Abstract
Hypertension is associated with the development of atrial fibrillation; however, the electrophysiological consequences of this condition remain poorly understood. ATP-sensitive K+ (KATP) channels, which contribute to ventricular arrhythmias, are also expressed in the atria. We hypothesized that salt-induced elevated blood pressure (BP) leads to atrial KATP channel activation and increased arrhythmia inducibility. Elevated BP was induced in mice with a high-salt diet (HS) for 4 wk. High-resolution optical mapping was used to measure atrial arrhythmia inducibility, effective refractory period (ERP), and action potential duration at 90% repolarization (APD90). Excised patch clamping was performed to quantify KATP channel properties and density. KATP channel protein expression was also evaluated. Atrial arrhythmia inducibility was 22% higher in HS hearts compared with control hearts. ERP and APD90 were significantly shorter in the right atrial appendage and left atrial appendage of HS hearts compared with control hearts. Perfusion with 1 μM glibenclamide or 300 μM tolbutamide significantly decreased arrhythmia inducibility and prolonged APD90 in HS hearts compared with untreated HS hearts. KATP channel density was 156% higher in myocytes isolated from HS animals compared with control animals. Sulfonylurea receptor 1 protein expression was increased in the left atrial appendage and right atrial appendage of HS animals (415% and 372% of NS animals, respectively). In conclusion, KATP channel activation provides a mechanistic link between salt-induced elevated BP and increased atrial arrhythmia inducibility. The findings of this study have important implications for the treatment and prevention of atrial arrhythmias in the setting of hypertensive heart disease and may lead to new therapeutic approaches.
Keywords: action potentials, arrhythmia mechanisms, cardiac remodeling, potassium channel, salt sensitivity hypertension
atrial fibrillation (AF) is the most prevalent cardiac arrhythmia and is a major risk factor for stroke, congestive heart failure, and mortality (8, 33, 46). Although remodeling in response to AF has been a subject of intense investigation (1, 62), relatively few studies have focused on the mechanisms responsible for the substrate changes that underlie the initiation of atrial tachyarrhythmias. Hypertension is widely recognized as an important independent risk factor for AF (7, 46). Animal models of hypertension have been shown to have significant structural and electrophysiological remodeling and increased atrial arrhythmia inducibility (19, 24, 49, 53). However, the cellular mechanisms responsible for these effects remain poorly understood. Importantly, the contribution of ATP-sensitive K+ (KATP) channel currents (IK,ATP) to hypertension-induced electrophysiological remodeling has not been investigated.
KATP channels were first discovered in cardiac muscle (64) and couple the metabolic state of the cell with its electrical activity. These channels are expressed throughout the body in metabolically active cell types and are found in the sarcolemmal and mitochondrial membranes. Differences in the molecular composition of the channel determine its biophysical and pharmacological properties. The KATP channel complex is composed of inward rectifier K+ channel (Kir6.x) and sulfonylurea receptor (SURx) subunits (63). In ventricular myocytes, KATP channels are composed of Kir6.2 and SUR2A (3, 56, 79). Activation of ventricular KATP channels results in profound abbreviation of action potential (AP) duration (APD), which protects the heart during myocardial ischemia by limiting Ca2+ entry (9, 36, 51, 90). The reduction of Ca2+ entry is thought to reduce energy consumption and limit cellular damage. However, APD abbreviation shortens wavelength, which can facilitate the formation of ventricular arrhythmias (47, 73, 81, 82). Recent studies (29, 32) have demonstrated that atrial KATP channel complexes are predominantly composed of Kir6.2 and SUR1 subunits. Activation of these channels in isolated atrial preparations has been shown to be proarrhythmic (54). However, the physiological role and contribution to electrophysiological remodeling of atrial KATP channels in response to cardiac disease remain unknown.
Here, we investigated the electrophysiological consequences of salt-induced elevated blood pressure (BP) in adult mice. Our findings demonstrate that elevated BP is associated with a reduction in atrial effective refractory period (ERP), APD, and increased arrhythmia inducibility. These electrophysiological effects are reversible with KATP channel blockers. In addition, salt-induced elevated BP is associated with increased SUR1 levels in atrial myocytes and increased sarcolemmal KATP channel density. The findings of this study highlight the KATP channel as a novel molecular link between salt-induced elevated BP and atrial arrhythmias.
MATERIALS AND METHODS
Mice.
All studies were performed using 2- to 3-mo-old male CD-1 mice. Two groups of mice were fed ad libitum. The normal salt (NS) group received tap water, and the high-salt (HS) group received 1.0% NaCl and 0.3% KCl in the drinking water for a period of 4 wk (92). All procedures complied with the standards for the care and use of animal subjects as stated in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996), and protocols were approved by the Institutional Animal Care and Use Committee of the New York University School of Medicine.
BP and systolic function.
BP and fractional shortening (FS) measurements were obtained from a subset of animals before euthanization. BP measurements were obtained from conscious, restrained mice using tail-cuff plethysmography after a period of training on a warmed (37°C) platform (Kent Scientific) as previously described (95). At least five measurements were obtained from each animal and averaged. FS measurements were obtained from anesthetized animals (1.5% inhaled isoflurane, VetEquip) using a Philips HDI 5000 ultrasound system and a 15-MHz probe. The heart was visualized at the short axis at the level of the papillary muscle in the two-dimensional mode, and FS measurements were calculated from M-mode recordings as the percent decrease from diastolic to systolic diameter at the level of the papillary muscle (20). Heart and body weight measurements were performed immediately after euthanization.
In vivo electrophysiological experiments.
Animals were anesthetized with inhaled isoflurane. The jugular vein was dissected and cannulated with a 1.1-Fr octapolar electrophysiological catheter (Millar Instruments), which was advanced and positioned in the right heart chambers. The esophagus was cannulated with a 4-Fr octapolar electrophysiological catheter (St. Jude Medical) and positioned adjacent to the left atrium (LA). Electrode locations were determined from the surface ECG while the hearts was paced with different pairs of electrodes. Atrial pacing was performed using 2-ms stimuli at twice diastolic threshold. ERP measurements were obtained using an S1–S2 protocol consisting of a 10-beat S1 drive cycle at a basic cycle length (BCL) of 100 ms followed by a single S2 extrastimulus. The S1–S2 coupling interval was reduced by 2 ms until the S2 stimulus failed to elicit a conducted response. ERP was defined as the shortest S1–S2 interval that resulted in successful capture. This process was performed twice for each atrium, and an average ERP for each atrium was obtained. Animals were considered inducible if an arrhythmic episode lasting longer than 1 s was observed during the ERP measurement protocol.
Isolated heart preparation.
Hearts were Langendorff perfused as previously described (60, 86). Briefly, mice were administered heparin (1.0 U/g ip heparin sodium, Alfa Aesar) 15 min before being euthanized with 100% CO2 inhalation followed by cervical dislocation. Hearts were excised through a sternotomy and rinsed in ice-cold modified Tyrode solution containing (in mM) 1.8 CaCl2, 1.0 MgCl2, 1.2 KH2PO4, 130.0 NaCl, 4.7 KCl, 11.1 glucose, and 24.0 NaHCO3 with 0.052 g/l albumin equilibrated with a 95% O2-5% CO2 gas mixture. Aortas were cannulated, and hearts were perfused with warm (37°C) Tyrode solution at a constant pressure of 68–74 mmHg. Hearts were submerged in warm oxygenated Tyrode solution during the experiments to limit transmural temperature gradients. Hearts were included in the study if they remained in sinus rhythm with a heart rate of >300 beats/min after a 20-min equilibration period. Hearts were stained with the voltage-sensitive dye di-4-ANEPPS (Invitrogen) as previously described (55). Volume-conducted ECGs were recorded using Ag-AgCl electrodes flanking the heart. Whenever possible, one electrode was located near the right atrium (RA), and the other was positioned near the apex of the right ventricle. Signals were amplified and low-pass filtered with a differential amplifier (cutoff: 300 Hz, CyberAmp 380, Molecular Devices), digitized at 5 kHz (Digidata1322A, Molecular Devices), and stored for offline analysis.
High-resolution optical mapping.
Optical mapping experiments were performed using isolated Langendorff perfused hearts as previously described (60). Briefly, experiments were conducted on an upright Olympus microscope (MVX10) with a reflected light fluorescence attachment (BX-FLA) equipped with a CMOS camera (MiCAM Ultima-L, SciMedia USA). Excitation light from a 100-W mercury arc lamp (Olympus) entered a filter cube that reflected green excitation light (480–550 nm, dichroic mirror: 570 nm) to the heart and passed the emitted light (>610 nm) to the camera. Images were acquired at 1,000 frames/s with 14-bit resolution from a 100 × 100-pixel array, which provided a spatial resolution of 31 μm. Blebbistatin (5 μM, BioMol) was used to limit motion artifacts during optical recordings (28).
Electrophysiological parameters and arrhythmia inducibility.
