditions, measurements were made at two sarcomere lengths, 1.8 and 2.2 μm, performed in random order.
The following mechanical measurements were made: maximal Ca2+ saturated developed force normalized to the cross section (Fmax), Ca2+ concentration at which force was half-maximal (pCa50), and Hill coefficient (an index of myofilament cooperativity).
Materials.
The following ntibodies were used: 1° MyBPC3 (F-1, sc-137181 and sc-137237, Santa Cruz Biotechnology, 1:2,500), desmin (ab6322, Abcam, 1:1,000), TnT (T6277, Sigma, 1:500), cardiac TnI (Fitzgerald, 1:574), cardiac TnI p22/23 (Abcam, 1:10,000), PP1 (sc-7482, Santa Cruz Biotechnology, 1:1,000), PP2 (sc-14020, Santa Cruz Biotechnology, 1:1,000), actin (Sigma, 1:5,000), 2° anti-mouse (Sigma, 1:50,000), and anti-rabbit (Sigma, 1:50,000). Adult bovine tissue was from Innovative Research (Novi, MI).
Statistical analysis.
For biochemical measurements, control and pressure-overloaded groups were compared using Student's t-test. Significance was set as P < 0.05. For mechanical measurements, the experiments followed a split plot experimental design with sarcomere length nested within myocytes, which were nested within treatment levels (control vs. pressure overload). Data were analyzed using a mixed effects model using the R statistical language. Main effects and interactions were reported as significant if P < 0.05. Effect plots show means and 95% confidence intervals.
RESULTS
In vivo hemodynamic data.
Table 1 shows hemodynamic data from the animals used in this study. Systemic pressure was unaffected by the hypobaric atmospheric (HA) condition (39); however, pulmonary artery pressures were markedly increased (mean pulmonary artery pressure was 21.4 ± 2.7 mmHg in the controls vs. 104.5 ± 5.5 in the experimental cohort). Echocardiograms done on a subset of the animals (data not shown) as well as previous autopsy studies showed that the RV in animals exposed to high-altitude hypobaric hypoxia dilates over the 2-wk interval in response to pressure overload, as previously seen in this model (22, 25). Speckle tracking done on echo images suggested a marked decrease in wall strain throughout the entire RV, suggesting that the ability of the RV to shorten is impaired.
Table 1.
Hemodynamic measurements in 2-wk neonatal calves
| Systolic PAP, mmHg | Diastolic PAP, mmHg | Mean PAP, mmHg | Pulse Pressure, mmHg | |
|---|---|---|---|---|
| Control | 32.8 ± 3.4 | 11.1 ± 2.7 | 21.4 ± 2.7 | 21.7 ± 1.3 |
| HA | 136.8 ± 8.0* | 82.0 ± 4.9* | 104.5 ± 5.5* | 54.8 ± 3.8* |
Values are means ± SE; n = 5 control animals and 7 high-altitude hypertensive (HA) animals. PAP, pulmonary artery pressure. Pulse pressure is the difference between systolic and diastolic PAP.
P < 0.05.
Biochemical responses to RV pressure overload.
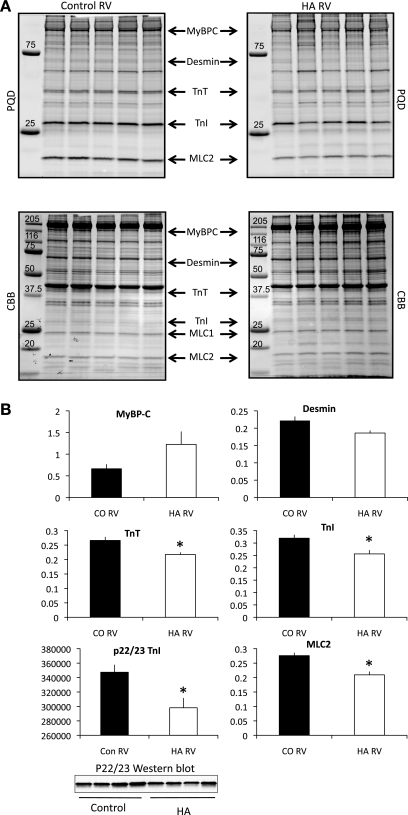
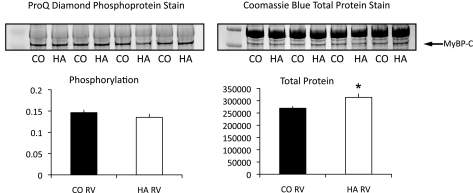
LV failure in both humans and rodent models is associated with changes in phosphorylation of the myofilament proteins, but little is known about myofilament phosphorylation and RV failure. In this model, phosphorylation of the myofilament proteins TnT, TnI, and MLC2 (Fig. 1, A and B) was significantly decreased in the pressure-overloaded animals, and desmin phosphorylation was unchanged. The decrease in TnI phosphorylation was largely evident at the canonical PKA site as the phosphorylation of Ser22/23 was significantly reduced (Fig. 1B). Interestingly, MyBP-C phosphorylation appeared to be increased when normalized to MLC1 total protein (see materials and methods) (Fig. 1, A and B). However, on the 12.5% SDS-PAGE gels, the phosphosignal for MyBP-C was difficult to distinguish from the nonspecific staining of the MHC band. Therefore, to better quantify the changes in phosphorylation of MyBP-C, we ran 7.5% SDS-PAGE gels and normalized the phosphosignal to the total protein signal for MyBP-C itself. When analyzed in this fashion, it was evident that total MyBP-C protein expression was significantly