Abstract
Reactive oxygen species (ROS) induce matrix metalloproteinase (MMP) activity that mediates hypertrophy and cardiac remodeling. Adiponectin (APN), an adipokine, modulates cardiac hypertrophy, but it is unknown if APN inhibits ROS-induced cardiomyocyte remodeling. We tested the hypothesis that APN ameliorates ROS-induced cardiomyocyte remodeling and investigated the mechanisms involved. Cultured adult rat ventricular myocytes (ARVM) were pretreated with recombinant APN (30 μg/ml, 18 h) followed by exposure to physiologic concentrations of H2O2 (1–200 μM). ARVM hypertrophy was measured by [3H]leucine incorporation and atrial natriuretic factor (ANF) and brain natriuretic peptide (BNP) gene expression by RT-PCR. MMP activity was assessed by in-gel zymography. ROS was induced with angiotensin (ANG)-II (3.2 mg·kg−1·day−1 for 14 days) in wild-type (WT) and APN-deficient (APN-KO) mice. Myocardial MMPs, tissue inhibitors of MMPs (TIMPs), p-AMPK, and p-ERK protein expression were determined. APN significantly decreased H2O2-induced cardiomyocyte hypertrophy by decreasing total protein, protein synthesis, ANF, and BNP expression. H2O2-induced MMP-9 and MMP-2 activities were also significantly diminished by APN. APN significantly increased p-AMPK in both nonstimulated and H2O2-treated ARVM. H2O2-induced p-ERK activity and NF-κB activity were both abrogated by APN pretreatment. ANG II significantly decreased myocardial p-AMPK and increased p-ERK expression in vivo in APN-KO vs. WT mice. ANG II infusion enhanced cardiac fibrosis and MMP-2-to-TIMP-2 and MMP-9-to-TIMP-1 ratios in APN-KO vs. WT mice. Thus APN inhibits ROS-induced cardiomyocyte remodeling by activating AMPK and inhibiting ERK signaling and NF-κB activity. Its effects on ROS and ultimately on MMP expression define the protective role of APN against ROS-induced cardiac remodeling.
Keywords: reactive oxygen species, adenosine 5′-monophosphate-activated protein kinase, matrix metalloproteinase, cardiomyocyte hypertrophy
an imbalance between antioxidant defenses and excessive reactive oxygen species (ROS), or oxidative stress, may modulate intracellular signaling pathways that are important in cardiac hypertrophy or the failing heart (35). Elevated concentrations of ROS induce cell death and perpetuate more highly reactive radicals that lead to adverse cardiac remodeling (35). In contrast, lower, physiological concentrations of ROS, including the relatively more stable and diffusible hydrogen peroxide (H2O2), serve as second messengers by targeting highly specific intracellular signaling molecules and have been reported to induce cardiomyocyte hypertrophy (28, 54). ROS also mediate the activation of various mitogen-activated protein kinases (MAPK) and nuclear factor-κB (NF-κB), which are implicated in cardiac hypertrophy (18, 44).
Cardiomyocyte hypertrophy occurs in response to cardiac stress (such as hypertension or myocardial infarction) and neurohormonal activation (e.g., ANG II and endothelin) and may initially serve as a compensatory response. However, prolonged cardiac remodeling, in particularly left ventricular (LV) hypertrophy (LVH), leads to LV dilation, contractile dysfunction, and subsequent heart failure (45). ANG II increases ROS production by activating NAD(P)H oxidase, a major source of ROS generation (9, 27).
Adiponectin (APN) is a cytokine (“adipokine”) produced predominantly in adipose tissue and is abundantly present in the plasma especially in lean, healthy individuals (4, 48). However, APN has been reported as being expressed in cardiac myocytes under pathological conditions (11). APN exerts anti-inflammatory effects and is downregulated in obesity, hypertension, and ischemic heart disease (40). Additionally, APN exerts antihypertrophic effects in the cardiomyocyte and protects against ischemia-reperfusion (I/R) injury (50, 51). However, it is unknown if and by what mechanism(s) APN modulates oxidative stress. Recently, Fujita et al. (15) demonstrated that APN protects against ANG II-induced cardiac fibrosis possibly by AMP-activated protein kinase (AMPK)-dependent peroxisome proliferator-activated receptor-α activation. Conversely, others (58, 63) have suggested that the globular APN isoform mediates superoxide-suppressing effects in I/R injury through the reduction of oxidative/nitrative stress independent of AMPK.
We thus sought to test the hypothesis that APN is cardioprotective against ROS-induced cardiac remodeling and also to identify the involved signaling mechanisms. Our results indicate that APN protects cardiomyocytes against in vitro and in vivo adverse cardiac remodeling associated with oxidative stress via an AMPK-extracellular regulated protein kinase (ERK)-NF-κB signaling axis.
MATERIALS AND METHODS
The Boston University School of Medicine Institutional Animal Care and Use Committee approved all of the study procedures related to the handling and surgery of rodents.
Isolation and treatment of adult rat cardiac myocytes.
Adult rat ventricular myocytes (ARVM) were isolated as previously described (46). Briefly, ARVM (90–95% purity) were harvested from adult Sprague-Dawley male rats (∼200–220 g) and nonconfluently plated on laminin-coated (1 μg/cm2; Invitrogen, Carlsbad, CA) plastic culture dishes (Fisher Scientific, Pittsburgh, PA) at a density of 30–50 cells/mm2. Cells were maintained in DMEM (Invitrogen) containing 2 mg/ml BSA, 2 mmol/l l-carnitine, 5 mmol/l creatinine, 5 mmol/l taurine (Sigma-Aldrich), 100 IU/ml penicillin, and 10 g/ml streptomycin (Invitrogen) at 37°C before treatment. ARVM were subjected to physiologic amounts of H2O2 (1–200 μM), which has previously been described to induce hypertrophy (28, 54). In some experiments, ARVM were pretreated with recombinant APN (30 μg/ml) for 18 h.
In vivo murine model.
Male wild-type (WT) and APN-deficient (APN-KO) mice in a C57/BL6 background were used in this study, as described by Fujita et al. (15). WT and APN-KO mice were subjected to subcutaneous ANG II (3.2 mg·kg−1·day−1) or saline infusion using an implanted osmotic minipump (Durect, Cupertino, CA). Mice were killed after 14 days, hearts were dissected, and the LV was snap frozen in liquid nitrogen. Tail cuff blood pressure, noninvasive heart rate, morphology, and echocardiography measurements were measured as previously described (15).
Histology analysis.
Cardiac fibrosis was assessed in mouse hearts using a Masson trichrome stain kit (Sigma-Aldrich, St. Louis, MO). Briefly, tissue samples were frozen in optimum cutting temperature compound. Samples were sectioned at a thickness of 5–10 μM and fixed in 4% paraformaldehyde for 2–5 min at room temperature. Cell nuclei were first stained with Weigert's iron hematoxylin, followed by Beibrich scarlet-acid fuchsin staining for cytoplasm and muscle cells. Slides were then treated with phosphotungstic and phosphomolydic acid and finally stained for collagen with aniline blue. Slides were observed under light microscopy (Olympus 4, ×400 magnification), and images were taken using the MagnaFIRE software. Fibrosis was quantified using ImageJ measuring software (National Institutes of Health).
Chemicals and reagents.