Conduction velocity (CV) and APD measurements were acquired from isolated perfused hearts by pacing the LA appendage (LAA) and RA appendage (RAA) with a platinum bipolar electrode (FHC). CV measurements were obtained at a BCL of 100 ms with 4-ms stimuli at twice diastolic threshold. Movies were filtered and signal averaged to improve the signal-to-noise ratio of the recordings. Average CVs were calculated as previously described (60). CV was measured as the average of all conduction vector magnitudes. APD measurements were obtained using an S1-S2 protocol consisting of a 10-beat S1 drive cycle at a BCL of 100 ms followed by a single S2 extrastimulus at coupling intervals of 25 and 100 ms. APD values were calculated from the single S2 beat. APDs were measured for each pixel at 50% (APD50), 70% (APD70), and 90% repolarization (APD90). Atrial ERP and arrhythmia inducibility were assessed with programmed stimulation of the LAA and RAA consisting of 10 S1 stimuli at 100-ms intervals followed by a single S2 extrastimulus. Capture was confirmed with optical mapping. The interval between the last S1 stimulus and the S2 stimulus was gradually reduced in 2-ms intervals until the S2 stimulus failed to elicit a conducted response. This process was performed once for each appendage. ERP was defined as the shortest S1-S2 interval that resulted in successful capture. Hearts were considered inducible if an arrhythmic episode lasting longer than 1 s was observed. Arrhythmia dynamics were assessed using dominant frequency (DF) maps calculated from optical recordings (23). The power spectrum of the time series for each pixel was calculated using fast Fourier transform. DF was defined for each pixel as the frequency having the maximum power. Average DF was calculated as the mean value of all pixels during an arrhythmic episode. The contribution of IK,ATP to APD, ERP, and arrhythmia inducibility was evaluated in separate groups of NS and HS animals after 45 min of perfusion with Tyrode solution containing 1 μM glibenclamide (Sigma-Aldrich) (75) or 300 μM tolbutamide (Sigma-Aldrich) (35).
Atrial myocyte isolation.
Mice were heparinized and euthanized, and hearts were excised, cannulated, and Langendorff perfused with a modified Ca2+-free Tyrode solution containing (in mM) 113.0 NaCl, 4.7 KCl, 0.6 KH2PO4, 0.6 NaHPO4, 1.2 MgSO4, 12.0 NaHCO3, 10.0 KHCO3, 10.0 HEPES, 10.0 taurine, 10.0 2,3-butanedione monoxime, and 10.0 d-glucose for 2 min. Next, hearts were perfused with modified Tyrode solution containing 12.5 μM CaCl, 2.0 g/l collagenase type 2 (Worthington), and 0.14 g/l trypsin (Invitrogen) for 10 min. LAs were dissected, chopped, and mechanically dissociated with a micropipette. Atrial myocytes were stored in modified Tyrode solution, which included 12.5 μM CaCl and 5% bovine calf serum at 4°C, and used for patch clamp experiments within 9 h of isolation.
Patch-clamp experiments.
Myocytes isolated from the LAs of NS and HS mice were plated on laminin-coated coverslips, mounted in a recording chamber, and placed on the stage of an inverted microscope (Nikon TE2000-V). Single channel recordings were performed in the inside-out configuration at room temperature using standard patch-clamp techniques (38). Patch pipettes were pulled using borosilicate glass and had resistances between 3 and 4 MΩ when filled with pipette solution, which contained (in mM) 140.0 KCl, 2.0 CaCl2, 1.0 MgCl2, and 10.0 HEPES (pH 7.4). The bath solution contained (in mM) 140.0 KCl, 1.0 EGTA, 10.0 HEPES, and 1.2 MgCl2 (pH 7.2). Current was filtered (low-pass Bessel response with a cutoff frequency of −3 dB at 1 kHz), digitized at 5 kHz, and stored for off line analysis with pCLAMP software (Clampex 9.0, Axon Instruments). Unless otherwise stated, the pipette potential was +80 mV (membrane potential of −80mV). At this potential, current passing through the KATP channel out of the pipette (inward membrane currents) is represented as downward deflections in all figures. Unitary conductance measurements were made in 100 or 300 μM ATP in the bath solution from −80 to −20 mV. KATP channel unitary conductance has been shown to be unaffected by ATP concentration (85). Recordings were made while slowly changing the patch potential from −100 to 100 mV. ATP sensitivity was determined by applying ATP from 1,000 to 1 μM using a rapid solution changer. Exposure to ATP ramps was bracketed by recordings in zero ATP to ensure the absence of significant rundown, which was corrected if necessary. Current was normalized to the maximal channel current amplitude in zero ATP and the minimum current amplitude in 1,000 μM ATP. The IC50 was defined as the ATP concentration that resulted in the half-maximal inhibition of excised patch current. Patches typically contained several channels, and IK,ATP was calculated as the mean patch current relative to zero current in the presence of 1,000 μM ATP when all KATP channels were blocked. IK,ATP was plotted as a function of ATP concentration, and ATP-inhibitory curves were obtained by fitting a pseudo-Hill function to the experimental data using a nonlinear least-squares method. The apparent number of channels per patch was estimated as the maximal number of coincident channel openings observed in the absence of bath ATP, as determined from a multiple Gaussian fit to all-points current amplitude histograms. In patches that contained too many channels to perform this procedure, the number of channels per patch was estimated immediately after patch excision as the mean patch current divided by the average measured unitary conductance.
Histology.
Animals were euthanized, and hearts were immediately excised via a midline sternotomy. Aortas were cannulated, and hearts were perfused with Tyrode solution to clear the blood. Spontaneously contracting tissue was arrested in diastole by perfusing 50 mM KCl at 20°C. The solution was then switched to 4% paraformaldehyde and kept at 4°C for 8 h. Hearts were stored in PBS for 1 h (4°C), dehydrated in ethanol (20°C), fixed in xylene (20°C), and embedded in paraffin. Tissue was sectioned (5 μm) using a microtome. Sections were stained with Gomori's trichrome, following the manufacturer's instructions. Fibrosis content was determined from four-chamber views in a blinded fashion using Image-Pro Plus 5.0 software (Media Cybernetics). Data were acquired from at least 5 sections/heart, which were analyzed by quantifying blue pixel content as a percentage of total tissue area.
Western blot analysis.
Purification and enrichment of membrane proteins were performed as previously described (78). Briefly, atria were minced, snap frozen in liquid nitrogen, and stored at −80°C. A total of 15 animals/group were analyzed in 3 independent preparations. For each preparation, five atria were pooled and homogenized in 2 ml of cold lysis buffer containing (in mM) 250.0 sucrose, 2.0 EGTA, 20.0 HEPES, 1.0 NaVO4, 50.0 NaF, and 1.0 PMSF (pH 7.4) with 2× Complete proteinase inhibitor (Roche) using a mechanical tissue homogenizer (Ultra-Turrax T8, IKA Labortechnik). The homogenate was dounced on ice and centrifuged at 4,000 g for 5 min at 4°C. Supernatants were centrifuged at 190,000 g and 4°C for 1 h. Membrane pellets were dissolved in 25 μl of SDS buffer containing 62.5 mM Tris·HCl, 3% SDS, and 10% glycerol (pH 6.8) and sonicated for 10 s on ice. Protein concentrations were determined using the Lowry method. β-Mercaptoethanol (5%) and bromphenol blue (0.01%) were added, and equal amounts of protein (100 μg) were subjected to SDS-PAGE on 10% acrylamid gels and transferred to polyvinylidene difluoride membranes. Blots were blocked for 1.5 h in blocking solution, which contained 0.085 mM Tris·HCl, 0.17 mM Tris base, 0.5 mM NaCl, 0.1% Tween 25, 5% and low-fat milk, and incubated with primary antibodies diluted in blocking buffer overnight at 4°C. Immunoreactive proteins were visualized by species-specific secondary horseradish peroxidase-conjugated antibodies and subsequent enhanced chemiluminescence (Supersignal West Pico or Femto Chemiluminescent Substrate, Pierce Chemical) as recommended by the manufacturer. To confirm equal loading, signals were normalized to N-cadherin levels using computer-assisted densitometry (Scion Image Software, Scion, NHI). Values for HS LAs and RAs were normalized to the same chambers in NS animals. Additionally, equal loading was confirmed with Ponceau staining. Briefly, data were collected for three Ponceau-stained signals of different molecular weights and averaged. Densitometric values for respective KATP channel subunits were normalized to these signals.
Antibodies.
Primary antibodies used for immunoblot analyses included sera raised in the rabbit (1:250 SUR1, custom made by Dr. William Coetzee) and goat (1:200 gtKir6.2, G-16, Santa Cruz Biotechnology). Secondary antibodies included horseradish peroxidase-conjugated donkey anti-rabbit (1:5,000, Santa Cruz Biotechnology) and donkey anti-goat (1:5,000, Santa Cruz Biotechnology).
Statistical analysis.
