Abstract
Cardiac tissue from mice that do not express secreted protein acidic and rich in cysteine (SPARC) have reduced amounts of insoluble collagen content at baseline and in response to pressure overload hypertrophy compared with wild-type (WT) mice. However, the cellular mechanism by which SPARC affects myocardial collagen is not clearly defined. Although expression of SPARC by cardiac myocytes has been detected in vitro, immunohistochemistry of hearts demonstrated SPARC staining primarily associated with interstitial fibroblastic cells. Primary cardiac fibroblasts isolated from SPARC-null and WT mice were assayed for collagen I synthesis by [3H]proline incorporation into procollagen and by immunoblot analysis of procollagen processing. Bacterial collagenase was used to discern intracellular from extracellular forms of collagen I. Increased amounts of collagen I were found associated with SPARC-null versus WT cells, and the proportion of total collagen I detected on SPARC-null fibroblasts without propeptides [collagen-α1(I)] was higher than in WT cells. In addition, the amount of total collagen sensitive to collagenase digestion (extracellular) was greater in SPARC-null cells than in WT cells, indicating an increase in cell surface-associated collagen in the absence of SPARC. Furthermore, higher levels of collagen type V, a fibrillar collagen implicated in collagen fibril initiation, were found in SPARC-null fibroblasts. The absence of SPARC did not result in significant differences in proliferation or in decreased production of procollagen I by cardiac fibroblasts. We conclude that SPARC regulates collagen in the heart by modulating procollagen processing and interactions with fibroblast cell surfaces. These results are consistent with decreased levels of interstitial collagen in the hearts of SPARC-null mice being due primarily to inefficient collagen deposition into the extracellular matrix rather than to differences in collagen production.
Keywords: extracellular matrix, fibrosis, matricellular, osteonectin, secreted protein acidic and rich in cysteine
the collagen content of the cardiac interstitium is tightly regulated to preserve the form and function of the heart. Collagen in the healthy heart aligns myocytes and provides structural support; however, increased collagen deposition in response to myocardial infarction or in some types of cardiac hypertrophy can impair heart function (2, 18, 31). For example, an increase in collagen concentration is associated with chronic pressure overload, such as that seen in individuals with arterial hypertension, and is frequently associated with impaired diastolic function (14). The increase in cardiac collagen has been shown to be a significant contributor to greater diastolic stiffness (31). Increases in interstitial fibrillar collagens (collagen types I, III, and V) as well as basal lamina-associated collagen IV have been observed in the hypertrophic myocardium (9, 12).
Cardiac fibroblasts produce the majority of fibrillar collagen in the heart, of which collagen I is the most abundant (12). Procollagen I is synthesized and secreted by fibroblasts with NH2- and COOH-terminal propeptides, which are cleaved by specific enzymes to yield fibril-forming collagen I monomers (22). Collagen units are then incorporated into the insoluble extracellular matrix (ECM) as collagen fibers that provide structural support to cardiac myocytes and contribute to the tensile properties of the tissue (12). Regulation of collagen levels in the cardiac interstitium can occur at the transcriptional level through effects on mRNA production and also by degradation of insoluble collagen, due primarily to the activity of collagenases belonging to the matrix metalloproteinase family (27, 28). However, regulation at the level of incorporation of collagen into the ECM, or postsynthetic processing, is another important point of control. In adult hearts, as much as 92% of newly synthesized procollagen protein was found to be degraded before ECM incorporation to the insoluble ECM (1). In addition, the proportion of newly synthesized collagen degraded was significantly decreased in the hypertrophic myocardium. Hence, the identification of factors that influence procollagen processing and deposition by cardiac fibroblasts is of significant importance to better understand the mechanisms of cardiac collagen fibrosis and for the design of novel therapeutic strategies.
Secreted protein acidic and rich in cysteine (SPARC; also called osteonectin or basement membrane protein 40) is a collagen-binding, counteradhesive protein belonging to the matricellular group of proteins (20). Matricellular proteins associate with the ECM and facilitate cell-ECM interactions particularly in development and in response to ECM remodeling in adult organisms but do not serve as structural components of the ECM (3). In SPARC-deficient animals, there is a failure to generate a robust fibrotic response to a number of different insults (4). In the heart, lack of SPARC resulted in an increased incidence of rupture after myocardial infarction (26). The infarct scar formed in SPARC-null mice exhibited a higher incidence of immature collagen fibers than the scars of wild-type (WT) mice. Similarly, pressure overload hypertrophy induced by transaortic constriction (TAC) revealed decreased collagen deposition in the hearts of SPARC-null mice compared with WT mice with TAC (5). Whereas WT TAC hearts displayed a significant increase in collagen concentrations and muscle stiffness, the increases in collagen and associated muscle stiffness was substantially less in SPARC-null animals.