H2O2 (30%, wt/wt) and trichloroacetic acid were purchased from (Sigma-Aldrich). Recombinant APN was prepared as previously described (39). Mouse APN (amino acids 15–247) was cloned into the bacterial expression vector pTrcHisB (Amersham). The histidine-tagged proteins were purified using nickel ion-agarose column, mono Q column, and for removal of lipopolysaccharide, Detoxi-Gel Affinity Pak column (Pierce Scientific, Rockford, IL). NEMO-binding domain binding peptide (NBD) for NF-κB inhibition was purchased from Calbiochem (Rockland, MA).
[3H]leucine incorporation assay.
Protein synthesis as an indication of cardiomyocyte hypertrophy was measured using a [3H]leucine incorporation assay described previously (60). ARVM were plated on sixwell plastic culture dishes (Fisher) and pretreated with or without 30 μg/ml APN for 18 h. Culture media were replaced with media containing 0.5 μC/ml of l-[4,5-3H]leucine (Amersham Biosciences, Piscataway, NJ) with or without 1 μM H2O2 for 48 h. Cells were then washed with PBS and precipitated in 10% TCA for 30 min at 4°C and solubilized in 0.4 M NaOH for 30 min at room temperature. Samples were diluted in Ultima Gold-XR liquid scinitillation cocktail (Perkin Elmer Life Sciences, Waltham, MA), and samples were measured for radioactivity using Beckman LS 3801 liquid scintillation counter. Data are expressed as count per minute.
Protein concentration measurement.
To assess for hypertrophy of ARVMs in the presence of H2O2, total protein concentration was measured as previously described (26). Cells were plated on sixwell plastic culture dishes and pretreated with or without 30 μg/ml APN for 18 h. Cells were then treated with 10 μM H2O2 for 24 h. Following exposure, cell numbers were counted on a 1-mm2 grid under light microscopy. Cells were lysed, scraped, and collected in cold lysis buffer, and total protein concentration was measured by Bradford assay (Bio-Rad Laboratories, Hercules, CA). Protein amounts were divided by the number of viable cells in each well, and data were expressed as a protein-to-cell ratio and normalized to control. To assess the role of NF-κB activity on H2O2-mediated hypertrophy, a similar experiment was conducted using NBD (6.25 μM/30-min pretreatment) to inhibit NF-κB to activation.
In gel zymography to assess MMP activity.
MMP activity was determined by in-gel zymography as described previously (46). Briefly, isolated ARVM were plated in serum free media and pretreated with or without 30 μg/ml APN for 18 h, followed by treatment with 10 μM H2O2 for an additional 18 h. Media was then collected and centrifuged (5000 g/3 min) to remove debris, and the supernatant was concentrated in Centricon YM-30 concentrator (Millipore, Billerica, MA). Protein concentration was determined by Bradford assay (Bio-Rad), and MMP activity (50–75 μg total protein/sample) was measured by in-gel zymography using 10% precast gel with a gelatin substrate (Bio-Rad). Gels were washed in 2.5% Triton X-100 for 30 min, followed by an additional 30-min wash in H2O before digestion at 37°C for 18 h in enzyme buffer (50 mM Tris·HCl, 5 mM CaCl2, and 0.02% NaN3 pH 8.0). Gels were stained in Coomassie Brilliant Blue (Sigma-Aldrich), and unstained regions were quantified by densitometry using ImageJ measuring software (National Institutes of Health). MMP identity was determined by estimated molecular weights against prestained molecular weight markers.
RNA isolation and RT-PCR to assess gene expression.
Atrial natriuretic factor (ANF), brain natriuretic peptide (BNP), and GAPDH mRNA levels from isolated ARVMs were quantified by RT-PCR. Following experimental procedures, total RNA was extracted from ARVM with Qiagen RNeasy Micro kit (Valencia, CA) according to the manufacturer's instructions. cDNA was generated from total RNA using SuperScript III first-strand synthesis kit purchased from Invitrogen. ANF, BNP, and GAPDH transcript expression levels were quantified by StepOne Plus real-time PCR detection systems (Applied Biosystems, Warrington, UK) using SYBR Green Master Mix (Applied Biosystems). ANF and BNP transcript levels were then adjusted relative to GAPDH expression. The PCR primers were manufactured by Integrated DNA Technologies (Coralville, IA), and the rat sequences were as follows: ANF forward: 5′-GGGGGTAGGATTGACAGGAT-3′ and ANF reverse: 5′-GGATCTTTTGCGATCTGCTC-3′; BNP forward: 5′-GCTGCTTTGGGCAGAAGATA-3′ and BNP reverse: 5′-AGAGTCTGCAGCCAGGAGGT-3′; GAPDH forward: 5′-CTGCACCACCAACTGCTTAG-3′ and GAPDH reverse: 5′-CTTCTGAGTGGCAGTGATGG-3′. Additionally, mRNA levels of the NAD(P)H oxidase subunits p22phox and p47phox from mouse ventricles were also measured by RT-PCR. Total RNA was extracted from mouse tissue as described above. Transcript levels of p22phox and p47phox were then adjusted relative to the expression of GAPDH. The PCR primers were manufactured by Integrated DNA Technologies, and the mouse sequences were as follows: p22phox forward: 5′- GTCCACCATGGAGCGATGTG-3′ and p22phox reverse: 5′-CAATGGCCAAGCAGACGGTC-3′; p47phox forward: 5′-GATGTTCCCCATTGAGGCCG-3′ and p47phox reverse: 5′-GTTTCAGGTCATCAGGCCGC-3′; GAPDH forward: 5′-CCAAGGTCATCCATGACAACT-3′ and GAPDH reverse: 5′- GGGCCATCCACAGTCTTCT-3′.
Western blot analysis.
Homogenized cardiac tissue from mouse hearts were subjected to SDS-PAGE (12% Tris-glycine gels, Lonza, Rockland, ME) and Western blotting for MMP-2, MMP-9, tissue inhibitor of MMP-2 (TIMP-2), and TIMP-1 expression. Phosphorylated p-AMPK and p-ERK signaling proteins were also measured. In addition, following H2O2 treatment of ARVM, samples were prepared and subjected to 12% SDS-PAGE and Western blotting for phosphorylated and total ERK, phosphorylated and total p38, and phosphorylated and total JNK, and phosphorylated and total AMPK. The following primary antibodies were used: monoclonal rabbit antibody against p-p44/42 extracellular signal regulated kinase (ERK) (1:1,000), polyclonal rabbit total p44/42 ERK (1:1,000), polyclonal rabbit p-p38 MAPK (1:1,000), polyclonal rabbit total p38 MAPK (1:1,000), polyclonal rabbit p-JNK (1:1,000), polyclonal rabbit total JNK (1:1,000), polyclonal rabbit p-AMPKα (Thr172; 1:1,000), and polyclonal rabbit AMPKα (Cell Signaling Technology, Danvers, MA); mouse anti-MMP-2 monoclonal antibody (1:1,000; Chemicon International, Temecula, CA); rabbit anti-rat MMP-9 polyclonal antibody (1:1,000), rabbit anti-human TIMP-1 polyclonal antibody (1:400), and rabbit anti-TIMP-2 polyclonal antibody (1:5,000; all from Millipore); and mouse anti-GAPDH monoclonal antibody (Thermo Scientific. Rockford, IL). Membranes were then probed with either goat anti-rabbit or goat anti-mouse horseradish peroxidase-conjugated secondary antibodies (1:5,000; Santa Cruz Biotechnology, Santa Cruz, CA). Blots were detected with ECL Western blotting detection reagent (Amersham), and chemiluminescence was quantified by densitometry using ImageJ measuring software (National Institutes of Health). Protein expression was normalized for equal protein loading, and data is expressed in arbitrary units relative to control.