Ex vivo arrhythmia inducibility data were compared using the Freeman-Halton extension of the Fisher's exact test. In vivo arrhythmia inducibilty data were compared without the Freeman-Halton extension. The Mann-Whitney rank-sum test was used for the comparison of the number of channels per patch. Two-way ANOVA with replication was used for the comparison of atrial fibrosis followed by two-tailed Student's t-tests. Two-tailed Student's t-tests were used for all other comparisons. Values are reported as means ± SE. Differences were considered significant if P < 0.05.
RESULTS
BP and systolic function.
Systolic BP was quantified using tail-cuff plethysmography, and cardiac function was assessed using echocardiography. BP was significantly elevated (P = 0.003) in HS animals (121.2 ± 4.3 mmHg, n = 11) compared with NS animals (103.4 ± 3.3 mmHg, n = 11). Average FS was not significantly different between groups. The ratio of heart weight to body weight was significantly increased (P = 0.013) by 22.6% in HS animals (n = 5) compared with NS animals (n = 10).
Atrial interstitial fibrosis.
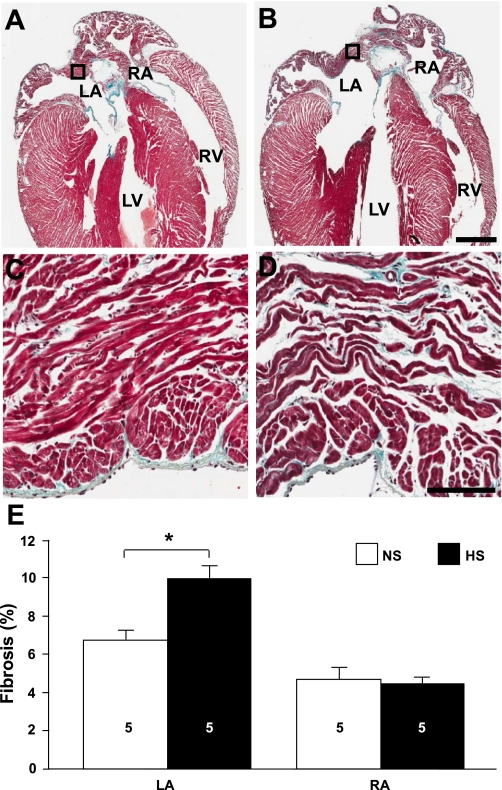
The development of atrial interstitial fibrosis has been demonstrated in several animal models of hypertension (19, 49, 53). Figure 1 shows representative NS and HS sections stained with Gomori's trichrome. In HS hearts, the percentage of interstitial fibrosis was significantly higher (P = 0.006) in the LA (9.97 ± 0.70%, n = 5) compared with NS hearts (6.75 ± 0.51%, n = 5). The percentage of interstitial fibrosis was not significantly changed in the RA.
Fig. 1.
Increased left atrial (LA) fibrosis in high-salt diet-fed (HS) animals. A and B: trichrome-stained sections of hearts from normal-salt diet-fed (NS; A) and HS (B) animals. Bar = 1 mm. RA, right atrium; LV, left ventricle; RV, right ventricle. C and D: high-magnification images of the boxed regions indicated in A and B, respectively. Bar = 0.1 mm. E: average fibrosis values for LA and RA free walls and appendages (LAA and RAA, respectively). Numbers in bars indicate numbers of animals in each group. *P < 0.05.
Atrial arrhythmia inducibility.
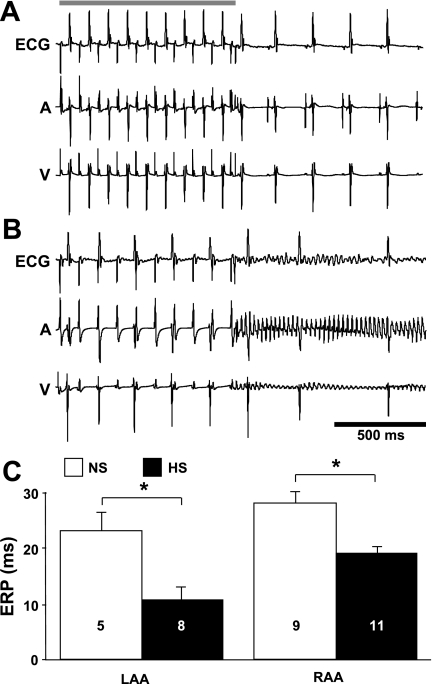
To determine whether salt-induced elevated BP is associated with atrial electrophysiological changes and increased arrhythmia inducibility in vivo, electrophysiological experiments were conducted on intact anaesthetized animals. Figure 2 shows representative surface and intracardiac electrograms and average LA and RA ERP measurements. Using transesophageal and transvenous pacing techniques for stimulating the LA and RA, significantly more atrial arrhythmias were observed in HS animals (6 of 11) compared with NS animals (1 of 10, P < 0.05). Average ERP values in HS animals were significantly shorter in the LA (46.1 ± 10.1% of NS animals, P = 0.009) and RA (67.9 ± 4.3% of NS animals, P = 0.001). These data demonstrate that salt-induced elevated BP is associated with a significant reduction in the duration of refractoriness and increased atrial arrhythmia inducibility in vivo.
Fig. 2.
Altered electrophysiological characteristics in vivo. A and B: representative surface (ECG), intra-atrial (A), and intraventricular (V) electrograms obtained from NS (A) and HS (B) animals. The shaded bar indicates when the programmed stimulation was delivered. C: average LA and RA effective refractory period (ERP) values. Numbers in bars indicate numbers of animals in each group. *P < 0.05.
Atrial arrhythmia dynamics.
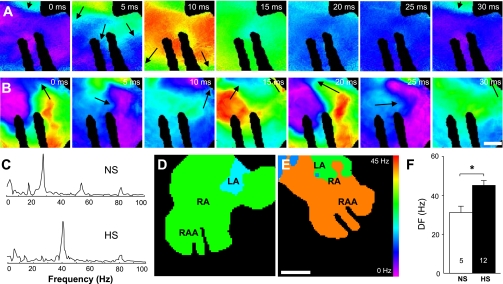
Atrial arrhythmia dynamics were evaluated in isolated Langendoff-perfused hearts. Atrial arrhythmias were induced in a small percentage of NS hearts (5 of 36). In contrast, programmed electrical stimulation induced atrial arrhythmias in a significantly higher (P = 0.04) percentage of HS hearts (16 of 45). Figure 3, A and B, shows representative voltage maps showing the progression of polymorphic reentrant activity in the RAA of NS and HS hearts, respectively. Reentrant activity cycle length in the NS and HS hearts were ∼32 and 20 ms, respectively. The power spectra for these atrial arrhythmias are shown in Fig. 3C, and Fig. 3, D and E, shows the respective DF maps. Arrhythmias in NS and HS atria had average DFs of 28 and 41 Hz, respectively. Figure 3F shows the average DFs for atrial arrhythmias recorded from NS and HS atria. These data indicate that arrhythmias in HS hearts are characterized by significantly higher (P = 0.008) DFs (45.1 ± 2.7 Hz, n = 12) compared with NS hearts (31.2 ± 3.5 Hz, n = 5).
Fig. 3.
Increased atrial arrhythmia dominant frequencies (DFs). A and B: voltage maps showing RAA activity recorded during a sustained atrial arrhythmia in NS (A) and HS (B) hearts. Arrows indicate the locations of the pivot points for reentrant activation. Bar = 1 mm. C: power spectra of the atrial arrhythmias shown in A and B. D and E: DF maps for the arrhythmias shown in A and B, respectively. Bar = 2.5 mm. F: average atrial DFs in NS and HS hearts. Numbers in bars indicate numbers of animals in each group. *P < 0.05.
Atrial CV and repolarization.
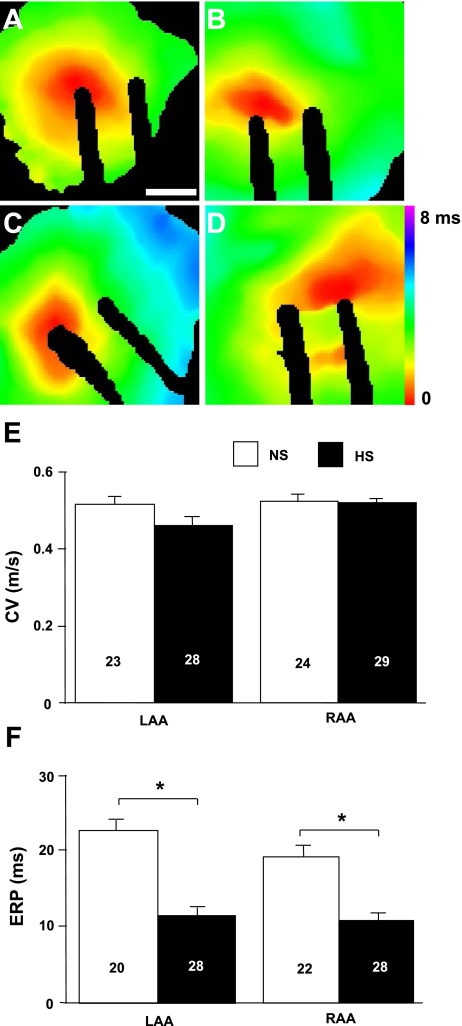
CV and ERP represent important parameters for arrhythmia inducibility and complexity (14, 17, 18, 50). Figure 4 shows representative activation maps and average CV and ERP measurements obtained from the LAA and RAA of isolated NS and HS hearts. Atrial CVs were not found to be significantly altered in HS hearts compared with NS hearts in either the LAA or RAA. Average ERP values were significantly shorter in HS hearts for both the LAA (53.6 ± 5.0% of NS animals, P = 2.68 × 10−7) and RAA (59.7 ± 5.3% of NS animals, P = 1.67 × 10−5). These data are consistent with the in vivo electrophysiological changes and demonstrate that salt-induced elevated BP is associated with minimal changes in conduction parameters and a significant reduction in the duration of refractoriness.