To uncover the cellular mechanisms that function to decrease collagen content in SPARC-null hearts, we cultured primary cardiac fibroblasts from WT and SPARC-null mice and assessed whether differences in proliferation, collagen production, and/or cell surface collagen association were influenced by the lack of SPARC expression. These results strongly implicate SPARC as an important mediator of extracellular procollagen processing and fibrillar collagen deposition in cardiac tissue.
MATERIAL AND METHODS
Reagents.
Cell culture reagents were from Invitrogen (Carlsbad, CA). Recombinant SPARC (rSPARC) was generated by baculovirus infection of insect cells and purified as described in Ref. 7. Anti-murine collagen I antibodies were from MD Biosciences (Zurich, Switzerland), anti-collagen V antibodies were a kind gift of Dr. David Birk (University of South Florida), anti-α-smooth muscle actin (α-SMA) antibodies were from Sigma (St. Louis, MO), anti-murine SPARC antibodies were from R&D Systems (Minneapolis, MN), and isolectin GS-IB4 (from Griffonia simplicifolia) conjugated to Alexa fluor 594 was from Molecular Probes (Carlsbad, CA).
Animals.
Transgenic mice (age: 3–4 mo) that did not express SPARC (SPARC-null mice) were compared with age-matched WT mice on the same background (C57Bl6) (21). All procedures performed were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina in accordance with National Institutes of Health guidelines.
Cardiac tissue immunofluorescence.
WT and SPARC-null hearts were isolated, and the aorta was cannulated. After 5 min of perfusion with cardioplegic buffer, the tissue was fixed by perfusion with 4% paraformaldehyde for 15 min. Heart tissue was fixed either for 1 h by submersion in fresh 4% paraformaldehyde on ice or by immersion in methyl Carnoy's fixative. Tissue fixed in paraformaldehyde was washed in cardioplegic buffer and then cryoprotected in buffer containing 20% sucrose overnight at 4°C. Hearts were then perfused with buffer-diluted OCT embedding medium (TBS Tissue freezing medium no. H-TFM, 50:50), embedded in OCT embedding medium, and stored at −70°C. Tissue fixed in methyl Carnoy's fixative was embedded in paraffin. Tissue sections were taken using either a Leica cryostat at 8 μm (frozen sections) or a Leica microtome at 7 μm (paraffin embedded) and mounted on glass slides (Superfrost/Plus, Fisher). Immunofluorescence was performed with antibodies generated against murine SPARC (R&D Systems), isolectin GS-IB4 (from G. simplicifolia) conjugated to Alexa fluor 594 [α-SMA conjugated to FITC (Sigma) or anti-murine collagen I (MD Biosciences)]. Secondary antibody-fluorescent conjugates were anti-mouse TRITCRedx100 (Jackson ImmunoResearch Laboratories, Bar Harbor, ME) and anti-Alexa fluor (Molecular Probes). Images were captured by sequentially scanning three channels using a Leica SP5 laser scanning confocal microscope. Images shown are representative of hearts taken from three separate WT and SPARC-null age-matched mice.
Primary cell culture.
Primary cardiac fibroblasts were isolated from age-matched (3–4 mo) WT and SPARC-null mice. Hearts were removed, rinsed in PBS, minced, and subjected to collagenase digestion in 1/10 Blendzyme 3 (Roche, Indianapolis, IN) in DMEM (Invitrogen) at 37°C for 1–3 h. Tissue was triturated, and the resulting cell suspensions were rinsed three times in growth media (DMEM containing 10% FCS and antibiotic-antimycotic solution) before final plating. Cardiac fibroblasts were cultured in growth media supplemented with 50 μg/ml sodium ascorbate and 10 ng/ml transforming growth factor (TGF)-β1 to induce collagen production and secretion as indicated. All experiments were performed with cardiac fibroblasts between passages 1 and 4.
Immunoblot analysis.
Cell layers were extracted in 300 μl of 1% deoxycholate with protease inhibitors (Roche) and tumbled overnight at 4°C. Proteins soluble in deoxycholate were extracted from the cell layer and separated by SDS-PAGE on 3–8% gradient gels under reducing conditions. Separated proteins were transferred to nitrocellulose membranes for detection with the appropriate primary antibodies. Chemiluminescence was used to detect secondary antibodies conjugated to horseradish peroxidase. Quantification of protein bands was performed using ImageJ software. Immunoblots from at least four primary independent isolates were evaluated. Values generated from scanned images were analyzed using Student's t-test analysis. P values of <0.05 were considered significant.
Metabolic labeling.
Primary cells were incubated in growth media containing 25 μCi/ml [3H]proline (2,3,4,5-proline, Perkin-Elmer, Wellesley, MA) for 18 h. Conditioned media and cell layers were collected as described above. After separation by SDS-PAGE, metabolically labeled proteins were detected by fluorography on X-OMAT film. Data resulting from the metabolic labeling of four separate primary isolates was quantified from scanned films using ImageJ software analysis.
Primary cell immunofluorescence.
