Abstract
There is an increased risk of cardiac events after exercise, which may, in part, be mediated by the sympathoexcitation that accompanies exercise. The duration and extent of this sympathoexcitation following moderate exercise is unknown, particularly in those with coronary artery disease (CAD). Twenty control subjects (mean age, 51 years) and 89 subjects with CAD (mean age, 58 years) underwent two 16-min bicycle exercise sessions followed by 30–45 min of recovery. Session 1 was performed under physiological conditions to peak workloads of 50–100 W. In session 2, parasympathetic blockade with atropine (0.04 mg/kg) was achieved at end exercise at the same workload as session 1. RR interval was continually recorded, and plasma catecholamines were measured at rest and selected times during exercise and recovery. Parasympathetic effect, measured as the difference in RR interval with and without atropine, did not differ between controls and CAD subjects in recovery. At 30 and 45 min of recovery, RR intervals were 12% and 9%, respectively, shorter than at rest. At 30 and 45 min of recovery, plasma norepinephrine levels were 15% and 12%, respectively, higher than at rest. A brief period of moderate exercise is associated with a prolonged period of sympathoexcitation extending >45 min into recovery and is quantitatively similar among control subjects and subjects with CAD, with or without left ventricular dysfunction. Parasympathetic reactivation occurs early after exercise and is also surprisingly quantitatively similar in controls and subjects with CAD. The role of these autonomic changes in precipitating cardiac events requires further evaluation.
Keywords: sympathetic activity, catecholamines
regular physical activity is known to be associated with lower rates of sudden cardiac death and overall mortality in individuals without established coronary artery disease (CAD) (25). Based on meta-analyses, which have extolled the benefit of exercise in patients with CAD, it is now routinely prescribed for secondary prevention (26). Although exercise is thought to have an overall beneficial effect, vigorous exercise has been demonstrated as a potential trigger for sudden cardiac death (1, 4), sustained ventricular tachyarrhythmias (12, 24), and myocardial infarction (30). Although the exact mechanism by which this occurs is not completely understood, the autonomic nervous system is thought to play an important role.
The increase in heart rate that accompanies exercise is due to a combination of parasympathetic withdrawal and sympathetic activation (2). The early, rapid decrease in heart rate after cessation of exercise is believed to be predominantly due to parasympathetic reactivation (15, 22), although withdrawal of sympathoexcitation has also been suggested to be the predominant factor (36). Several findings relate the autonomic milieu of exercise and the postexercise recovery period to outcomes. A blunted decrease in heart rate 1 min after cessation of exercise has been reported to be predictive of overall mortality (8, 9, 18, 39). Abnormal heart rate recovery after exercise has also been linked to an increase in sudden death (20). Diminished heart rate recovery in the postexercise recovery period may be due to blunted parasympathetic reactivation or persistent sympathetic activation. Circulating catecholamines from increased sympathetic activity are felt to be a possible trigger for rupture of vulnerable plaques, which may precipitate sudden cardiac death (4).
The Determinants of Myocardial Infarction Onset Study examined the relative risks of myocardial infarction, a common precipitant of sudden cardiac death, in 1-h increments for 5 h consecutively after a bout of heavy physical exertion (30). Although they determined that individuals were at greater susceptibility for only the 1 h immediately after exertion, it is unknown whether the risk persists for the entire first hour or only a portion of it. The Physicians′ Health Study (1) considered the 30 min after an episode of vigorous exercise to be a period of risk associated with exercise, but no rationale or data were provided for the choice of this time period. Thus the purpose of this study was to characterize sympathetic and parasympathetic nervous system contributions toward heart rate recovery after submaximal exercise in subjects with established CAD and to assess when heart rate recovers completely.
METHODS
Study population.
A control group of 20 subjects without cardiac disease were studied and a group of 89 subjects with CAD were studied. Control subjects older than 45 years were recruited to have a similar age profile to the patients with CAD. Control subjects had to have normal physical examinations, ECGs, hematocrits, and serum electrolytes. Subjects with cardiac complaints (chest pain, shortness of breath, palpitations, dyspnea, on exertion), a cardiac history, or those taking cardioactive medications were excluded. Subjects with other major systemic illnesses (i.e., asthma, diabetes mellitus) were also excluded. In addition, only subjects who indicated that they participated in regular aerobic exercise a minimum of 60 min per week were studied. Highly trained endurance athletes were excluded.
For the CAD group, 89 patients underwent the study protocol. CAD was defined as >50% stenosis of at least one coronary artery on angiography or a documented history of myocardial infarction. Subjects were excluded if they had an acute coronary syndrome, percutaneous coronary intervention or coronary artery bypass graft surgery within the past 3 mo, objective evidence of stress-induced myocardial ischemia without revascularization, ongoing arrhythmias (e.g., atrial fibrillation or frequent atrial or ventricular ectopy), pacemaker dependency, diabetes mellitus, or other conditions that could affect autonomic function. Subjects must have been on a stable medication regimen for the 3 mo preceding their participation in the study.
For proper dosing of atropine, all subjects must have weighed <90 kg to be included. All subjects provided written informed consent. The study was approved by the Northwestern University Institutional Review Board.
Study protocol.
The study protocol consisted of three separate visits. On the first visit, subjects performed a symptom limited cardiopulmonary exercise stress test using the Bruce protocol to determine maximal exercise capacity. Standard 12-lead ECGs and systolic and diastolic blood pressures, measured using a standard cuff sphygmomanometer, were obtained at rest, at the end of each stage of exercise, and during the recovery period. Breath-by-breath respiratory gas analysis for measurement of oxygen and carbon dioxide was obtained online at rest, throughout exercise, and during the recovery period, using a Medical Graphics Cardio-2 metabolic exercise cart (Minneapolis, MN). The subject's rating of perceived exertion (RPE) on the Borg Scale and respiratory exchange ratio were recorded at the end of each stage and at peak exercise. The peak exercise V̇o2 value was defined as the highest V̇o2 value achieved at end-exercise after reaching anaerobic threshold. The V̇o2 at anaerobic threshold values were determined as the point at which expired carbon dioxide increased in a nonlinear fashion relative to the rate of oxygen consumption by the V-slope method. The V̇o2 at anaerobic threshold value for each subject was visually noted by an independent cardiopulmonary exercise-testing specialist. The RPE, peak exercise V̇o2, and exercise duration were recorded.