Fig. 4.
Decreased atrial ERP. A and B: representative activation maps from the LAA (A) and RAA (B) of a NS heart. C and D: representative activation maps from the LAA (C) and RAA (D) of a HS heart. E and F: average conduction velocity (CV; E) and ERP (F) values. Numbers in bars indicate numbers of animals in each group. Bar = 1 mm. *P < 0.05.
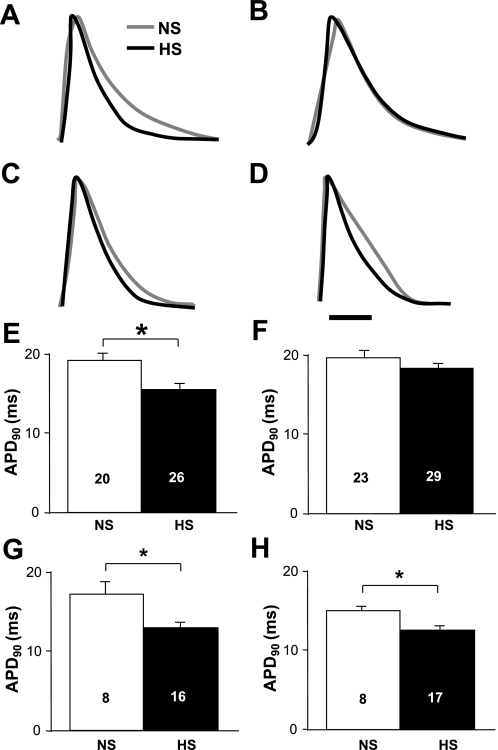
ERP abbreviation suggests that APD may be reduced in HS atria. Figure 5 shows representative optical AP traces from NS and HS atria and average APD90 values. The optical traces (Fig. 5, A and B) and average APD90 values (Fig. 5, E and F) recorded at a 100-ms S1–S2 coupling interval showed significantly shorter (P = 0.003) APDs in the LAA of HS hearts (15.63 ± 0.73, n = 26) compared with NS hearts (19.30 ± 1.02 ms, n = 20). The reduction in APD was not observed in the RAA. APD90 values measured at an S1-S2 coupling interval of 25 ms (Fig. 5, C, D, G, and H) were significantly shorter in both the LAA (13.04 ± 0.67, n = 16) and RAA (12.63 ± 0.55, n = 17) in HS hearts compared with the LAA (17.29 ± 1.56, n = 8, P = 0.009) and RAA (15.14 ± 0.53, n = 8, P = 0.009) in NS hearts. Similar differences were obtained at APD50 and APD70 (data not shown).
Fig. 5.
Decreased atrial action potential (AP) duration (APD). A and B: representative LAA (A) and RAA (B) single-pixel optical AP traces obtained at an S2 coupling interval of 100 ms. C and D: representative LAA (C) and RAA (D) single-pixel optical AP traces obtained at an S2 coupling interval of 25 ms. E and F: average LAA (E) and RAA (F) APD at 90% repolarization (APD90) values obtained at an S2 coupling interval of 100 ms. G and H: average LAA (G) and RAA (H) APD90 values obtained at an S2 coupling interval of 25 ms. Numbers in bars indicate numbers of animals in each group. *P < 0.05.
Effects of KATP channel blockers on arrhythmia inducibility and APD.
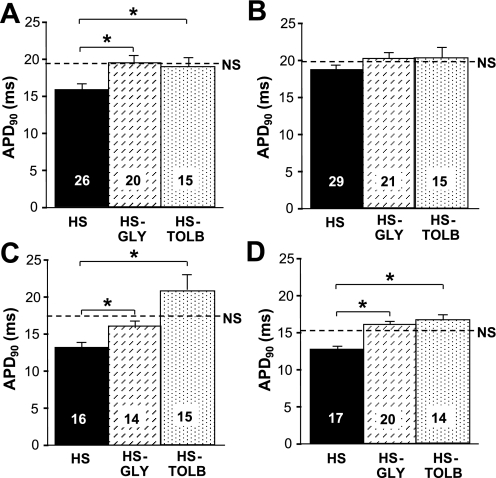
The contribution of IK,ATP to atrial arrhythmia inducibility was determined in NS and HS hearts after perfusion with the KATP channel blocker glibenclamide. Atrial arrhythmia inducibility was significantly reduced (P = 0.04) in HS hearts during glibenclamide perfusion (2 of 21) compared with untreated hearts (16 of 45). A similar decrease in arrhythmia inducibility was also observed for HS hearts treated with tolbutamide (P = 0.006). Arrhythmia inducibility was unaffected by glibenclamide or tolbutamide in the NS group.
The contribution of IK,ATP to atrial APD was evaluated during perfusion with glibenclamide and tolbutamide. Consistent with a previous study (32), atrial APD values in NS hearts were unaffected by perfusion of either drug (data not shown), suggesting that atrial KATP channels play a limited role in membrane excitability under physiological conditions. Figure 6 shows average APD values in HS mice during perfusion of glibenclamide and tolbutamide. At an S1-S2 coupling interval of 100 ms, glibenclamide (P = 0.004) and tolbutamide (P = 0.02) significantly prolonged LAA APDs compared with untreated HS atria. This effect was not observed in the RAA. At the 25-ms S1-S2 coupling interval, glibenclamide and tolbutamide significantly prolonged LAA (P = 0.007 and 0.001, respectively) and RAA APD values (P = 3.59 × 10−5 and 0.008, respectively) compared with untreated HS hearts. These data demonstrate that activation of atrial IK,ATP contributes to elevated BP-induced APD shortening.
Fig. 6.
Glibenclamide and tolbutamide increase APD. A and B: average LAA (A) and RAA (B) APD90 values obtained at an S2 coupling interval of 100 ms. C and D: average LAA (C) and RAA (D) APD90 values obtained at an S2 coupling interval of 25 ms, respectively. HS-GLY, HS group perfused with 1 μM glibenclamide; HS-TOLB, HS group perfused with 300 μM tolbutamide. The dashed lines indicate untreated NS average APD90 values. Numbers in bars indicate numbers of animals in each group. *P < 0.05.
Sarcolemmal KATP channel properties and density.
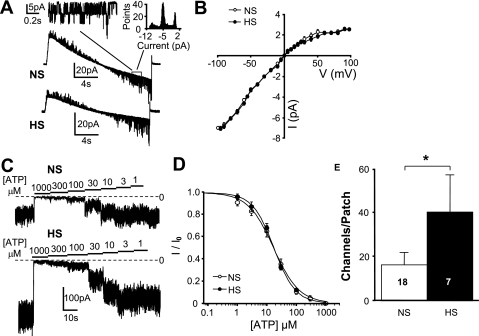
Figure 7 shows KATP channel biophysical properties and channel densities evaluated in isolated LA myocytes from NS and HS animals measured using patch-clamp techniques. Inside-out single channel recordings showed unitary deflections with inward rectification properties (Fig. 7A). The slope conductance of the unitary current (Fig. 7B) was consistent with a previous study (71) of KATP channels and was not significantly different in HS myocytes (77.2 ± 1.37 pS, n = 6) compared with NS myocytes (75.8 ± 0.71 pS, n = 7). Channel activity in excised patches from both NS and HS myocytes decreased with increasing concentrations of ATP (Fig. 7C). The IC50 for ATP (Fig. 7D) was not significantly different in patches from HS myocytes (14.6 ± 2.13 μM, n = 6) compared with NS myocytes (17.7 ± 3.31 μM, n = 12). The average number of functional KATP channels per patch (Fig. 7E) was significantly greater (P = 0.046) in HS myocytes (41 ± 17.2, n = 7) compared with NS myocytes (16 ± 5.7, n = 18). Excised patch membrane areas, as estimated from pipette resistances, were unchanged between the groups (3.2 ± 0.13 and 3.3 ± 0.22MΩ for the NS and HS group, respectively).