WT and SPARC-null fibroblasts were plated in equal numbers on glass coverslips in growth media. Cells were treated with 50 μg/ml ascorbate and grown for the indicated number of days. Cell layers were fixed in 4% paraformaldehyde in PBS (pH 7.5) for 30–45 min. Coverslips were blocked in 1% normal goat serum in Tris-buffered saline (blocking solution) with and without 0.5% Triton X-100 before the addition of anti-murine collagen I antibodies (1:200 dilution in blocking solution) or anti-α-SMA (1:200 in blocking solution, Sigma). Primary antibodies were detected with the appropriate fluorescein-conjugated secondary antibodies (Jackson ImmunoResearch). Coverslips were mounted in anti-fade reagent with 4′,6-diamidino-2-phenylindole (Molecular Probes) and viewed on an Olympus IX71 microscope equipped for epifluorescence. Images were captured using a Hamamatsu digital camera with the accompanying Slidebook software. The number of α-SMA-positive cells as a percentage of total cell nuclei was quantified in three separate primary isolates of WT and SPARC-null cells at passages 2 or 3 plated on glass cover slips for 24 h. Five fields per primary isolate were counted.
RESULTS
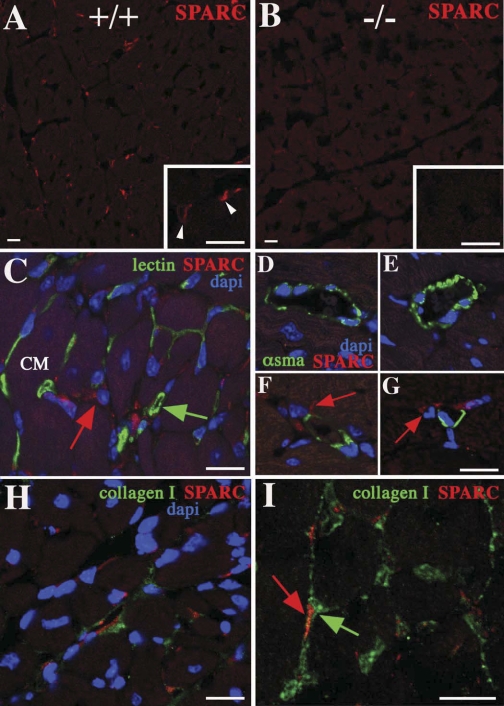
As SPARC expression has been reported in cardiac myocytes as well as cardiac fibroblasts in vitro, immunohistochemistry using anti-SPARC antibodies was performed to determine whether cell-specific production of SPARC protein was significantly higher in interstitial fibroblasts or cardiac myocytes in vivo (10, 26). As shown in Fig. 1, SPARC immunoreactivity in WT mice (Fig. 1A, white arrowheads) was predominantly associated with cell bodies of interstitial cells associated with the surface of myocyte bundles. Limited SPARC immunoreactivity was found associated with cardiac myocyte cell bodies. No SPARC staining was observed in SPARC-null hearts (Fig. 1B). To determine the cell type-specific expression of SPARC in cardiac interstitial cells, markers against endothelial cells (Fig. 1C, lectin) and vascular smooth muscle cells (Fig. 1, D–G, α-SMA) were used to identify cell type-specific SPARC expression. Although α-SMA expression has also been associated with myofibroblast differentiation, in normal mouse hearts, no detectable levels were found in the interstitium that were not associated with blood vessels. The α-SMA-positive cells were therefore consistent with vascular smooth muscle cells. The majority of interstitial cells that expressed SPARC were not positive for either lectin or α-SMA expression. In contrast, cells immunopositive for SPARC were closely associated with collagen I expression (Fig. 1, H and I). Hence, in normal hearts, fibroblasts appear to represent the primary cell type producing SPARC.
Fig. 1.
Immunofluorescence of secreted protein acidic and rich in cysteine (SPARC) immunoreactivity in murine hearts. A: SPARC staining was primarily detected in the cardiac interstitium associated with fibroblastic cells in wild-type (WT) mice [red staining indicated by arrowheads within the magnified region (inset)]. B: sections generated from the SPARC-null myocardium demonstrated no detectable SPARC immunoreactivity. C–I: WT sections. C: lectin staining (green), indicative of endothelial cells, labeled a significant proportion of the interstitium surrounding cardiomyocyte (CM) profiles but was not colocalized with the cells immunoreactive for SPARC (red arrow, green arrow: lectin-positive cell). D–G: labeling of coronary vasculature smooth muscle cells with α-smooth muscle actin (α-SMA; green staining) revealed that this cell population does not express SPARC; however, interstitial cells expressing SPARC could be found juxtaposed to these presumptive vessels (red arrows in F and G). H: SPARC-positive fibroblasts localized to regions of the cardiac interstitium rich in collagen I protein expression (green staining). I: higher-magnification image demonstrating a SPARC-positive fibroblast (red arrow) within the collagen I-labeled interstitium (green arrow). Nuclei were revealed with 4′,6-diamidino-2-phenylindole (dapi) counterstaining (blue) in C–H. Bars = 20 μm.