On the second and third visits, subjects underwent a submaximal exercise protocol while seated on a bicycle ergometer (SciFit Pro II; Tulsa, OK). Indwelling intravenous catheters were placed in each arm for drug infusions and blood draws, as needed. The 12-lead ECG and blood pressure were recorded at rest after assuming a seated resting position for at least 5 min. Continuous 12-lead ECG monitoring was performed for a 5-min rest period, a 16-min exercise period, and a 30–45 min recovery period. Subjects were instructed to exercise, maintaining pedal speed at 80 revolutions per minute. The exercise protocol began with a workload of 50 watts, followed by increases to 75 watts at 4 min and 100 watts at 8 min, as tolerated by the subject. Blood samples for determination of plasma epinephrine and norepinephrine levels were taken during the rest period, at minutes 8 and 15 of exercise, and during the recovery period. The third visit was identical to the second visit except that intravenous atropine (0.04 mg/kg) was administered in four divided doses (0.01 mg/kg every 30 s) beginning at minute 12 of exercise to achieve parasympathetic blockade (19).
After completion of 25 subjects, a persistent elevation in the heart rate was observed at the end of the 30-min postexercise recovery period. In addition, elevation in the plasma norepinephrine level at minute 10 of the postexercise recovery period was also present. Therefore, the monitored time in recovery was extended to 45 min and additional catecholamine samples were taken at minutes 20 and 30 in the postexercise recovery period. After completion of an additional 31 subjects with CAD, persistent elevation in the plasma norepinephrine level 30 min into the postexercise recovery period prompted the addition of plasma epinephrine and norepinephrine samples at 45 min postexercise. All 20 control subjects had a 45-min recovery period and a blood catecholamine sample 45 min postexercise.
Data analysis.
Continuous 12-lead ECG recording was performed using a commercially available system (Quest Exercise Stress System; Burdick, Deerfield, WI). ECG data were analyzed with custom software using the MATLAB program (Mathworks, Natick, MA). QRS detection was performed using a template matching algorithm. First, median templates of the QRS complexes were generated from a 10-s segment for each of the ECG leads using a slope based detection algorithm with the point of maximum negative slope chosen as the fiducial point. The cross-correlations of the templates with their respective signals were then summed across all leads, and the QRS complexes were detected by finding the peaks of the resulting signal that exceeded a third of the maximum value. After manually identifying premature atrial and ventricular beats, the RR interval preceding the premature beat and the two RR intervals following the premature beat were excluded from further analysis.
The %RR interval recovery was calculated at 30 and 45 min of recovery using the RR interval at the end of the respective recovery period (RRe), the RR interval at peak exercise (RRp), and the RR interval at rest (RRr) as reference points: %RRRecovery = (RRe − RRp)/(RRr − RRp).
The parasympathetic effect on the RR interval during recovery was quantified by the difference between the RR interval during the baseline test and the RR interval noted after parasympathetic blockade with atropine (22): ΔRR = RRbaseline − RRatropine.
Heart rate recovery at 1 min (HRR) was defined as: HRR = heart rate at peak exercise − heart rate at 1 min of recovery.
Heart rate variability (HRV) was calculated from the resting (seated) 5-min ECG data. Time domain measures calculated were the standard deviation of all normal RR intervals and the root mean square of successive RR interval differences. Frequency-domain measures of HRV were calculated for the recordings obtained during the 5-min resting period. First, the RR intervals were resampled at 4 Hz and then linearly detrended. After a Hanning window was applied, the power spectrum was calculated using the fast Fourier transform. Low frequency (LF) power was measured in the 0.04–0.15-Hz band. High frequency (HF) power was measured in the 0.15- to 0.4-Hz band. The natural logarithm of LF and HF were used for analysis.
Statistical analysis.
For purpose of analysis, subjects with CAD were divided into two groups: those with preserved left ventricular ejection fraction (LVEF ≥ 50%; n = 70) and those with depressed LVEF (LVEF < 50%; n = 17); one subject was excluded from analysis in the preserved LVEF group due to frequent atrial ectopy and one was excluded from the depressed LVEF group since only baseline data were obtained.
Baseline demographics among the controls and the two CAD groups were compared with ANOVA or χ2-tests, as appropriate. Wilcoxon nonparametric test was used for non-normally distributed continuous variables, and Fisher's Exact test was used for categorical outcomes with low cell counts. RR interval and catecholamine data were modeled using mixed effects repeated-measures methodology. Nineteen of the 61 RR measurements that were obtained during the study were selected for modeling: rest; exercise minutes 1, 4, 8, and 12-16; as well as recovery minutes 1-5, 10, 20, 30, and 45. To better approximate a normal distribution, catecholamine data were transformed using the natural logarithm. Results of the mixed effects models are reported as means ± SE. Conventional multiple least-squares regression was used to model %RR recovery and HRR. Missing data were imputed with CAD group means for multiple regression analyses. We evaluated the subject characteristics as predictors of HRR and %RR recovery at 30 min to assess whether the predictors for HRR and %RR recovery at 30 min were similar. Variables were included in the multivariable model only if their univariate associations yielded P values less than 0.20.
P values less than 0.05 were considered statistically significant.
RESULTS
Baseline characteristics.
The baseline characteristics of all subjects are shown in Table 1. The control subjects were slightly younger than the two CAD groups (P = 0.02). On cardiopulmonary stress testing, control subjects were able to exercise to a higher peak heart rate (P < 0.001), achieving a higher peak V̇o2 (P < 0.001). The time domain measures of HRV and the HF power were significantly lower in the CAD group with depressed LVEF compared with controls and the CAD group with preserved LVEF. The LF power differed significantly among all three groups, with the controls exhibiting the highest value and the CAD group with depressed LVEF exhibiting the lowest. The LF-to-HF ratio did not differ among the groups.
Table 1.