Luciferase reporter assay.
Recombinant adenoviral vector expressing NF-κB transcriptional activation (Ad.NF-κB-Luc) was prepared from pNF-κB-Luc plasmid (Clontech Laboratories, Mountain View, CA). The adenoviral titer was 1013 DNA particles/ml. ARVM were plated on 35-mm tissue culture plates and transfected with Ad.NF-κBLuc at a multiplicity of infection of 50 particles per cell. At 18 h after transfection, cell culture media were changed and cells were pretreated with APN (30 μg/ml for 18 h; control cells were not pretreated with APN). Cells were then stimulated with or without H2O2, and samples were collected and subjected to luciferase assay following the manufacturer's instructions (Stratagene, La Jolle, CA). Briefly, cells were lysed in lysis buffer (25 mM Tris-phosphate, 2 mM DTT, 2 mM 1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid, 10% glycerol, and 1% Triton X-100), scraped into microcentrifuge tubes, and centrifuged at 14,000 g for 10 min. The supernatant was collected, and a sample volume of each lysate was mixed with equal volume of luciferase substrate-assay buffer. Light production was measured using a TD-20e luminometer (Turner Designs, Sunnyvale CA) with an integration time of 5–30 s. Data are expressed in relative light units and normalized to protein concentration of each sample.
Statistical analysis.
All data are expressed as means ± SE; differences among multiple conditions were determined by ANOVA followed by a paired t-test with the Bonferroni correction for multiple comparisons. P values <0.05 were considered significant.
RESULTS
APN attenuates H2O2-induced hypertrophy in ARVM in vitro.
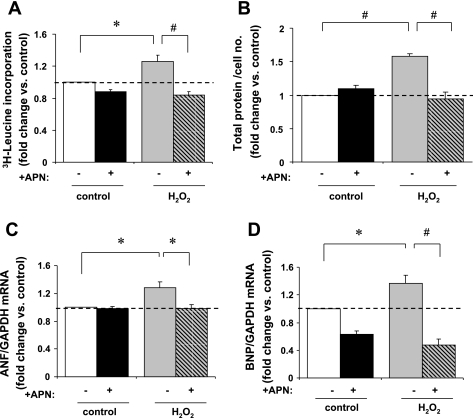
To assess cardiomyocyte hypertrophy in vitro, protein synthesis was measured by [3H]leucine incorporation and total protein content by Bradford Assay. H2O2 significantly increased [3H]leucine incorporation after 48 h by 26 ± 8% vs. controls (P < 0.05). This H2O2-induced [3H]leucine incorporation was completely abrogated by APN pretreatment (P < 0.01 vs. H2O2; Fig. 1A). Total protein content was measured by Bradford assay after H2O2 stimulation, in the presence or absence of APN pretreatment, as an independent measure of cardiomyocyte hypertrophy. H2O2 increased protein quantity per cell by 58 ± 4% vs. baseline control (P < 0.01). This was reduced to baseline levels with APN pretreatment (P < 0.01 vs. non-APN preteated H2O2-stimulated cells; Fig. 1B).
Fig. 1.
In vitro assessment of cardiomyocyte hypertrophy. A: H2O2 stimulation (1 μM/48 h) increased [3H]leucine incorporation in adult rat ventricular myocytes (ARVM) by 26 ± 8% vs. non-H2O2-treated controls (*P < 0.05). Adiponectin (APN) pretreatment (30 μg·ml−1·18 h−1) abrogated [3H]leucine incorporation vs. non-APN pretreated cells (#P < 0.01). Data are expressed relative to the control group as count per minutes per cell (n = 5). B: in myocytes, H2O2-stimulation (10 μM/24 h) increased total protein quantity per cell by 58 ± 4% vs. baseline controls (#P < 0.01). APN pretreatment (30 μg·ml−1·18 h−1) completely abrogated this increase in protein quantity per cell in response to H2O2-stimulation (#P < 0.01; n = 3). C: atrial natriuretic factor (ANF) mRNA expression was increased by 28 ± 9% in response to H2O2 (200 μM/2 h) vs. control (*P < 0.05). Pretreatment with APN completely abrogated ANF mRNA expression in response to H2O2 vs. non-APN pretreated controls (*P < 0.05; n = 3). D: similarly, brain natriuretic peptide (BNP) mRNA expression was increased 36 ± 12% in response to H2O2, (*P < 0.05 vs. control) and decreased to below basal levels by APN pretreatment vs. non-APN pretreated controls (#P < 0.01; n = 4). ANF and BNP levels were normalized to GAPDH mRNA expression, and results are expressed relative to control.
H2O2 also increased ANF and BNP gene expression (Fig. 1, C and D), molecular markers of cardiomyocyte hypertrophy, by 28 ± 8 and 36 ± 12% (P < 0.05 vs. respective controls for both). APN pretreatment abrogated ANF and BNP expression in response to H2O2. Thus APN prevents cardiomyocyte hypertrophy in response to physiologic levels of ROS in vitro.
APN diminishes H2O2-induced MMP-2 and MMP-9 activity in vitro.
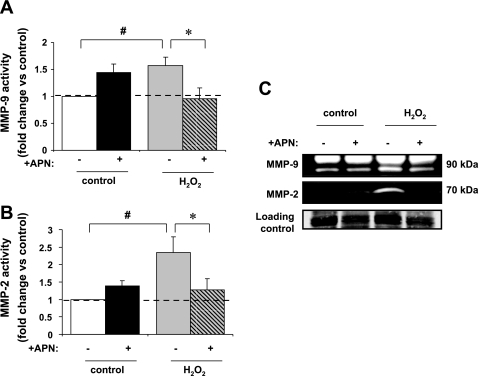
With the use of in-gel zymography, H2O2 treatment increased MMP-9 and MMP-2 activity by a factor of 1.6 ± 0.2 and 2.4 ± 0.5, respectively (P < 0.01 vs. control for both; Fig. 2, A–C). Pretreatment of ARVM with APN (30 μg·ml−1·18 h−1) inhibited both MMP-9 and MMP-2 activities (P < 0.05 vs. H2O2 for both).
Fig. 2.
In-gel zymography assessment of matrix metalloproteinase (MMP)-9 and MMP-2 activity. A: MMP-9 gelatinase activity was localized to ∼90 kDa. MMP gelatinase activity in left ventricular (LV) myocyte-conditioned media was increased 57 ± 16% after treatment with H2O2 (#P < 0.01 vs. control). APN pretreatment completely inhibited the increase in MMP-9 activity in response to H2O2 (*P < 0.05 vs. H2O2 alone; n = 5). B: MMP-2 gelatinase activity was localized to ∼70 kDa. MMP gelatinase activity was increased by 1.4 ± 0.5-fold after treatment with H2O2 (#P < 0.01 vs. control). Increase in MMP-2 activity seen with H2O2 was significantly reduced by APN pretreatment (*P < 0.05 vs. H2O2 alone; n = 6). C: representative gelatin zymogram of MMP activity in conditioned media taken from untreated LV myocytes and those treated with 10 μM H2O2 and/or pretreated with APN (30 μg·ml−1·18 h−1; 105 total cells; n = 5–6). MMP gelatinolytic activity was observed between 100 and 50 kDa, which is consistent with MMP-2 and MMP-9.
APN diminishes H2O2-induced p-ERK MAPK expression in vitro but had no effect on stress-activated kinases.