Fig. 7.
Increased atrial ATP-sensitive K+ (KATP) channel density. A: representative single channel currents from NS and HS atrial myocytes recorded during a slow ramp (−100 to 100 mV). Inset, representative single channel recording and activity histogram. B: average current (I)-voltage (V) curve. C: representative responses of excised patches to decreasing concentrations of ATP. D: average responses to varying concentrations of ATP. E: average numbers of KATP channels per patch. Numbers in bars indicate numbers of animals in each group. *P < 0.05.
KATP subunit protein levels.
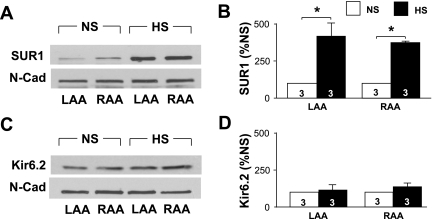
Atrial tissue from NS and HS animals was analyzed to determine KATP channel subunit levels using immunoblot analysis (Fig. 8). SUR1 protein expression was significantly increased in HS LAs (414.6 ± 87.6% of NS LAs, n = 3, P = 0.02) and RAs (371.7 ± 10.5% of NS RAs, n = 3, P = 0.001; Fig. 8, A and B). Kir6.2 protein expression was unchanged (Fig. 8, C and D). N-cadherin expression was not significantly altered in HS atria compared with NS atria and was used as a loading control. Similar results were obtained using three Ponceau-stained signals of different molecular weights as a loading control (data not shown).
Fig. 8.
Elevated sulfonylurea receptor 1 (SUR1) protein levels. A and B: representative immunoblot (A) and average SUR1 expression levels (B). N-cadherin (N-Cad) was used as a loading control. Protein expression levels were normalized to the NS group for each chamber. C and D: representative immunoblot (C) and average Kir6.2 expression levels (D). Numbers in bars indicate numbers of animals in each group. *P < 0.05.
DISCUSSION
Salt-induced hypertension is highly relevant given the dramatic rise of salt consumption in Western countries over the last three decades (12). Increased risk of cardiovascular morbidity and mortality due to chronic high-salt intake has been attributed to both BP-dependent and -independent effects on the heart and vasculature (59). Moreover, hypertension is widely recognized as an independent risk factor for AF (7, 46); however, the electrophysiological consequences of this condition are poorly understood. This is the first study to demonstrate elevated BP with a HS diet leads to significant atrial electrophysiological remodeling and an increase in atrial arrhythmia inducibility.
After the induction of elevated BP with a HS diet, we observed a modest increase in LAA interstitial fibrosis, consistent with previous observations using other models of hypertension (19, 49, 53). On average, HS animals and isolated hearts were more susceptible to atrial arrhythmias induced by programmed stimulation compared with NS animals. Atrial arrhythmias were characterized by higher DFs in HS hearts compared with NS hearts. CV was unaffected by salt-induced elevated BP. LAA and RAA ERPs were significantly shorter in the HS group. In HS animals, APD was decreased in the LAA when measured at a 100-ms cycle length and decreased in both the LAA and RAA when measured at a 25-ms cycle length. Differences in RAA APDs measured at the two coupling intervals suggest that salt-induced elevated BP may lead to electrical remodeling, which includes altered ion channel gating kinetics. Importantly, perfusion with glibenclamide and tolbutamide resulted in the prolongation of APD and a reduction in atrial arrhythmia inducibility in HS animals, suggesting the basal activity of KATP channels. Analysis of the biophysical characteristics of atrial sarcolemmal KATP channels demonstrated that unitary conductance and ATP sensitivity were unchanged in LA HS myocytes, whereas the density of sarcolemmal channels was significantly increased. Finally, SUR1 levels were significantly increased in HS atria, suggesting that SUR1 is responsible for the increase in functional KATP channels.
The atrial myocardium has been shown to remodel in response to AF (1, 62). Structural remodeling has been extensively documented and is characterized by atrial dilation and an increase in interstitial fibrosis (66). Additionally, patients with AF show electrophysiological changes, including shorter atrial ERP, increased heterogeneity of atrial CV, and decreased local electrogram amplitude (77). Similar changes in electrophysiological parameters have been described in tachypaced animal models (1, 61, 74, 76). The principal cellular mechanisms that have been associated with AF-induced electrical remodeling include reduced L-type Ca2+ current (ICaL), transient outward K+ current (Ito), and ACh-activated K+ current (IK,ACh) and increased levels of inward rectifier K+ current (IK1) and the constitutively active component of IK,ACh (16, 25, 26, 93). Several studies have examined the role of the KATP channel in humans and animal models of AF. Although one study (91) found increased IK,ATP density associated with chronic AF, others have demonstrated that KATP subunit transcripts (13) and current density (4) are downregulated in AF patients. Studies (34, 43, 89) using animal models of rapid atrial pacing have suggested the electrophysiological changes are unaffected by KATP channel blockade.
A comparatively small number of studies have examined the structural and electrophysiological consequences of hypertension (19, 49, 53). In the ventricles, structural remodeling associated with hypertension is characterized by myocyte hypertrophy, increased myofibrillar disarray, and interstitial fibrosis (40). Macroscopic electrophysiological changes associated with hypertension include prolongation and increased dispersion of ventricular repolarization (40, 52, 57), which are thought to be mediated by reductions in IK1 and Ito (58).
Choisy et al. (19) investigated electrophysiological remodeling and susceptibility to atrial arrhythmias in spontaneously hypertensive rats at 3 and 11 mo. Comparisons of hypertensive and control animals indicated that APD and atrial ERP values were not significantly different. Arrhythmia inducibility was unchanged in 3-mo-old hypertensive animals and significantly increased in 11-mo-old animals compared with control animals. Patch-clamp recordings of LA myocytes demonstrated that ICaL densities were decreased in 3-mo-old hypertensive hearts compared with age-matched control hearts. These data indicate that acute hypertension induces electrophysiological remodeling in the LA myocardium that is consistent with APD shortening. A more recent study (24) demonstrated that atrial ERP is significantly decreased in spontaneously hypertensive rats compared with control animals, and this was associated with an increase in AF duration. Pharmacological studies have suggested that these changes may be associated with an increase in the Ca2+-activated K+ current. Another study by Lau et al. (53) characterized the atrial electrophysiological and structural changes using the one-kidney, one-clip ovine hypertensive model. After 7 wk of hypertension, significant hypertrophy and interstitial fibrosis were evident in the atria. Electrophysiological alterations were observed in the LA and RA and included increased atrial ERP, reduced and more heterogeneous CVs, and greater AF inducibility. None of the above studies investigated the contribution of IK,ATP to electrophysiological remodeling. There are several factors that could account for the model-specific differences in electrophysiological remodeling. The interventions used to increase BP may have different effects on the renin-angiotensin-aldosterone system. Circulating levels of angiotensin II can affect electrophysiological characteristics through direct interactions with ion channels (84) and transcriptional mechanisms (41), possibly resulting in the differences observed. Recent evidence has also suggested that differences in sympathetic tone can alter atrial repolarization kinetics through KATP channel-dependent mechanisms (42). Together, these studies suggest that the mode of hypertension induction may determine whether atrial repolarizing currents are remodeled.
Mechanoelectric feedback has long been suspected to modulate the hypertensive AF substrate (10, 69, 94). Acute atrial stretch has been demonstrated to affect ERP (10, 11, 48). Such changes can be spatially heterogeneous, contributing to arrhythmogenesis by shortening the reentrant wave length and favoring functional block (89). Stretch-activated channels are the primary ionic mediators of mechanoelectric feedback and have been found in the cardiac tissue of various species, including humans (37, 72, 83). It has been shown that activation of stretch-activated channel current accelerates repolarization during the AP plateau, resulting in APD shortening and afterdepolarizations (30, 39, 69). Despite the fact that irreversible atrial structural remodeling has been well documented in hypertension, the electrophysiological alterations caused by atrial stretch (decreased APD and atrial ERP) have been shown to be quickly reversible with the release of stretch (10). The present study demonstrated that altered electrophysiological parameters persist in isolated nonworking Langendorff-perfused hearts, where afterloads in the atria are negligible and would have been similar in all groups studied. Another possible effect of mechanical stretch would be through the modulation of SUR1 expression. The FOXO subfamily of forkhead transcription factors has been shown to regulate KATP channel gene transcription (68). As a downstream target of Akt (2), FOXOs are subject to regulation by the complex interplay of angiotensin II expression (70) and mechanical stress (67).