A number of activities associated with SPARC have been described in cultured cells, including effects on cell proliferation and adhesion (11). As shown in Fig. 2, primary cardiac fibroblasts from SPARC-null mice did not exhibit significant differences in rates of cell proliferation compared with WT cells in response to stimulation with 1% FCS. In addition, significant differences in cell adhesion and cytoskeletal architecture were not detected in SPARC-null versus WT fibroblasts (Fig. 3). Cardiac fibroblasts have been shown to spontaneously convert to a myofibroblast phenotype characterized by α-SMA expression in vitro (29). As shown in Fig. 3, the α-SMA cytoskeleton did not demonstrate significant differences in terms of abundance or structure in SPARC-null cultures. Quantification of α-SMA-positive cells per total cell nuclei demonstrated a population of 33.2 ± 3.1% positive α-SMA in WT cultures and 34.5 ± 2.9% positive α-SMA in SPARC-null cultures. Hence, there were no significant differences in the number of α-SMA-positive cells between SPARC-null and WT cultures.
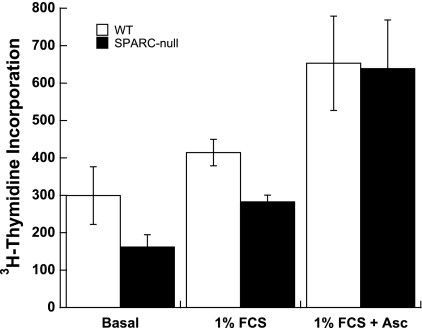
Fig. 2.
Significant differences in proliferation rates of SPARC-null versus WT cardiac fibroblasts in response to 1% FCS were not found. [3H]thymidine incorporation was used to monitor cell proliferation. Results shown are representative of three separate experiments using triplicate wells of pooled fibroblasts isolated from four SPARC-null hearts or four WT hearts. Error bars show SEs. Differences in [3H]thymidine incorporation between WT and SPARC-null cells were not found to be significant by Student's t-test. Asc, ascorbate.
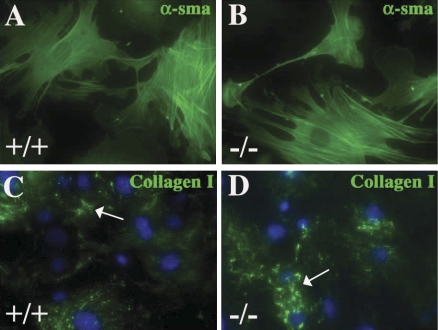
Fig. 3.
Cultures of SPARC-null cardiac fibroblasts exhibit increased cell-associated collagen I but not increases in numbers of α-SMA-positive myofibroblasts versus WT myofibroblasts. α-SMA staining of WT (A) and SPARC-null (B) primary cultures demonstrated no significant differences in the percentage of myofibroblast conversion in vitro between genotypes (see text). Cell-associated collagen I immunoreactivity (arrows) was, however, increased in SPARC-null (D) versus WT (C) fibroblasts.
However, differences in amounts of collagen on cell surfaces detected by immunofluorescence were apparent in SPARC-null versus WT cells (Fig. 3). Immunostaining of WT and SPARC-null cardiac fibroblasts with anti-murine collagen I antibodies revealed more robust staining of collagen I on SPARC-null cells versus WT cells (Fig. 3, C and D). Therefore, immunoblot analyses were performed to detect whether differences in amounts of collagen I and processing of procollagen I to collagen I were apparent in SPARC-null cardiac fibroblast cell layers extracted in detergent (detergent soluble; see materials and methods). As shown in Fig. 4A, anti-collagen I antibodies detected distinct forms of collagen I generated by processing of procollagen-α1(I) to collagen-α1(I), procollagen-α1(I), pC collagen-α1(I) and pN collagen-α1(I) [the collagen-α1(I) subunit lacking the NH2- and COOH-terminal propeptide, respectively], and collagen-α1(I) (both propeptides removed) in cell layers of WT and SPARC-null mice. β-Components are intermediates in triple helical collagen formation representing two of three cross-linked α-subunits and were detected as higher-molecular-weight bands (Fig. 4A). An increase in total collagen-α1(I) was found in SPARC-null versus WT cells, and, of the total collagen present on SPARC-null cells, a greater amount was represented as fully processed collagen-α1(I) than that on WT cells.
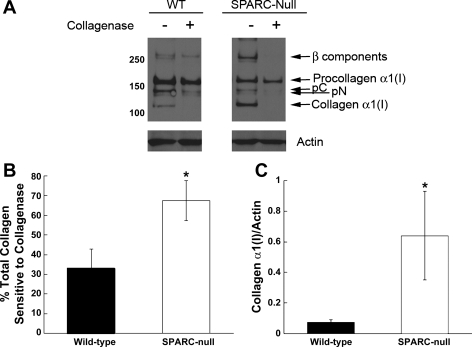
Fig. 4.