upregulated in the pulmonary arterial hypertension (PAH) samples (Fig. 2, control vs. failure, P = 0.00384), and when the phosphorylation of MyBP-C was normalized to itself, there was no change in phosphorylation (Fig. 2, left). None of the other myofilament proteins examined (total MHC, actin, desmin, TnT, TnI, MLC1, and MLC2) showed changes in protein expression in the pressure-overloaded ventricles compared with controls. To assess whether the increased MyBP-C protein was incorporated into the sarcomere, we calculated the ratio of MyBP-C to myosin in the skinned cell fragments that were used for the measurement of force (below). These cell fragments are extensively skinned, and free cytoplasmic proteins are lost. The ratio of MyBP-C to myosin in skinned myocytes from the HA RVs was significantly increased compared with controls (0.24 ± 0.1 vs. 0.19 ± 0.1, P < 0.05), whereas the myosin-to-actin ratio remained the same (2.6 ± 0.07 in HA myocytes vs. 2.5 ± 1.2 in control myocytes, P > 0.05). These data strongly suggest that the increased MyBP-C protein was associated with the myofilament lattice.
Fig. 1.
Phosphorylation changes in response to pulmonary hypertension. Shown is a summary of protein phosphorylation in control (CO) and high-altitude hypertensive animals (HA). A: 12.5% SDS-PAGE gel images representative of five control and five HA animals. B: phosphosignals [Pro-Q diamond gel stain (PQD)] for myosin-binding protein C (MyBP-C), desmin, troponin T (TnT), (total) troponin I (TnI), and myosin light chain (MLC)2 were normalized to total MLC1 protein expression to correct for loading differences. TnI p22/23 phosphorylation was measured using a phosphospecific antibody and was normalized to the total TnI signal after reprobing the same membrane with a pan-specific TnI antibody. n = 5 control and 7 HA. RV, right ventricle. *P < 0.05. Molecular weight standards are in kiloDaltons.
Fig. 2.
MyBP-C expression and phosphorylation. 7.5% SDS-PAGE was used to increase the separation of myosin and MyBP-C to more accurately quantify changes in MyBP-C expression and phosphorylation. Phosphorylated MyBP-C was quantified by dividing the PQD signal by the Coomassie brilliant blue (CBB) signal for MyBP-C. Total MyBP-C was quantified by densitometry of the CBB signal. Total protein (50 μg) was loaded in each lane. n = 5. *P < 0.005, control vs. failure.
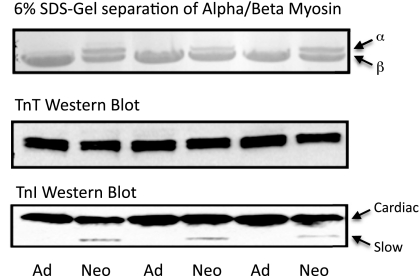
Heart failure is associated with a switch from α- to β-MHC in rodents, and even in humans (who normally express nearly all β-myosin), small changes in the amount of α-myosin expression are associated with ventricular dysfunction. Therefore, α- and β-myosin expression was determined by 6% SDS-PAGE. No significant differences were observed in α-myosin expression between control and pressure-overloaded samples (α-myosin: 28.5 ± 5.8% in control samples vs. 25.2 ± 12.6% in HA samples, P > 0.05). It is important to note that, although no significant differences were observed between groups, in this neonatal model there is still a significant amount of α-MHC at the time of death (Fig. 3). As with other large animal models, the adult bovine model expressed nearly all (>90%) β-myosin, whereas in the 2-wk calf model, there was ∼75% β-myosin and 25% α-myosin. Since TnT and TnI are also developmentally regulated, we examined expression differences in adult and neonatal animals (Fig. 3). There were no differences in TnT expression; however, slow TnI was still minimally expressed in the ventricle from neonatal animals, and treatment had no effect on expression levels (data not shown).
Fig. 3.
Comparison of adult (Ad) and neonatal (Neo) protein expression. Protein extracts from adult bovine hearts were compared with control neonatal extracts. Significantly more α-myosin heavy chain was expressed in the neonatal extracts, and a small amount of slow TnI remained in the neonatal hearts. No differences were seen in the isoform expression of TnT.