Our Western blot analysis data suggest that increased SUR1 expression may be sufficient to increase the density of functional KATP channels, since Kir6.2 expression was unchanged. The notion that SUR subunits are stoichiometrically limiting for the formation of cardiac KATP channels was first introduced by the work of Du et al. (27). Using a transgenic mouse where SUR2A expression was under the control of a cytomegalovirus promoter, they demonstrated that overexpression of SUR2A (in the absence of altered mRNA expression of other KATP channel proteins) resulted in increased sarcolemmal expression of functional KATP channels in ventricular myocytes (27). Moreover, available mRNA expression data suggest that SUR1 may be stoichiometrically limiting in the atria (31, 68), implying that overexpression of this subunit alone may be sufficient to increase the density of functional KATP channels. Other recent evidence has suggested that SUR1-containing KATP channels are preferentially localized to the plasmalemma, whereas SUR2 causes localization to intracellular vesicles (5). It is possible that the increased expression of SUR1 that we observed resulted in enhanced trafficking to the plasma membrane from an endosomal reservoir.
Despite the expression of functional KATP channels, excised hearts from NS animals did not demonstrate ERP or APD prolongation with KATP channel blockade. This suggests that, as in the ventricles, atrial KATP channels contribute little to repolarizing current under basal conditions, which is consistent with a previous study (32). However, APD in hypertensive animals was prolonged with KATP channel blockade, implying that, in addition to overexpression of sarcolemmal KATP channels, a HS diet also leads to increased channel function. There are a number of potential explanations for this observed KATP channel activation. It is possible that salt-induced hypertension results in a sufficient metabolic challenge, resulting in KATP channel activation. In addition, changes in the levels of phospholipid (phosphatydilinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate) (6), G protein (80), adenylate kinase (15), creatine kinase (22), and M-LDH (21) as well as GAPDH activity (44, 45) may play a role in KATP channel activation in HS animals. Although the patch-clamp data provide direct evidence supporting that sarcolemmal KATP channels contribute to the electrophysiological remodeling in hypertensive animals, a possible role of mitochondrial KATP channels was not excluded. Several studies (65, 87, 88) of ischemic preconditioning have suggested that mitochondrial KATP channels are involved in myocardial protection.
We have provided evidence supporting KATP channel activation as a mechanistic link between salt-induced elevated BP and atrial arrhythmia inducibility. These data also suggest that elevated systolic BP may be an important early risk factor for electrical remodeling leading to AF and emphasize the need for aggressive treatment at early stages. The findings of this study have important implications for the treatment and prevention of atrial arrhythmias in the setting of elevated BP and may lead to new therapeutic approaches.
GRANTS
This work was supported by National Institutes of Health Grants HL-076751 (to G. E. Morley), 1-T32-HL-098129 (to C. Vasquez), HL-105046 (to L. Bao), HL-82727, HL-081336, and 1-S10-RR-026681 (to G. I. Fishman), and HL-085820 and HL-093563 (to W. A. Coetzee), an American Heart Association (AHA) Health Sciences Student Research Fellowship (to J. M. Lader), AHA Grant 0725898T (to C. Vasquez), an Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship (to J. M. Lader), and a New York Academy of Medicine Glorney-Raisbeck Fellowship (to J. M. Lader).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Mark Alu and the New York University Histopathology Core (partially supported by National Cancer Institute Grant 5-P30-CA-16087-31) for contributions to the histology experiments.
REFERENCES
- 1. Allessie MA, Konings K, Kirchhof CJ, Wijffels M. Electrophysiologic mechanisms of perpetuation of atrial fibrillation. Am J Cardiol 77: 10A–23A, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Anderson MJ, Viars CS, Czekay S, Cavenee WK, Arden KC. Cloning and characterization of three human forkhead genes that comprise an FKHR-like gene subfamily. Genomics 47: 187–199, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Babenko AP, Gonzalez G, Aguilar-Bryan L, Bryan J. Reconstituted human cardiac KATP channels: functional identity with the native channels from the sarcolemma of human ventricular cells. Circ Res 83: 1132–1143, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Balana B, Dobrev D, Wettwer E, Christ T, Knaut M, Ravens U. Decreased ATP-sensitive K+ current density during chronic human atrial fibrillation. J Mol Cell Cardiol 35: 1399–1405, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Bao L, Hadjiolova K, Coetzee WA, Rindler MJ. Endosomal KATP channels as a reservoir after myocardial ischemia: a role for SUR2 subunits. Am J Physiol Heart Circ Physiol 300: H262–H270, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science 282: 1141–1144, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort: the Framingham Heart Study. JAMA 271: 840–844, 1994 [PubMed] [Google Scholar]
- 8. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 98: 946–952, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Billman GE. The cardiac sarcolemmal ATP-sensitive potassium channel as a novel target for anti-arrhythmic therapy. Pharmacol Ther 120: 54–70, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Bode F, Katchman A, Woosley RL, Franz MR. Gadolinium decreases stretch-induced vulnerability to atrial fibrillation. Circulation 101: 2200–2205, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Bode F, Sachs F, Franz MR. Tarantula peptide inhibits atrial fibrillation. Nature 409: 35–36, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Annu Rev Nutr 24: 401–431, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Brundel BJ, Van Gelder IC, Henning RH, Tuinenburg AE, Wietses M, Grandjean JG, Wilde AA, Van Gilst WH, Crijns HJ. Alterations in potassium channel gene expression in atria of patients with persistent and paroxysmal atrial fibrillation: differential regulation of protein and mRNA levels for K+ channels. J Am Coll Cardiol 37: 926–932, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Calkins H, el-Atassi R, Kalbfleisch S, Langberg J, Morady F. Effects of an acute increase in atrial pressure on atrial refractoriness in humans. Pacing Clin Electrophysiol 15: 1674–1680, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Carrasco AJ, Dzeja PP, Alekseev AE, Pucar D, Zingman LV, Abraham MR, Hodgson D, Bienengraeber M, Puceat M, Janssen E, Wieringa B, Terzic A. Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc Natl Acad Sci USA 98: 7623–7628, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cha TJ, Ehrlich JR, Chartier D, Qi XY, Xiao L, Nattel S. Kir3-based inward rectifier potassium current: potential role in atrial tachycardia remodeling effects on atrial repolarization and arrhythmias. Circulation 113: 1730–1737, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Chen YJ, Chen SA, Tai CT, Wen ZC, Feng AN, Ding YA, Chang MS. Role of atrial electrophysiology and autonomic nervous system in patients with supraventricular tachycardia and paroxysmal atrial fibrillation. J Am Coll Cardiol 32: 732–738, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Chen YJ, Tai CT, Chiou CW, Wen ZC, Chan P, Lee SH, Chen SA. Inducibility of atrial fibrillation during atrioventricular pacing with varying intervals: role of atrial electrophysiology and the autonomic nervous system. J Cardiovasc Electrophysiol 10: 1578–1585, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Choisy SC, Arberry LA, Hancox JC, James AF. Increased susceptibility to atrial tachyarrhythmia in spontaneously hypertensive rat hearts. Hypertension 49: 498–505, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Cittadini A, Stromer H, Katz SE, Clark R, Moses AC, Morgan JP, Douglas PS. Differential cardiac effects of growth hormone and insulin-like growth factor-1 in the rat. A combined in vivo and in vitro evaluation. Circulation 93: 800–809, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Crawford RM, Budas GR, Jovanovic S, Ranki HJ, Wilson TJ, Davies AM, Jovanovic A. M-LDH serves as a sarcolemmal KATP channel subunit essential for cell protection against ischemia. EMBO J 21: 3936–3948, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Crawford RM, Ranki HJ, Botting CH, Budas GR, Jovanovic A. Creatine kinase is physically associated with the cardiac ATP-sensitive K+ channel in vivo. FASEB J 16: 102–104, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Danik SB, Rosner G, Lader J, Gutstein DE, Fishman GI, Morley GE. Electrical remodeling contributes to complex tachyarrhythmias in connexin43-deficient mouse hearts. FASEB J 22: 1204–1212, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diness JG, Skibsbye L, Jespersen T, Bartels ED, Sorensen US, Hansen RS, Grunnet M. Effects on atrial fibrillation in aged hypertensive rats by Ca2+-activated K+ channel inhibition. Hypertension 57: 1129–1135 [DOI] [PubMed] [Google Scholar]
