Abstract
In the vasculature, nitric oxide (NO) is generated by endothelial NO synthase (eNOS) in a calcium/calmodulin-dependent reaction. In the absence of the requisite eNOS cofactor tetrahydrobiopterin (BH4), NADPH oxidation is uncoupled from NO generation, leading to the production of superoxide. Although this phenomenon is apparent with purified enzyme, cellular studies suggest that formation of the BH4 oxidation product, dihydrobiopterin, is the molecular trigger for eNOS uncoupling rather than BH4 depletion alone. In the current study, we investigated the effects of both BH4 depletion and oxidation on eNOS-derived superoxide production in endothelial cells in an attempt to elucidate the molecular mechanisms regulating eNOS oxidase activity. Results demonstrated that pharmacological depletion of endothelial BH4 does not result in eNOS oxidase activity, whereas BH4 oxidation gave rise to significant eNOS-oxidase activity. These findings suggest that the endothelium possesses regulatory mechanisms, which prevent eNOS oxidase activity from pterin-free eNOS. Using a combination of gene silencing and pharmacological approaches, we demonstrate that eNOS-caveolin-1 association is increased under conditions of reduced pterin bioavailability and that this sequestration serves to suppress eNOS uncoupling. Using small interfering RNA approaches, we demonstrate that caveolin-1 gene silencing increases eNOS oxidase activity to 85% of that observed under conditions of BH4 oxidation. Moreover, when caveolin-1 silencing was combined with a pharmacological inhibitor of AKT, BH4 depletion increased eNOS-derived superoxide to 165% of that observed with BH4 oxidation. This study identifies a critical role of caveolin-1 in the regulation of eNOS uncoupling and provides new insight into the mechanisms through which disease-associated changes in caveolin-1 expression may contribute to endothelial dysfunction.
Keywords: endothelial nitric oxide synthase, endothelium, oxidative stress, tetrahydrobiopterin
endothelium-derived nitric oxide (NO) is a potent vasodilator that plays a critical role in maintaining vascular homeostasis through its antiatherogenic and antiproliferative effects on the vascular wall. Reduced NO bioavailability is a key determinant of endothelial dysfunction and has been demonstrated to play a central role in the increased cardiovascular risks associated with metabolic syndrome (3, 26). Endothelial dysfunction has been observed in pre-diabetic stages of insulin resistance and subsequently contributes to smooth muscle cell proliferation and platelet and leukocyte adhesion as well as atherogenesis (4, 10, 22). Impaired endothelial function is apparent in experimental diabetes and in diabetic patients despite the fact that endothelial NO synthase (eNOS) expression is actually increased. The paradoxical finding of a concomitant increase in eNOS expression and reduced endothelium-dependent vasodilatation has drawn attention to the fact that eNOS itself, in pathological states, may be a source of superoxide anions, a process that has been termed eNOS uncoupling (2, 16, 23, 24, 28).
Our laboratory and others have demonstrated that when purified eNOS is depleted of its substrate l-arginine or the cofactor tetrahydrobiopterin (BH4), NADPH oxidation is uncoupled from NO synthesis and results in the reduction of O2 to form O2·− (1, 10, 11). BH4 is a critical NOS cofactor and required for efficient electron transfer and NO production. BH4 has been demonstrated to be redox sensitive and can be oxidized under pathological conditions resulting in decreased bioavailability and eNOS uncoupling. In support, Landmesser et al. (16) were the first to demonstrate this in a disease model in which oxidation of BH4 led to eNOS uncoupling in a rat model of hypertension. These findings have been further described in a variety of other disease states including atherosclerosis, myocardial infarction, and diabetes with recent experimental and clinical studies implicating eNOS as an important source of vascular reactive oxygen species (ROS) production (1, 8, 25).
Despite the expansive literature demonstrating eNOS uncoupling in cardiovascular pathology, fundamental questions remain regarding the molecular trigger for eNOS oxidase activity. Specifically, the role of BH4 in eNOS uncoupling has recently been challenged. Using purified enzyme, we and others have clearly demonstrated that BH4 depletion results in significant eNOS-derived superoxide with rates of production exceeding 300 nmol·mg−1·min−1. However, in the intact cell, BH4 depletion alone does not appear to be sufficient of an insult to trigger eNOS uncoupling. In support, Crabtree et al. (9) and Vasques-Vivar et al. (25) have independently demonstrated that increased levels of the BH4 oxidation product dihydrobiopterin (BH2), rather than BH4 depletion alone, is the molecular trigger for NO insufficiency and eNOS uncoupling. Specifically, the Crabtree et al. group (9) has demonstrated that BH4 and BH2 bind eNOS with equal affinity and BH2 can rapidly and efficiently replace BH4 in preformed eNOS-BH4 complexes. Moreover, the total biopterin pool of murine endothelial cells was unaffected by 48 h exposure to diabetic glucose levels (30 mM), whereas BH2 levels increased from undetectable to 40% of total biopterin. This BH2 accumulation was associated with diminished calcium ionophore-evoked NO activity and accelerated superoxide production (9). In support, we present data demonstrating that pharmacological depletion of endothelial BH4 does not result in eNOS uncoupling and in fact suppresses basal eNOS oxidase activity observed with BH4 oxidation. These findings together with our studies from purified enzyme suggest that the endothelium possesses some regulatory mechanism, which prevents eNOS oxidase activity from pterin-free eNOS. In the current study we provide evidence that caveolin-1 (Cav-1) is involved in regulating both eNOS-derived NO and superoxide and serves as a sink for sequestering pterin-free eNOS. These studies identify a novel role for Cav-1 in the regulation of eNOS uncoupling and provide new insight into the mechanisms through which disease-associated changes in Cav-1 expression may contribute to endothelial dysfunction and cardiovascular disease.