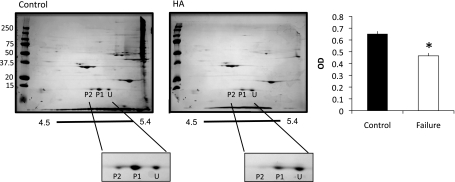
To quantify the absolute levels of MLC2 phosphorylation, two-dimensional electrophoresis was performed (Fig. 4). Phosphorylation was determined by adding the signal for the P1 and P2 sites and dividing by total MLC2 (U + P2 + P2). Total MLC2 phosphorylation in each group was quite high (as has been previously shown in other model systems). However, in pressure-overloaded animals, MLC2 phosphorylation was significantly reduced (46.5 ± 0.02% compared with 65.2 ± 0.02% in control animals).
Fig. 4.
Absolute quantitation of MLC2 phosphorylation. Two-dimensional SDS-PAGE was used to quantify changes in MLC2 phosphorylation in control and HA animals. Spots corresponding to MLC have been enlarged for clarity. Phosphorylation was calculated as P1 + P2/U + P1 + P2. n = 5 control and 5 HA. *P < 0.05.
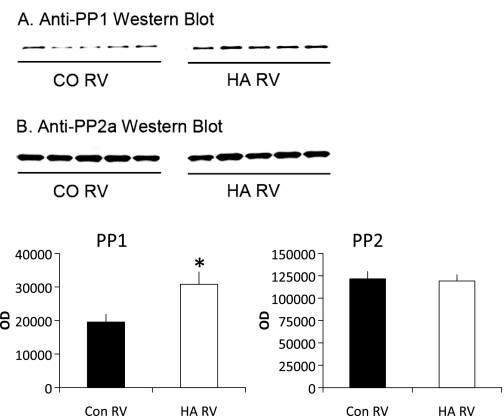
Because significant changes in phosphorylation of a number of myofilament proteins were seen, we examined the expression of the PP2 and PP1 catalytic subunits. Both PP1 and PP2 have been shown to be relevant myofilament phosphatases, and it has been suggested that the expression and activity of these enzymes influence contractility in the failing human heart (27, 52). In this model, expression of the PP2a catalytic subunit (Fig. 5A) was not changed in response to RV pressure overload. PP1 catalytic subunit expression (Fig. 5B) was significantly increased in pressure-overloaded animals.
Fig. 5.
Expression of protein phosphatase (PP)1 and PP2. Protein expression of PP1 (A) and PP2 (B) catalytic subunits was measured by Western blot. OD, optical density. n = 7 control and 5 HA. *P < 0.05.
Regional differences in myofilament protein expression and phosphorylation.
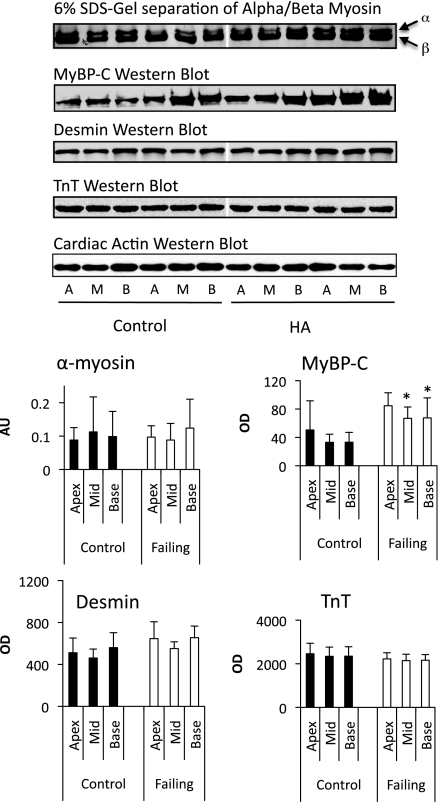
Because of the unique geometric properties of the RV, it is important to establish whether there are regional differences in contractile protein biology. Therefore, we took biopsies from the RV free wall near the base, in the midventricular wall, and near the apex of the heart (from both control and experimental animals). Total protein expression of MyBP-C, desmin, TnT, and α-myosin were determined by gel electrophoresis (α-myosin and MyBP-C) or Western blot analysis (desmin and TnT), as described above. There were no significant regional differences in total protein expression (normalized per gram of protein; Fig. 6) of any of these proteins in any of the sampled regions of the RV, although differences between control and experimental groups were similar to those seen in our previous set of experiments.
Fig. 6.
Regional protein expression. Shown is a summary of protein expression of MyBP-C, desmin, TnT, and α/β-myosin in three distinct regions of the bovine RV: the apex (A), midventricle (M), and base (B). Top: representative gels (α/β-myosin and MyBP-C) and Western blots (desmin and TnT). Bottom: histograms showing regional expression in five animals (means ± SE). AU, arbitrary units. *P < 0.05 from control.
As with total protein expression, there were no significant regional differences in the phosphorylation of MyBP-C, desmin, TnT, or TnI in samples from either control or experimental animals (data not shown). This finding affirms that sampling from any region of the RV is representative of the entire RV.
Single cell mechanics.