SPARC-null fibroblasts have increased amounts of extracellular cell-associated collagen I. A: immunoblot analysis of detergent-soluble cell extracts from primary fibroblasts treated with and without collagenase. More cell-associated collagen I was sensitive to digestion (i.e., extracellular) in SPARC-null cells versus WT cells. B and C: quantification of five separate immunoblot experiments carried out with five independent primary cell isolates. B: the percentage of total collagen sensitive to collagenase digestion was consistently elevated in SPARC-null cells. C: levels of cell-associated collagen-α1(I) [processed collagen-α1(I) with both propeptides removed] were also consistently increased in the absence of SPARC expression. Error bars show SEs. *P < 0.05 vs. WT. β-Components, bands representing two (of three) covalently linked α-subunits of collagen I; pC, procollagen-α1(I) with the NH2-propeptide removed; pN, procollagen-α1(I) with the COOH-propeptide removed.
As detergent-soluble collagen extracted from cells in culture includes intra- and extracellular collagen produced by fibroblasts, collagenase digestion of cell layers before extraction was performed to determine whether the increased levels of collagen-α1(I) on SPARC-null cells were extracellular in nature. As shown in Fig. 4A, collagen-α1(I) (and β-components) on SPARC-null and WT cells were sensitive to collagenase digestion. pC and pN intermediates demonstrated slightly diminished sensitivity to collagenase digestion in WT versus SPARC-null cells; however, the collagenase sensitivity of these intermediates was not consistent between cell preparations. In contrast, collagen-α1(I) and β-components [products of collagen-α1(I) assembly] were reproducibly sensitive to collagenase digestion and were thus considered extracellular in nature.
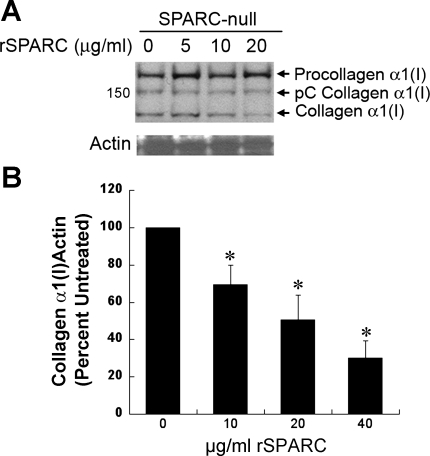
Quantification of the results generated from five separate primary cell isolates is shown in Fig. 4B. Total collagen in SPARC-null cell layers was more sensitive to collagenase digestion than that in WT cell layers. In addition, collagen-α1(I), the fibril-forming monomeric form of collagen I, was substantially increased on the surfaces of SPARC-null cells versus WT cells (Fig. 4C). The addition of rSPARC reduced collagen-α1(I) association with cell surfaces in a concentration-dependent manner (Fig. 5A), demonstrating at least a partial rescue of phenotype by the addition of exogenous SPARC protein. Quantification of four separate primary fibroblast isolates from SPARC-null animals treated with increasing concentrations of rSPARC is shown in Fig. 5B.
Fig. 5.
A: levels of detergent-soluble collagen I are diminished by the addition of recombinant (r)SPARC in a concentration-dependent manner in SPARC-null cardiac fibroblast cultures. B: quantification of immunoblot analysis from separate experiments carried out with five independent primary SPARC-null cell isolates. Error bars show SEs. *P < 0.05 vs. control.
Although significant differences in procollagen processing and cell association were found in SPARC-null cardiac fibroblasts, differences in the synthesis of procollagen, as measured by [3H]proline incorporation, were not detected in SPARC-null cells compared with WT cells (Fig. 6). Given the high levels of hydroxylated prolines in the primary sequence of collagen I, collagen protein is preferentially labeled in fibroblast cultures incubated with [3H]proline (22). A representative fluorograph of SPARC-null and WT collagen production in the presence of [3H]proline for 4 h is shown in Fig. 6A. Quantification of four separate primary isolates is shown in Fig. 6B. No significant differences in total collagen protein produced by SPARC-null versus WT fibroblasts were found. Similar to results from immunoblot analysis, a trend toward enhanced processing of procollagen to collagen I was apparent as a greater percentage of the total labeled collagen was found in the form of processed collagen-α1(I) in SPARC-null cell layers in most experiments.
Fig. 6.
Production of procollagen by SPARC-null fibroblasts is not significantly different than WT cells. A: fluorography of [3H]proline-labeled procollagen, procollagen intermediates, and collagen-α1(I) was detected in detergent-soluble cell layers of SPARC-null and WT fibroblasts. B: quantification of radiolabeled collagen bands from four separate fibroblast isolations. Error bars show SEs.