MATERIALS AND METHODS
Cell culture.
Bovine aortic endothelial cells (BAECs) were purchased from Cell-Systems and cultured in DMEM (Sigma, St. Louis, MO) containing 10% FBS, 1% nonessential amino acid, 0.2% endothelial cell growth factor supplement, and 1% antibiotic-antimyotic (Gibco, Carlsbad, CA) and incubated at 37°C, 5% CO2. The PKC inhibitor chelerythrine chloride and the AKT inhibitor 5-(2-benzothiazolyl)-3-ethyl-2-[2-methylphenylamino)ethynyl]-1-phenyl-1H-benzimidazolium iodide were purchased from Sigma.
EPR spectroscopy and spin trapping.
Spin-trapping measurements of NO were performed using a Bruker E-scan spectrometer with Fe-N-methyl-D-glucamine dithiocarbamate (MGD) as the spin trap (7, 20). For measurements of NO produced by BAECs, cells were cultured as described above and spin-trapping experiments were performed on cells grown in six-well plates. Attached cells were studied since scraping or enzymatic removal leads to injury and membrane damage with impaired NO generation. The media from ∼1 × 106 cells attached to the surface of the six-well plates were removed, and the cells were washed three times in Krebs and incubated at 37°C, 5% CO2 in 0.2 ml of Krebs buffer containing the spin trap complex Fe-MGD (0.5 mM Fe2+, 5.0 mM) and the cells stimulated with calcium ionophore (1 μM). Subsequent measurements of NO production were performed following a 30-min incubation period. Spectra recorded from cellular preparations were obtained using the following parameters: microwave power, 20 mW; modulation amplitude, 3.00 G; and modulation frequency, 86k HZ. For the detection of O2− in intact cells, a spin-trap solution consisting of 50 mM 5,5-dimethyl-l-pyrroline-N-oxide (Dojindo Laboratories), 5 μl Lipofectamine 2000 (Invitrogen), 12 mM diethylenetriaminepentaacetic acid (Sigma Chemicals), 2 μM sodium diethyl dithio carbamate trihydrate (Sigma Chemicals), 10 μM calcium ionophore, and 1 mM NG-nitro-l-arginine methyl ester were added to each well and incubated for 20 min. Approximately 2 × 106 cells were suspended in a final volume of 400 μl Krebs and loaded into an aquaX electron paramagnetic resonance (EPR) tube, and O2− production was measured at room temperature. Spectra were obtained using the following parameters: microwave power, 20 mW; modulation amplitude, 1.06 G; and modulation frequency, 86 kHz.
Cav-1 gene silencing.
The sequence of the small interfering RNA (siRNA) specifically targeting the bovine Cav-1 gene was designed through siRNA Target Finder (Ambion, Austin, TX). Induced silent sites were selected from the Cav-1 gene where the G-to-C ratio was 30–50% with Ambion siRNA analysis software (http://www.invitrogen.com/rnai), and BLAST analysis (http://www.pubmed.gov) suggested that it had no homology with other genes. Two complementary oligonucleotide strands were designed based on the following sites: top strand, 5′-AUGAACGAGAAGCAAGUGUtt-3′ and bottom strand 3′-ACACUUGCUUCUCGUUCAUtt-5′. siRNAs were transfected in BAECs by using Lipofectamine RNAiMAX (Invitrogen). Endothelial cells at a density of 2 × 106 cells/well in a six-well plate were used for reverse transfection. For each transfection, 240 nM siRNA and 5 μl Lipofectamine RNAiMAX were diluted in 500 μl serum-free medium. The solutions were mixed gently and incubated for 20 min at room temperature. BAECs were plated using 1.5 ml serum-free medium. The siRNA-Lipofectamine complex was added drop-wise to each well and incubated for 24 h at 37°C in a humidifier CO2 incubator. The medium was replaced with growth medium 24 h after transfection. Forty-eight hours post-transfection (which resulted in a ∼70% reduction in Cav-1 expression), the cells were used for Western blot analysis using specific antibody against Cav-1.
Western blot analysis of eNOS, phosphorylated eNOS, Cav-1, and Cav-1/eNOS association.
BAECs were briefly washed with Krebs containing (in mM) 145 NaCl, 5.7 sodium phosphate, 4.86 KCl, 0.54 CaCl2, 1.2 MgSO4, and 5.5 glucose (pH 7.4), scraped in IP buffer containing 10 mM Tris (pH 8.0), 60 mM n-octyl-β-D-glucopyranoside, 150 mM NaCl, 1 mM sodium orthovanadate, 20 mM NaF, 1 mM Na4P2O7, 1 mM Pefabloc, 10 μg/ml aprotinin, and 10 μg/ml leupeptin and briefly sonicated. Immunoprecipitation was carried out by standard protocols in the presence of protease inhibitors. About 200 μg of whole cell lysates were incubated with anti-eNOS antibody (Santa Cruz Biotechnology). After 1 h at 4°C, protein A/G plus agarose beads (Santa Cruz Biotechnology) 25 μl were added to the lysates for a further overnight incubation at 4°C. Bound immune complexes were washed three times with 50 mM Tris-HCl (pH 7.4). The immunoprecipitates were eluted by boiling in Laemmli sample buffer. For Western analysis, protein was separated by 4–20% SDS-PAGE, transferred to nitrocellulose membranes, and probed with anti-Cav-1 antibody (Cell signaling Technology). For eNOS phosphorylation, BAECs were washed twice with ice-cold Krebs buffer and scraped in Krebs-okadaic acid (5 nM) buffer, followed by mild sonication on ice. Protein concentration was determined by the Bradford assay from Bio-Rad using bovine serum albumin standard. Protein separation was performed on a 4–20% SDS-PAGE, transferred to nitrocellulose membranes, and probed with anti-phospho-eNOS (Ser1177 and Thr495; Cell signaling Technology) antibodies. Horseradish peroxidase-conjugated secondary antibody was used to visualize bound primary antibody by enhanced chemiluminescence. As loading control, blots were stripped and exposed to β-actin antibody.
