Abstract
Current rodent models of ischemia/infarct or pressure-volume overload are not fully representative of human heart failure. We developed a new model of congestive heart failure (CHF) with both ischemic and stress injuries combined with fibrosis in the remote myocardium. Sprague-Dawley male rats were used. Ascending aortic banding (Ab) was performed to induce hypertrophy. Two months post-Ab, ischemia-reperfusion (I/R) injury was induced by ligating the left anterior descending (LAD) artery for 30 min. Permanent LAD ligation served as positive controls. A debanding (DeAb) procedure was performed after Ab or Ab + I/R to restore left ventricular (LV) loading properties. Cardiac function was assessed by echocardiography and in vivo hemodynamic analysis. Myocardial infarction (MI) size and myocardial fibrosis were assessed. LV hypertrophy was observed 4 mo post-Ab; however, systolic function was preserved. LV hypertrophy regressed within 1 mo after DeAb. I/R for 2 mo induced a small to moderate MI with mild impairment of LV function. Permanent LAD ligation for 2 mo induced large MI and significant cardiac dysfunction. Ab for 2 mo followed by I/R for 2 mo (Ab + I/R) resulted in moderate MI with significantly reduced ejection fraction (EF). DeAb post Ab + I/R to reduce afterload could not restore cardiac function. Perivascular fibrosis in remote myocardium after Ab + I/R + DeAb was associated with decreased cardiac function. We conclude that Ab plus I/R injury with aortic DeAb represents a novel model of CHF with increased fibrosis in remote myocardium. This model will allow the investigation of vascular and fibrotic mechanisms in CHF characterized by low EF, dilated LV, moderate infarction, near-normal aortic diameter, and reperfused coronary arteries.
Keywords: echocardiography, myocardial fibrosis, experimental models heart failure, hypertrophy and myocardial infarction.
congestive heart failure (CHF) is caused by conditions that reduce cardiac output through ischemic damage, increased afterload, or restrictive disease such as myocardial infarction (MI), hypertension, and amyloidosis, respectively (16, 24). Multiple factors, including MI size and location, myocardial conduction, calcium handling disturbances, neurohumoral activation, altered preload/afterload, and inflammation, can all contribute to the development of heart failure (16, 32).
Left anterior descending (LAD) coronary artery ligation to induce MI and aortic constriction to induce pressure overload are the most widely used models of heart failure in rats (2). Left ventricular (LV) performance after ischemia-reperfusion (I/R) injury or permanent LAD ligation is directly related to infarct size. Small infarcts induced by I/R injury are characterized by minimal impairment of LV function, whereas moderate to large infarcts from LAD ligation exhibit systolic failure with substantial scarring (32). However, the pathophysiology of permanent LAD ligation is far different from atherosclerotic-induced coronary artery narrowing in human patients. Many of these patients have a long-standing history of hypertension (18, 24).
In parallel, aortic constriction induces LV pressure overload, leading to compensatory hypertrophy (9). Unfortunately, the proportion of rats progressing to CHF varies widely because of banding severity, location, rodent strain, age, and time course (21, 26, 29, 34, 37). Moderate constriction (>50% of aortic diameter) does induce hypertrophy, but not CHF, whereas severe aortic banding produces acute heart failure and death following the surgical procedure, which makes it difficult to study long-term, chronic processes of myocardial remodeling, fibrosis, and subsequent systolic failure. It is therefore critical to develop a new model of CHF, with low ejection fraction, near-normal afterload, perfusable coronary arteries, and small MI. These clinical characteristics reflect chronic ischemic heart disease (31).
In the present study, we hypothesized that aortic banding coupled with myocardial I/R injury could significantly and reproducibly accelerate heart failure progression. We developed a CHF model reflecting the clinical paradigm seen in CHF patients with hypertension and ischemic heart disease. We characterized the morphological and hemodynamic alterations in rats with aortic banding, debanding, myocardial I/R, or a combination of these techniques. Additionally, this novel CHF model enables the study of fibrotic mechanisms involved in the development of CHF. Finally, we also explored the role of perivascular fibrosis in remote myocardium in the development of CHF.
METHODS
All procedures were followed by the recommendations of the Guide for the Care and Use of Laboratory Animals (Department of Health and Human Services publication no. NIH 78–23, 1996) and were approved by the Mount Sinai School of Medicine Animal Care and Use Committee.
Animal Protocol
Aortic banding (Ab) was performed in male Sprague-Dawley rats (180–200 g) by constricting the ascending aorta with a 4–0 suture against a PE-50 tube (outside diameter = 0.965 mm) through the right thoracotomy at the second intercostal space. Two months after Ab, rats underwent an LAD ligation for 30 min followed by reperfusion to induce I/R injury (5). Briefly, after a left thoracotomy, the LAD was firmly ligated with a 6–0 silk suture placed 4 mm below the tip of the left atrium. Successful ligation of the LAD was verified by visual inspection of the LV apex. After 30 min of occlusion, the suture was released to achieve reperfusion. For permanent ligation (8), the suture was kept in place. One month after I/R, the rats underwent a third thoracotomy at the upright side of the sternum. The roots of two to approximately four ribs were cut along the right side of the sternum to expose the aorta. The Ab suture was cut with microdissection scissors and separated with cotton-tipped applicators. For all surgical procedures, anesthesia was induced by intraperitoneal administration of pentobarbital (60 mg/kg). Animals underwent intratracheal intubation and mechanical ventilation. Postsurgery, 0.02 mg/kg buprenorphine was administered one time daily for 3 days after the procedure, and the frequency of medicine was increased if the animals had the symptom of serious pain.
Rats were divided into seven groups as follows: 1) control, sham operation (n = 13), 2) Ab for 4 mo (Ab-4m, n = 10), 3) Ab for 3 mo followed by aortic debanding for 1 mo (Ab + DeAb, n = 9), 4) 30 min ischemia and 2 mo reperfusion (I/R-2m, n = 11), 5) permanent LAD ligation for 2 mo (MI-2m, n = 11), 6) Ab for 2 mo followed by I/R for 2 mo (Ab + I/R, n = 18), and 7) Ab for 2 mo plus 30 min of I/R for 1 mo followed by aortic debanding for 1 mo (Ab + I/R + DeAb, n = 13). The surgical mortalities are summarized in Table 1. Ab + I/R + DeAb combined mortality was 62%.