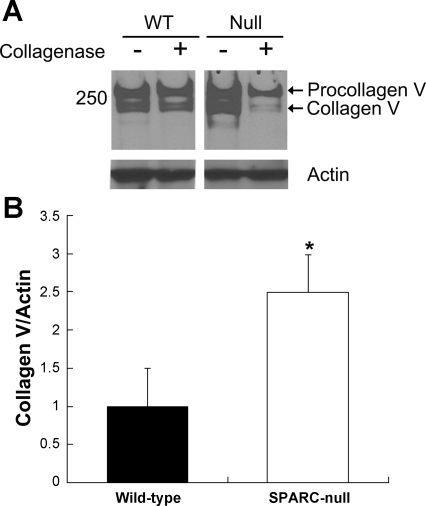
SPARC has been shown to bind other collagen family members in addition to collagen I (24). As collagen V has been implicated in collagen I fibril assembly, experiments to determine whether SPARC influenced cell association of collagen V, in addition to collagen I, were performed (33). As shown in Fig. 7A, increased levels of collagen V were also found associated with SPARC-null cell layers compared with WT cell layers. Collagenase treatment revealed that a significant portion of the collagen V on SPARC-null cell surfaces was sensitive to collagenase digestion and was thus extracellular cell surface-associated collagen V. Quantification from five separate primary isolates demonstrated a consistent increase in collagen V on SPARC-null fibroblast cell surfaces (Fig. 7B). Quantification of the collagenase sensitivity of collagen V in four primary isolates demonstrated a slight increase in the percentage of total collagen V that was associated with SPARC-null cell surfaces (39.6 ± 3% was sensitive to digestion) versus that associated with WT cells (34 ± 3% sensitive to digestion). Although the increase in the percentage of extracellular collagen V on SPARC-null fibroblasts did not reach statistical significance compared with that of WT cells, an overall increase in the amount of collagen V on SPARC-null cells was clearly evident.
Fig. 7.
Extracellular cell-associated collagen V is increased on SPARC-null fibroblast surfaces. A: representative immunoblot analysis of detergent-soluble SPARC-null and WT fibroblast layers with collagen V antibodies revealed increased levels of extracellular collagen V in the absence of SPARC (noncontiguous lanes from one blot are shown). B: quantification of immunoblot analysis from separate experiments carried out with five independent primary cell isolates. Error bars show SEs. *P = 0.03 vs. WT.
DISCUSSION
A number of different activities have been attributed to SPARC in a variety of cells, tissues, and cancers, including effects on cell proliferation, migration, growth factor efficacy, and matrix metalloproteinase activity (11). The phenotypic alterations in SPARC-null mice have been attributed primarily to differences in ECM production and assembly (4). Notably, a significant decrease in fibrillar collagen and a diminished capacity to mount a robust fibrotic response have been found in these mice. In the heart, the function of the matricellular protein SPARC has been recognized as a critical component in the control of interstitial collagen content under normal conditions and in fibrotic conditions such as response to infarction and pressure overload hypertrophy (5, 26). This study represents the first examination of the cellular mechanisms by which SPARC influences cardiac fibroblast proliferation and collagen deposition. A number of phenotypic differences in cardiac fibroblast behavior relative to fibroblasts from other tissues have been noted, including increased resistance to apoptosis and a high propensity for cardiac fibroblasts to convert to myofibroblasts in vitro (8, 30). In light of these differences, and the knowledge that significant amounts of procollagen are degraded before ECM incorporation in the heart, experiments were performed to characterize collagen production and processing in cardiac fibroblasts in the absence of SPARC (13).
In this study, we found an increase in cell surface-associated collagen in addition to enhanced processing of procollagen-α1(I) to collagen-α1(I) in the absence of SPARC. These findings compare favorably with similar experiments carried out on dermal fibroblasts (23). However, the present experiments go further by showing that the forms of collagen I that were increased in SPARC-null cells were extracellular in nature by virtue of sensitivity to collagenase digestion. We also found that murine cardiac fibroblasts produced low levels of collagen and that the collagen fibers in cardiac fibroblast cultures were transient in nature, and, in this regard, cardiac fibroblast differed significantly from dermal fibroblasts. Whereas dermal fibroblasts produced detectable collagen in the presence of ascorbate and 1% FCS, cardiac fibroblasts had to be treated with TGF-β and ascorbate to induce collagen production consistently detectable by immunohistochemistry or immunoblot analysis. Primary cultures of dermal fibroblasts deposited an insoluble ECM that could be used to measure collagen deposition by hydroxyproline analysis (23). In addition, collagen fibers produced by dermal fibroblasts persisted on WT cell surfaces and were readily detected up to 5 days after induction with ascorbate. In contrast, no detectable detergent-insoluble ECM pellet was recovered from murine cardiac fibroblast cultures, and no collagen fibers on cardiac fibroblast cell surfaces were detected >24 h after the addition of TGF-β and ascorbate. The relative decrease in collagen deposition and stability associated with cardiac versus dermal fibroblasts in vitro likely reflects the different kinetic properties of the collagenous ECM and resident fibroblast activity in hearts compared with skin. Indeed, cardiac collagen is dynamically turned over at significantly higher rates than most other tissues, including dermis (19).