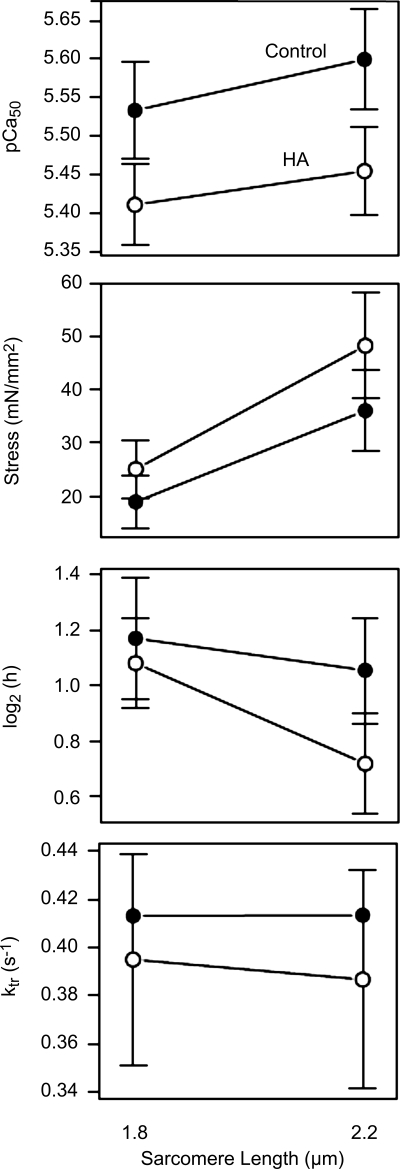
Skinned single cell preparations were used to evaluate ventricular function at the level of the isolated skinned myocyte. As was seen with the biochemical data, myocytes isolated from the various regions of the RV showed no regional differences in the force-pCa relationship (data not shown). Measurements from control and pressure-overloaded (HA) RVs were made at two different sarcomere lengths (1.8 and 2.2 μm) to evaluate whether length-dependent activation changed in response to pressure overload. As shown in Fig. 7, there was a significant decrease in the Ca2+ sensitivity (pCa50) of contraction in animals exposed to hypobaric hypoxia compared with control animals without a significant change in maximal force generation or cross-bridge turnover rate (Fig. 7, bottom). Interestingly, there was a good correlation between mean arterial pressure and Ca2+ sensitivity, suggesting a graded mechanical response to the afterload pressure. An increase in sarcomere length from 1.8 to 2.2 μm produced a significant (P < 0.01) increase in Ca2+ sensitivity, supporting the existence of length-dependent activation in both groups (Fig. 7). However, there was no significant interaction between sarcomere length and treatment, suggesting that length-dependent activation had a similar dependence on length in both control and HA groups. Finally, to establish the differential impact of pressure overload and hypoxia on contractile performance, we evaluated isolated cell mechanics in LV tissue from HA and control animals and found no differences in pCA50, maximal force, or cooperativity between the two groups (data not shown).
Fig. 7.
Mechanical characterization of control and HA RVs. The dependence of parameters of the Hill equation on HA and sarcomere length is shown. Error bars denote 95% confidence intervals. Nonoverlap indicates significant differences at P < 0.05. Top: significant reduction in pCa50 in HA compared with control (P = 0.03). There was a significant increase in Ca2+ sensitivity with an increase in sarcomere length (P < 0.01) but no significant interaction of HA with sarcomere length (P = 0.37). Middle top: significant increase in developed tension with increase in sarcomere length (P < 0.01) but no significant effect of HA (P = 0.30) or interaction between sarcomere length and HA (P = 0.10). Middle bottom: log2 of the Hill coefficient, a normally distributed measure of cooperativity (46) in muscle, which showed a significant decline in cooperativity with increases in sarcomere length (P = 0.002) but no significant change in the HA group compared with the control group (P = 0.14) and no significant interaction (P = 0.10). Bottom: cross-bridge turnover rates (ktr), which showed no significant differences with treatment.
DISCUSSION
The goals of this study were twofold: 1) to quantify adaptations in bovine RV biochemistry and mechanics in response to acute pressure overload and 2) to define the biochemical and mechanical characteristics of the bovine RV by region. The questions are of substantial importance as the function of the RV in clinical disease is one of the strongest predictors of outcome and there are few therapies that acknowledge and target the unique biology of the RV. Understanding the early stages of RV failure may help to identify adaptive and maladaptive responses and help to identify therapeutic targets with the aim of preventing progression of the disease.
The neonatal calf is an appealing model for several of the reasons articulated above, including the fact that it is a large animal model that has considerable fidelity to human heart disease. However, it is important to acknowledge that there are some innate limitations. One is that the animals are neonates, not adults, and another is that the RV pressure overload is not purely mechanical but occurs in the context of hypobaric hypoxia and is associated with both vascular inflammation and activation of inflammatory cytokines (10, 38). The neonatal calf contains significantly greater amounts of α-MHC than the adult bovine and TnI in this model is still ∼15% slow TnI. These differences in isoform expression, while distinguishing fetal from adult animals, still significantly resemble human isoform expression, suggesting that this large animal model may more closely depict human disease than available rodent models. However, we cannot rule out the possibility that isoform differences in the neonates may have contributed to the response of the ventricle to pressure overload, particularly the presence of slow TnI. Despite these limitations, the calf provides a unique large animal model of PAH that accurately and rapidly recapitulates the clinical presentation of PAH and concomitant right heart dysfunction.