Table 1.
Surgical mortality in different groups
| Mortality, % |
|||||
|---|---|---|---|---|---|
| Surgical Procedure | Animal No. | Surgical Death | Single procedure | Accumulative | |
| Control | Sham operate | 13 | 0 | 0 | |
| Ab | Aortic banding | 86 | 17 | 20 | |
| Ab + DeAb | Debanding after Ab | 11 | 2 | 18 | 34 |
| I/R-2m | Ischemia-reperfusion | 14 | 3 | 21 | |
| MI-2m | LAD permanent ligation | 18 | 7 | 39 | |
| Ab + I/R | I/R on Ab rats | 45 | 14 | 31 | 45 |
| Ab + I/R + DeAb | DeAb on Ab + I/R rats | 19 | 6 | 32 | 62 |
MI, myocardial infarction; LAD, left anterior descending artery. Calculation for accumulative mortality of multiprocedures is as follows: Ab + DeAb, (11/0.8 − 11 × 0.82)/(11/0.8) × 100 = 34%; Ab + I/R, (45/0.8–45 × 0.69)/(45/0.8) × 100 = 45%; and Ab + I/R + DeAb, (19/0.69/0.8 − 19 × 0.68)/(19/0.69/0.8) × 100 = 62%.
Echocardiographic measurements.
The animals were sedated by intraperitoneal injection of ketamine (40 mg/kg) for all measurements. At intervals of 1, 2, and 4 mo post-Ab, before I/R, DeAb, and hemodynamics, serial echocardiograms were performed using a 14-MHz transducer as previously described (5, 20). LV volume was obtained in two-dimensional mode by taking the measurements of short-axis cross-sectional areas (A) and LV length (L) (V = 5/6AL, diastolic and systolic separately) (13).
In vivo hemodynamics.
Hemodynamic measurements were acquired and analyzed using IOX software (EMKAtech). LV pressure-volume (PV) analysis was conducted as previously described (30). Briefly, rats were anesthetized and maintained on isoflurane (1–2%). A thoracotomy was performed to expose the heart and provide access for an apical approach for PV conductance catheter placement (1.9 Fr; Scisense, Ontario, Canada). The inferior vena cava was transiently occluded to reduce preload to obtain load-independent PV relationships. Finally, myocardial parallel conductance was calculated via intravenous infusion of 30% NaCl (50 μl) to determine absolute ventricular volumes.
Histological analysis.
Hearts were harvested after the final hemodynamic measurement. The hearts were perfused with 30 ml of cold PBS with 0.1 ml of 1% heparin. The LV was cut from the root of the pulmonary artery to the ventricular apex and photographed. The longitudinal left half of the LV and aorta were embedded in optimum-cutting temperature (OCT) medium. The right half of the LV was cut along the border area of infarction, frozen, and then stored at −80°C. MI size and the cross-sectional area of the aorta were quantified using Image-Pro software (Media Cybernetics, Bethesda, MD). Two slides (collected at a 100-μm interval) from each frozen OCT sample were stained with Masson's Trichrome (7) and digitally imaged. The fibrosis in perivascular space in the remote myocardium was measured according to Ito et al. (15). Digital images were processed for 8–10 vessels (luminal diameter 20∼100 μm) in normal/remote areas of each section. The fibrotic areas within the perivascular space and vessel diameter were quantified using an Applied Image Analysis Micrometer.
Quantitative Real-Time PCR
Atrial natriuretic factor (ANF) and brain natriuretic peptide (BNP) mRNA levels were measured by quantitative real-time PCR of cDNA (8). Total RNA (2 μg) was extracted from remote regions by using Trizol (GIBCO-BRL) and reverse transcribed to cDNA (Applied Biosystems). Real-time PCR was performed using an ABI Prism 7500 Sequence Detection system with SYBR Green (Bio-Rad). Fragments were amplified for 40 cycles with the following specific primers: ANF forward: ACCTGCTAGACCACCTGGAGGAG; ANF reverse: CCTTGGCTGTTATCTTCGGTACCG; BNP forward: GCTGCTTTGGGCACAAGATAG; and BNP reverse: GGTCTTCCTACAACAACTTCA.
Western Blot
Western blotting of myocardial sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) 2a expression levels was performed by using a primary anti-SERCA2a polyclonal antibody (1:3,000; 21st Century Biochemicals) and a secondary antibody conjugated with alkaline phosphatase (1:10,000; Sigma). Alkaline phosphatase activity was visualized with nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate substrate (Boehringer-Mannheim) (6).
Statistics
Variables are expressed as means ± SE. One-Way ANOVA followed by Tukey's Multiple-Comparison Test was performed to compare experimental groups. Correlation data were analyzed by regression, using GraphPad Prism software. P values <0.05 were considered statistically significant.
