Abstract
Prenatal exposure to polybrominated diphenyl ethers (PBDEs) may disrupt thyroid function and contribute to adverse neurodevelopmental outcomes. We conducted a pilot study to explore the relationship between serum concentrations of lower-brominated PBDEs (BDE-17 to -154), higher-brominated PBDEs (BDE-183 to -209), and hydroxylated PBDE metabolites (OH-PBDEs) with measures of thyroid function in pregnant women. Concentrations of PBDEs, OH-PBDEs, thyroid-stimulating hormone (TSH), total thyroxine (T4), and free T4 were measured in serum samples collected between 2008 and 2009 from 25 second trimester pregnant women in California. Median concentrations of lower-brominated PBDEs and OH-PBDEs were the highest reported to date in pregnant women. Median concentrations of BDE-47 and the sum of lower-brominated PBDEs (ΣPBDE5) were 43.1 ng/g lipid and 85.8 ng/g lipid; and 0.084 ng/mL for the sum of OH-PBDEs (ΣOH-PBDE4). We observed a positive association between the weighted sum of chemicals known to bind to transthyretin (ΣTTR binders) and TSH levels. We also found positive associations between TSH and ΣPBDE5, ΣOH-PBDE4, BDE-47, BDE-85, 5-OH-BDE47, and 4′-OH-BDE49; and an inverse association with BDE-207. Relationships with free and total T4 were weak and inconsistent. Our results indicate that PBDE exposures are elevated in pregnant women in California, and suggest a relationship with thyroid function. Further investigation is warranted to characterize the risks of PBDE exposures during pregnancy.
INTRODUCTION
Polybrominated diphenyl ethers (PBDEs) are persistent organic chemicals widely used as flame retardants in consumer products since the 1970s. The commercial mixture penta-BDE has been most often added to polyurethane foam used in furniture, while octa- and deca-BDE commercial mixtures have been used in electronics and other plastic products. Although PBDEs are being phased out, human exposure in the U.S. continues due to the slow replacement time of PBDE-containing products and ingestion of contaminated foods [1].
Concentrations of lower-brominated PBDE congeners, characteristic of penta-BDE, measured in household dust, breast milk, and serum from North America are an order of magnitude higher than those measured in Europe and Asia [2]. Few biomonitoring studies have measured higher brominated PBDEs which are commonly found in octa- and deca-BDE. Serum penta-BDEs levels are approximately two-fold higher in California residents compared to the rest of the U.S., likely a result of California’s unique furniture flammability standards [3]. Higher serum PBDE levels are also correlated with lower socioeconomic status (SES) [4]. Thus, low-income California residents may encounter elevated exposures to PBDEs.
PBDE exposures may exert adverse effects by interfering with the thyroid system. Thyroid hormones (TH) in serum are regulated through negative feedback such that when TH levels decline, the pituitary gland secretes thyroid-stimulating hormone (TSH), which stimulates the thyroid to increase secretion of TH. When TH levels increase above a “set point”, TSH secretion is effectively suppressed [5]. In animal studies, penta-BDE mixture, as well as individual PBDE congeners, reduce serum free and total thyroxine (T4) in developing and adult rodents probably by activating liver enzymes that increase TH clearance from serum [6–8].
However, associations of penta-BDEs and TH in humans show more variability. Yuan et al. [9] found higher serum TSH in highly-exposed Chinese electronic-waste workers relative to controls, which would suggest lower serum T4 in the exposed population. In contrast, other studies among adults show null associations or positive correlations between PBDE exposure and T4 [10, 11]; and T3 [12]. Few studies have focused on pregnant women. A recent study of 270 pregnant women found inverse associations between PBDE levels and TSH but not T4 [13]. Two smaller studies of pregnant women reported null associations between PBDE levels and T4 [14, 15], and a study of newborns found an inverse association between PBDE levels in umbilical cord blood and total T4 in the subset of babies delivered vaginally [16].
These studies indicate that PBDEs may interfere with thyroid function in humans, and if so, pregnant women may be particularly vulnerable. Pregnancy represents a period of increased demand on the thyroid gland. Serum TH levels increase by almost 50 percent during the first trimester due to an increase in thyroxine-binding globulin (TBG) and the direct action of human chorionic gonadotropin on the thyroid gland [17]. TH insufficiency during pregnancy can impair the health of mother and offspring [18]. Overt and subclinical hypothyroidism are associated with increased risk of miscarriage and preterm birth [19]. Even modest reductions in maternal T4 during early pregnancy are associated with long lasting developmental deficits in their children, such as reduced IQ [20]. Thus, PBDE exposure may represent an added stressor on the thyroid gland of pregnant women that could have lifelong effects on the offspring.