Baseline characteristics of study subjects
| CAD |
||||
|---|---|---|---|---|
| Controls | Preserved LVEF | Depressed LVEF | P Value | |
| n | 20 | 70 | 17 | |
| Age, years | 50.7 ± 1.4 | 58.4 ± 1.4 | 57.1 ± 2.8 | 0.02a |
| Males, n (%) | 8 (40) | 50 (71.4) | 13 (76.5) | 0.03b |
| LVEF, % median (IQR) | 60.0 (55–62.5) | 35.0 (32.5–42.5) | <0.001 | |
| BMI, kg/m2 | 26.5 ± 0.7 | 27.6 ± 0.4 | 28.1 ± 0.7 | 0.30 |
| Systolic blood pressure, mmHg | 119.9 ± 2.5 | 116.7 ± 1.3 | 113.4 ± 3.2 | 0.22 |
| Medical history, n (%) | ||||
| Hypertension | 0 (0) | 36 (52) | 7 (41) | <0.001b |
| Hyperlipidemia | 2 (10) | 51 (74) | 13 (76) | <0.001b |
| Prior myocardial infarction | 0 (0) | 34 (49) | 15 (88) | <0.001d |
| Percutaneous coronary intervention | 0 (0) | 37 (53) | 16 (94) | <0.001d |
| Coronary artery bypass graft | 0 (0) | 25 (36) | 4 (24) | 0.002b |
| Current smoker | 3 (15) | 8 (11) | 4 (24) | 0.35 |
| Medications, n (%) | ||||
| β-Blocker | 0 (0) | 56 (80) | 17 (100) | <0.001b |
| Angiotensin-converting enzyme inhibitors or ANG II receptor blockers | 0 (0) | 37 (53) | 12 (71) | <0.001b |
| Ca-channel blocker | 0 (0) | 12 (17) | 0 (0) | 0.03e |
| Statin | 1 (5) | 68 (97) | 14 (82) | <0.001d |
| Aspirin | 0 (0) | 67 (96) | 15 (88) | <0.001b |
| Cardiopulmonary stress test | ||||
| Exercise time, min | 11.8 ± 0.7 | 10.5 ± 0.4 | 10.3 ± 0.7 | 0.19 |
| HR, peak exercise, beats/min | 172.9 ± 2.4 | 146.0 ± 2.4 | 144.8 ± 3.4 | <0.001b |
| Max V̇o2, ml·kg−1·min−1 | 34.1 ± 1.7 | 27.0 ± 0.9 | 24.0 ± 1.4 | <0.001b |
| AT, ml·kg−1·min−1, median (IQR) | 18.9 (16.5–21.1) | 16.5 (14.9–19.3) | 15.6 (13.5–17.2) | 0.03b |
| RPE, median (IQR) | 6.5 (5.5–8.0) | 6.0 (5.0–8.0) | 8.0 (6.0–8.0) | 0.18 |
| Rest heart rate variability, median (IQR) | ||||
| LF, ms2 | 122.9 (88.1–226.2) | 70.1 (34.3–137.6) | 43.3 (30.1–57.8) | <0.001d |
| HF, ms2 | 10.4 (7.5–19.9) | 11.1 (3.9–27.4) | 4.7 (3.4–10.0) | 0.022c |
| LF/HF | 10.5 (4.5–22.7) | 7.4 (3.9–10.8) | 7.9 (4.2–12.7) | 0.31 |
| Root mean square of successive RR interval differences, ms | 23.5 (19.0–33.0) | 26.0 (14.0–36.0) | 16.0 (13.0–20.0) | 0.021c |
| SD of normal RR intervals, ms | 41.5 (35.0–47.5) | 41.5 (33.0–56.0) | 32.0 (24.0–38.0) | 0.002c |
Values are means ± SE unless stated otherwise. CAD, coronary artery disease; LVEF, left ventricular ejection fraction; IQR, interquartile range, 25th to 75th percentiles or the middle 50% of the data used for nonnormally distributed variables along with Kruskal-Wallis nonparametric test; BMI, body mass index; HR, heart rate; max V̇o2, peak oxygen uptake; AT, anaerobic threshold; RPE, rating of perceived exertion; LF, low frequency power; HF, high frequency power.
Controls vs. CAD-depressed LVEF, P < 0.05;
controls vs. CAD-depressed LVEF and controls vs. CAD-preserved LVEF, P < 0.05;
controls vs. CAD-depressed LVEF and CAD-depressed LVEF vs. CAD-preserved LVEF, P < 0.05;
all three pair-wise comparisons, P < 0.05;
controls vs. CAD-preserved LVEF, P < 0.05.
The vast majority of CAD subjects were on medical therapy including β-blockers, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, aspirin, and statins. Overall fitness levels, as noted by the peak V̇o2, were excellent in all three groups. During the 16-min study exercise protocol, 80% of controls attained 100 W, whereas 59% of the CAD subjects with preserved LVEF and 56% of the CAD subjects with depressed LVEF attained 100 W (P = 0.19).
RR intervals during exercise and the postexercise recovery period.
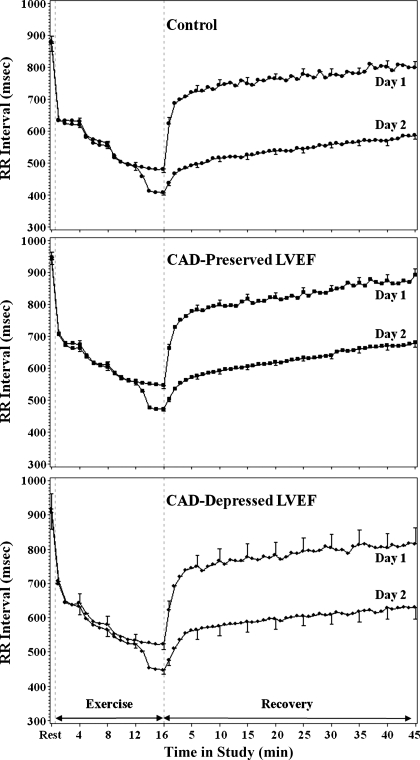
Figure 1 shows the change in RR intervals from rest to exercise and then in recovery for the three groups of subjects. All groups show the typical shortening of the RR interval with exercise, followed by rapid lengthening in early recovery, and more gradual lengthening in later recovery. The RR intervals at rest were slightly longer for CAD subjects (946.3 ± 16.3 ms and 917.0 ± 32.7 ms for preserved and depressed LVEF, respectively) than for controls (879.7 ± 30.1 ms; P = 0.15). The peak exercise RR interval was shorter in the control subjects (481.5 ± 15.2 ms) when compared with CAD subjects with preserved LVEF (545.7 ± 8.2; P < 0.001) and CAD subjects with depressed LVEF (523.4 ± 16.5; P = 0.065). CAD groups with preserved and depressed LVEF did not differ between them (P = 0.23). The 1-min HRR was 27.6 ± 1.4 beats/min in the controls (P < 0.001 vs. both CAD groups), 19.0 ± 0.8 beats/min in the CAD subjects with preserved LVEF, and 17.5 ± 1.5 beats/min in the CAD subjects with depressed LVEF. CAD subjects with preserved and depressed LVEF did not differ (P = 0.36).