RESULTS
Cardiac Morphological Changes Due to Concomitant Pressure Overload and I/R Injury
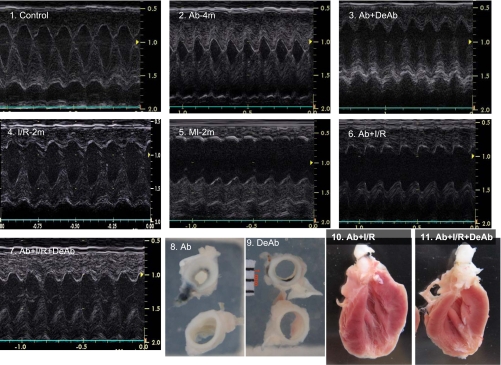
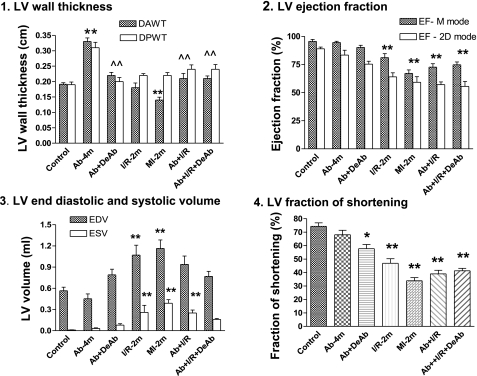
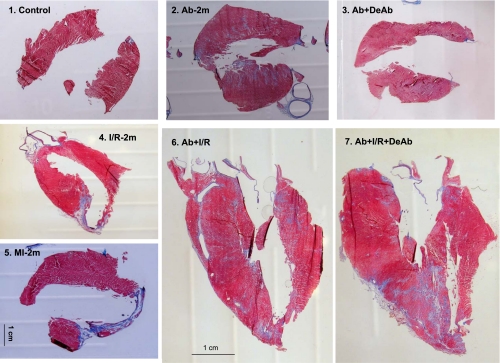
After banding surgery, the aortas were constricted respectively to 52% in diameter and 28% in cross-sectional area relative to the normal aorta with a body weight of 543 ± 20 g. Deconstriction partly restored the ascending aortic diameter and cross-sectional area to 71 and 54% of normal (Tables 2 and 3). Constriction of the ascending aorta for 4 mo induced myocardial hypertrophy. Deconstriction of the aorta generated a regression of the hypertrophic LV (Table 3 and Figs. 1 and 2.1). Although the LV wall thickness was not changed significantly in the I/R-2m group, it did significantly decrease in MI-2m rats. Echocardiography in M-mode indicated that Ab + I/R and Ab + I/R + DeAb had no significant LV wall thinning, even though direct imaging evidenced the MI (Figs. 1 and 2). Direct imaging on longitudinally opened LV indicated that LAD permanent ligation for 2 mo resulted in a MI encompassing 21% of the LV area. The MI size was 15, 15, and 13% of the LV area in I/R-2m, Ab + I/R, and Ab + I/R + DeAb groups, respectively (Table 3).
Table 2.
Heart functions by echocardiography
| Control | Ab-4m | Ab + DeAb | I/R-2m | MI-2m | Ab + I/R | Ab + I/R + DeAb | |
|---|---|---|---|---|---|---|---|
| n | 13 | 10 | 9 | 11 | 11 | 18 | 13 |
| HR, beats/min | 419 ± 6 | 402 ± 12 | 405 ± 12 | 411 ± 9 | 390 ± 7 | 393 ± 8 | 404 ± 7 |
| Banded AO, cm | 0.36 ± 0.01 | 0.19 ± 0.01** | 0.26 ± 0.01*∧ | 0.37 ± 0.01 | 0.34 ± 0.01 | 0.17 ± 0.01** | 0.25 ± 0.01*∧ |
| Normal AO, cm | 0.36 ± 0.01 | 0.35 ± 0.01 | 0.36 ± 0.01 | 0.37 ± 0.01 | 0.34 ± 0.01 | 0.36 ± 0.01 | 0.37 ± 0.01 |
| AO banded ratio | 100 ± 0.01 | 52 ± 2.6** | 71 ± 3.8**∧∧ | 100 ± 0.01 | 100 ± 0.01 | 47 ± 1.1** | 68 ± 2.4**∧∧ |
| LVIDd, cm | 0.61 ± 0.02 | 0.56 ± 0.03 | 0.71 ± 0.03 | 0.78 ± 0.04** | 0.78 ± 0.02** | 0.73 ± 0.03 | 0.69 ± 0.02 |
| LVIDs, cm | 0.14 ± 0.01 | 0.19 ± 0.03 | 0.31 ± 0.03* | 0.42 ± 0.04** | 0.50 ± 0.02** | 0.42 ± 0.04** | 0.39 ± 0.01** |
| DPWT, cm | 0.19 ± 0.01 | 0.31 ± 0.02** | 0.20 ± 0.01∧∧ | 0.22 ± 0.01 | 0.21 ± 0.01 | 0.24 ± 0.02∧ | 0.24 ± 0.02∧ |
| SPWT, cm | 0.40 ± 0.01 | 0.46 ± 0.03 | 0.38 ± 0.02 | 0.37 ± 0.02 | 0.31 ± 0.01 | 0.38 ± 0.02 | 0.39 ± 0.03 |
Values are means ± SE; n, no. of rats. Ab-4m, aortic banding for 4 mo; I/R-2m, 30 min ischemia and 2 mo reperfusion; MI-2m, myocardial-infarcted areas in I/R-2m and permanent LAD ligation for 2 mo; HR, heart rate; AO, aorta diameter; LVIDd, left ventricular (LV) internal diameter, diastole, LVIDs, LV internal diameter, systole; DPWT, diastolic posterior wall thickness; SPWT, systolic posterior wall thickness.
P < 0.05 and
P < 0.01 compared with control.
P < 0.05 and
P < 0.01 compared with Ab-4m.
Table 3.
Cardiac morphology by direct imaging
| Control | Ab-4m | Ab + DeAb | I/R-2m | MI-2m | Ab + I/R | Ab + I/R + DeAb | |
|---|---|---|---|---|---|---|---|
| n | 8 | 8 | 9 | 9 | 11 | 11 | 11 |
| MI, % LV area | 0 ± 0 | 0 ± 0 | 0 ± 0 | 15 ± 1.3** | 21 ± 0.9**# | 15 ± 1.3** | 13 ± 1.2** |
| AO, mm2 | 4.4 ± 0.3 | 5.0 ± 0.3 | 4.9 ± 0.4 | 4.6 ± 0.2 | 4.9 ± 0.3 | 5.3 ± 0.3 | 6.1 ± 0.3 |
| Ab, mm2 | 4.4 ± 0.3 | 1.4 ± 0.2** | 2.8 ± 0.3**∧∧ | 4.6 ± 0.3 | 4.9 ± 0.3 | 1.3 ± 0.1** | 3.5 ± 0.2**∧∧ |
| Ab-to-AO ratio | 1.00 ± 0 | 0.28 ± 0.01** | 0.54 ± 0.02**∧∧ | 1.00 ± 0 | 1.00 ± 0 | 0.25 ± 0.01** | 0.58 ± 0.02**∧∧ |
| LV, g | 1.1 ± 0.1 | 1.8 ± 0.1** | 1.2 ± 0.1∧∧ | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.8 ± 0.1** | 1.4 ± 0.1*∧ |
| Body wt, g | 542 ± 48 | 609 ± 16 | 550 ± 14 | 541 ± 24 | 530 ± 13 | 578 ± 22 | 605 ± 29 |
| LV-to-body wt ratio | 2.0 ± 0.1 | 3.0 ± 0.1** | 2.2 ± 0.1∧∧ | 2.2 ± 0.1 | 2.2 ± 0.2 | 3.1 ± 0.2** | 2.4 ± 0.1∧∧ |
Values are means ± SE; n, no. of rats. AO, normal aortic cross-sectional area; Ab, banded aortic cross-sectional area.