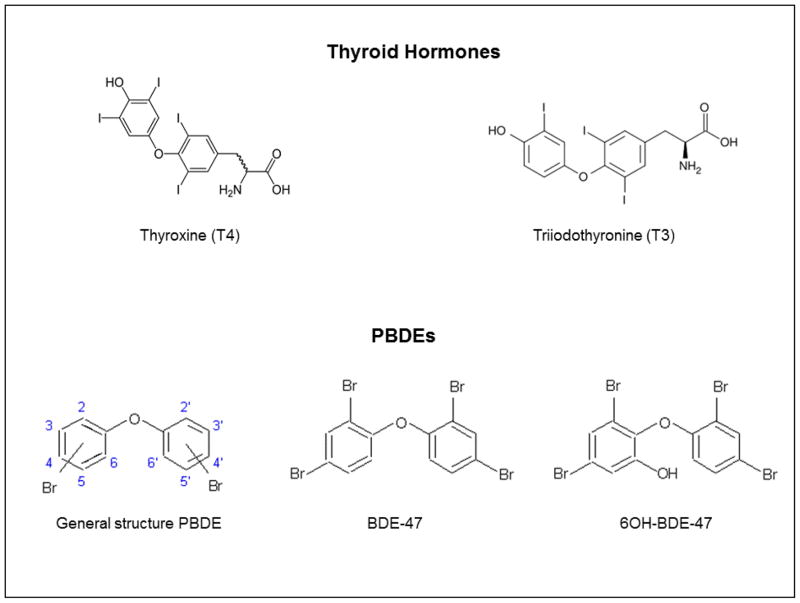
PBDEs can be metabolized via cytochrome P450 mediated oxidation to form OH-PBDEs [21]; these hydroxylated forms may be more potent on the thyroid system. As halogenated diphenyl ethers, PBDEs are structurally similar to T4 (Figure 1) and hydroxylation improves their ability to interact with TH-binding proteins in serum and perhaps in cells [22]. For example, 5-OH-BDE-47 has a three times stronger binding affinity to serum binding protein transthyretin (TTR) than T4 and three orders of magnitude stronger binding affinity than BDE-47 [23]. Additionally, some OH-PBDEs can bind to the thyroid hormone receptor [24, 25]. It is possible that both of these mechanisms may result in altered thyroid status. However, OH-PBDEs have rarely been studied in pregnant women.
Figure 1.
Molecular structures of thyroid hormones (TH) and PBDEs.
Given the growing evidence of the role of PBDEs and their metabolites in TH functioning and the opportunity to study these effects in a population with relatively high exposures, we conducted a pilot study to measure exposures to a large suite of PBDE flame retardants in an ethnically diverse and predominately low-income population of pregnant women in California; and examine associations of PBDEs and OH-PBDEs with measures of thyroid function.
MATERIALS AND METHODS
Study Population
We recruited pregnant women (n=25) prior to second trimester pregnancy termination procedures at the San Francisco General Hospital Women’s Options Center (WOC) in San Francisco, California between 2008 and 2009. The WOC is an outpatient clinic serving an ethnically diverse and predominantly low-income population from the San Francisco Bay area and other parts of Northern and Central California. We excluded participants if they were currently smoking, using illicit drugs, taking thyroid medication; or obtained an abortion because of fetal anomalies. Because TH fluctuate throughout pregnancy [26], we limited pregnancy gestation from 19 to 23 weeks. We also abstracted information on pregnancy and demographic characteristics from medical records. Study protocols were approved by the University of California, San Francisco Committee on Human Research.