Fig. 1.
Mean (± SE; shown only for selected time periods) RR interval shown for each study group at rest, during exercise, and during recovery. Day 1 data represent baseline (no blockade), and during day 2 testing parasympathetic blockade with atropine was administered beginning at 12 min of exercise. The RR intervals after atropine were significantly shorter in all study groups (P < 0.001). CAD, coronary artery disease; LVEF, left ventricular ejection fraction.
At 30 and 45 min of recovery, the RR interval remained shorter than the resting value (P < 0.001) with no significant difference among the groups in the relative change. The RR interval at 30 min was still significantly shorter than the rest RR interval in controls (776.2 ± 28.0 ms; P < 0.001), CAD subjects with preserved LVEF (843.6 ± 15.2 ms; P < 0.001), and CAD subjects with depressed LVEF (804.0 ± 30.3 ms; P < 0.001). The RR interval at 45 min was still significantly shorter than the rest RR interval in controls (799.4 ± 29.2 ms; P < 0.001), CAD subjects with preserved LVEF (891.3 ± 19.7 ms; P < 0.001), and CAD subjects with depressed LVEF (815.8 ± 32.7 ms; P < 0.001).
At 30 min, the RR interval was 11.5% shorter than at rest (11.8% in controls; 11.9% in CAD with depressed LVEF; 10.9% in CAD with preserved LVEF); at 45 min, the RR interval was 8.8% shorter than at rest (9.1% in controls; 9.5% in CAD with depressed LVEF; 7.9% in CAD with preserved LVEF). Relative to the peak exercise RR interval, the %RR recovery at 30 min was 75.1 ± 3.4% for controls, 75.4 ± 1.8% in the CAD subjects with preserved LVEF, and 71.0 ± 3.7% in the CAD subjects with depressed LVEF. The %RR recovery at 45 min was 80.4 ± 3.6% for controls, 84.1 ± 2.4% in the CAD subjects with preserved LVEF, and 74.6 ± 4.0% in the CAD subjects with depressed LVEF. There were no differences among the three groups in %RR recovery.
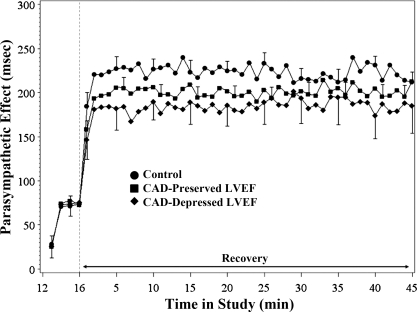
Parasympathetic effect.
Figure 2 demonstrates the parasympathetic effect in all three groups. Toward the end of exercise, after atropine was administered, parasympathetic effect on the RR interval was evident in all three groups at minute 13 of exercise (P = 0.06) and is clearly observable for the rest of the study (P < 0.001). The magnitude of the parasympathetic effect is comparable among the three groups. In early recovery, there is a rapid increase in parasympathetic effect in all three groups. By 2 min into recovery, the parasympathetic effect has reached its plateau level and remains relatively stable for the remaining duration of the recovery period. The mixed effects model estimates of parasympathetic effect at 45 min in recovery were 212.0 ± 20.7 ms in the controls, 206.7 ± 12.1 ms in the CAD group with preserved LVEF, and 189.9 ± 22.7 ms in the CAD group with depressed LVEF.
Fig. 2.
Mean (± SE; shown only for selected time periods) parasympathetic effect for each study group during late exercise and recovery. See Parasympathetic effect for details of significant changes.
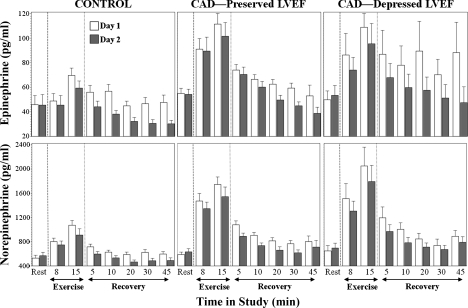
Plasma catecholamine levels.
Figure 3 demonstrates the results of the mixed effects model analysis for the plasma catecholamines. Resting plasma epinephrine levelswere 43.8 pg/ml (95% confidence interval 39.8–48.1) and norepinephrine levels were 559.9 pg/ml (521.6–601.1). There were no significant differences among the three groups.
Fig. 3.
Mean (± SE) plasma epinephrine and norepinephrine levels for each study group at rest and during selected times during exercise and recovery. Day 1 data represent baseline (no blockade). During day 2 testing, parasympathetic blockade with atropine was administered beginning at 12 min of exercise. See Plasma catecholamine levels for details of significant changes in plasma epinephrine and norepinephrine levels.
At 8 and 15 min of exercise, plasma epinephrine and norepinephrine increased relative to rest (P < 0.001) in the CAD group with preserved LVEF and in the CAD group with depressed LVEF. In the controls, all but the epinephrine level at 8 min were also significantly increased (P < 0.001).
During early recovery, there was a rapid decrease in plasma epinephrine and norepinephrine levels followed by a slower decline over the 45-min course of recovery. Throughout exercise and recovery, plasma catecholamine levels were lower in controls than in subjects with CAD (P < 0.03); there were no differences in plasma catecholamine levels between the CAD subjects with preserved versus depressed LVEF (P > 0.51).
At 30 min of recovery, epinephrine levels remained above their resting values for CAD subjects with depressed LVEF (P < 0.04) and subjects with preserved LVEF (P = 0.07) but were not different for healthy controls (P = 0.7). At 45 min in recovery, CAD subjects with depressed LVEF showed a strong trend toward higher epinephrine levels compared with baseline (P = 0.05), whereas healthy controls and CAD patients with preserved LVEF did not differ from baseline (P > 0.6). Norepinephrine levels remained above the resting levels for all three groups at both 30 and 45 min of recovery (P < 0.04). At 30 min of recovery, the norepinephrine levels were 15% higher than at rest; at 45 min, the norepinephrine levels were 12% higher than at rest.