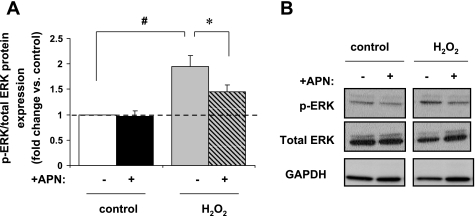
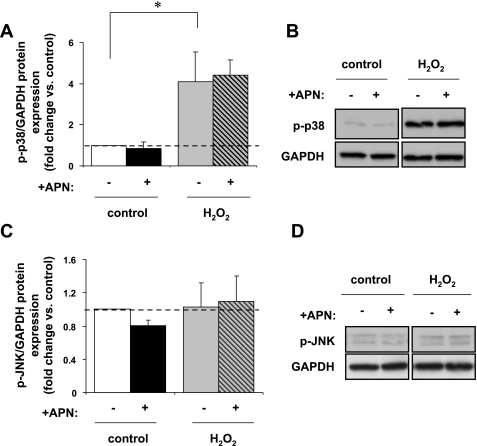
H2O2 treatment increased p-ERK expression in ARVM by a factor 2.2 ± 0.3 (P < 0.01 vs. control). APN pretreatment diminished this p-ERK expression (P < 0.05 vs. H2O2 alone; Fig. 3, A and B). H2O2 enhanced p-p38 expression by a factor of 3.3 ± 0.8 (P < 0.05 vs. control), but APN pretreatment did not affect this p-p38 expression (Fig. 4, A and B). Neither H2O2 nor APN pretreatment had any effect on p-JNK expression (Fig. 4, C and D). Thus APN modulates p-ERK expression but not the stress-activated kinases p38 or JNK in response to physiologic oxidative stress.
Fig. 3.
In vitro protein expression of p-ERK MAPK. A: H2O2 (10 μM/15 min) increased p-ERK protein expression by 2.2 ± 0.3-fold (#P < 0.01 vs. control). This increase was diminished to 1.5-fold with APN pretreatment (*P < 0.05 vs. H2O2 alone; n = 7). Results are expressed relative to control. B: representative Western blot of p-ERK expression. Protein expression of p-ERK is normalized to total ERK.
Fig. 4.
In vitro protein expression of p-p38 and p-JNK MAPK. A and B: H2O2 (10 μM/15 min) increased p-p38 expression 3.1 ± 1.4-fold vs. control (*P < 0.05; n = 5) but was unaffected by APN pretreatment. C and D: additionally, neither H2O2 nor APN pretreatment had any effect on p-JNK protein expression (n = 5). p-p38 and p-JNK are normalized to GAPDH protein expression for equal loading. Results are expressed relative to control.
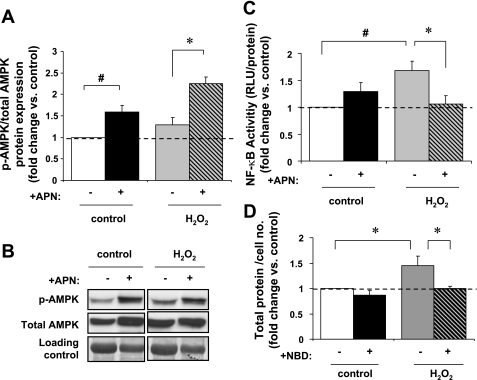
APN increases p-AMPK expression and inhibits H2O2-induced NF-κB activity in vitro.
ARVM were pretreated with APN before stimulation with H2O2. Interestingly, APN increased p-AMPK by 59 ± 15% (P < 0.01) and 81 ± 22% (P < 0.05) in both untreated and H2O2 treated ARVM, respectively (Fig. 5, A and B). H2O2 alone had no effect on p-AMPK expression vs. non-H2O2 controls.
Fig. 5.
In vitro p-AMPK protein expression and NF-κB activity. A: APN pretreatment increased p-AMPK expression in both nonstimulated and H2O2-stimulated (10 μM/60 min) cells by 59 ± 15% (#P < 0.01 vs. control) and 81 ± 22%, respectively (*P < 0.05 vs. H2O2 alone; n = 4). Data are expressed relative to control. B: representative Western blot of p-AMPK expression. Protein expression of p-AMPK is normalized to total AMPK and Coomassie Blue staining for equal loading. C: H2O2 (10 μM/6 h) leads to a 68 ± 17% increase in NF-κB activity as measured by the luciferase reporter assay (#P < 0.01 vs. control). APN pretreatment reduced this activity to near baseline levels (*P < 0.05 vs. H2O2 alone; n = 4). D: ARVM pretreated with NBD (6.25 μM/30 min) had 29 ± 6% less protein quantity/cell (*P < 0.05 vs. non-NBD-H2O2 treated).
To further investigate how APN modulates intracellular signaling pathways that influence cardiomyocyte hypertrophy, we examined in vitro NF-κB activity, a transcription factor implicated in both in vitro and in vivo cardiomyocyte hypertrophy and that is reportedly mediated by ROS (22, 44). NF-κB-Luc expressing ARVM were pretreated with APN before stimulation with H2O2 (Fig. 5C). H2O2 increased NF-κB activity by 68 ± 17% (P < 0.01 vs. control). APN pretreatment diminished H2O2-induced NF-κB activity to almost baseline levels (∼6% above baseline levels; P < 0.05 vs. non-APN-H2O2-stimulated ARVM). In addition, we further investigated the relationship between NF-κB activity and H2O2-mediated hypertrophy in ARVM. Pretreatment of ARVMs with NBD (6.25 μM/30 min) before H2O2 stimulation resulted in 29 ± 6% decrease in protein content per cell (Fig. 5D; P < 0.05 vs. non-NBD-H2O2-stimulated ARVM), therefore demonstrating that the role of NF-κB in H2O2-mediated cardiomyocyte hypertrophy.
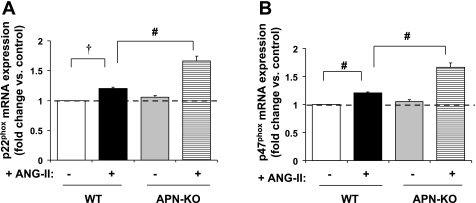
We sought to determine the significance of these findings in an established in vivo animal model of increased oxidative stress using chronic ANG II infusion in APN-KO mice (15). ANG II increases gene expression of NAD(P)H oxidase subunits and induces severe cardiac fibrosis in APN-KO mice (15). APN replacement protected against ANG II-induced cardiac fibrosis (15). As expected, ANG II (3.2 mg·kg−1·day−1) for 14 days induced hypertension and cardiac hypertrophy in APN-KO vs. WT mice. The ANG II-induced cardiac hypertrophy seen in the APN-KO mice may be independent of systolic blood pressure as there was no difference in systolic blood pressure between the APN-KO vs. WT mice (Table 1). The marked increase in cardiac hypertrophy was LVH, since LV wall thickness was measured by echocardiography and was most marked in APN-KO ANG II hearts (Table 2). ANG II increased expression of the NAD(P)H oxidase subunits: p22phox and p47phox in WT mice and was further increased in APN-KO mice (P < 0.01; Fig. 6, A and B) similar to the findings of Fujita et al. (15).
Table 1.