RESULTS
Effects of BH4 depletion and oxidation on eNOS-derived NO and superoxide.
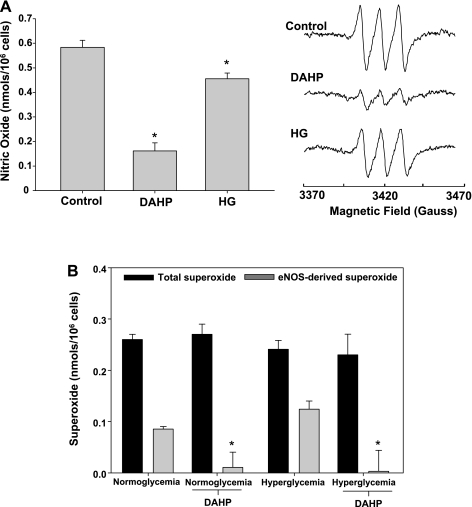
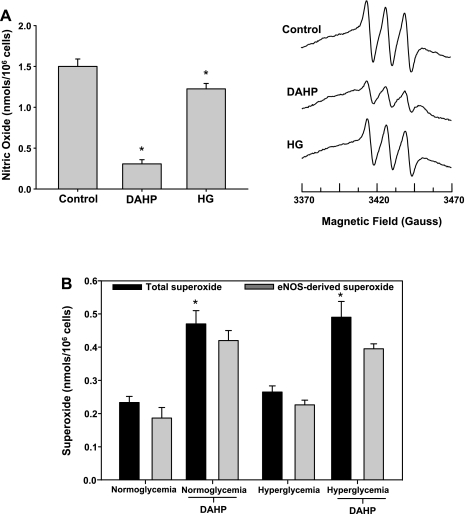
Studies were undertaken to assess the effects of endothelial BH4 depletion or oxidation on basal eNOS-derived NO and superoxide production. To induce a state of BH4 depletion, endothelial cells were treated with the GTP cyclohydrolase I inhibitor, DAHP (10 mM) for 24 h. BH4 oxidation was initiated by exposing endothelial cells to hyperglycemic conditions (30 mM glucose, 48 h) as previously reported (9). Results demonstrated that following BH4 depletion, endothelial NO generation was reduced by 72%, whereas under hyperglycemic conditions endothelial NO was inhibited by 22% (Fig. 1A). Based on the degree of NO inhibition, we would predict that BH4 depletion (DAHP treatment) would be associated with greater eNOS uncoupling than that which occurs following hyperglycemia-induced BH4 oxidation. However, EPR-based measurements of eNOS-derived superoxide clearly indicate that BH4 depletion is not associated with any eNOS oxidase activity, whereas the hyperglycemic insult gave rise to significant eNOS oxidase activity with 51% of the total cellular ROS production attributed to eNOS (Fig. 1B). Moreover, BH4 depletion of hyperglycemic endothelial cells resulted in a complete attenuation of the observed eNOS-dependent superoxide generation, suggesting that BH4 oxidation products are required for eNOS uncoupling. This is in clear contrast with the in vitro phenomenon wherein we and others have reported that BH4-depleted recombinant eNOS has robust oxidase activity (10, 24, 28). Subsequent experiments were carried out to assess whether the effects of BH4 depletion/oxidation hold true under conditions where calcium is not limiting. BAECs were treated with DAHP or exposed to hyperglycemic conditions to induce BH4 depletion or oxidation, respectively, and activated with calcium ionophore (1 μM). As expected, EPR-based measurement of calcium ionophore-stimulated NO production demonstrated a significant increase from the control cells. Both BH4 depletion and BH4 oxidation produced similar decreases in NO generation as seen in cells not treated with calcium ionophore, decreasing NO generation by 79% and 19%, respectively (Fig. 2A). However, in stark contrast with unstimulated cells, measurements of superoxide production from cells when calcium is not limiting demonstrated robust eNOS oxidase activity under all conditions tested (Fig. 2B). Thus the observed differences in oxidase activity with and without calcium ionophore activation clearly indicate that the endothelium possesses a calcium-dependent regulatory mechanism for suppressing basal eNOS oxidase activity. Given the known ability of Cav-1 to tonically suppress eNOS-derived NO generation together with its calcium dependence (13, 14), we sought to determine whether this scaffolding protein was involved in the regulation of eNOS uncoupling.
Fig. 1.
Effects of tetrahydrobiopterin (BH4) depletion and oxidation on basal endothelial nitric oxide (NO) synthase (eNOS)-derived NO and superoxide production. A: basal (unstimulated) endothelial NO production under normoglycemic (control), BH4-depleted (DAHP; 10 mM), and hyperglycemic (HG; 30 mM) conditions. B: total and eNOS-dependent [NG-nitro-l-arginine methyl ester (l-NAME) inhibitable] endothelial superoxide production under basal (unstimulated) conditions. Experimental groups consisted of normoglycemic and hyperglycemic conditions with and without BH4 depletion (DAHP). Results are means ± SD. *Significance at P < 0.05 as compared with the respective control.
Fig. 2.