After the administration of atropine, plasma epinephrine and norepinephrine levels declined relative to those obtained without atropine (Fig. 3). Even at peak exercise, plasma norepinephrine was reduced after parasympathetic blockade (P = 0.03), but the difference was not significant for epinephrine. During recovery after parasympathetic blockade with atropine, plasma epinephrine and norepinephrine levels generally declined to resting or below resting levels by 10 min into recovery. Norepinephrine levels for CAD patients with preserved LVEF remained above resting levels at 10 min of recovery (P = 0.02).
Determinants of late (30 min) RR interval recovery and 1-min HRR.
To evaluate predictors of late RR interval recovery, we considered a variety of clinical factors (age, sex, body mass index, CAD, LVEF, HRV), results from the cardiopulmonary stress test (exercise time, peak heart rate, peak V̇o2, anaerobic threshold, RPE), and a variety of parameters from the study stress test (resting RR, RR at peak exercise, change in norepinephrine level at peak exercise to 10 min of recovery, mean parasympathetic effect during recovery, 1-min HRR). Since parasympathetic effect plateaued after 2 min, we calculated the mean parasympathetic effect for each subject between minutes 5 and 10 for this analysis. Sympathetic effects in recovery were estimated by the change in plasma norepinephrine levels from peak exercise to the 10th min of recovery.
Univariate predictors of %RR recovery at 30 min are shown in Table 2. In the multivariable model for %RR recovery at 30 min, peak heart rate on the cardiolpulmonary stress test (P = 0.006), maximum V̇o2 (P = 0.04), RR interval at peak submaximal bicycle exercise (P = 0.002), and HRR during the first minute after exercise (P = 0.05) were independently associated with %RR recovery at 30 min. All of these variables showed positive association with the outcome except for maximum V̇o2.
Table 2.
Univariate and multivariable predictors of %RRRecovery at minute 30 of recovery
| Univariate Model |
Multivariable Model |
|||||
|---|---|---|---|---|---|---|
| Variable | β | SE | P Value | β | SE | P Value |
| Clinical variables | ||||||
| Age, years | −0.002 | 0.001 | 0.25 | |||
| Male | −0.021 | 0.031 | 0.49 | |||
| BMI, kg/m2 | −0.005 | 0.005 | 0.31 | |||
| CAD | −0.006 | 0.037 | 0.87 | |||
| Control vs. CAD-depressed | 0.041 | 0.050 | 0.41 | |||
| CAD-preserved vs. control | 0.003 | 0.038 | 0.95 | |||
| CAD: preserved vs. depressed | 0.044 | 0.041 | 0.29 | |||
| LVEF2, % | −5.8E-7 | 1.5E-5 | 0.97 | |||
| Cardiopulmonary stress test | ||||||
| Exercise time, min | 0.002 | 0.005 | 0.62 | |||
| HR, peak exercise, beats/min | 0.001 | 0.001 | 0.11 | 0.003 | 0.001 | 0.006 |
| Max V̇o2, ml·kg−1·min−1 | 0.002 | 0.002 | 0.20 | −0.005 | 0.003 | 0.04 |
| ln(AT), ml·kg−1·min−1 | 0.042 | 0.066 | 0.53 | |||
| ln(RPE) | 0.015 | 0.051 | 0.77 | |||
| Protocol bicycle exercise studies | ||||||
| RR, rest, ms | −5.8E-5 | 1.1E-4 | 0.59 | |||
| RR, peak exercise, ms | 4.2E-4 | 2.0E-4 | 0.04 | 9.82E-4 | 3.07E-4 | 0.002 |
| ΔNE, pg/ml | −0.036 | 0.014 | 0.01 | −0.003 | 0.016 | 0.86 |
| PSE, 5–10 min, ms | 0.001 | 1.7E-4 | 0.002 | 1.22E-4 | 2.20E-4 | 0.58 |
| Heart rate recovery from peak exercise (16 min) and recovery minute 1, beats/min | 0.004 | 0.002 | 0.03 | 0.005 | 0.002 | 0.05 |
| Rest heart rate variability | ||||||
| ln(LF), ms2 | 0.002 | 0.014 | 0.87 | |||
PSE, 5–10 min, average parasympathetic effect during minutes 5–10 of recovery; ln, natural logarithm; ΔNE, change in plasma norepinephrine level from exercise to 10 min of recovery.
Univariate predictors of HRR during the first minute after exercise are shown in Table 3. In the multivariable model for HRR, RR interval during peak bicycle exercise and parasympathetic effect at 1 min in recovery emerged as the strongest independent predictors of HRR (P < 0.001). Multivariable relationship was negative with RR at peak exercise and positive with parasympathetic effect at 1 min.
Table 3.
Univariate and multivariable predictors of HR recovery at 1 min in recovery
| Univariate Model |
Multivariable Model |
|||||
|---|---|---|---|---|---|---|
| Variable | β | SE | P Value | SE | β | P Value |
| Clinical variables | ||||||
| Age, years | −0.20 | 0.061 | 0.002 | 0.054 | 0.067 | 0.42 |
| Male | −3.15 | 1.46 | 0.03 | −1.364 | 1.340 | 0.31 |
| BMI, kg/m2 | −0.46 | 0.22 | 0.04 | −0.062 | 0.168 | 0.71 |
| CAD vs. control | 8.90 | 1.58 | <0.001 | 1.980 | 1.674 | 0.24 |
| Control vs. CAD-depressed | 10.16 | 2.10 | <0.001 | |||
| CAD-preserved vs. control | −8.58 | 1.62 | <0.001 | |||
| CAD: preserved vs. depressed | 1.59 | 1.73 | 0.36 | |||
| LVEF2, % | 3.3E-4 | 7.1E-4 | 0.64 | |||
| Cardiopulmonary stress test | ||||||
| Exercise time, min | 0.72 | 0.23 | 0.002 | −0.174 | 0.270 | 0.52 |
| Peak HR, beats/min | 0.16 | 0.03 | <0.001 | −0.029 | 0.049 | 0.55 |
| Max V̇o2, ml·kg−1·min−1 | 0.41 | 0.08 | <0.001 | 0.275 | 0.162 | 0.09 |
| ln(AT), ml·kg−1·min−1 | 8.43 | 3.12 | 0.008 | −3.829 | 3.114 | 0.22 |
| ln(maximum RPE) | 0.85 | 2.45 | 0.73 | |||
| Protocol bicycle exercise studies | ||||||
| RR, rest, ms | 3.9E-4 | 5.2E-3 | 0.94 | |||
| RR, peak exercise, min | −0.03 | 0.010 | 0.004 | −0.066 | 0.013 | <0.001 |
| ln(NE), peak exercise, pg/ml | −3.65 | 1.24 | 0.004 | 0.180 | 1.319 | 0.89 |
| PSE, 1 min, ms | 0.039 | 0.008 | <0.001 | 0.066 | 0.010 | <0.001 |
| Rest heart rate variability | ||||||
| ln(LF) (ms2) | 1.53 | 0.64 | 0.02 | 0.241 | 0.536 | 0.65 |
Other findings.