Characteristics of WT and APN-KO mice at 14 days after ANG II infusion
| WT |
APN-KO |
|||
|---|---|---|---|---|
| Saline (n = 3) | ANG II (n = 6) | Saline (n = 3) | ANG II (n = 5) | |
| HR, beats/min | 548 ± 31 | 551 ± 19 | 549 ± 29 | 557 ± 22 |
| SBP, mmHg | 93.1 ± 2.1 | 131.6 ± 3.3* | 94.6 ± 3.1 | 135.2 ± 3.9‡ |
| HW, mg | 107 ± 4 | 170 ± 6* | 112 ± 9 | 206 ± 11†‡ |
| BW, g | 25.6 ± 0.5 | 26.1 ± 0.8 | 25.9 ± 0.6 | 25.7 ± 0.7 |
| HW/BW, mg/g | 4.35 ± 0.06 | 6.71 ± 0.27* | 4.49 ± 0.13 | 8.02 ± 0.12†‡ |
Data are means ± SE. ANG II, angiotensin II; WT, wild type; APN-KO, adiponectin knockout mice; HR, heart rate; SBP, systolic blood pressure; HW, heart weight; BW, body weight.
P < 0.001 vs. WT saline.
P < 0.05 vs. WT ANG II.
P < 0.001 vs. APN-KO saline.
Table 2.
Echocardiography measurements of LV wall thickness in WT and APN-KO mice at 14 days after ANG II infusion
| WT |
APN-KO |
|||
|---|---|---|---|---|
| Saline (n = 5) | ANG II (n = 8) | Saline (n = 5) | ANG II (n = 6) | |
| IVS, mm | 0.72 ± 0.02 | 1.14 ± 0.02* | 0.74 ± 0.03 | 1.43 ± 0.05†‡ |
| LVPW, mm | 0.76 ± 0.03 | 1.18 ± 0.02* | 0.73 ± 0.03 | 1.44 ± 0.06†‡ |
| FS, % | 57 ± 2.6 | 57 ± 2.8 | 57 ± 2.2 | 57 ± 3.3 |
Data are means ± SE. IVS, interventricular septum; LVPW, left ventricular (LV) posterior wall; FS, fractional shortening.
P < 0.001 vs. WT saline.
P < 0.001 vs. APN-KO saline.
P < 0.01 vs. WT ANG II
Fig. 6.
In vivo NAD(P)H oxidase expression. A: ANG II infusion (3.2 30 mg·kg−1·day−1 for 14 days) increased expression of NAD(P)H oxidase subunit p22phox by 20 ± 2% in WT mouse LV (†P < 0.001 vs. WT saline). p22phox expression was further enhanced by an additional 38 ± 6% in APN-KO (#P < 0.01 vs. WT ANG II). B: similarly, ANG II infusion increased p47phox expression by 31 ± 5% in WT mouse LV (#P < 0.01 vs. WT saline) and was further increased by 28 ± 0.5% in APN-KO (#P < 0.01 vs. WT ANG II).
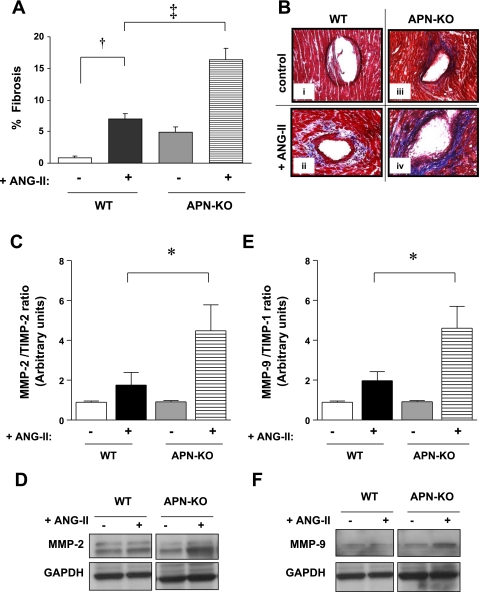
Loss of APN potentiates cardiac fibrosis and MMP activity in the LV of ANG II-infused mice.
Cardiac fibrosis was quantified after Masson trichrome stain was performed. Chronic ANG II infusion increased interstitial and perivascular fibrosis in the LV of WT mice by 7.9-fold (P < 0.001 vs. WT alone; Fig. 7, A and Bii). This increased fibrosis was further enhanced in the LV of APN-KO ANG II hearts by 2.4-fold (P < 0.001 vs. WT ANG II; Fig. 7Biv). Because ROS induces MMP activity (24) and both play a role in pathological cardiac remodeling, we sought to determine myocardial MMP-2 and MMP-9 expression. ANG II infusion increased MMP-2 and MMP-9 expression by 63 ± 3 and 133.3 ± 3%, respectively, in WT hearts (P < 0.05 vs. WT saline for both; Fig. 7, D and F) and further augmented this increase in APN-KO hearts by 44 and 56%, respectively (P < 0.05 vs. APN-KO-saline for both). Neither ANG II infusion nor APN deficiency affected LV TIMP-1 or TIMP-2 expression (data not shown). Since TIMP-2 is the tissue inhibitor of MMP-2 and TIMP-1 for MMP-9, the ratio of the MMP and its inhibitor was reported to indicate an index of net MMP activation. Therefore, the ratio of MMP-2 to TIMP-2 (4.5 ± 1.3 vs. 1.8 ± 0.6) and MMP-9 to TIMP-1 (4.6 ± 1.1 vs. 1.9 ± 0.5) was significantly increased in APN-KO hearts in response to ANG II infusion vs. WT ANG II (P < 0.05 for both; Fig. 7, C and E).
Fig. 7.
In vivo cardiac fibrosis. A: ANG II infusion (3.2 mg·kg−1·day−1 for 14 days) increased fibrosis in WT LV by 7.9-fold (†P < 0.001 vs. WT saline; 7.0 ± 1% vs. 0.9 ± 0.2%). ANG II infusion further enhanced fibrosis in APN-KO (16.4 ± 1.8%) by 2.4-fold (‡P < 0.001 vs. WT ANG II). Data reflect measurements of three sections each for 3 WT saline, 3 APN-KO saline, 6 WT ANG II, and 5 APN-KO ANG II mice. B: representative light microscopy images (×400 magnification); ANG II infusion increased fibrosis in WT ventricles (ii, indicated by aniline blue stain) compared with control (i). LV from APN-KO mice exhibit enhanced fibrosis in response to ANG II infusion (iv) vs. ANG II-infused WT mice (ii). C: ANG II infusion significantly increased the MMP-2-to-TIMP-2 ratio in the LV of APN-KO (*P < 0.05 vs. WT ANG II). D: representative Western blot of MMP-2 expression. E: ANG II infusion significantly increased the MMP-9-to-TIMP-1 ratio in the LV of APN-KO. F: representative Western blot of MMP-9 expression. (*P < 0.05 vs. WT ANG II; n = 3–6).
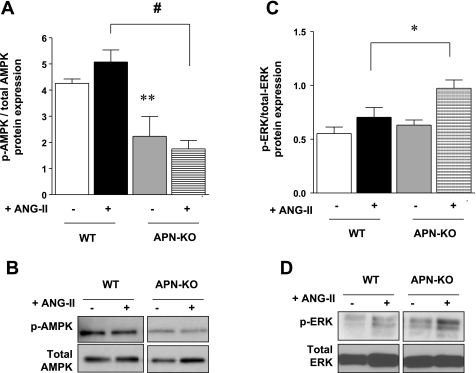
ANG II effects on p-ERK and p-AMPK expression in the LV of APN-deficient mice.