One important finding of the study was that there were no regional differences in contractile protein isoform expression, phosphorylation, or sarcomere dynamics in the RV either in control conditions and in response to pressure overload. This result mirrors a similar study (45) done using tissue samples from the human LV, which also showed the absence of regional differences in contractile protein biochemistry, even in portions of the LV that evidenced asymmetric hypertrophy. This result affirms that studies defining functional properties of the failing RV can assume that muscle sampled from any part of the chamber free wall will be representative of the whole. A caveat of both our study and the previously published work is that the samples included the entire free wall and were not intended to contrast the endocardium versus epicardium. There is literature that suggests both that the pressure gradient varies across the muscle wall (6) and that expression of Ca2+ handling proteins, atrial natriuretic peptide, and phosphorylation of at least one contractile protein (MLC2) reflects this gradient (5, 29).
Sarcomere mechanics of the RV were significantly influenced by the coincidence of hypobaric hypoxia and pressure overload and were characterized by a striking decrease in Ca2+ sensitivity of contraction with preserved Fmax. This was primarily due to the mechanical pressure load and not the global impact of the hypobaric hypoxia, as preliminary data suggested that there were no mechanical differences in cells isolated from the LV (which was subjected to an equivalent hypoxia but without increased pressure load). Decreases in Ca2+ sensitivity of skinned muscle preparations have been described in response to a number of conditions, including treatment with PKA and PKD, and have been postulated to primarily reflect an increase in TnI phosphorylation at Ser22/23 sites (1, 20). In fact, the overall decrease in Ca2+ sensitivity seen in cardiocytes from the pathological RV contrasts with a number of studies evaluating sarcomere mechanics in the failing LV, which generally demonstrates an increase in Ca2+ sensitivity, also in the context of decreases in sarcomeric protein phosphorylation (3, 44, 50).
The Frank-Starling effect, an increase in the force of contraction as the muscle moves from a shorter to a longer length, is characteristic of cardiac performance. It has been suggested that patients with heart failure lack a strong Frank-Starling effect (31), although this effect has been found in failing human RV tissue (49) and in failing rat RV myocytes (9). Here, we demonstrate a Frank-Starling effect in the pressure-overloaded calf RV and show that in the acute pressure-overloaded RV there is no change in the degree of length-dependent activation.
We were struck by the overall decline in protein phosphorylation in the HA RV. However, the decline in phosphorylation of multiple contractile proteins, including TnI, TnT, and MLC2, seen in this model of RV failure is similar to data seen in end-stage LV heart disease in human and animal models (42, 54). When studied in isolation, these changes in protein phosphorylation have distinct and occasionally contradictory effects on sarcomere dynamics. For example, dephosphorylation of TnI at Ser22/23 is associated with an increase in Ca2+ sensitivity (44), whereas increased phosphorylation of TnT has been shown to significantly decrease the sensitivity of the filaments to Ca2+ in vitro (40). Chemical dephosphorylation of MLC2 results in decreased tension development of isolated skinned cardiac fibers (24), and genetic modification of the molecule to render it nonphosphorylatable impairs in situ cardiac performance (32). In vitro dephosphorylation of MLC2 by PP1 shifts the force-Ca relationship to the right (43), which mirrors the results seen in the present study, and, indeed, increases in PP1 were seen in the severely overloaded RV.
These mechanically distinct effects that have been described in isolated muscle preparations or in transgenic models in which a single protein is modulated are difficult to extrapolate into a complex in vivo pathological circumstance (such as RV pressure overload), both because it's hard to describe a functional hierarchy of effects and also because, in addition to the separable effects of each modification, there are clearly cooperative interactions whereby a modification of one protein influences the phosphorylation of a second. For example, a decrease in phosphorylation of MLC2 has been shown to have secondary effects to decrease overall TnI phosphorylation (32), and mutations in TnT (e.g., a deletion at Lys201) can alter the phosphorylation of several of the other sarcomeric proteins (33). Furthermore, it has recently been shown that there is an uncoupling of TnI phosphorylation and Ca2+ sensitivity in two different models of dilated cardiomyopathy (8, 36). This suggests that there is a hierarchy of both phosphorylation propensity and site-specific phosphorylation (48), and how this manifests in disease is unclear. It is possible, for example, that there are competing time- and disease-specific domains that impact contractile protein phosphorylation and muscle performance, and sorting this out will require a “systems biology” approach to myofilament function.
Expression of MBP-C protein was increased in the overloaded RV, whereas phosphorylation of MyBP-C was unchanged with regard to total protein. The importance of alterations in MyBP-C in the regulation of contraction is increasingly being recognized (37, 41). Expression of nonphosphorylatable protein in transgenic mouse hearts results in contractile dysfunction (26, 30); however, both increases (53) and decreases (2, 18) in overall protein phosphorylation have been reported in human heart disease. As a result, no clear consensus has emerged as to how this might impact contractile performance. In contrast to the other proteins analyzed, MBP-C phosphorylation appears to exert a mechanical effect indirectly by rendering the protein more or less vulnerable to proteolysis (PKA sites at Ser282 and Ser302 have been implicated) with mechanical sequellae that either reflect haploinsufficiency or the generation of a poison peptide (51). This might imply that the increase in protein expression seen is a compensatory response to β-adrenergic downregulation and might serve to preserve muscle performance.