A subgroup analysis was performed to evaluate the effect of β-blocker treatment on both parasympathetic effect and plasma catecholamines. This was performed only in the CAD group with preserved LVEF since there were subjects who were taking and not taking β-blockers. Although β-blocker therapy was associated with a longer resting RR interval (966.7 ± 16.8 ms vs. 851.5 ± 33.5 ms; P = 0.003), there was no significant effect on parasympathetic effect in recovery (P = 0.08). There was no significant difference in the plasma catecholamine response to exercise and recovery.
DISCUSSION
Our findings demonstrate that a brief 16-min period of moderate exercise is associated with a prolonged period of sympathoexcitation in the postexercise recovery period extending more than 45 min. Surprisingly, this physiology is apparent and quantitatively similar among controls, subjects with CAD and preserved LVEF, and subjects with CAD and depressed LVEF. Parasympathetic reactivation occurs early after exercise and is also surprisingly quantitatively similar in controls and subjects with CAD. Contrary to expectations, even subjects with CAD and left ventricular dysfunction had similar parasympathetic effects during recovery compared with controls and CAD subjects with normal left ventricular function, despite having lower resting HRV. In addition, these subjects had similar sympathoexcitation in late recovery to those with CAD and normal left ventricular function. Clinical factors and markers of fitness do not predict the degree of sympathoexcitation in the late postexercise recovery period. These findings have important implications regarding the potential role of exercise and the postexercise recovery period in precipitating cardiac events.
Autonomic changes that occur with exercise and in the postexercise recovery period are well described. Exercise is associated with parasympathetic withdrawal and sympathoexcitation. Interestingly, it has been unclear how complete the parasympathetic withdrawal that occurs during exercise is. In a prior study of normal subjects performing maximal symptom limited exercise tests, it was found that even during peak exercise there was residual parasympathetic effect (22). In the present study, similar parasympathetic effect was noted after submaximal exercise in all three groups, even among CAD subjects with left ventricular dysfunction.
In the postexercise recovery period, there is typically parasympathetic reactivation and sympathetic withdrawal. Although the relative timing of these changes has been debated (15, 22, 36), it has become clear that parasympathetic reactivation occurs predominantly early in recovery. The present study demonstrates that beyond the first few minutes of recovery, parasympathetic reactivation is essentially complete and that the degree of parasympathetic reactivation is similar in all three groups. Surprisingly, the group of CAD subjects with left ventricular dysfunction that were studied did not have depressed parasympathetic effect in recovery. Although these subjects did not have active congestive heart failure, they did have lower resting HRV. Specifically, the time domain parameters and the HF power, considered to be a marker of parasympathetic effect, were significantly lower in this group compared with the other two groups. The normal (same as controls) parasympathetic effect observed during exercise and recovery in CAD subjects, whether or not left ventricular dysfunction was present, could reflect a selection bias of well-treated individuals who are clinically stable or may generally characterize parasympathetic effects in these groups. If the former is correct, this highlights the potential benefits of good medical therapy. Prior studies from our group evaluating the parasympathetic effects on a variety of cardiac electrophysiological parameters using noninvasive programmed stimulation in patients with implanted devices during exercise and recovery have also noted no major differences between those with preserved LVEF (21) and those with depressed LVEF (7). Further studies will be needed to place this novel finding into perspective in the larger context of patients with CAD.
With regard to the sympathoexcitation associated with exercise and recovery, there was an increase in catecholamine levels in the subjects with CAD. However, these subjects were mostly being treated with β-blockers; therefore, direct comparisons between the CAD subjects and controls cannot be made. Nevertheless, the time-dependent changes with exercise and recovery are revealing. Persistent elevation in the plasma catecholamines was noted at 45 min compared with rest, accompanied by a persistent elevation in the heart rate. Although other studies have demonstrated persistent (up to 60 min) elevation of the heart rate after more prolonged and heavy exercise (11, 17, 37), this is the first study, to our knowledge, to demonstrate this finding in the clinically important CAD population with a relatively short duration of exercise of moderate intensity and persisting for 45 min. Persistent elevation of norepinephrine levels beyond 10 min after exercise has also been reported (40), although multiple small studies using a variety of different exercise protocols have yielded variable results (3, 13, 14, 16, 33, 35).
The precise duration of sympathoexcitation following exercise has not been well characterized. Several studies (11, 17) that have monitored the heart rate after exercise to 30 min of recovery have revealed that the heart rate does not recover to baseline values within this time frame, although these studies have incorporated more intense exercise. Interestingly, persistent elevation in the heart rate at 30 min is even noted in well-trained marathon runners. In the present study, at 30 min of recovery, the RR interval was still 11% to 12% shorter than at baseline and at 45 min was 8% to 9% shorter than at baseline. The persistent elevation of plasma norepinephrine levels at this time confirms the persistent sympathoexcitation for at least 45 min after brief, moderate exertion. The identification of the postexercise recovery period as a time for increased risk of sudden cardiac death (1) and myocardial infarction (30) highlights the importance of this finding. However, the pathophysiological consequences of this persistent sympathoexcitation need to be further explored.