ANG II infusion demonstrated nonsignificant trends to increased expression of p-AMPK and p-ERK in WT hearts (Fig. 8, A–D). Additionally, in the LV of APN-KO ANG II mice, p-AMPK protein expression was significantly decreased by 105 ± 4% (P < 0.001), while p-ERK expression was increased by 38 ± 5% (P < 0.05) vs. WT ANG II. These results suggest that endogenous APN positively regulates AMPK activity, while negatively regulating ERK activity in vivo, supporting the above in vitro data.
Fig. 8.
In vivo protein expression of p-AMPK. A: ventricular p-AMPK expression was significantly decreased in APN-KO ANG II mice by 105 ± 4% (#P < 0.001 vs. WT ANG II), although there was no difference in p-AMPK expression between saline and ANG II-infused hearts of both WT and APN-KO mice. B: representative Western blot of p-AMPK and total AMPK expression. C: ventricular p-ERK expression was increased in APN-KO ANG II mice by 38 ± 5%. D: representative Western blot of p-ERK and total ERK expression. (*P < 0.05 vs. WT ANG II; n = 3–6).
DISCUSSION
Here, we demonstrate that: 1) APN plays a protective role against ROS-induced, adverse cardiomyocyte remodeling; and 2) APN mediates these cardioprotective effects via both positive and negative regulation of AMPK and ERK signaling, respectively, and downstream regulation of NF-κB activity.
Physiological ROS induces hypertrophy.
Multiple factors including systemic hypertension, I/R injury, myocardial infarction, and diabetes all contribute to increased systemic and myocardial ROS production (16, 23, 43). NAD(P)H oxidase is the major enzymatic source for ROS production in cardiomyocytes (18, 52), and its activity is increased in human heart failure (20, 37). Studies (3, 36) indicate that factors such as ANG II, TNFα, and α1-adrenergic receptor agonists, lead to enhanced ROS generation and induce hypertrophy in isolated cardiomyocytes. However, physiological levels of oxidative stress is sufficient to induce cardiomyocyte hypertrophy (1, 28, 57). The dose of H2O2 used in in vitro experiments ranged from 1 to 200 μM, which has previously been described to induce cardiac hypertrophy without immediately affecting cell survival (1, 28, 57). However, others (59) have used greater doses of “physiological” H2O2 (>103-fold) in cell culture when BSA is used because serum albumin scavenges free radicals (19). Additionally, our in vivo studies also demonstrate that ANG II infusion increased two of the subunits of NAD(P)H oxidase (p22phox and p47phox expression), a source of ROS, and was associated with LVH. Thus our data are consistent in that a physiologic level of H2O2 induced cardiomyocyte hypertrophy.
APN ameliorates the effects of ROS on cardiac remodeling.
APN mediates cardioprotective roles against adverse changes in the heart such as fibrosis and hypertrophy (15, 17, 40, 49, 50, 58), but the mechanism may be more complex (38). ANG II induces NAD(P)H oxidase activation, leading to a significant increase in MMP-2, suggesting that ROS play a role in cardiac fibrosis and remodeling (34, 53, 61). Consistent with these findings, our data indicate that APN (in addition to ameliorating LVH) protected against the development of fibrosis and limited matrix remodeling in response to ANG II infusion in vivo by suppressing expression of MMP-2 and MMP-9. There was no effect on TIMP expression, the endogenous inhibitors of MMPs, which is in agreement with our prior findings in aldosterone-infused APN-KO hearts (47). Therefore, the increased MMP-to-TIMP ratio seen in ANG II-infused APN-KO hearts is a measure of net MMP activation and results in an MMP/TIMP stoichiometry that would favor increased myocardial matrix degradation and a prolonged/persistent matrix proteolytic state. Similarly, the loss of endogenous MMP inhibitory control favors prolonged MMP activity within the APN-KO ANG II hearts. This abnormal MMP-to-TIMP balance seen in ANG II-infused hearts is exacerbated in APN-KO ANG II hearts and is associated with the greatest degree of LV remodeling (41, 55).
Our in vitro data indicates that APN prevented ROS-induced cardiomyocyte hypertrophy. Oxidative stress itself induces MMP activity (2, 29), and our in vitro data show that APN modulates H2O2-induced MMP-9 and -2 activities. Taken together, these data provide a link among ROS production (induced by ANG II), cardiac hypertrophy, and MMP activity, suggesting that APN exerts cardioprotective benefits by modulating ROS-mediated signaling.
Physiological ROS induces ERK and not the stress-activated kinases.
ROS can function as second messenger molecules and affect intracellular signaling cascades that subsequently alter cardiomyocyte phenotypes, inducing changes in the myocardium and eventual heart failure (12). ROS activate the ERK, p38 kinase, and JNK members of the MAPK signaling cascades in cardiomyocytes (56). In addition to H2O2 increasing ERK activity and cardiomyocyte hypertrophy (28), in vivo studies with transgenic mice have linked ERK activation with adaptive cardiomyocyte hypertrophy (5). Conversely, APN has been shown to have an inhibitory effect on ERK activity in cardiomyocytes (50). Lack of APN in pressure overload increased cardiac p-ERK expression compared with WT controls, and the addition of recombinant APN reduced norepinephrine-induced ERK phosphorylation in neonatal rat ventricular myocytes (NRVM) (50). In our study, APN prevented ROS-induced ERK phosphorylation in ARVMs and mice deficient in APN had increased p-ERK expression in the LV. JNK and p38 expression was not involved in the signaling pathway in our system, and previous reports (28) indicate that JNK mediates only a proapoptotic response at much higher H2O2 concentrations than that utilized in our system in isolated cardiomyocytes.
APN increases p-AMPK expression and inhibits H2O2-induced NF-κB activity in vitro.
AMPK activity has been described as a negative regulator of cell growth and hypertrophy (25). AMPK inhibits cardiac hypertrophy in phenylephrine-stimulated NRVM (8), as well as in rats subjected to pressure overload (31). APN stimulates AMPK activity in cardiomyocytes both in vitro and in vivo (33, 50). In the present study, although ANG II infusion had no significant effect on AMPK activity in vivo, AMPK activity was significantly diminished in APN-KO mice at baseline. Similarly, the presence of H2O2 at a physiologic, prohypertrophic dose had no effect on AMPK activity, while APN pretreatment induced AMPK phosphorylation. Taken together, this suggests that APN inhibits cardiac hypertrophy as ROS gradually accumulate. It has been reported that APN-mediated activation of AMPK inhibits ERK phosphorylation (15), suggesting a possible APN/AMPK/ERK signaling pathway involved in the inhibition of cardiac hypertrophy (13, 42, 50, 65).
Several reports (10, 21, 22, 30, 44, 62, 66) have implicated NF-κB activation and nuclear translocation in the development of cardiomyocyte hypertrophy in vitro in response to TNF-α, ANG II, endothelin-1, phenylephrine stimulation, and also ROS. Likewise, NF-κB activation is involved in the development of LVH in pressure overload and during ANG II infusion (14, 32). Contradictory studies (6, 10) suggest that ROS may either activate or inhibit NF-κB, indicating perhaps a dual effect of these oxidants on the signaling pathways that mediate NF-κB activation. While higher concentrations of H2O2 have been suggested not to cause NF-κB activation and may even lead to its inhibition, lower concentrations may actually stimulate NF-κB activity (64). Using a luciferase reporter, we showed that physiologic concentrations of H2O2 led to NF-κB activation in ARVM, while pretreatment with APN significantly attenuated NF-κB activity. Others (31) have reported that long-term activation of the AMPK signaling pathway with 5-aminoimidazole 1 carboxamide ribonucleoside mediates downstream inhibition of both ERK signaling and NF-κB activation and attenuates LVH in vivo. Both in vitro and in vivo studies indicate that NF-κB activation and subsequent cardiac hypertrophy and fibrosis occur in response to an ANG II/ROS/MEK/ERK signaling pathway (7, 66). Also, APN inhibited ANG II-mediated NRVM hypertrophy via AMPK-mediated suppression of NF-κB (62). Nevertheless, the involvement of NF-κB activity in response to oxidative stress remains controversial. Further experiments are needed to determine cross-talk between NF-κB and other key signaling factors including ERK and AMPK.