In summary, the spectrum of changes observed in the phosphorylation of these several contractile proteins in this model of RV failure are consistent with data observed in other studies (13, 48) of the hypertrophied and failing LV and most likely reflect, at least in part, β-adrenergic receptor downregulation. It is certainly plausible that, when integrated, the biochemical findings result in a decline in the Ca2+ sensitivity of the sarcomere and provide a mechanistic explanation for the overall decline in RV performance in the context of pressure overload. However, similar changes in sarcomeric protein phosphorylation in the hypertrophied and failing LV appear to produce different mechanical sequellae, a result that underscores the fact that there are chamber-specific differences in muscle mechanics and that these differences may provide targets for tailored therapies for RV and LV failure.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Training Grant 1-R25-HL-103286-01 (to A. Glazier), Specialized Centers for Clinically Oriented Research Grant HL-084923-03, Program Project Grant HL-014985-36 and by the Temple Hoyne Buell Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Bardswell SC, Cuello F, Rowland AJ, Sadayappan S, Robbins J, Gautel M, Walker JW, Kentish JC, Avkiran M. Distinct sarcomeric substrates are responsible for protein kinase D-mediated regulation of cardiac myofilament Ca2+ sensitivity and cross-bridge cycling. J Biol Chem 285: 5674–5682, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barefield D, Sadayappan S. Phosphorylation and function of cardiac myosin binding protein-C in health and disease. J Mol Cell Cardiol 48: 866–875, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bodor GS, Oakeley AE, Allen PD. Troponin I phosphorylation in the normal and failing adult human heart. Circulation 96: 1495–1500, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Bristow MR, Minobe W, Rasmussen R, Larrabee P, Skerl L, Klein JW, Anderson FL, Murray J, Mestroni L, Karwande SV. Beta-adrenergic neuroeffector abnormalities in the failing human heart are produced by local rather than systemic mechanisms. J Clin Invest 89: 803–815, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davis JS, Hassanzadeh S, Winitsky S, Wen H, Aletras A, Epstein ND. A gradient of myosin regulatory light-chain phosphorylation across the ventricular wall supports cardiac torsion. Cold Spring Harb Symp Quant Biol 67: 345–352, 2002 [DOI] [PubMed] [Google Scholar]
- 6. de Simone G, Devereux RB. Rationale of echocardiographic assessment of left ventricular wall stress and midwall mechanics in hypertensive heart disease. Eur J Echocardiogr 3: 192–198, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Dell'Italia LJ. The right ventricle: anatomy, physiology, and clinical importance. Curr Probl Cardiol 16: 653–720, 1991 [DOI] [PubMed] [Google Scholar]
- 8. Dyer EC, Jacques AM, Hoskins AC, Ward DG, Gallon CE, Messer AE, Kaski JP, Burch M, Kentish JC, Marston SB. Functional analysis of a unique troponin c mutation, GLY159ASP, that causes familial dilated cardiomyopathy, studied in explanted heart muscle. Circ Heart Fail 2: 456–464, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Fan D, Wannenburg T, de Tombe PP. Decreased myocyte tension development and calcium responsiveness in rat right ventricular pressure overload. Circulation 95: 2312–2317, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Frid MG, Aldashev AA, Dempsey EC, Stenmark KR. Smooth muscle cells isolated from discrete compartments of the mature vascular media exhibit unique phenotypes and distinct growth capabilities. Circ Res 81: 940–952, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Frid MG, Li M, Gnanasekharan M, Burke DL, Fragoso M, Strassheim D, Sylman JL, Stenmark KR. Sustained hypoxia leads to the emergence of cells with enhanced growth, migratory, and promitogenic potentials within the distal pulmonary artery wall. Am J Physiol Lung Cell Mol Physiol 297: L1059–L1072, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol 37: 183–188, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Hamdani N, Kooij V, van Dijk S, Merkus D, Paulus WJ, Remedios CD, Duncker DJ, Stienen GJ, van der Velden J. Sarcomeric dysfunction in heart failure. Cardiovasc Res 77: 649–658, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Herron TJ, Korte FS, McDonald KS. Loaded shortening and power output in cardiac myocytes are dependent on myosin heavy chain isoform expression. Am J Physiol Heart Circ Physiol 281: H1217–H1222, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Ho SY, Nihoyannopoulos P. Anatomy, echocardiography, and normal right ventricular dimensions. Heart 92, Suppl 1: i2–i13, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hunter KS, Albietz JA, Lee PF, Lanning CJ, Lammers SR, Hofmeister SH, Kao PH, Qi HJ, Stenmark KR, Shandas R. In vivo measurement of proximal pulmonary artery elastic modulus in the neonatal calf model of pulmonary hypertension: development and ex vivo validation. J Appl Physiol 108: 968–975, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Inscore SC, Stenmark KR, Orton C, Irvin CG. Neonatal calves develop airflow limitation due to chronic hypobaric hypoxia. J Appl Physiol 70: 384–390, 1991 [DOI] [PubMed] [Google Scholar]