Effects of BH4 depletion and oxidation on agonist stimulated eNOS-derived NO and superoxide production. A: calcium ionophore (1 μM; A23187) evoked endothelial NO production under normoglycemic (control), BH4-depleted (DAHP; 10 mM), and hyperglycemic (HG; 30 mM) conditions. B: calcium ionophore (1 μM; A23187) evoked total and eNOS-dependent endothelial superoxide production under normoglycemic and hyperglycemic conditions with and without BH4 depletion (DAHP). Results are means ± SD. *Significance at P < 0.05 as compared with the respective control.
Effects of BH4 depletion and oxidation on eNOS-Cav-1 association.
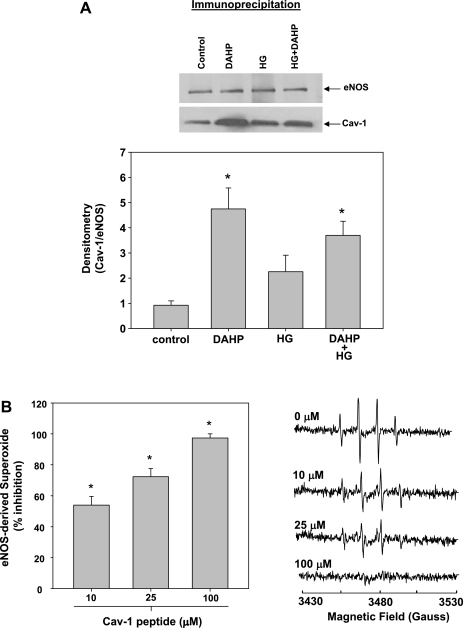
Initial cellular experiments were carried out to determine whether BH4 regulates eNOS-Cav-1 binding. Endothelial cells were exposed to conditions of both BH4 depletion (DAHP) or BH4 oxidation (hyperglycemia), and immunoprecipitation Western blot analysis was performed to assess eNOS-Cav-1 binding as described in materials and methods. Results demonstrated that following BH4 depletion, eNOS-Cav-1 association was increased by greater than fivefold (Fig. 3A). Hyperglycemia also increased eNOS-Cav-1 association, although to a lesser degree. These results suggest that Cav-1 may be sequestering BH4-depleted eNOS thus preventing its oxidase activity. To directly test this, purified recombinant BH4-free human eNOS was incubated in the presence of increasing concentrations of a Cav-1 peptide containing the eNOS binding domain. Results demonstrated that the Cav-1 peptide dose dependently inhibited eNOS oxidase activity with >50% inhibition observed at 10 μM Cav-1 (Fig. 3B). These results indicate that Cav-1 indeed inhibits eNOS oxidase activity.
Fig. 3.
Effects of BH4 depletion and oxidation on eNOS-caveolin-1 (Cav-1) association. A: immunoprecipitation Western blot of eNOS-Cav-1 association from normoglycemic (control), normoglycemic with BH4 depletion (DAHP), hyperglycemic (HG), and hyperglycemic with BH4 depletion (HG + DAHP) endothelial cells. Homogenates were immunoprecipitated with eNOS antibody and probed with Cav-1 antibody. B: dose-dependent effects of Cav-1 peptide (10 μM-100 μM) on eNOS-derived superoxide from human recombinant eNOS (2 μg) measured by EPR spin trapping. Results are means ± SD. *Significance at P < 0.05 as compared with the respective control.
Effects of Cav-1 on eNOS-derived superoxide.
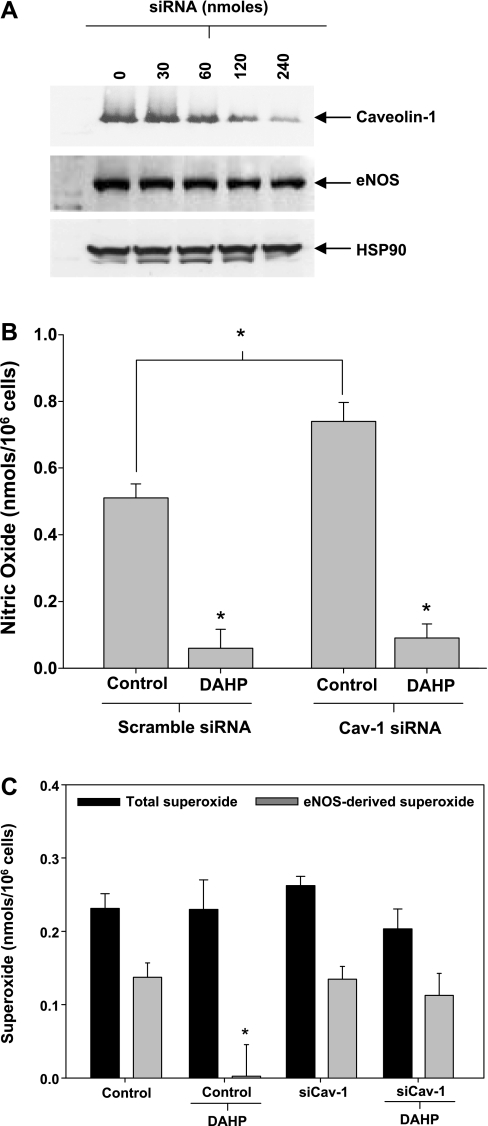
Subsequent in vitro studies were carried out to directly assess the cellular effects of Cav-1 on eNOS-derived superoxide production. Using an siRNA approach, we investigated the effects of Cav-1 silencing on eNOS uncoupling from hyperglycemic and hyperglycemic DAHP-treated endothelial cells. Initial studies were performed to optimize the siRNA titer. Results demonstrated that 240 nmol of Cav-1 siRNA were sufficient to reduce Cav-1 expression by >70% (Fig. 4A). This concentration was used for all subsequent siRNA experiments. Initial studies investigated the effects of Cav-1 silencing on endothelial NO production. Results demonstrated that siRNA-mediated silencing of Cav-1 increased endothelial NO production by 35%, consistent with previously published findings (Fig. 4B) (5). As expected BH4 depletion inhibited NO generation from the endothelial cells independent of Cav-1 expression status (Fig. 4B). BH4 depletion of hyperglycemic endothelial cells completely suppressed eNOS oxidase activity (Fig. 1B and Fig. 4C). However, with Cav-1 silencing, BH4 depletion did not inhibit eNOS oxidase activity from hyperglycemic cells, and these cells exhibited 85% of the eNOS oxidase activity observed in the hyperglycemic control group (Fig. 4C). In addition, these results demonstrate that Cav-1 silencing alone had no effect on eNOS-derived superoxide under BH4 oxidizing conditions (hyperglycemia). Together, these studies suggest that Cav-1 tonically suppresses eNOS oxidase activity in BH4-depleted cells by sequestering the fraction of eNOS that is devoid of all biopterins (biopterin-free eNOS).