P < 0.05 and
P < 0.01 compared with control.
P < 0.05 compared with I/R-2m, Ab + I/R, and Ab + I/R + DeAb.
P < 0.05 and
P < 0.01 compared with Ab-4m.
Fig. 1.
Echocardiography and histological imaging of hearts and aortas. 1–7: Examples of LV echocardiography in different animal groups. 8 and 9: Cross-sectional aortic area. 10 and 11: Imaging on myocardial infarction with aortic banding and debanding. Ab-4m, aortic banding for 4 mo; Ab + DeAb, Ab for 3 mo followed by aortic debanding for 1 mo; I/R-2m, 30 min ischemia and 2 mo reperfusion; MI-2m, permanent left anterior descending artery ligation for 2 mo; Ab + I/R, aortic banding for 2 mo followed by I/R for 2 mo; Ab + I/R + DaAb, aortic banding for 2 mo plus 30 min of I/R for 1 mo followed by aortic debanding for 1 mo.
Fig. 2.
Cardiac functions by echocardiography. 1: Left ventricular (LV) wall thickness, diastolic anterior wall thickness (DAWT), and diastolic posterior wall thickness (DPWT); 2: LV ejection fraction; 3: LV end diastolic volume (EDV) and end systolic volume (ESV); 4: LV fraction of shortening. EF, ejection fraction; 2D, two dimensional. Means ± SE. *P < 0.05 and **P < 0.01 compared with control. ∧∧P < 0.01 compared with Ab-4m.
Echocardiographic Assessment of Heart Function
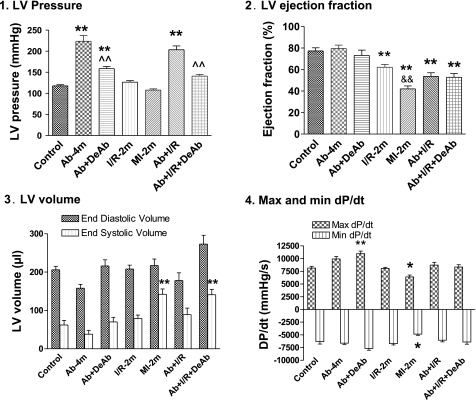
Ejection fraction (EF) and fractional shortening (FS) were preserved in Ab-4m rats, with a significant increase of LV pressure (Ab-4m = 224 ± 14 mmHg vs. control rats 118 ± 3 mmHg; Figs. 2 and 3). DeAb caused a slight decrease in EF and FS. LV pressure in the DeAb group reflected the character of the partial release of aortic constriction (158 ± 6 mmHg; Fig. 1.9 and 1.11). I/R-2m resulted in a moderate decrease in heart function. MI-2m and Ab + I/R had significantly decreased EF and FS. Ab + I/R + DeAb exhibited continual low cardiac function (Figs. 2 and 3).
Fig. 3.
Heart functions by in vivo hemodynamics. 1: LV pressure. 2: LV ejection fraction; 3: LV end diastolic and systolic volume. 4: LV maximum (Max) and minimum (min) dP/dt. Means ± SE. *P < 0.05 and **P < 0.01 compared with control. ∧∧P < 0.01 compared with Ab-4m. &&P < 0.01 compared with I/R-2m.
Ab + I/R + DeAb Injury Leads to Sustained Ventricular Dilatation and Systolic Dysfunction
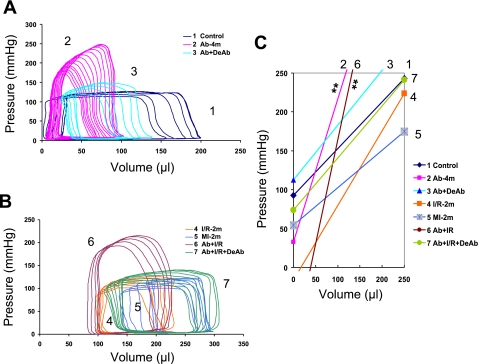
The cardiac hemodynamic changes could be characterized by the LV PV relationship: end systolic pressure-volume relationship (ESPVR, LV contractility), peak systolic LV pressure, stroke volume [end diastolic volume (EDV) − end systolic volume (ESV)], EF (stroke volume/EDV), and LV geometric and mechanic integrity (ESV and EDV). Figure 4 illustrates the complex changes of ESPVR in all groups that can be summarized by: 1) ESPVR slope increases in the Ab-4m group with higher pressure and lower LV systolic and diastolic volume; 2) ESPVR slope shifts toward normal after DeAb in the Ab + DeAb group; 3) ESPVR slope decreases in the I/R-2m group and is the lowest in MI-2m animals having increased LV systolic and diastolic volume; 4) Ab + I/R has elevated ESPVR slopes and higher LV pressures relative to the I/R-2m group and higher systolic and diastolic volume than Ab-4m; and 5) ESPVR slope shifts toward normal after DeAb in the Ab + I/R + DeAb group with a significant increase in LV end systolic and diastolic volume. The LV pressure remained higher in the Ab + I/R + DeAb group relative to the I/R-2m, MI-2m, and control groups (Figs. 3 and 4 and Table 4).
Fig. 4.
LV end systolic pressure-volume relationship. A and B: pressure-volume (P-V) loop during transient occlusion of thoracic inferior vena cava. C: ESPVR, mean slope, and V0. **P < 0.01 compared with control. V0, ESPVR intercept on x-axis when no pressure or no volume is generated.