- 25. Dobrev D, Friedrich A, Voigt N, Jost N, Wettwer E, Christ T, Knaut M, Ravens U. The G protein-gated potassium current IK,ACh is constitutively active in patients with chronic atrial fibrillation. Circulation 112: 3697–3706, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Dobrev D, Graf E, Wettwer E, Himmel HM, Hala O, Doerfel C, Christ T, Schuler S, Ravens U. Molecular basis of downregulation of G-protein-coupled inward rectifying K+ current (IK,ACh) in chronic human atrial fibrillation: decrease in GIRK4 mRNA correlates with reduced IK,ACh and muscarinic receptor-mediated shortening of action potentials. Circulation 104: 2551–2557, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Du Q, Jovanovic S, Clelland A, Sukhodub A, Budas G, Phelan K, Murray-Tait V, Malone L, Jovanovic A. Overexpression of SUR2A generates a cardiac phenotype resistant to ischemia. FASEB J 20: 1131–1141, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fedorov VV, Lozinsky IT, Sosunov EA, Anyukhovsky EP, Rosen MR, Balke CW, Efimov IR. Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm 4: 619–626, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Flagg TP, Kurata HT, Masia R, Caputa G, Magnuson MA, Lefer DJ, Coetzee WA, Nichols CG. Differential structure of atrial and ventricular KATP: atrial KATP channels require SUR1. Circ Res 103: 1458–1465, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Franz MR, Burkhoff D, Yue DT, Sagawa K. Mechanically induced action potential changes and arrhythmia in isolated and in situ canine hearts. Cardiovasc Res 23: 213–223, 1989 [DOI] [PubMed] [Google Scholar]
- 31. Gaborit N, Le Bouter S, Szuts V, Varro A, Escande D, Nattel S, Demolombe S. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J Physiol 582: 675–693, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Glukhov AV, Flagg TP, Fedorov VV, Efimov IR, Nichols CG. Differential KATP channel pharmacology in intact mouse heart. J Mol Cell Cardiol 48: 152–160, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA 285: 2370–2375, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Goette A, Honeycutt C, Langberg JJ. Electrical remodeling in atrial fibrillation. Time course and mechanisms. Circulation 94: 2968–2974, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Gribble FM, Tucker SJ, Ashcroft FM. The interaction of nucleotides with the tolbutamide block of cloned ATP-sensitive K+ channel currents expressed in Xenopus oocytes: a reinterpretation. J Physiol 504: 35–45, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gross GJ, Peart JN. KATP channels and myocardial preconditioning: an update. Am J Physiol Heart Circ Physiol 285: H921–H930, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Hagiwara N, Masuda H, Shoda M, Irisawa H. Stretch-activated anion currents of rabbit cardiac myocytes. J Physiol 456: 285–302, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- 39. Hansen DE, Craig CS, Hondeghem LM. Stretch-induced arrhythmias in the isolated canine ventricle. Evidence for the importance of mechanoelectrical feedback. Circulation 81: 1094–1105, 1990 [DOI] [PubMed] [Google Scholar]
- 40. Ichkhan K, Molnar J, Somberg J. Relation of left ventricular mass and QT dispersion in patients with systematic hypertension. Am J Cardiol 79: 508–511, 1997 [DOI] [PubMed] [Google Scholar]
- 41. Isidoro Tavares N, Philip-Couderc P, Baertschi AJ, Lerch R, Montessuit C. Angiotensin II and tumour necrosis factor alpha as mediators of ATP-dependent potassium channel remodelling in post-infarction heart failure. Cardiovasc Res 83: 726–736, 2009 [DOI] [PubMed] [Google Scholar]
- 42. James AF, Kim SJ, Zhang H, Choisy SC, Lin H, Hancox JC, Suleiman MS. Anti-arrhythmic action of an ATP-sensitive potassium channel blocker against atrial fibrillation associated with β-adrenergic stress in rat hearts. Biophys J; doi:10.1016/j.bpj.2010.12.1290 [Google Scholar]
- 43. Jayachandran JV, Zipes DP, Weksler J, Olgin JE. Role of the Na+/H+ exchanger in short-term atrial electrophysiological remodeling. Circulation 101: 1861–1866, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Jovanovic S, Du Q, Crawford RM, Budas GR, Stagljar I, Jovanovic A. Glyceraldehyde 3-phosphate dehydrogenase serves as an accessory protein of the cardiac sarcolemmal KATP channel. EMBO Rep 6: 848–852, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jovanovic S, Jovanovic A. High glucose regulates the activity of cardiac sarcolemmal ATP-sensitive K+ channels via 1,3-bisphosphoglycerate: a novel link between cardiac membrane excitability and glucose metabolism. Diabetes 54: 383–393, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 82: 2N–9N, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Kantor PF, Coetzee WA, Carmeliet EE, Dennis SC, Opie LH. Reduction of ischemic K+ loss and arrhythmias in rat hearts. Effect of glibenclamide, a sulfonylurea. Circ Res 66: 478–485, 1990 [DOI] [PubMed] [Google Scholar]
- 48. Kaseda S, Zipes DP. Contraction-excitation feedback in the atria: a cause of changes in refractoriness. J Am Coll Cardiol 11: 1327–1336, 1988 [DOI] [PubMed] [Google Scholar]
- 49. Kistler PM, Sanders P, Dodic M, Spence SJ, Samuel CS, Zhao C, Charles JA, Edwards GA, Kalman JM. Atrial electrical and structural abnormalities in an ovine model of chronic blood pressure elevation after prenatal corticosteroid exposure: implications for development of atrial fibrillation. Eur Heart J 27: 3045–3056, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Klein LS, Miles WM, Zipes DP. Effect of atrioventricular interval during pacing or reciprocating tachycardia on atrial size, pressure, and refractory period. Contraction-excitation feedback in human atrium. Circulation 82: 60–68, 1990 [DOI] [PubMed] [Google Scholar]
- 51. Koumi SI, Martin RL, Sato R. Alterations in ATP-sensitive potassium channel sensitivity to ATP in failing human hearts. Am J Physiol Heart Circ Physiol 272: H1656–H1665, 1997 [DOI] [PubMed] [Google Scholar]
- 52. Kulan K, Ural D, Komsuoglu B, Agacdiken A, Goldeli O, Komsuoglu SS. Significance of QTc prolongation on ventricular arrhythmias in patients with left ventricular hypertrophy secondary to essential hypertension. Int J Cardiol 64: 179–184, 1998 [DOI] [PubMed] [Google Scholar]
- 53. Lau DH, Mackenzie L, Kelly DJ, Psaltis PJ, Worthington M, Rajendram A, Kelly DR, Nelson AJ, Zhang Y, Kuklik P, Brooks AG, Worthley SG, Faull RJ, Rao M, Edwards J, Saint DA, Sanders P. Short-term hypertension is associated with the development of atrial fibrillation substrate: a study in an ovine hypertensive model. Heart Rhythm 7: 396–404, 2010 [DOI] [PubMed] [Google Scholar]
- 54. Le Grand B, Hatem S, Le Heuzey JY, Deroubaix E, Benitah JP, Coraboeuf E. Pro-arrhythmic effect of nicorandil in isolated rabbit atria and its suppression by tolbutamide and quinidine. Eur J Pharmacol 229: 91–96, 1992 [DOI] [PubMed] [Google Scholar]
- 55. Leaf D, Feig JE, Vasquez C, Riva PL, Yu C, Lader JM, Kontogeorgis A, Peters NS, Fisher EA, Gutstein DE, Morley GE. Connexin40 imparts conduction heterogeneity to atrial tissue. Circ Res 103: 1001–1008, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lorenz E, Terzic A. Physical association between recombinant cardiac ATP-sensitive K+ channel subunits Kir6.2 and SUR2A. J Mol Cell Cardiol 31: 425–434, 1999 [DOI] [PubMed] [Google Scholar]
- 57. Mayet J, Shahi M, McGrath K, Poulter NR, Sever PS, Foale RA, Thom SA. Left ventricular hypertrophy and QT dispersion in hypertension. Hypertension 28: 791–796, 1996 [DOI] [PubMed] [Google Scholar]
- 58. McIntosh MA, Cobbe SM, Kane KA, Rankin AC. Action potential prolongation and potassium currents in left-ventricular myocytes isolated from hypertrophied rabbit hearts. J Mol Cell Cardiol 30: 43–53, 1998 [DOI] [PubMed] [Google Scholar]