Fig. 4.
Effects of Cav-1 on eNOS-derived superoxide. A: Western blot analysis of endothelial Cav-1, eNOS, and heat shock protein (HSP)90 expression following reverse transfection with small interfering RNA (siRNA) targeting bovine Cav-1. B: effects of Cav-1 silencing (siCav-1; 240 nmol siRNA) on NO production from hyperglycemic endothelial cells in the presence and absence of BH4 depletion (DAHP). Control groups consisted of transfection of hyperglycemic cells with scrambled siRNA (240 nmol). C: effects of Cav-1 silencing (240 nmol siRNA) on basal total and eNOS-derived superoxide production from hyperglycemic endothelial cells with and without BH4 depletion (DAHP). Control groups consisted of transfection of hyperglycemic cells with scrambled siRNA (240 nmol). Results are means ± SD. *Significance at P < 0.05 as compared with the respective control.
Effects of Cav-1 and BH4 status on eNOS phosphorylation and uncoupling.
Previous work has demonstrated that in addition to directly modulating eNOS activity, Cav-1 can also regulate eNOS phosphorylation (5, 13, 14). Given the known regulation of eNOS function by phosphorylation, we sought to investigate whether the observed regulation of eNOS oxidase activity by Cav-1 was regulated by changes in eNOS phosphorylation.
Western blot analysis of eNOS phosphorylation at Ser1177 was measured from control and Cav-1-silenced endothelial cells with and without BH4 depletion. Results demonstrated a detectable basal level of Ser1177 phosphorylation in the control group, which was largely suppressed with pharmacological inhibition of AKT. Silencing of Cav-1 increased Ser1177 phosphorylation in the non-DAHP-treated group (Fig. 5A). Pharmacological depletion of BH4 resulted in a marked increase in eNOS phosphorylation at Ser1177, which was inhibited by AKT inhibition. When the cells were depleted of BH4, Cav-1 silencing suppressed the phosphorylation of eNOS (Fig. 5A). These results suggest that both biopterin bioavailability and Cav-1 may modulate endothelial cell eNOS oxidase activity through phosphorylation events wherein a reduction in total BH4 increases eNOS phosphorylation at Ser1177.
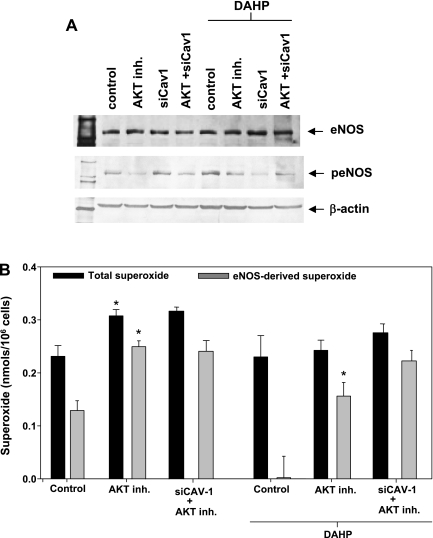
Fig. 5.
Effects of eNOS phosphorylation on eNOS-derived superoxide. A: Western blot analysis of total eNOS and phosphorylated (Ser1177) eNOS (peNOS) under conditions of hyperglycemia (control), AKT inhibition (AKT inh.), siCav-1, and dual AKT inhibition and Cav-1 silencing (AKT + siCav-1) with and without DAHP treatment. B: effects of AKT inhibition with 5-(2-benzothiazolyl)-3-ethyl-2-[2-methylphenylamino)ethynyl]-1-phenyl-1H-benzimidazolium iodide (20 μM) on basal total and eNOS-derived superoxide production from hyperglycemic endothelial cells with and without Cav-1 silencing (240 nM) and BH4 depletion (DAHP). Control groups consisted of hyperglycemic cells without kinase inhibitor treatment or Cav-1 silencing. Results are means ± SD. *Significance at P < 0.05 as compared with the respective control.
The effects of pharmacological inhibition of AKT on uncoupled eNOS activity were assessed under conditions of BH4 oxidation (hyperglycemia) and BH4 depletion (DAHP) in BAECs. AKT inhibition in cells with increased BH4 oxidation increased both total and eNOS-derived superoxide by 36% and 95%, respectively. Moreover, like Cav-1 silencing, AKT inhibition prevented the observed inhibitory effects of BH4 depletion on eNOS oxidase activity (Fig. 5B).
When Cav-1 silencing is combined with AKT inhibition, eNOS oxidase activity following BH4 depletion is upregulated, and the amount of eNOS-derived superoxide produced in the BH4-depleted setting is increased twofold as compared with the control (120 pmol superoxide/106 cells vs. 270 pmol superoxide/106 cells; Fig. 5B).