In summary, the findings in this current study indicate that APN protects against adverse cardiomyocyte hypertrophy and LVH in response to the accumulation of ROS and mediates inhibitory effects on MMP expression and activity. Additionally, APN has anti-hypertrophic functions in cardiomyocytes via an interaction with an AMPK/ERK/NF-κB signaling axis.
GRANTS
Supported for this study was provided by National Heart, Lung, and Blood Institute Grants T32-HL-007224 (to E. Essick) and HL-079099, HL-095891, and HL-102631 (to F. Sam).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The recombinant adenoviral vector expressing NF-κB transcriptional activation (Ad.NF-κB-Luc) was provided as a gift by Bingbing Jiang.
REFERENCES
- 1. Aikawa R, Komuro I, Yamazaki T, Zou Y, Kudoh S, Tanaka M, Shiojima I, Hiroi Y, Yazaki Y. Oxidative stress activates extracellular signal-regulated kinases through Src and Ras in cultured cardiac myocytes of neonatal rats. J Clin Invest 100: 1813–1821, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ali MA, Schulz R. Activation of MMP-2 as a key event in oxidative stress injury to the heart. Front Biosci 14: 699–716, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Amin JK, Xiao L, Pimental DR, Pagano PJ, Singh K, Sawyer DB, Colucci WS. Reactive oxygen species mediate alpha-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes. J Mol Cell Cardiol 33: 131–139, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257: 79–83, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, Kitsis RN, Molkentin JD. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J 19: 6341–6350, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Byun MS, Jeon KI, Choi JW, Shim JY, Jue DM. Dual effect of oxidative stress on NF-kappakB activation in HeLa cells. Exp Mol Med 34: 332–339, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Cai J, Yi FF, Bian ZY, Shen DF, Yang L, Yan L, Tang QZ, Yang XC, Li H. Crocetin protects against cardiac hypertrophy by blocking MEK-ERK1/2 signalling pathway. J Cell Mol Med 13: 909–925, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem 279: 32771–32779, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Das S, Otani H, Maulik N, Das DK. Redox regulation of angiotensin II preconditioning of the myocardium requires MAP kinase signaling. J Mol Cell Cardiol 41: 248–255, 2006 [DOI] [PubMed] [Google Scholar]
- 10. de Oliveira-Marques V, Cyrne L, Marinho HS, Antunes F. A quantitative study of NF-kappaB activation by H2O2: relevance in inflammation and synergy with TNF-alpha. J Immunol 178: 3893–3902, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Ding G, Qin Q, He N, Francis-David SC, Hou J, Liu J, Ricks E, Yang Q. Adiponectin and its receptors are expressed in adult ventricular cardiomyocytes and upregulated by activation of peroxisome proliferator-activated receptor-γ. J Mol Cell Cardiol 43: 73–84, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dworakowski R, Anilkumar N, Zhang M, Shah AM. Redox signalling involving NADPH oxidase-derived reactive oxygen species. Biochem Soc Trans 34: 960–964, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Esposito G, Prasad SV, Rapacciuolo A, Mao L, Koch WJ, Rockman HA. Cardiac overexpression of a G(q) inhibitor blocks induction of extracellular signal-regulated kinase and c-Jun NH(2)-terminal kinase activity in in vivo pressure overload. Circulation 103: 1453–1458, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Freund C, Schmidt-Ullrich R, Baurand A, Dunger S, Schneider W, Loser P, El-Jamali A, Dietz R, Scheidereit C, Bergmann MW. Requirement of nuclear factor-kappaB in angiotensin II- and isoproterenol-induced cardiac hypertrophy in vivo. Circulation 111: 2319–2325, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Fujita K, Maeda N, Sonoda M, Ohashi K, Hibuse T, Nishizawa H, Nishida M, Hiuge A, Kurata A, Kihara S, Shimomura I, Funahashi T. Adiponectin protects against angiotensin II-induced cardiac fibrosis through activation of PPAR-alpha. Arterioscler Thromb Vasc Biol 28: 863–870, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Gao L, Mann GE. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res 82: 9–20, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med 6: 27–35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494–501, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Gum ET, Swanson RA, Alano C, Liu J, Hong S, Weinstein PR, Panter SS. Human serum albumin and its N-terminal tetrapeptide (DAHK) block oxidant-induced neuronal death. Stroke 35: 590–595, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, Shah AM. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol 41: 2164–2171, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Higuchi Y, Otsu K, Nishida K, Hirotani S, Nakayama H, Yamaguchi O, Matsumura Y, Ueno H, Tada M, Hori M. Involvement of reactive oxygen species-mediated NF-kappa B activation in TNF-alpha-induced cardiomyocyte hypertrophy. J Mol Cell Cardiol 34: 233–240, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Hirotani S, Otsu K, Nishida K, Higuchi Y, Morita T, Nakayama H, Yamaguchi O, Mano T, Matsumura Y, Ueno H, Tada M, Hori M. Involvement of nuclear factor-kappaB and apoptosis signal-regulating kinase 1 in G-protein-coupled receptor agonist-induced cardiomyocyte hypertrophy. Circulation 105: 509–515, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Hori M, Nishida K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc Res 81: 457–464, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, Uchida K, Arimura K, Egashira K, Takeshita A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res 85: 357–363, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577–590, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Jeong MY, Walker JS, Brown RD, Moore RL, Vinson CS, Colucci WS, Long CS. AFos inhibits phenylephrine-mediated contractile dysfunction by altering phospholamban phosphorylation. Am J Physiol Heart Circ Physiol 298: H1719–H1726, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Suzuki T, Maeta H, Abe Y. Role of NAD(P)H oxidase- and mitochondria-derived reactive oxygen species in cardioprotection of ischemic reperfusion injury by angiotensin II. Hypertension 45: 860–866, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Kwon SH, Pimentel DR, Remondino A, Sawyer DB, Colucci WS. H(2)O(2) regulates cardiac myocyte phenotype via concentration-dependent activation of distinct kinase pathways. J Mol Cell Cardiol 35: 615–621, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Leon H, Bautista-Lopez N, Sawicka J, Schulz R. Hydrogen peroxide causes cardiac dysfunction independent from its effects on matrix metalloproteinase-2 activation. Can J Physiol Pharmacol 85: 341–348, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Li HL, Huang Y, Zhang CN, Liu G, Wei YS, Wang AB, Liu YQ, Hui RT, Wei C, Williams GM, Liu DP, Liang CC. Epigallocathechin-3 gallate inhibits cardiac hypertrophy through blocking reactive oxidative species-dependent and -independent signal pathways. Free Radic Biol Med 40: 1756–1775, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Li HL, Yin R, Chen D, Liu D, Wang D, Yang Q, Dong YG. Long-term activation of adenosine monophosphate-activated protein kinase attenuates pressure-overload-induced cardiac hypertrophy. J Cell Biochem 100: 1086–1099, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Li Y, Ha T, Gao X, Kelley J, Williams DL, Browder IW, Kao RL, Li C. NF-κB activation is required for the development of cardiac hypertrophy in vivo. Am J Physiol Heart Circ Physiol 287: H1712–H1720, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Liao Y, Takashima S, Maeda N, Ouchi N, Komamura K, Shimomura I, Hori M, Matsuzawa Y, Funahashi T, Kitakaze M. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc Res 67: 705–713, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Luchtefeld M, Grote K, Grothusen C, Bley S, Bandlow N, Selle T, Struber M, Haverich A, Bavendiek U, Drexler H, Schieffer B. Angiotensin II induces MMP-2 in a p47phox-dependent manner. Biochem Biophys Res Commun 328: 183–188, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Murdoch CE, Zhang M, Cave AC, Shah AM. NADPH oxidase-dependent redox signalling in cardiac hypertrophy, remodelling and failure. Cardiovasc Res 71: 208–215, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Nakamura K, Fushimi K, Kouchi H, Mihara K, Miyazaki M, Ohe T, Namba M. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-alpha and angiotensin II. Circulation 98: 794–799, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Nediani C, Borchi E, Giordano C, Baruzzo S, Ponziani V, Sebastiani M, Nassi P, Mugelli A, d′Amati G, Cerbai E. NADPH oxidase-dependent redox signaling in human heart failure: relationship between the left and right ventricle. J Mol Cell Cardiol 42: 826–834, 2007 [DOI] [PubMed] [Google Scholar]
- 38. O'Shea KM, Chess DJ, Khairallah RJ, Rastogi S, Hecker PA, Sabbah HN, Walsh K, Stanley WC. Effects of adiponectin deficiency on structural and metabolic remodeling in mice subjected to pressure overload. Am J Physiol Heart Circ Physiol 298: H1639–H1645, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem 279: 1304–1309, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ouchi N, Shibata R, Walsh K. Cardioprotection by adiponectin. Trends Cardiovasc Med 16: 141–146, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peterson JT, Li H, Dillon L, Bryant JW. Evolution of matrix metalloprotease and tissue inhibitor expression during heart failure progression in the infarcted rat. Cardiovasc Res 46: 307–315, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Pimentel DR, Amin JK, Xiao L, Miller T, Viereck J, Oliver-Krasinski J, Baliga R, Wang J, Siwik DA, Singh K, Pagano P, Colucci WS, Sawyer DB. Reactive oxygen species mediate amplitude-dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ Res 89: 453–460, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Puddu P, Puddu GM, Cravero E, Rosati M, Muscari A. The molecular sources of reactive oxygen species in hypertension. Blood Press 17: 70–77, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Purcell NH, Tang G, Yu C, Mercurio F, DiDonato JA, Lin A. Activation of NF-kappa B is required for hypertrophic growth of primary rat neonatal ventricular cardiomyocytes. Proc Natl Acad Sci USA 98: 6668–6673, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rohini A, Agrawal N, Koyani CN, Singh R. Molecular targets and regulators of cardiac hypertrophy. Pharmacol Res 61: 269–280, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Rude MK, Duhaney TA, Kuster GM, Judge S, Heo J, Colucci WS, Siwik DA, Sam F. Aldosterone stimulates matrix metalloproteinases and reactive oxygen species in adult rat ventricular cardiomyocytes. Hypertension 46: 555–561, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Sam F, Duhaney TA, Sato K, Wilson RM, Ohashi K, Sono-Romanelli S, Higuchi A, De Silva DS, Qin F, Walsh K, Ouchi N. Adiponectin deficiency, diastolic dysfunction, and diastolic heart failure. Endocrinology 151: 322–331, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270: 26746–26749, 1995 [DOI] [PubMed] [Google Scholar]
- 49. Shibata R, Izumiya Y, Sato K, Papanicolaou K, Kihara S, Colucci WS, Sam F, Ouchi N, Walsh K. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J Mol Cell Cardiol 42: 1065–1074, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med 10: 1384–1389, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through. Nat Med 11: 1096–1103, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sirker A, Zhang M, Murdoch C, Shah AM. Involvement of NADPH oxidases in cardiac remodelling and heart failure. Am J Nephrol 27: 649–660, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol 280: C53–C60, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Siwik DA, Tzortzis JD, Pimental DR, Chang DL, Pagano PJ, Singh K, Sawyer DB, Colucci WS. Inhibition of copper-zinc superoxide dismutase induces cell growth, hypertrophic phenotype, and apoptosis in neonatal rat cardiac myocytes in vitro. Circ Res 85: 147–153, 1999 [DOI] [PubMed] [Google Scholar]
- 55. Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev 87: 1285–1342, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Sugden PH, Clerk A. Regulation of mitogen-activated protein kinase cascades in the heart. Adv Enzyme Regul 38: 87–98, 1998 [DOI] [PubMed] [Google Scholar]
- 57. Tanaka H, Sakurai K, Takahashi K, Fujimoto Y. Requirement of intracellular free thiols for hydrogen peroxide-induced hypertrophy in cardiomyocytes. J Cell Biochem 89: 944–955, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, Lopez BL, Koch W, Chan L, Goldstein BJ, Ma XL. Adiponectin Cardioprotection After Myocardial Ischemia/Reperfusion Involves the Reduction of Oxidative/Nitrative Stress. Circulation 115: 1408–1416, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Tao R, Karliner JS, Simonis U, Zheng J, Zhang J, Honbo N, Alano CC. Pyrroloquinoline quinone preserves mitochondrial function and prevents oxidative injury in adult rat cardiac myocytes. Biochem Biophys Res Commun 363: 257–262, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Thaik CM, Calderone A, Takahashi N, Colucci WS. Interleukin-1 beta modulates the growth and phenotype of neonatal rat cardiac myocytes. J Clin Invest 96: 1093–1099, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Viappiani S, Nicolescu AC, Holt A, Sawicki G, Crawford BD, Leon H, van MT, Schulz R. Activation and modulation of 72kDa matrix metalloproteinase-2 by peroxynitrite and glutathione. Biochem Pharmacol 77: 826–834, 2009 [DOI] [PubMed] [Google Scholar]
- 62. Wang C, Li L, Zhang ZG, Fan D, Zhu Y, Wu LL. Globular adiponectin inhibits angiotensin II-induced nuclear factor kappaB activation through AMP-activated protein kinase in cardiac hypertrophy. J Cell Physiol 222: 149–155, 2010 [DOI] [PubMed] [Google Scholar]
- 63. Wang Y, Tao L, Yuan Y, Lau WB, Li R, Lopez BL, Christopher TA, Tian R, Ma XL. Cardioprotective effect of adiponectin is partially mediated by its AMPK-independent antinitrative action. Am J Physiol Endocrinol Metab 297: E384–E391, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wu M, Bian Q, Liu Y, Fernandes AF, Taylor A, Pereira P, Shang F. Sustained oxidative stress inhibits NF-kappaB activation partially via inactivating the proteasome. Free Radic Biol Med 46: 62–69, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xiao L, Pimental DR, Amin JK, Singh K, Sawyer DB, Colucci WS. MEK1/2-ERK1/2 mediates alpha1-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes. J Mol Cell Cardiol 33: 779–787, 2001 [DOI] [PubMed] [Google Scholar]
- 66. Zou XJ, Yang L, Yao SL. Propofol depresses angiotensin II-induced cardiomyocyte hypertrophy in vitro. Exp Biol Med (Maywood) 233: 200–208, 2008 [DOI] [PubMed] [Google Scholar]