The observation that increased amounts of extracellular collagen V were also detected in SPARC-null cardiac cells was notable as collagen V has been proposed to serve a nucleating or seed function in the formation of collagen I-rich fibers (32). Collagen V-null mice do not survive gestation; however, analysis of collagen V-null embryos revealed a paucity of collagen I fibers in the absence of collagen V expression (33). As SPARC has been shown to bind a number of collagen types in addition to type I, including type V, the absence of SPARC appears to enhance cell association of several collagen types and might consequently contribute to the substantial decrease in overall collagen content in SPARC-null tissues, perhaps due to effects on multiple types of collagen that influence ECM assembly (25). Hence, an increase in detergent-soluble, cell-associated collagen is found in SPARC-null cells that is associated with a decrease in insoluble collagen I incorporation in vivo.
Recently, the SPARC binding sites within collagens I and III were mapped, and, interestingly, they were found to be identical to the binding site for the collagen cell surface receptor discoidin domain receptor 2 (DDR2) (15, 16). Perhaps SPARC and DDR2 compete for collagen binding, and thus, in the absence of SPARC, an increase in collagen binding to DDR2 traps collagen at the cell surface. Experiments to address this interaction are currently under investigation.
That SPARC serves as a critical regulator of collagen processing and deposition in cardiac fibroblasts suggests that SPARC is key to the collagen accumulation in aging and disease. In fact, increased expression of SPARC within older mammalian hearts has been shown to contribute to increased collagen content and associated increases in stiffness indicative of the aged myocardium (6, 17). Previous reports (5, 26) have demonstrated the importance of SPARC in the fibrotic response to pressure overload and myocardial infarction. These studies highlight the dichotomy of collagen content in the heart: whereas the absence of SPARC improved diastolic function in response to pressure overload, SPARC-null mice had increased rupture after myocardial infarction. Hence, the maintenance of homeostatic levels of cardiac collagen is clearly essential to cardiac function, and SPARC is an essential modulator of cardiac collagen content.
In conclusion, the results presented here suggest that SPARC serves a vital role in regulating extracellular procollagen processing in the pericellular environment that gives rise to efficient incorporation of collagen to the ECM.
GRANTS
This work was supported by National Institutes of Health Grants P20-RR-16434-10 (to B. S. Harris), R01-CA-118240 (to R. A. Brekken), P20-RR-017696 (to A. D. Bradshaw), and HL-094517 (to A. D. Bradshaw) and by Veterans Administration Merit Award (to A. D. Bradshaw).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Bishop JE, Rhodes S, Laurent GJ, Low RB, Stirewalt WS. Increased collagen synthesis and decreased collagen degradation in right ventricular hypertrophy induced by pressure overload. Cardiovasc Res 28: 1581–1585, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Boluyt MO, O'Neill L, Meredith AL, Bing OH, Brooks WW, Conrad CH, Crow MT, Lakatta EG. Alterations in cardiac gene expression during the transition from stable hypertrophy to heart failure. Marked upregulation of genes encoding extracellular matrix components. Circ Res 75: 23–32, 1994 [DOI] [PubMed] [Google Scholar]
- 3. Bornstein P. Matricellular proteins: an overview. J Cell Commun Signal 3: 163–165, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bradshaw AD. The role of SPARC in extracellular matrix assembly. J Cell Commun Signal 3: 239–246, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Boggs J, Lacy JM, Zile MR. Pressure overload-induced alterations in fibrillar collagen content and myocardial diastolic function: role of secreted protein acidic and rich in cysteine (SPARC) in post-synthetic procollagen processing. Circulation 119: 269–280, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Bonnema DD, Zile MR. Age-dependent alterations in fibrillar collagen content and myocardial diastolic function: role of SPARC in postsynthetic procollagen processing. Am J Physiol Heart Circ Physiol 298: H614–H622, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bradshaw AD, Bassuk JA, Francki A, Sage EH. Expression and purification of recombinant human SPARC produced by baculovirus. Mol Cell Biol Res Commun 3: 345–351, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol 45: 657–687, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Chapman D, Weber KT, Eghbali M. Regulation of fibrillar collagen types I and III and basement membrane type IV collagen gene expression in pressure overloaded rat myocardium. Circ Res 67: 787–794, 1990 [DOI] [PubMed] [Google Scholar]