These results demonstrate that Cav-1 and AKT independently regulate eNOS uncoupling in the endothelium and serve to suppress eNOS oxidase activity under conditions of reduced biopterin-bioavailability.
DISCUSSION
Enzyme kinetic studies of purified eNOS have clearly and convincingly demonstrated that BH4 depletion results in eNOS uncoupling with rates of eNOS-derived superoxide production reaching values similar to that of NO production from coupled eNOS and implicate eNOS as a major potential source of superoxide under uncoupling conditions (10). However, results from cellular and in vivo studies indicate that BH4 depletion may not be the molecular trigger for eNOS uncoupling. Rather it is an increased level of the BH4 oxidation product BH2, rather than BH4 depletion alone, that is the molecular trigger for the induction of eNOS oxidase activity (9, 25).
As such, there are three states of eNOS in regard to the biopterin cofactor: BH4-eNOS (the functional NO synthase), BH2-eNOS, and biopterin-free eNOS, both of which are uncoupled-eNOS, which have oxidase activity. There are well-known mechanisms that serve to regulate the BH4-to-BH2 ratio in cells, and thus regulate potential eNOS oxidase activity by preventing the formation of BH2-eNOS [for review see: Clinical Science (2007) 113, (47–63)]. Although the level of biopterin-free eNOS could be regulated by the de novo synthesis of BH4, this is a multistep enzymatic process. Thus we hypothesized that the endothelium would possess regulatory mechanisms to prevent or limit eNOS oxidase activity under conditions of reduced total BH4 bioavailability. To investigate this potential phenomenon, we studied eNOS uncoupling under conditions of total BH4 depletion and BH4 oxidation. Depletion was achieved through pharmacological means using the GTP cyclohydrolase-1 inhibitor DAHP. To induce BH4 oxidation, we chose hyperglycemic conditions similar to those described by Crabtree et al. (9), which result in reduced BH4 and increased BH2. In fact, at 48 h postexposure to high glucose, BH4 and BH2 reach equimolar concentrations, a large shift when compared with normal biopterin levels wherein BH2 is undetectable.
Using these two approaches, we assessed the effects of both BH4 depletion and BH4 oxidation on basal eNOS-derived NO and superoxide production. Results demonstrated that DAHP reduced endothelial NO production by 80%, consistent with significant BH4 depletion. Hyperglycemia-induced BH4 oxidation effects were less pronounced with only 20% inhibition of NO observed. Based on these results we would predict more eNOS uncoupling with DAHP treatment, given the robust decrease in NO production. However, measurements of eNOS-derived endothelial superoxide production in the DAHP group (cells in which total biopterin levels are decreased) revealed no eNOS oxidase activity despite the loss of NO production. Moreover, although hyperglycemia, which induces the oxidation of BH4 to BH2 (but total biopterin remains the same), only reduced NO production by 20%, this group exhibited robust eNOS oxidase activity with levels of superoxide generation approaching 150 pmol/106 cells. These results clearly indicate that the endothelium possesses some regulatory pathway for preventing eNOS oxidase activity from biopterin-free NOS.
When these studies were repeated with calcium ionophore, the effect of BH4 depletion to decrease NOS oxidase activity was lost and resulted in significant eNOS-derived superoxide production. These results indicated that the mechanisms responsible for suppressing eNOS oxidase activity in the unstimulated biopterin-depleted endothelium were calcium dependent. Agonists that promote an elevation in intracellular calcium trigger a Ca-CaM-dependent dissociation of eNOS from Cav-1 that results in eNOS activation and NO production (15, 18). Based on these studies and our observations we hypothesized that Cav-1 is involved in inhibiting eNOS-derived superoxide from biopterin-free eNOS.
Cav−/− mice have hyperactive eNOS, as would be expected due to the removal of the negative regulatory Cav protein (21). Interestingly, these mice also have severe heart failure and lung fibrosis; treatment with BH4 is able to rescue these animals, resulting in substantially improved heart and lung function (27). These authors argue that the eNOS-mediated dysfunction may be caused by an imbalance between cellular BH4 levels and eNOS, leading to eNOS-dependent ROS generation. In contrast, eNOS overexpression, which according to the arguments of Bendall et al.(6) would also lead to a BH4 insufficiency and eNOS-dependent ROS, has been found to be protective (12). So, both Cav-1 knockout and eNOS overexpression both lead to increased NOS activity; however, the former is detrimental, whereas the latter is protective. Our hypothesis that Cav-1 is involved in the sequestration of uncoupled eNOS would fit these data; the lack of Cav-1 in the Cav−/− animals leads to an inability to sequester BH4-free eNOS and thus excess ROS production leading to disease. These conclusions are supported by a recent study from Zhao et al. (29) in which the authors demonstrate that knockout of both eNOS and Cav-1 suppresses the pulmonary vascular remodeling observed in the Cav-1 null mouse.
If, as we predict, Cav-1 is involved in sequestering BH4-free eNOS, then DAHP treatment would be expected to increase eNOS-Cav-1 association. To test this, we performed a series of immunoprecipitation studies to assess eNOS-Cav-1 interactions under both conditions of BH4 depletion and hyperglycemia. Our results indicated a robust fivefold increase in eNOS-Cav-1 association with DAHP treatment thus supporting our hypothesis that BH4 depletion leads to sequestration of BH4-free eNOS by Cav-1. Hyperglycemia also increased this association albeit to a lesser degree. To directly examine the ability of Cav-1 to inhibit eNOS oxidase activity, we measured the dose-dependent effects of a Cav-1 peptide on eNOS-derived superoxide. Results clearly demonstrated that Cav-1 inhibits superoxide generation from biopterin-free eNOS.