The role of adrenergic activation in arrhythmogenesis is well established (10, 28, 29). Circadian changes coincide with a morning increase in sudden cardiac death (31, 41), which is blunted by propranolol (34). This is further supported by the effects of β-blockade to blunt the diurnal variation in ventricular refractoriness (23). Sympathetic nerve remodeling following myocardial infarction is postulated to be another mechanism, which increases the probability of ventricular tachycardia, ventricular fibrillation, and sudden cardiac death (5, 6). Adrenergic activation is known to occur in the face of mental or physical stress. In patients with exercise-induced ventricular tachycardia and normal coronary arteries, HRV analysis has suggested increased sympathetic tone as a possible precipitant (32). Anger and modest physical activity have been shown to be associated with shocks in patients with implantable cardioverter-defibrillators (24). In another study, physical stress provided the potential trigger for ventricular arrhythmias detected and treated by an implantable defibrillator in 26% of cases. Mental stress and sexual activity were potential triggers in 24% and 2%, respectively (12). Circulating catecholamines released during exertion are thought to be a potential trigger for rupture of vulnerable plaques with thin caps, thereby leading to sudden death (4).
The decline in plasma catecholamine levels after parasympathetic blockade is an interesting finding. Atropine is a muscarinic receptor blocker. Because muscarinic blockade has been shown to increase norepinephrine release in the setting of nicotinic agonists (27), it is unlikely that atropine, a muscarinic blocker, exerts a direct effect to decrease plasma catecholamine levels. Thus the observed reduction in plasma catecholamines is likely related to reflex inhibition due to a change in a physiological parameter. The changes are most likely mediated by the carotid artery and aortic arch baroreceptors, which are involved in important feedback loops that regulate heart rate and blood pressure.
Limitations.
This study focused only on relatively brief, modest intensity exercise. It is possible that the findings would be even more dramatic with longer duration or higher intensity exercise. It is also possible that longer duration or higher intensity exercise would uncover group differences that were not noted with the intensity and duration of exercise used in this study. However, the type of exercise studied is more characteristic of typical levels of exertion performed by individuals in the community, heightening the relevance of these findings. In addition, subjects were studied on medications, including 86% on β-blockers. Although this most certainly affects sympathetic activation, evaluation of time-dependent effects compared each individual's heart rate and plasma catecholamine levels to his or her baseline. Furthermore, this is the clinically most relevant state to assess.
The data presented have been interpreted as showing complete or near complete parasympathetic reactivation early in recovery and persistent sympathoexcitation in later recovery. Because the parasympathetic effect at rest was not assessed in this study, it is difficult to rule out incomplete parasympathetic reactivation during the 45-min recovery period. However, it is unlikely to be of major significance for the following reasons. In a prior study of nine subjects with a mean age of 59 years and normal left ventricular function, parasympathetic effect, as measured in the present study, was 247 ± 140 ms at rest and 209 ± 114 ms at 1 min of recovery (21). As noted in Fig. 2, there is substantially more parasympathetic effect at 2 min of recovery compared with 1 min and no further change over the course of recovery. This plateau of parasympathetic effect therefore likely represents complete or near complete parasympathetic reactivation.
Finally, the assessment of sympathoexcitation in exercising humans can be challenging (38). Although plasma catecholamines are certainly a marker of sympathoexcitation, they provide only a rudimentary assessment. However, coupled with the elevated heart rate, the data are convincing. Nevertheless, regional differences in sympathetic innervation and adrenergic receptor density and/or responsiveness could result in variable effects at different sites in the heart.
Implications.
The findings from our study have several important implications. First, it demonstrates that the period of increased risk for postexercise cardiac events may be as far out as 45 min after submaximal exercise. Our subjects exercised for only 16 min and achieved a peak heart rate of only 112 ± 13 beats/min. With more vigorous exercise, the period of risk could be longer. Future studies examining cardiac event risk after exercise need to consider these factors when determining the period for which monitoring will continue. Given that the relative risk for sudden death during and after vigorous exercise is 16.9 relative to sedentary periods, and because this risk is likely due to increased sympathetic activation, further studies are needed to address the pathophysiological link between sympathoexcitation after exercise and cardiac events.
GRANTS
This research was supported by Grant No. 1RO1-HL-70179-01A2 from the National Heart, Lung, and Blood Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, Manson JE. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med 343: 1355–1361, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Arai Y, Saul JP, Albrecht P, Hartley H, Lilly LS, Cohen RJ, Colucci WS. Modulation of cardiac autonomic activity during and immediately after exercise. Am J Physiol Heart Circ Physiol 256: H132–H141, 1989 [DOI] [PubMed] [Google Scholar]
- 3. Bonanni E, Pasquali L, Manca ML, Maestri M, Prontera C, Fabbrini M, Berrettini S, Zucchelli G, Siciliano G, Murri L. Lactate production and catecholamine profile during aerobic exercise in normotensive OSAS patients. Sleep Med 5: 137–145, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Burke AP, Farb A, Malcom GT, Liang YH, Smialek JE, Virmani R. Plaque rupture and sudden death related to exertion in men with coronary artery disease. JAMA 281: 921–926, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Cao JM, Chen LS, KenKnight BH, Ohara T, Lee MH, Tsai J, Lai WW, Karagueuzian HS, Wolf PL, Fishbein MC, Chen PS. Nerve sprouting and sudden cardiac death. Circ Res 86: 816–821, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Chen PS, Chen LS, Cao JM, Sharifi B, Karagueuzian HS, Fishbein MC. Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc Res 50: 409–416, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Chicos AB, Kannankeril PJ, Kadish AH, Goldberger JJ. Parasympathetic effects on cardiac electrophysiology during exercise and recovery in patients with left ventricular dysfunction. Am J Physiol Heart Circ Physiol 297: H743–H749, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cole C, Blackstone E, Pashkow F, Snader C, Lauer M. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 341: 1351–1357, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Cole C, Foody J, Blackstone E, Lauer M. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med 132: 552–555, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Corr PB, Gillis RA. Autonomic neural influences on the dysrhythmias resulting from myocardial infarction. Circ Res 43: 1–9, 1978 [DOI] [PubMed] [Google Scholar]