- 18. Jacques AM, Copeland O, Messer AE, Gallon CE, King K, McKenna WJ, Tsang VT, Marston SB. Myosin binding protein C phosphorylation in normal, hypertrophic and failing human heart muscle. J Mol Cell Cardiol 45: 209–216, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Jweied EE, McKinney RD, Walker LA, Brodsky I, Geha AS, Massad MG, Buttrick PM, de Tombe PP. Depressed cardiac myofilament function in human diabetes mellitus. Am J Physiol Heart Circ Physiol 289: H2478–H2483, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Kentish JC, McCloskey DT, Layland J, Palmer S, Leiden JM, Martin AF, Solaro RJ. Phosphorylation of troponin I by protein kinase A accelerates relaxation and crossbridge cycle kinetics in mouse ventricular muscle. Circ Res 88: 1059–1065, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Lammers SR, Kao PH, Qi HJ, Hunter K, Lanning C, Albietz J, Hofmeister S, Mecham R, Stenmark KR, Shandas R. Changes in the structure-function relationship of elastin and its impact on the proximal pulmonary arterial mechanics of hypertensive calves. Am J Physiol Heart Circ Physiol 295: H1451–H1459, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lemler MS, Bies RD, Frid MG, Sastravaha A, Zisman LS, Bohlmeyer T, Gerdes AM, Reeves JT, Stenmark KR. Myocyte cytoskeletal disorganization and right heart failure in hypoxia-induced neonatal pulmonary hypertension. Am J Physiol Heart Circ Physiol 279: H1365–H1376, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Mendes LA, Dec GW, Picard MH, Palacios IF, Newell J, Davidoff R. Right ventricular dysfunction: an independent predictor of adverse outcome in patients with myocarditis. Am Heart J 128: 301–307, 1994 [DOI] [PubMed] [Google Scholar]
- 24. Morano I. Effects of different expression and posttranslational modifications of myosin light chains on contractility of skinned human cardiac fibers. Basic Res Cardiol 87, Suppl 1: 129–141, 1992 [DOI] [PubMed] [Google Scholar]
- 25. Morrell NW, Danilov SM, Satyan KB, Morris KG, Stenmark KR. Right ventricular angiotensin converting enzyme activity and expression is increased during hypoxic pulmonary hypertension. Cardiovasc Res 34: 393–403, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Nagayama T, Takimoto E, Sadayappan S, Mudd JO, Seidman JG, Robbins J, Kass DA. Control of in vivo left ventricular [correction] contraction/relaxation kinetics by myosin binding protein C: protein kinase A phosphorylation dependent and independent regulation. Circulation 116: 2399–2408, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Neviere R, Hassoun SM, Decoster B, Bouazza Y, Montaigne D, Marechal X, Marciniak C, Marchetti P, Lancel S. Caspase-dependent protein phosphatase 2A activation contributes to endotoxin-induced cardiomyocyte contractile dysfunction. Crit Care Med 38: 2031–2036, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Polak JF, Holman BL, Wynne J, Colucci WS. Right ventricular ejection fraction: an indicator of increased mortality in patients with congestive heart failure associated with coronary artery disease. J Am Coll Cardiol 2: 217–224, 1983 [DOI] [PubMed] [Google Scholar]
- 29. Prestle J, Dieterich S, Preuss M, Bieligk U, Hasenfuss G. Heterogeneous transmural gene expression of calcium-handling proteins and natriuretic peptides in the failing human heart. Cardiovasc Res 43: 323–331, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Sadayappan S, Gulick J, Osinska H, Martin LA, Hahn HS, Dorn GW, 2nd, Klevitsky R, Seidman CE, Seidman JG, Robbins J. Cardiac myosin-binding protein-C phosphorylation and cardiac function. Circ Res 97: 1156–1163, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schwinger RH, Bohm M, Koch A, Schmidt U, Morano I, Eissner HJ, Uberfuhr P, Reichart B, Erdmann E. The failing human heart is unable to use the Frank-Starling mechanism. Circ Res 74: 959–969, 1994 [DOI] [PubMed] [Google Scholar]
- 32. Scruggs SB, Hinken AC, Thawornkaiwong A, Robbins J, Walker LA, de Tombe PP, Geenen DL, Buttrick PM, Solaro RJ. Ablation of ventricular myosin regulatory light chain phosphorylation in mice causes cardiac dysfunction in situ and affects neighboring myofilament protein phosphorylation. J Biol Chem 284: 5097–5106, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sfichi-Duke L, Garcia-Cazarin ML, Sumandea CA, Sievert GA, Balke CW, Zhan DY, Morimoto S, Sumandea MP. Cardiomyopathy-causing deletion K210 in cardiac troponin T alters phosphorylation propensity of sarcomeric proteins. J Mol Cell Cardiol 48: 934–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Solaro RJ. Modulation of cardiac myofilament activity by protein phosphorylation. In: Handbook of Physiology. The Cardiovascular System. The Heart. Hoboken, NJ: Wiley, 2002, p. 264–300 [Google Scholar]