- 10. Chen H, Huang XN, Stewart AF, Sepulveda JL. Gene expression changes associated with fibronectin-induced cardiac myocyte hypertrophy. Physiol Genomics 18: 273–283, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Clark CJ, Sage EH. A prototypic matricellular protein in the tumor microenvironment–where there's SPARC, there's fire. J Cell Biochem 104: 721–732, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Eghbali M, Weber KT. Collagen and the myocardium: fibrillar structure, biosynthesis and degradation in relation to hypertrophy and its regression. Mol Cell Biochem 96: 1–14, 1990 [DOI] [PubMed] [Google Scholar]
- 13. Eleftheriades EG, Durand JB, Ferguson AG, Engelmann GL, Jones SB, Samarel AM. Regulation of procollagen metabolism in the pressure-overloaded rat heart. J Clin Invest 91: 1113–1122, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gaasch WH, Zile MR. Left ventricular diastolic dysfunction and diastolic heart failure. Annu Rev Med 55: 373–394, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Giudici C, Raynal N, Wiedemann H, Cabral WA, Marini JC, Timpl R, Bachinger HP, Farndale RW, Sasaki T, Tenni R. Mapping of SPARC/BM-40/osteonectin-binding sites on fibrillar collagens. J Biol Chem 283: 19551–19560, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hohenester E, Sasaki T, Giudici C, Farndale RW, Bachinger HP. Structural basis of sequence-specific collagen recognition by SPARC. Proc Natl Acad Sci USA 105: 18273–18277, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jugdutt BI, Jelani A, Palaniyappan A, Idikio H, Uweira RE, Menon V, Jugdutt CE. Aging-related early changes in markers of ventricular and matrix remodeling after reperfused ST-segment elevation myocardial infarction in the canine model: effect of early therapy with an angiotensin II type 1 receptor blocker. Circulation 122: 341–351, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Lindsey ML, Mann DL, Entman ML, Spinale FG. Extracellular matrix remodeling following myocardial injury. Ann Med 35: 316–326, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Mays PK, McAnulty RJ, Campa JS, Laurent GJ. Age-related changes in collagen synthesis and degradation in rat tissues. Importance of degradation of newly synthesized collagen in regulating collagen production. Biochem J 276: 307–313, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCurdy S, Baicu CF, Heymans S, Bradshaw AD. Cardiac extracellular matrix remodeling: fibrillar collagens and secreted protein acidic and rich in cysteine (SPARC). J Mol Cell Cardiol 48: 544–549, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Norose K, Clark JI, Syed NA, Basu A, Heber-Katz E, Sage EH, Howe CC. SPARC deficiency leads to early-onset cataractogenesis. Invest Ophthalmol Vis Sci 39: 2674–2680, 1998 [PubMed] [Google Scholar]
- 22. Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem 64: 403–434, 1995 [DOI] [PubMed] [Google Scholar]
- 23. Rentz TJ, Poobalarahi F, Bornstein P, Sage EH, Bradshaw AD. SPARC regulates processing of procollagen I and collagen fibrillogenesis in dermal fibroblasts. J Biol Chem 282: 22062–22071, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Sasaki T, Gohring W, Mann K, Maurer P, Hohenester E, Knauper V, Murphy G, Timpl R. Limited cleavage of extracellular matrix protein BM-40 by matrix metalloproteinases increases its affinity for collagens. J Biol Chem 272: 9237–9243, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Sasaki T, Hohenester E, Gohring W, Timpl R. Crystal structure and mapping by site-directed mutagenesis of the collagen-binding epitope of an activated form of BM-40/SPARC/osteonectin. EMBO J 17: 1625–1634, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schellings MW, Vanhoutte D, Swinnen M, Cleutjens JP, Debets J, van Leeuwen RE, d'Hooge J, Van de Werf F, Carmeliet P, Pinto YM, Sage EH, Heymans S. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med 206: 113–123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev 87: 1285–1342, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Villarreal FJ, Dillmann WH. Cardiac hypertrophy-induced changes in mRNA levels for TGF-β1, fibronectin, and collagen. Am J Physiol Heart Circ Physiol 262: H1861–H1866, 1992 [DOI] [PubMed] [Google Scholar]
- 29. Wang J, Chen H, Seth A, McCulloch CA. Mechanical force regulation of myofibroblast differentiation in cardiac fibroblasts. Am J Physiol Heart Circ Physiol 285: H1871–H1881, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Wang J, Seth A, McCulloch CA. Force regulates smooth muscle actin in cardiac fibroblasts. Am J Physiol Heart Circ Physiol 279: H2776–H2785, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Weber KT. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol 13: 1637–1652, 1989 [DOI] [PubMed] [Google Scholar]
- 32. Wenstrup RJ, Florer JB, Brunskill EW, Bell SM, Chervoneva I, Birk DE. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem 279: 53331–53337, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Wenstrup RJ, Florer JB, Davidson JM, Phillips CL, Pfeiffer BJ, Menezes DW, Chervoneva I, Birk DE. Murine model of the Ehlers-Danlos syndrome. col5a1 haploinsufficiency disrupts collagen fibril assembly at multiple stages. J Biol Chem 281: 12888–12895, 2006 [DOI] [PubMed] [Google Scholar]