- 11. Du N, Bai SQ, Oguri K, Kato Y, Matsumoto I, Kawase H, Matsuoka T. Heart rate recovery after exercise and neural regulation of heart rate variability in 30–40 year old female marathon runners. J Sci Med Sport 4: 9–17, 2005 [PMC free article] [PubMed] [Google Scholar]
- 12. Fries R, Konig J, Schafers HJ, Bohm M. Triggering effect of physical and mental stress on spontaneous ventricular tachyarrhythmias in patients with implantable cardioverter-defibrillators. Clin Cardiol 25: 474–478, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goto K, Takahashi K, Yamamoto M, Takamatsu K. Hormone and recovery responses to resistance exercise with slow movement. J Physiol Sci 58: 7–14, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Ikeda N, Yasu T, Tsuboi K, Sugawara Y, Kubo N, Umemoto T, Arao K, Kawakami M, Momomura S. Effects of submaximal exercise on blood rheology and sympathetic nerve activity. Circ J 74: 730–734, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Imai K, Sato H, Hori M, Kusuoka H, Ozaki H, Yokoyama H, Takeda H, Inoue M, Kamada T. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol 24: 1529–1535, 1994 [DOI] [PubMed] [Google Scholar]
- 16. Jabbour G, Lemoine-Morel S, Casazza GA, Hala Y, Moussa E, Zouhal H. Catecholamine response to exercise in obese, overweight, and lean adolescent boys. Med Sci Sports Exerc 43: 408–415, 2011 [DOI] [PubMed] [Google Scholar]
- 17. Javorka M, Zila I, Balharek T, Javorka K. Heart rate recovery after exercise: relations to heart rate variability and complexity. Braz J Med Biol Res 35: 991–1000, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Johnson NP, Holly TA, Goldberger JJ. QT dynamics early after exercise as a predictor of mortality. Heart Rhythm 7: 1077–1084, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jose A. Effect of combined sympathetic and parasympathetic blockade on heart rate and cardiac function in man. Am J Cardiol 18: 476–478, 1966 [DOI] [PubMed] [Google Scholar]
- 20. Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med 352: 1951–1958, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Kannankeril PJ, Goldberger JJ. Parasympathetic effects on cardiac electrophysiology during exercise and recovery. Am J Physiol Heart Circ Physiol 282: H2091–H2098, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Kannankeril PJ, Le FK, Kadish AH, Goldberger JJ. Parasympathetic effects on heart rate recovery after exercise. J Investig Med 52: 394–401, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Kong T, Goldberger J, Parker M, Wang T, Kadish A. Circadian variation in human ventricular refractoriness. Circulation 92: 1507–1516, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Lampert R, Joska T, Burg MM, Batsford WP, McPherson CA, Jain D. Emotional and physical precipitants of ventricular arrhythmia. Circulation 106: 1800–1805, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Leon AS, Connett J, Jacobs DR, Rauramaa R. Leisure-time physical-activity levels and risk of coronary heart-disease and death—the multiple risk factor intervention trial. JAMA 258: 2388–2395, 1987 [PubMed] [Google Scholar]
- 26. Leon AS, Franklin BA, Costa F, Balady GJ, Berra KA, Stewart KJ, Thompson PD, Williams MA, Lauer MS. Cardiac rehabilitation and secondary prevention of coronary heart disease: an American Heart Association scientific statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity), in collaboration with the American association of Cardiovascular and Pulmonary Rehabilitation. Circulation 111: 369–376, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Lindmar R, Loffelholz K, Muscholl E. A muscarinic mechanism inhibiting the release of noradrenaline from peripheral adrenergic nerve fibres by nicotinic agents. Br J Pharmacol Chemother 32: 280–294, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lown B, Verrier RL. Neural activity and ventricular-fibrillation. N Engl J Med 294: 1165–1170, 1976 [DOI] [PubMed] [Google Scholar]
- 29. Malliani A, Schwartz P, Zanchetti A. Neural mechanisms in life-threatening arrhythmias. Am Heart J 100: 705–715, 1980 [DOI] [PubMed] [Google Scholar]
- 30. Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. Determinants of Myocardial Infarction Onset Study Investigators. N Engl J Med 329: 1677–1683, 1993 [DOI] [PubMed] [Google Scholar]
- 31. Muller JE, Ludmer PL, Willich SN, Tofler GH, Aylmer G, Klangos I, Stone PH. Circadian variation in the frequency of sudden cardiac death. Circulation 75: 131–138, 1987 [DOI] [PubMed] [Google Scholar]
- 32. Ozdemir O, Soylu M, Demir AD, Topaloglu S, Alyan O, Geyik B, Kutuk E. Increased sympathetic nervous system activity as cause of exercise-induced ventricular tachycardia in patients with normal coronary arteries. Tex Heart Inst J 30: 100–104, 2003 [PMC free article] [PubMed] [Google Scholar]
- 33. Perini R, Orizio C, Comande A, Castellano M, Beschi M, Veicsteinas A. Plasma norepinephrine and heart-rate dynamics during recovery from submaximal exercise in man. Eur J Appl Physiol Occup Physiol 58: 879–883, 1989 [DOI] [PubMed] [Google Scholar]
- 34. Peters RW. Propranolol and the morning increase in sudden cardiac death: (the beta-blocker heart-attack trial experience). Am J Cardiol 66: G57–G59, 1990 [DOI] [PubMed] [Google Scholar]
- 35. Ramel A, Wagner KH, Elmadfa I. Acute impact of submaximal resistance exercise on immunological and hormonal parameters in young men. J Sports Sci 21: 1001–1008, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Savin WM, Davidson DM, Haskell WL. Autonomic contribution to heart rate recovery from exercise in humans. J Appl Physiol 53: 1572–1575, 1982 [DOI] [PubMed] [Google Scholar]
- 37. Short KR, Sedlock DA. Excess postexercise oxygen consumption and recovery rate in trained and untrained subjects. J Appl Physiol 83: 153–159, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 54: 1747–1762, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Watanabe J, Thamilarasan M, Blackstone EH, Thomas JD, Lauer MS. Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality: the case of stress echocardiography. Circulation 104: 1911–1916, 2001 [PubMed] [Google Scholar]
- 40. Watson RD, Hamilton CA, Jones DH, Reid JL, Stallard TJ, Littler WA. Sequential changes in plasma noradrenaline during bicycle exercise. Clin Sci (Lond) 58: 37–43, 1980 [DOI] [PubMed] [Google Scholar]
- 41. Willich SN, Levy D, Rocco MB, Tofler GH, Stone PH, Muller JE. Circadian variation in the incidence of sudden cardiac death in the Framingham heart-study population. Am J Cardiol 60: 801–806, 1987 [DOI] [PubMed] [Google Scholar]