Table 4.
In vivo hemodynamics
| Control | AB-4m | Ab + DeAb | I/R-2m | MI-2m | Ab + I/R | AB + I/R + DeAb | |
|---|---|---|---|---|---|---|---|
| n | 8 | 7 | 7 | 9 | 9 | 10 | 10 |
| Heart rate, beats/min | 328 ± 7 | 363 ± 25 | 372 ± 7 | 382 ± 16 | 329 ± 7 | 331 ± 12 | 344 ± 13 |
| Minimum pressure, mmHg | 3 ± 1 | 6 ± 0* | 5 ± 1 | 4 ± 0 | 4 ± 1 | 4 ± 1 | 2 ± 1 |
| EDP, mmHg | 8 ± 1 | 10 ± 2 | 9 ± 2 | 6 ± 1 | 8 ± 1 | 10 ± 3 | 7 ± 1 |
| Tau (Weiss), ms | 9.4 ± 0.3 | 8.5 ± 0.6 | 8.3 ± 0.2 | 8.3 ± 0.3 | 10.2 ± 0.5 | 10.2 ± 0.7 | 9.0 ± 0.3 |
| Stroke volume, μl | 187 ± 19 | 99 ± 11** | 164 ± 19 | 130 ± 6 | 95 ± 7** | 102 ± 7** | 151 ± 20 |
| CO, ml/min | 60.5 ± 5 | 37.5 ± 5** | 60.9 ± 7 | 63.3 ± 8 | 31.4 ± 3** | 36.3 ± 3* | 58.5 ± 7 |
| EDPVR slope | 0.04 ± 00 | 0.09 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.08 ± 0.02** | 0.16 ± 0.02** | 0.06 ± 0.2 |
| EDPVR intercept | −9 ± 24 | 126 ± 25 | 72 ± 28 | 168 ± 14 | 108 ± 29 | 128 ± 17 | 177 ± 33 |
| Emax, mmHg/μl | 2.0 ± 0.53 | 4.4 ± 1.14* | 3.4 ± 0.98 | 3.3 ± 0.42 | 1.8 ± 0.34 | 7.2 ± 1.42 | 3.2 ± 0.63 |
| Ea, mmHg/μl | 0.67 ± 0.06 | 2.34 ± 0.23** | 1.02 ± 0.11 | 0.83 ± 0.10 | 1.00 ± 0.12 | 1.32 ± 0.26* | 0.74 ± 0.12 |
Values are means ± SE; n, no. of rats. EDP, end diastolic pressure; Tau, time of isovolumic relaxation; CO, cardiac output; EDPVR, end diastolic pressure-volume relationship; Emax, maximal ventricular systolic elastance; Ea, arterial systolic elastance.
P < 0.05 and
P < 0.01 compared with control.
Perivascular Fibrosis is Associated With Systolic Deterioration
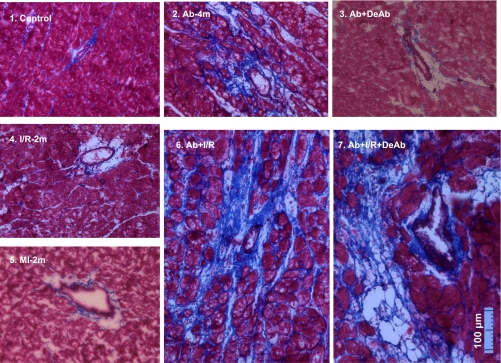
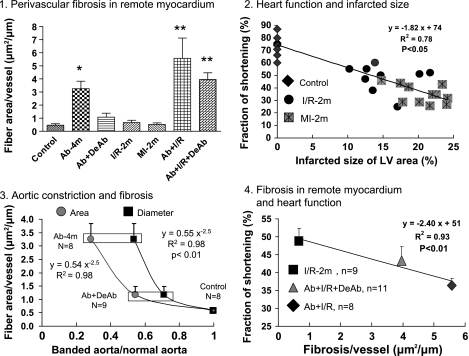
Ab for 4 mo resulted in collagen fiber development around small vessels and in myocardial extracellular matrix. In the Ab + DeAb group, collagen fiber accumulation was decreased. Fibrous expression in I/R-2m and MI-2m groups was mainly located in infarcted and border areas but not in remote myocardium. Ab + I/R and Ab + I/R + DeAb groups also developed elevated collagen fiber levels. However, in contrast to the I/R-2m and MI-2m groups, this effect was not only detected in infarcted and border areas but more importantly in remote myocardium as well (Figs. 5 and 6). Fibrosis in the remote myocardium increased nonlinearly as the aorta was constricted. LV performance after temporary or permanent LAD occlusion is related to infarct size; meanwhile, low LV fraction of shortening also correlated with increasing of perivascular fibrosis in remote myocardial myocardium (Fig. 7).
Fig. 5.
Masson's trichrome staining on whole heart slides. Blue, fibrillar collagen; red, myocardium. Collagen fibrosis was seen in myocardial-infarcted areas in I/R-2m and MI-2m groups. In the no ischemic/remote area, collagen fibrosis was significantly increased in Ab-4m, Ab + I/R, and Ab + I/R + DeAb hearts.
Fig. 6.
Perivascular fibrosis in remote area in different groups. Fibrosis was significantly increased in remote myocardium in Ab-4m, Ab + I/R, and Ab + I/R + DeAb hearts. Neointima formation was seen in the coronary small artery in the Ab + I/R + DeAb heart.
Fig. 7.
Perivascular fibrosis and heart function. 1: Perivascular fibrosis in noninfarcted/remote myocardium, means ± SE. *P < 0.05 and **P < 0.01 compared with control. 2: LV function was correlated with myocardial infarcted size. 3: Myocardial perivascular fibrosis was correlated with aortic constriction in a manner of nonlinear regression:  aortic cross-sectional area measured by direct digital photo: ■ aortic diameter measured by echocardiography. 4: Perivascular fibrosis was inversely correlated with LV function, means ± SE.
aortic cross-sectional area measured by direct digital photo: ■ aortic diameter measured by echocardiography. 4: Perivascular fibrosis was inversely correlated with LV function, means ± SE.