To further confirm our peptide studies, we undertook a series of experiments using siRNA-mediated silencing of Cav-1 in endothelial cells. All experiments were performed under conditions of either hyperglycemia or hyperglycemia with BH4 depletion. Under hyperglycemic conditions, Cav-1 silencing increased basal NO by 20%, as would be expected with removal of the negative regulatory effects, and no effect on eNOS-derived superoxide. In contrast, Cav-1 silencing in the DAHP-treated group led to a robust increase in eNOS-dependent oxidase activity thus unmasking the previously observed inhibitory effects of BH4 depletion. These results clearly implicate Cav-1 in the regulation of eNOS-derived superoxide and support its role in sequestering and preventing eNOS oxidase activity.
Because Cav-1 has been shown to regulate the AKT-driven phosphorylation of eNOS and this phosphorylation has been shown to regulate superoxide production from uncoupled eNOS, we sought to investigate this as a possible mechanism (8, 17). We found that Cav-1 silencing in hyperglycemic cells increased eNOS phosphorylation, consistent with the previous work showing that Cav-1 silencing increased AKT-driven eNOS phosphorylation, and that re-expression of Cav-1 reversed the observed AKT activation and eNOS phosphorylation (17). In contrast, Cav-1 silencing in cells depleted of biopterin leads to a decrease in eNOS phosphorylation. Thus the effect of Cav-1 on the AKT/eNOS axis is differentially regulated by the cellular biopterin status.
Chen et al. (7a) clearly demonstrated increased eNOS-derived superoxide production in response to AKT-driven phosphorylation of the purified recombinant BH4-free eNOS. As such, our observed increase in eNOS-derived superoxide from cells in response to AKT inhibition is not via a direct effect on eNOS activity. However, Chen et al. (7a) also demonstrated that AKT-dependent phosphorylation increased Cav-1-associated inhibition of superoxide generation from eNOS. These data support our hypothesis and indicate that the increased eNOS-derived superoxide we observed from cells exposed to AKT inhibition likely involves a dephosphorylation-driven reduction in Cav-1-eNOS association and thus a release of the inhibitory effects elicited by Cav-1. Thus, in addition to increasing eNOS-Cav-1 association, BH4 depletion may also result in increased eNOS phosphorylation with both events modulating eNOS oxidase activity. Subsequent studies were performed to investigate whether the Cav-1 and phosphorylation effects were synergistic. When Cav-1 silencing was combined with AKT inhibition, the loss of eNOS oxidase activity observed with BH4 depletion was fully unmasked. In fact, eNOS-derived superoxide production increased twofold as compared with the hyperglycemic controls. These results demonstrate that Cav-1 and AKT cooperate in the regulation of the activity of uncoupled eNOS in the endothelium and serve to suppress eNOS oxidase activity under conditions of reduced biopterin-bioavailability. However, the delineation of the specifics of this cooperative regulation will require additional work.
Current literature describes both increased and reduced Cav-1 expression in diabetes. Reports from Li et al. (16a) and Komers et al. (15a) independently describe decreased Cav-1 expression in the diabetic kidney, which is associated with nephropathy. In contrast, recent studies from Penumathsa et al. (19) demonstrated increased Cav-1/eNOS association in the diabetic rat heart. Based on our data, increased Cav-1 would be expected to elicit a decrease in eNOS-derived O2·−; alternatively, a reduction in Cav-1 would potentiate eNOS-derived O2·−. The pathological implications are difficult to predict a priori since Cav-1 effects on NO must also be considered. Overall, these studies demonstrate a novel role for Cav-1 in the regulation of the activity of uncoupled eNOS and implicate increased Cav-1 expression as a potential compensatory mechanism to prevent oxidative injury of the endothelium in response to reduced BH4 bioavailability.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-081734 (to A. J. Cardounel) and HL-63744 (to J. L. Zweier).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Alp NJ, McAteer MA, Khoo J, Choudhury RP, Channon KM. Increased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTP-cyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in ApoE-knockout mice. Arterioscler Thromb Vasc Biol 24: 445–450, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Alp NJ, Mussa S, Khoo J, Cai S, Guzik T, Jefferson A, Goh N, Rockett KA, Channon KM. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I overexpression. J Clin Invest 112: 725–735, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arcaro G, Cretti A, Balzano S, Lechi A, Muggeo M, Bonora E, Bonadonna RC. Insulin causes endothelial dysfunction in humans: sites and mechanisms. Circulation 105: 576–582, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Arteaga RB, Chirinos JA, Soriano AO, Jy W, Horstman L, Jimenez JJ, Mendez A, Ferreira A, de Marchena E, Ahn YS. Endothelial microparticles and platelet and leukocyte activation in patients with the metabolic syndrome. Am J Cardiol 98: 70–74, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Bauer PM, Yu J, Chen Y, Hickey R, Bernatchez PN, Looft-Wilson R, Huang Y, Giordano F, Stan RV, Sessa WC. Endothelial-specific expression of caveolin-1 impairs microvascular permeability and angiogenesis. Proc Natl Acad Sci USA 102: 204–209, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bendall JK, Alp NJ, Warrick N, Cai S, Adlam D, Rockett K, Yokoyama M, Kawashima S, Channon KM. Stoichiometric relationships between endothelial tetrahydrobiopterin, endothelial NO synthase (eNOS) activity, and eNOS coupling in vivo: insights from transgenic mice with endothelial-targeted GTP cyclohydrolase 1 and eNOS overexpression. Circ Res 97: 864–871, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Cardounel AJ, Xia Y, Zweier JL. Endogenous methylarginines modulate superoxide as well as nitric oxide generation from neuronal nitric-oxide synthase: differences in the effects of monomethyl- and dimethylarginines in the presence and absence of tetrahydrobiopterin. J Biol Chem 280: 7540–7549, 2005 [DOI] [PubMed] [Google Scholar]