- 59. Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev 85: 679–715, 2005 [DOI] [PubMed] [Google Scholar]
- 60. Morley GE, Vaidya D, Samie FH, Lo CW, Delmar M, Jalife J. Characterization of conduction in the ventricles of normal and heterozygous connexin43 knockout mice using optical mapping. J Cardiovasc Electrophysiol 10: 1361–1375, 1999 [DOI] [PubMed] [Google Scholar]
- 61. Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol 1: 62–73, 2008 [DOI] [PubMed] [Google Scholar]
- 62. Nattel S, Shiroshita-Takeshita A, Brundel BJ, Rivard L. Mechanisms of atrial fibrillation: lessons from animal models. Prog Cardiovasc Dis 48: 9–28, 2005 [DOI] [PubMed] [Google Scholar]
- 63. Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature 440: 470–476, 2006 [DOI] [PubMed] [Google Scholar]
- 64. Noma A. ATP-regulated K+ channels in cardiac muscle. Nature 305: 147–148, 1983 [DOI] [PubMed] [Google Scholar]
- 65. Pain T, Yang XM, Critz SD, Yue Y, Nakano A, Liu GS, Heusch G, Cohen MV, Downey JM. Opening of mitochondrial KATP channels triggers the preconditioned state by generating free radicals. Circ Res 87: 460–466, 2000 [DOI] [PubMed] [Google Scholar]
- 66. Pappone C, Oreto G, Rosanio S, Vicedomini G, Tocchi M, Gugliotta F, Salvati A, Dicandia C, Calabro MP, Mazzone P, Ficarra E, Di Gioia C, Gulletta S, Nardi S, Santinelli V, Benussi S, Alfieri O. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation 104: 2539–2544, 2001 [DOI] [PubMed] [Google Scholar]
- 67. Petroff MG, Kim SH, Pepe S, Dessy C, Marban E, Balligand JL, Sollott SJ. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat Cell Biol 3: 867–873, 2001 [DOI] [PubMed] [Google Scholar]
- 68. Philip-Couderc P, Tavares NI, Roatti A, Lerch R, Montessuit C, Baertschi AJ. Forkhead transcription factors coordinate expression of myocardial KATP channel subunits and energy metabolism. Circ Res 102: e20–e35, 2008 [DOI] [PubMed] [Google Scholar]
- 69. Ravelli F, Allessie M. Effects of atrial dilatation on refractory period and vulnerability to atrial fibrillation in the isolated Langendorff-perfused rabbit heart. Circulation 96: 1686–1695, 1997 [DOI] [PubMed] [Google Scholar]
- 70. Saad MJ, Velloso LA, Carvalho CR. Angiotensin II induces tyrosine phosphorylation of insulin receptor substrate 1 and its association with phosphatidylinositol 3-kinase in rat heart. Biochem J 310: 741–744, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Saegusa N, Sato T, Saito T, Tamagawa M, Komuro I, Nakaya H. Kir6.2-deficient mice are susceptible to stimulated ANP secretion: KATP channel acts as a negative feedback mechanism? Cardiovasc Res 67: 60–68, 2005 [DOI] [PubMed] [Google Scholar]
- 72. Sato R, Koumi S. Characterization of the stretch-activated chloride channel in isolated human atrial myocytes. J Membr Biol 163: 67–76, 1998 [DOI] [PubMed] [Google Scholar]
- 73. Shigematsu S, Sato T, Abe T, Saikawa T, Sakata T, Arita M. Pharmacological evidence for the persistent activation of ATP-sensitive K+ channels in early phase of reperfusion and its protective role against myocardial stunning. Circulation 92: 2266–2275, 1995 [DOI] [PubMed] [Google Scholar]
- 74. Shinagawa K, Shi YF, Tardif JC, Leung TK, Nattel S. Dynamic nature of atrial fibrillation substrate during development and reversal of heart failure in dogs. Circulation 105: 2672–2678, 2002 [DOI] [PubMed] [Google Scholar]
- 75. Shinohara T, Takahashi N, Kohno H, Yamanaka K, Ooie T, Wakisaka O, Murozono Y, Taniguchi Y, Torigoe Y, Hara M, Shimada T, Saikawa T, Yoshimatsu H. Mitochondria are targets for geranylgeranylacetone-induced cardioprotection against ischemia-reperfusion in the rat heart. Am J Physiol Heart Circ Physiol 293: H1892–H1899, 2007 [DOI] [PubMed] [Google Scholar]
- 76. Shiroshita-Takeshita A, Mitamura H, Ogawa S, Nattel S. Rate-dependence of atrial tachycardia effects on atrial refractoriness and atrial fibrillation maintenance. Cardiovasc Res 81: 90–97, 2009 [DOI] [PubMed] [Google Scholar]
- 77. Stiles MK, John B, Wong CX, Kuklik P, Brooks AG, Lau DH, Dimitri H, Roberts-Thomson KC, Wilson L, De Sciscio P, Young GD, Sanders P. Paroxysmal lone atrial fibrillation is associated with an abnormal atrial substrate: characterizing the “second factor”. J Am Coll Cardiol 53: 1182–1191, 2009 [DOI] [PubMed] [Google Scholar]
- 78. Suarez E, Bach D, Cadefau J, Palacin M, Zorzano A, Guma A. A novel role of neuregulin in skeletal muscle. Neuregulin stimulates glucose uptake, glucose transporter translocation, and transporter expression in muscle cells. J Biol Chem 276: 18257–18264, 2001 [DOI] [PubMed] [Google Scholar]
- 79. Suzuki M, Li RA, Miki T, Uemura H, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Ogura T, Seino S, Marban E, Nakaya H. Functional roles of cardiac and vascular ATP-sensitive potassium channels clarified by Kir6.2-knockout mice. Circ Res 88: 570–577, 2001 [DOI] [PubMed] [Google Scholar]
- 80. Terzic A, Tung RT, Inanobe A, Katada T, Kurachi Y. G proteins activate ATP-sensitive K+ channels by antagonizing ATP-dependent gating. Neuron 12: 885–893, 1994 [DOI] [PubMed] [Google Scholar]
- 81. Tosaki A, Engelman DT, Engelman RM, Das DK. Diabetes and ATP-sensitive potassium channel openers and blockers in isolated ischemic/reperfused hearts. J Pharmacol Exp Ther 275: 1115–1123, 1995 [PubMed] [Google Scholar]
- 82. Tosaki A, Hellegouarch A. Adenosine triphosphate-sensitive potassium channel blocking agent ameliorates, but the opening agent aggravates, ischemia/reperfusion-induced injury. Heart function studies in nonfibrillating isolated hearts. J Am Coll Cardiol 23: 487–496, 1994 [DOI] [PubMed] [Google Scholar]
- 83. Tseng GN. Cell swelling increases membrane conductance of canine cardiac cells: evidence for a volume-sensitive Cl channel. Am J Physiol Cell Physiol 262: C1056–C1068, 1992 [DOI] [PubMed] [Google Scholar]
- 84. Tsuchiya K, Horie M, Watanuki M, Albrecht CA, Obayashi K, Fujiwara H, Sasayama S. Functional compartmentalization of ATP is involved in angiotensin II-mediated closure of cardiac ATP-sensitive K+ channels. Circulation 96: 3129–3135, 1997 [DOI] [PubMed] [Google Scholar]
- 85. Tucker SJ, Gribble FM, Proks P, Trapp S, Ryder TJ, Haug T, Reimann F, Ashcroft FM. Molecular determinants of KATP channel inhibition by ATP. EMBO J 17: 3290–3296, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Vaidya D, Morley GE, Samie FH, Jalife J. Reentry and fibrillation in the mouse heart: a challenge to the critical mass hypothesis. Circ Res 85: 174–181, 1999 [DOI] [PubMed] [Google Scholar]
- 87. Wang S, Cone J, Liu Y. Dual roles of mitochondrial KATP channels in diazoxide-mediated protection in isolated rabbit hearts. Am J Physiol Heart Circ Physiol 280: H246–H255, 2001 [DOI] [PubMed] [Google Scholar]
- 88. Wang Y, Takashi E, Xu M, Ayub A, Ashraf M. Downregulation of protein kinase C inhibits activation of mitochondrial KATP channels by diazoxide. Circulation 104: 85–90, 2001 [DOI] [PubMed] [Google Scholar]
- 89. Wijffels MC, Kirchhof CJ, Dorland R, Power J, Allessie MA. Electrical remodeling due to atrial fibrillation in chronically instrumented conscious goats: roles of neurohumoral changes, ischemia, atrial stretch, and high rate of electrical activation. Circulation 96: 3710–3720, 1997 [DOI] [PubMed] [Google Scholar]
- 90. Wolleben CD, Sanguinetti MC, Siegl PK. Influence of ATP-sensitive potassium channel modulators on ischemia-induced fibrillation in isolated rat hearts. J Mol Cell Cardiol 21: 783–788, 1989 [DOI] [PubMed] [Google Scholar]
- 91. Wu G, Huang CX, Tang YH, Jiang H, Wan J, Chen H, Xie Q, Huang ZR. Changes of IK,ATP current density and allosteric modulation during chronic atrial fibrillation. Chin Med J (Engl) 118: 1161–1166, 2005 [PubMed] [Google Scholar]
- 92. Yu Q, Larson DF, Slayback D, Lundeen TF, Baxter JH, Watson RR. Characterization of high-salt and high-fat diets on cardiac and vascular function in mice. Cardiovasc Toxicol 4: 37–46, 2004 [DOI] [PubMed] [Google Scholar]
- 93. Yue L, Feng J, Gaspo R, Li GR, Wang Z, Nattel S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res 81: 512–525, 1997 [DOI] [PubMed] [Google Scholar]
- 94. Zarse M, Stellbrink C, Athanatou E, Robert J, Schotten U, Hanrath P. Verapamil prevents stretch-induced shortening of atrial effective refractory period in langendorff-perfused rabbit heart. J Cardiovasc Electrophysiol 12: 85–92, 2001 [DOI] [PubMed] [Google Scholar]
- 95. Zheng S, Noonan WT, Metreveli NS, Coventry S, Kralik PM, Carlson EC, Epstein PN. Development of late-stage diabetic nephropathy in OVE26 diabetic mice. Diabetes 53: 3248–3257, 2004 [DOI] [PubMed] [Google Scholar]