Expression of ANF, BNP, and SERCA2a in Remote Myocardium
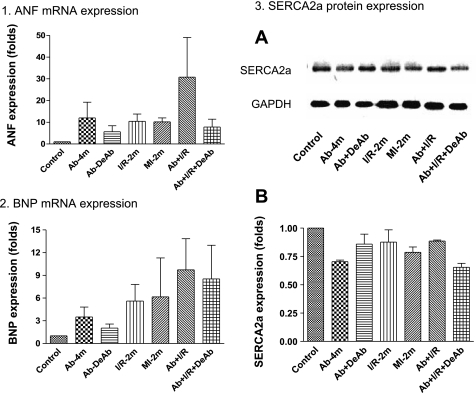
Real-time PCR confirmed that ANF and BNP mRNA contents were increased in myocardium in Ab-4, MI-2m, Ab + I/R, and Ab + I/R + DeAb. Western blotting showed that SERCA2a was decreased in Ab-4m, MI-2m, Ab + I/R, and also in Ab + I/R + DeAb groups (Fig. 8).
Fig. 8.
Atrial natriuretic factor (ANF), brain natriuretic peptide (BNP), and sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) 2a expression in remote myocardium. 1: ANF mRNA expression; 2: BNP mRNA expression; 3: SERCA2a protein expression, n = 3 rats in each group.
DISCUSSION
The current study describes a CHF model in rats that combines Ab, I/R, and DeAb. This Ab + I/R + DeAb model is characterized by dilated LV, low EF, near-normal LV outflow resistances, opened coronary arterial systems, and small MIs. LV function depends not only on geometric integrity and myocardial contractility but also on nonmyocardial fibrotic elements in the remote region. Ab + I/R + DeAb presents with significant perivascular fibrosis, which is associated with decreased cardiac function. This model may be useful for studying the role of vascular remodeling in the remote myocardium in the development of CHF.
LV pressure overload can be induced by constricting the ascending aorta (22), aortic arch (34), or the abdominal aorta (29) in rats, mice, and dogs (35). However, the occurrence of CHF post-aortic constriction depends on the degree of aortic stenosis and the overload duration (4, 26, 27). Our results indicated that LV function was preserved up to 4 mo post-Ab (aortic cross-sectional area constricted to 28% of normal) with collagen fiber accumulation in the interstitial and perivascular space. DeAb of the aorta led to regression of LV hypertrophy and reduction of fibrosis, consistent with other reports (15, 36). This indicates that ascending Ab leads to an adaptive, reversible hypertrophic response and not to CHF at 4 mo. Persistence of elevated LV pressure after DeAb also results from persisting neurohormonal activation or from distal vascular tension with partial DeAb.
The most widely used method to induce myocardial injury is temporary or permanent ligation of the LAD. Cardiac dysfunction correlates closely with MI size (33); however, it is difficult to control the MI size in small animal models because of the natural variability in patterns of LAD bifurcation (25). Ischemia for 30 min followed by reperfusion for 24 h is another useful model used to investigate myocardial ischemia. In the I/R 24-h model, the ratio of infarct/risk area, in contrast to absolute MI size, was used as the key index for ischemic injury (5, 19, 25). In the present study, ischemia for 30 min followed by 2 mo of reperfusion resulted in 15% infarcted LV and moderately impaired systolic function, including reduced EF and FS. As a positive control, complete LAD ligation for 2 mo resulted in significant cardiac function decreases. Importantly, in I/R-2m and MI-2m rats, fibrosis appears mainly in the infarct and border zones, with minimal accumulation in the remote myocardium.
LV remodeling occurs in response to pressure overload or myocardial injury (12, 23). Fibrosis is one common response to pressure overload or infarction to overcome elevated ventricular wall stress (17). The threshold and mechanisms of compensated hypertrophy to decompensated heart failure still remain unclear. When a large MI is present, the affected ventricular wall develops thin scar tissue. Subsequently, ventricular volumes increase as myocardial mass is replaced by the noncontractile scar tissue. The remaining noninjured myocardium becomes overloaded and decompensates, resulting in CHF (14, 32). In the present study, Ab for 2 mo plus I/R for 2 mo leads to low EF heart failure. These results suggest the pressure overload with I/R can significantly accelerate ventricular decompensation.
Heart failure is a complex syndrome where no single indicator can fully characterize the numerous changes that take place in CHF. Therefore, we used both echocardiography and in vivo hemodynamics to characterize cardiac functions. The altered heart function in Ab-4m hearts is attributable to compensatory hypertrophic responses leading to enhanced myocardial contractility. Upon DeAb, LV pressure and ESPVR returned to near baseline values, whereas the EF and FS were slightly lower than in Ab-4m rats. In contrast, I/R-2m and MI-2m rats exhibited dilated LV with increased ventricular volumes and reduced ESPVR. Cardiac function correlated inversely with MI size. The sequential 2 mo of Ab and 30 min coronary artery ischemia with 2 mo of reperfusion in Ab + I/R hearts resulted in high ESPVR and LV pressure similar to Ab-4m rats, but with increased ventricular volumes similar to I/R-2m rats. The LVEF, LVFS, and stroke volume were significantly reduced in the Ab + I/R hearts. DeAb of Ab + I/R significantly reduced LV pressures and the ESPVR. However, the end systolic and diastolic volumes remained elevated. EF and FS were dramatically impaired. The DeAb procedure in the Ab + I/R + DeAb group produces a model of heart failure with normal outflow resistance but, importantly, with a relatively small MI.
Histological data show significant fibrosis in the infarct, border area, and remote myocardium in Ab + I/R rats. Furthermore, excessive fibrotic deposits around small vessels in the remote myocardium may reduce oxygen and nutrient exchange rates between the blood supply and the surrounding myocardium. The result of these changes could be rapid onset of CHF. Extensive fibrosis in the remote myocardium also impedes vascular (15) and myocardial relaxation, increased stiffness in the ventricular wall, and reduced LV compliance, all contributing to impaired cardiac function (11). Importantly, aortic deconstriction after Ab + I/R results in a sustained low EF and increased fibrosis in the remote myocardium. This model does not exhibit LV hypertrophic or fibrotic regression after DeAb. Perivascular fibrosis continues to accumulate in the remote, noninfarcted myocardium of the Ab + I/R + DeAb rats. Additionally, perivascular fibrosis is associated with decreased ventricular function and CHF development in our model. This provides us with a useful tool to explore the impact of noninfarct fibrotic mechanisms in the transition from adaptive hypertrophy to CHF.