- 35. Solaro RJ. Myosin light chain phosphatase: a Cinderella of cellular signaling. Circ Res 87: 173–175, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Song W, Dyer E, Stuckey D, Leung MC, Memo M, Mansfield C, Ferenczi M, Liu K, Redwood C, Nowak K, Harding S, Clarke K, Wells D, Marston S. Investigation of a transgenic mouse model of familial dilated cardiomyopathy. J Mol Cell Cardiol 49: 380–389, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Stelzer JE, Patel JR, Moss RL. Acceleration of stretch activation in murine myocardium due to phosphorylation of myosin regulatory light chain. J Gen Physiol 128: 261–272, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Stenmark KR, Fasules J, Hyde DM, Voelkel NF, Henson J, Tucker A, Wilson H, Reeves JT. Severe pulmonary hypertension and arterial adventitial changes in newborn calves at 4,300 m. J Appl Physiol 62: 821–830, 1987 [DOI] [PubMed] [Google Scholar]
- 40. Sumandea MP, Pyle WG, Kobayashi T, de Tombe PP, Solaro RJ. Identification of a functionally critical protein kinase C phosphorylation residue of cardiac troponin T. J Biol Chem 278: 35135–35144, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Tong CW, Stelzer JE, Greaser ML, Powers PA, Moss RL. Acceleration of crossbridge kinetics by protein kinase A phosphorylation of cardiac myosin binding protein C modulates cardiac function. Circ Res 103: 974–982, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van der Velden J, Merkus D, Klarenbeek BR, James AT, Boontje NM, Dekkers DH, Stienen GJ, Lamers JM, Duncker DJ. Alterations in myofilament function contribute to left ventricular dysfunction in pigs early after myocardial infarction. Circ Res 95: e85–e95, 2004 [DOI] [PubMed] [Google Scholar]
- 43. van der Velden J, Papp Z, Boontje NM, Zaremba R, de Jong JW, Janssen PM, Hasenfuss G, Stienen GJ. The effect of myosin light chain 2 dephosphorylation on Ca2+-sensitivity of force is enhanced in failing human hearts. Cardiovasc Res 57: 505–514, 2003 [DOI] [PubMed] [Google Scholar]
- 44. van der Velden J, Papp Z, Zaremba R, Boontje NM, de Jong JW, Owen VJ, Burton PB, Goldmann P, Jaquet K, Stienen GJ. Increased Ca2+-sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovasc Res 57: 37–47, 2003 [DOI] [PubMed] [Google Scholar]
- 45. van Dijk SJ, Holewijn RA, Tebeest A, Dos Remedios C, Stienen GJ, van der Velden J. A piece of the human heart: variance of protein phosphorylation in left ventricular samples from end-stage primary cardiomyopathy patients. J Muscle Res Cell Motil 30: 299–302, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Walker JS, Li X, Buttrick PM. Analyzing force-pCa curves. J Muscle Res Cell Motil 31: 59–69, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Walker LA, Buttrick PM. The right ventricle: biologic insights and response to disease. Curr Cardiol Rev 5: 22–28, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Walker LA, Walker JS, Ambler SK, Buttrick PM. Stage-specific changes in myofilament protein phosphorylation following myocardial infarction in mice. J Mol Cell Cardiol 48: 1180–1186, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weil J, Eschenhagen T, Hirt S, Magnussen O, Mittmann C, Remmers U, Scholz H. Preserved Frank-Starling mechanism in human end stage heart failure. Cardiovasc Res 37: 541–548, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Wolff MR, Buck SH, Stoker SW, Greaser ML, Mentzer RM. Myofibrillar calcium sensitivity of isometric tension is increased in human dilated cardiomyopathies: role of altered beta-adrenergically mediated protein phosphorylation. J Clin Invest 98: 167–176, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang Q, Sanbe A, Osinska H, Hewett TE, Klevitsky R, Robbins J. A mouse model of myosin binding protein C human familial hypertrophic cardiomyopathy. J Clin Invest 102: 1292–1300, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yin X, Cuello F, Mayr U, Hao Z, Hornshaw M, Ehler E, Avkiran M, Mayr M. Proteomics analysis of the cardiac myofilament subproteome reveals dynamic alterations in phosphatase subunit distribution. Mol Cell Proteomics 9: 497–509, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yuan C, Guo Y, Ravi R, Przyklenk K, Shilkofski N, Diez R, Cole RN, Murphy AM. Myosin binding protein C is differentially phosphorylated upon myocardial stunning in canine and rat hearts–evidence for novel phosphorylation sites. Proteomics 6: 4176–4186, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Zakhary DR, Moravec CS, Stewart RW, Bond M. Protein kinase A (PKA)-dependent troponin-I phosphorylation and PKA regulatory subunits are decreased in human dilated cardiomyopathy. Circulation 99: 505–510, 1999 [DOI] [PubMed] [Google Scholar]