- 7a. Chen CA, Druhan LJ, Varadharaj S, Chen YR, Zweier JL. Phosphorylation of endothelial nitric-oxide synthase regulates superoxide generation from the enzyme. J Biol Chem 283: 27038–27047, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cosentino F, Hurlimann D, Delli Gatti C, Chenevard R, Blau N, Alp NJ, Channon KM, Eto M, Lerch P, Enseleit F, Ruschitzka F, Volpe M, Luscher TF, Noll G. Chronic treatment with tetrahydrobiopterin reverses endothelial dysfunction and oxidative stress in hypercholesterolaemia. Heart 94: 487–492, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Crabtree MJ, Smith CL, Lam G, Goligorsky MS, Gross SS. Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO versus superoxide production by eNOS. Am J Physiol Heart Circ Physiol 294: H1530–H1540, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Druhan LJ, Forbes SP, Pope AJ, Chen CA, Zweier JL, Cardounel AJ. Regulation of eNOS-derived superoxide by endogenous methylarginines. Biochemistry 47: 7256–7263, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Dumitrescu C, Biondi R, Xia Y, Cardounel AJ, Druhan LJ, Ambrosio G, Zweier JL. Myocardial ischemia results in tetrahydrobiopterin (BH4) oxidation with impaired endothelial function ameliorated by BH4. Proc Natl Acad Sci USA 104: 15081–15086, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elrod JW, Duranski MR, Langston W, Greer JJ, Tao L, Dugas TR, Kevil CG, Champion HC, Lefer DJ. eNOS gene therapy exacerbates hepatic ischemia-reperfusion injury in diabetes: a role for eNOS uncoupling. Circ Res 99: 78–85, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Garcia-Cardena G, Fan R, Stern DF, Liu J, Sessa WC. Endothelial nitric oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. J Biol Chem 271: 27237–27240, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Garcia-Cardena G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J Biol Chem 272: 25437–25440, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Gratton JP, Fontana J, O′Connor DS, Garcia-Cardena G, McCabe TJ, Sessa WC. Reconstitution of an endothelial nitric-oxide synthase (eNOS), hsp90, and caveolin-1 complex in vitro. Evidence that hsp90 facilitates calmodulin stimulated displacement of eNOS from caveolin-1. J Biol Chem 275: 22268–22272, 2000 [DOI] [PubMed] [Google Scholar]
- 15a. Komers R, Schutzer WE, Reed JF, Lindsley JN, Oyama TT, Buck DC, Mader SL, Anderson S. Altered endothelial nitric oxide synthase targeting and conformation and caveolin-1 expression in the diabetic kidney. Diabetes 55: 1651–1659, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a. Li Z, Rodríguez-Iturbe B, Ni Z, Shahkarami A, Sepassi L, Vaziri ND. Effect of hereditary obesity on renal expressions of NO synthase, caveolin-1, AKt, guanylate cyclase, and calmodulin. Kidney Int 68: 2766–2772, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Murata T, Lin MI, Huang Y, Yu J, Bauer PM, Giordano FJ, Sessa WC. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med 204: 2373–2382, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murata T, Lin MI, Stan RV, Bauer PM, Yu J, Sessa WC. Genetic evidence supporting caveolae microdomain regulation of calcium entry in endothelial cells. J Biol Chem 282: 16631–16643, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Penumathsa SV, Thirunavukkarasu M, Zhan L, Maulik G, Menon VP, Bagchi D, Maulik N. Resveratrol enhances GLUT-4 translocation to the caveolar lipid raft fractions through AMPK/Akt/eNOS signalling pathway in diabetic myocardium. J Cell Mol Med 12: 2350–2361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pope AJ, Karuppiah K, Kearns PN, Xia Y, Cardounel AJ. Role of dimethylarginine dimethylaminohydrolases in the regulation of endothelial nitric oxide production. J Biol Chem 284: 35338–35347, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem 276: 38121–38138, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Schafer A, Alp NJ, Cai S, Lygate CA, Neubauer S, Eigenthaler M, Bauersachs J, Channon KM. Reduced vascular NO bioavailability in diabetes increases platelet activation in vivo. Arterioscler Thromb Vasc Biol 24: 1720–1726, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Settergren M, Bohm F, Malmstrom RE, Channon KM, Pernow J. l-arginine and tetrahydrobiopterin protects against ischemia/reperfusion-induced endothelial dysfunction in patients with type 2 diabetes mellitus and coronary artery disease. Atherosclerosis 204: 73–78, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA 95: 9220–9225, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vasquez-Vivar J, Martasek P, Whitsett J, Joseph J, Kalyanaraman B. The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogues controls superoxide release from endothelial nitric oxide synthase: an EPR spin trapping study. Biochem J 362: 733–739, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wheatcroft SB, Williams IL, Shah AM, Kearney MT. Pathophysiological implications of insulin resistance on vascular endothelial function. Diabet Med 20: 255–268, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Wunderlich C, Schober K, Schmeisser A, Heerwagen C, Tausche AK, Steinbronn N, Brandt A, Kasper M, Schwencke C, Braun-Dullaeus RC, Strasser RH. The adverse cardiopulmonary phenotype of caveolin-1 deficient mice is mediated by a dysfunctional endothelium. J Mol Cell Cardiol 44: 938–947, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem 273: 25804–25808, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Zhao YY, Zhao YD, Mirza MK, Huang JH, Potula HH, Vogel SM, Brovkovych V, Yuan JX, Wharton J, Malik AB. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest 119: 2009–2018, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]