Natriuretic peptides, ANF and BNP, were increased, whereas SERCA2a was decreased in our heart failure, as previously reported (3, 10, 38).
Nolan et al. (28) reported that increased afterload aggravated infarct expansion after acute MI. However, their studies focused on short-term effects of increased afterload (3 wk) on acute MI in rats. Additionally, simultaneous LAD ligation and abdominal Ab were conducted by Anthonio et al. (1) to induce CHF, but LV function was evaluated ex vivo (1). Here, we show that our rat model of longer-term LV pressure overload followed by I/R recapitulates the interactions of multiple comorbid conditions in the development of CHF. We now have established relatively comprehensive baseline cardiac function data to define our model (Table 5). These results are the foundation for understanding the critical impact of fibrosis on ventricular function.
Table 5.
Summary of different heart dysfunction groups
| Heart Function | Aorta Banded Ratio | Hypertrophy | Myocardial Infarct Size, % of LV | Fibrosis in Infarcted Area | Fibrosis in Remote Myocardium | |
|---|---|---|---|---|---|---|
| Control | FS = 74 | 1 | NA | NA | NA | Normal |
| Ab-4m | FS = 68 | 0.52 | ↑↑↑ | NA | NA | ↑↑ |
| Ab + DeAb | FS = 58↓ | 0.71 | Regression | NA | NA | Regression |
| I/R-2m | FS = 47↓↓ | 1 | NA | Moderate (15%) | +++ | Normal |
| MI-2m | FS = 34↓↓↓ | 1 | NA | Big (20%) | +++ | Normal |
| Ab + I/R | FS = 39↓↓ | 0.47 | ↑↑ | Moderate (15%) | +++ | ↑↑↑ |
| Ab + I/R + DeAb | FS = 41↓↓ | 0.68 | Regression | Moderate (14%) | +++ | ↑↑ |
FS, fractional shortening; NA, not available; ↑, increase; ↓, decrease; +, positive.
The present study explored the interaction of multiple comorbid conditions in the development of chronic heart failure in rats in vivo. We recognize that there are limitations in the use of this model. This combined model of Ab + I/R + DeAb is time consuming, technically challenging, requires multiple chest surgeries in ventilated animals, and is associated with high morbidity and mortality. Furthermore, even though we have measured some of the molecular characteristics in this new model, there are other cellular and molecular indicators that need to be performed, such as neurohormones, coronary arterial activity, vascular endothelial function, apoptosis, fibrillar collagen phenotype, matrix metalloproteinase activities, and gene expression profile, to assess the suitability of this model for wider investigation.
In summary, we have sequentially combined LV pressure overload, I/R, and relief of LV pressure overload in a rat model of CHF. This model is characterized by a dilated left ventricle, low EF, near-normal LV outflow resistance, moderate myocardial infarction, and significant fibrosis in the remote myocardium. This model will provide a useful platform to investigate the role and cellular and molecular mechanisms of cardiovascular fibrosis in CHF.
GRANTS
This work was supported, in part, by National Heart, Lung, and Blood Institute Grants R01HL-078731, R01HL-083156, R01HL-093183, R01HL-088434, and P20HL-100396 (R. J. Hajjar) and the Transatlantic Leducq Foundation (R. J. Hajjar).
DISCLOSURES
There are no conflicts of interest with any of the authors.
ACKNOWLEDGMENTS
We appreciate Dr. Kenneth Fish for contributions in the revision.
REFERENCES
- 1. Anthonio RL, van Veldhuisen DJ, Scholtens E, van Bekkum C, de Boer E, van Gilst WH. Myocardial infarction with aortic banding. A combined rat model of heart failure. Jpn Heart J 38: 697–708, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Balakumar P, Singh AP, Singh M. Rodent models of heart failure. J Pharmacol Toxicol Methods 56: 1–10, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Beeri R, Chaput M, Guerrero JL, Kawase Y, Yosefy C, Abedat S, Karakikes I, Morel C, Tisosky A, Sullivan S, Handschumacher MD, Gilon D, Vlahakes GJ, Hajjar RJ, Levine RA. Gene delivery of sarcoplasmic reticulum calcium ATPase inhibits ventricular remodeling in ischemic mitral regurgitation. Circ Heart Fail 3: 627–634, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boluyt MO, Robinson KG, Meredith AL, Sen S, Lakatta EG, Crow MT, Brooks WW, Conrad CH, Bing OH. Heart failure after long-term supravalvular aortic constriction in rats. Am J Hypertens 18: 202–212, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Chen J, Chemaly E, Liang L, Kho C, Lee A, Park J, Altman P, Schecter AD, Hajjar RJ, Tarzami ST. Effects of CXCR4 gene transfer on cardiac function after ischemia-reperfusion injury. Am J Pathol 176: 1705–1715, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen J, Kuhlencordt P, Urano F, Ichinose H, Astern J, Huang PL. Effects of chronic treatment with l-arginine on atherosclerosis in apoE knockout and apoE/inducible NO synthase double-knockout mice. Arterioscleros Thromb Vasc Biol 23: 97–103, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Chen J, Lee SK, Abd-Elgaliel WR, Liang L, Galende EY, Hajjar RJ, Tung CH. Assessment of cardiovascular fibrosis using novel fluorescent probes. PloS one 6: e19097, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen J, Tung CH, Allport JR, Chen S, Weissleder R, Huang PL. Near-infrared fluorescent imaging of matrix metalloproteinase activity after myocardial infarction. Circulation 111: 1800–1805, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Del Monte F, Butler K, Boecker W, Gwathmey JK, Hajjar RJ. Novel technique of aortic banding followed by gene transfer during hypertrophy and heart failure. Physiol Genomics 9: 49–56, 2002 [DOI] [PubMed] [Google Scholar]
- 10. del Monte F, Williams E, Lebeche D, Schmidt U, Rosenzweig A, Gwathmey JK, Lewandowski ED, Hajjar RJ. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase in a rat model of heart failure. Circulation 104: 1424–1429, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frohlich ED. Fibrosis and ischemia: the real risks in hypertensive heart disease. Am J Hypertens 14: 194S–199S, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Gaballa MA, Goldman S. Ventricular remodeling in heart failure. J Card Fail 8: S476–S485, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Gueret P, Meerbaum S, Zwehl W, Wyatt HL, Davidson RM, Uchiyama T, Corday E. Two-dimensional echocardiographic assessment of left ventricular stroke volume: experimental correlation with thermodilution and cineangiography in normal and ischemic states. Cathet Cardiovasc Diagn 7: 247–258, 1981 [DOI] [PubMed] [Google Scholar]
- 14. Hochman JS, Bulkley BH. Pathogenesis of left ventricular aneurysms: an experimental study in the rat model. Am J Cardiol 50: 83–88, 1982 [DOI] [PubMed] [Google Scholar]
- 15. Ito N, Isoyama S, Takahashi T, Takishima T. Coronary dilator reserve and morphological changes after relief of pressure-overload in rats. J Mol Cell Cardiol 25: 3–14, 1993 [DOI] [PubMed] [Google Scholar]
- 16. Jackson G, Gibbs CR, Davies MK, Lip GY. ABC of heart failure. Pathophysiology. Br Med J 320: 167–170, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Janicki JS, Brower GL. The role of myocardial fibrillar collagen in ventricular remodeling and function. J Card Fail 8: S319–S325, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Klocke R, Tian W, Kuhlmann MT, Nikol S. Surgical animal models of heart failure related to coronary heart disease. Cardiovasc Res 74: 29–38, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Knight RA, Chen-Scarabelli C, Yuan Z, McCauley RB, Di Rezze J, Scarabelli GM, Townsend PA, Latchman D, Saravolatz L, Faggian G, Mazzucco A, Chowdrey HS, Stephanou A, Scarabelli TM. Cardiac release of urocortin precedes the occurrence of irreversible myocardial damage in the rat heart exposed to ischemia/reperfusion injury. FEBS Lett 582: 984–990, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Lee SH, Yang DK, Choi BY, Lee YH, Kim SY, Jeong D, Hajjar RJ, Park WJ. The transcription factor Eya2 prevents pressure overload-induced adverse cardiac remodeling. J Mol Cell Cardiol 46: 596–605, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Litwin SE, Katz SE, Weinberg EO, Lorell BH, Aurigemma GP, Douglas PS. Serial echocardiographic-Doppler assessment of left ventricular geometry and function in rats with pressure-overload hypertrophy. Chronic angiotensin-converting enzyme inhibition attenuates the transition to heart failure. Circulation 91: 2642–2654, 1995 [DOI] [PubMed] [Google Scholar]
- 23. Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation 111: 2837–2849, 2005 [DOI] [PubMed] [Google Scholar]
- 24. McMurray JJ, Pfeffer MA. Heart failure. Lancet 365: 1877–1889, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Michael LH, Entman ML, Hartley CJ, Youker KA, Zhu J, Hall SR, Hawkins HK, Berens K, Ballantyne CM. Myocardial ischemia and reperfusion: a murine model. Am J Physiol Heart Circ Physiol 269: H2147–H2154, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Miyamoto MI, del Monte F, Schmidt U, DiSalvo TS, Kang ZB, Matsui T, Guerrero JL, Gwathmey JK, Rosenzweig A, Hajjar RJ. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc Natl Acad Sci USA 97: 793–798, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Molina EJ, Gupta D, Palma J, Torres D, Gaughan JP, Houser S, Macha M. Novel experimental model of pressure overload hypertrophy in rats. J Surg Res 153: 287–294, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Nolan SE, Mannisi JA, Bush DE, Healy B, Weisman HF. Increased afterload aggravates infarct expansion after acute myocardial infarction. J Am Coll Cardiol 12: 1318–1325, 1988 [DOI] [PubMed] [Google Scholar]
- 29. Norton GR, Woodiwiss AJ, Gaasch WH, Mela T, Chung ES, Aurigemma GP, Meyer TE. Heart failure in pressure overload hypertrophy. The relative roles of ventricular remodeling and myocardial dysfunction. J Am Coll Cardiol 39: 664–671, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc 3: 1422–1434, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patten RD, Hall-Porter MR. Small animal models of heart failure: development of novel therapies, past and present. Circ Heart Fail 2: 138–144, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 81: 1161–1172, 1990 [DOI] [PubMed] [Google Scholar]
- 33. Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadaro J, Kloner RA, Braunwald E. Myocardial infarct size and ventricular function in rats. Circ Res 44: 503–512, 1979 [DOI] [PubMed] [Google Scholar]
- 34. Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J, Jr, Chien KR. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci USA 88: 8277–8281, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shen YT, Malik FI, Zhao X, Depre C, Dhar SK, Abarzua P, Morgans DJ, Vatner SF. Improvement of cardiac function by a cardiac myosin activator in conscious dogs with systolic heart failure. Circ Heart Fail 3: 522–527, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Stansfield WE, Rojas M, Corn D, Willis M, Patterson C, Smyth SS, Selzman CH. Characterization of a model to independently study regression of ventricular hypertrophy. J Surg Res 142: 387–393, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Szymanska G, Stromer H, Kim DH, Lorell BH, Morgan JP. Dynamic changes in sarcoplasmic reticulum function in cardiac hypertrophy and failure. Pflugers Arch 439: 339–348, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Yoon PO, Lee MA, Cha H, Jeong MH, Kim J, Jang SP, Choi BY, Jeong D, Yang DK, Hajjar RJ, Park WJ. The opposing effects of CCN2 and CCN5 on the development of cardiac hypertrophy and fibrosis. J Mol Cell Cardiol 49: 294–303, 2000 [DOI] [PubMed] [Google Scholar]