Data Collection and Thyroid Hormone Analysis
Blood samples were collected from each participant prior to medical procedures. Approximately, 3–5 ml of serum was isolated from these samples and stored in aliquots of 2 ml in polypropylene storage tubes at −70°C until analysis. To prevent unnecessary thawing and freezing, one tube of serum per participant was sent to Quest Diagnostics (San Jose, CA, USA) where it was analyzed for TSH, free and total T4, and lipid content immediately upon arrival. TSH was measured by third-generation immunochemiluminometric assay, and total T4 was measured by solid-phase immunochemiluminometric assay. Free T4 was measured by direct equilibrium dialysis followed by radioimmunoassay at Quest Diagnostics Nichols Institute (San Juan Capistrano, CA, USA). Equilibrium dialysis is considered the preferred method for free T4 analysis since it increases the accuracy of measurements in samples with normal or high transport proteins [27]. The coefficients of variation (CV) for the TH assays were 4%, 5%, and 11% for TSH, total T4, and free T4, respectively. Total lipid content was calculated based on measurements of total cholesterol and triglycerides using Phillip’s formula [28].
PBDEs and OH-PBDEs Analysis
We analyzed 37 PBDE analytes in serum at the Department of Toxic Substances Control (DTSC) (Berkeley, CA, USA). Our suite of analytes consisted of 19 PBDE congeners and 18 OH-PBDEs and included lower-brominated (BDE-17 to -154) and higher-brominated PBDEs (BDE-183 to -209). These analytes were representative of commercial mixtures penta-BDE (e.g. BDE-47), octa-BDE (e.g. BDE-183), and deca-BDE (e.g. BDE-209) [29] (Analyte list provided in Table S1). The serum preparation and extraction method, phase separation technique, and analysis were adopted from our earlier methods to separate phenolic compounds (e.g., OH-PCBs, PCP) from maternal serum [30, 31].
13C12 labeled PBDEs (13C12-BDE-28, 47, 99, 153, 154, 183, 197, and 207) and 13C12 6-OH-BDE-47 (Wellington Laboratory, Guelph, Ontario, Canada) were used as internal standards. 13C12-PCB-209 (Cambridge Isotope Laboratory, Andover, MA, USA) was used as a recovery standard. Native PBDE standards were purchased from Wellington Laboratories (Guelph, Ontario, Canada). MeO-BDE standards: 6′-MeO-BDE17, 4′-MeO-BDE17, 2′-MeO-BDE28, 6′-MeO-BDE49, 2′-MeO-BDE68, 6-MeO-BDE47, 3-MeO-BDE47, 5-MeO-BDE47, 4′-MeO-BDE49, 6-MeO-BDE99, 5′-MeO-BDE100, 6-MeO-BDE90, 5′-MeO-BDE99, 4′-MeO-BDE101, 2-MeO-BDE123, 6-MeO-BDE85, 4′-MeO-BDE103, and 6-MeO-BDE137 were purchased from Wellington Laboratories or donated by Dr. Robert Letcher. Diazomethane was synthesized in hexane by using N-nitroso-N-methylurea (Sigma-Aldrich, USA) as described elsewhere [32]. Other chemicals and solvents used for the gas chromatography (GC) analysis were of the highest quality available and included dichloromethane and hexane (trace analysis, Burdick and Jackson), methanol, methyl-tert butyl ether (MTBE), and water (HPLC grade, Fisher Scientific, USA), 2-propanol (99.9%, pesticide grade, Fisher Scientific, USA), hydrochloric acid, sulfuric acid (98%), potassium hydroxide, potassium chloride, sodium hydroxide, and ethyl alcohol (94–96%, 200 proof) (Fisher Scientific, USA), silica (200–400 mesh) (Sigma-Aldrich, USA), and Florisil (60–100 mesh) (Mallinckrodt, USA).
Serum preparation and extraction procedures were completed in the ultra-clean laboratory of DTSC. Before extraction, we spiked surrogate internal standards: 13C12 labeled PBDEs (2.5–10 ng) and 13C12 6-OH-BDE47 (1 ng). Maternal serum (1 mL) was denatured with 1 mL of 6 M hydrochloric acid and 6 mL of 2-propanol and extracted using 6 mL of hexane:MTBE (1:1; re-extraction with 3 mL). After potassium chloride (1%) wash, the extracts were phase-separated by adding 2 mL of potassium hydroxide solution (0.5 M). After the neutral fraction was extracted, the alkaline aqueous solutions (potassium hydroxide) were acidified with 2 M hydrochloric acid and extracted for phenolic compounds with 4 mL of hexane:MTBE (9:1; re-extraction with 3 mL). Phenolic extracts and five calibration standards (< 50 μL) were methylated with 1 mL of diazomethane for 24 hours. Co-extracted lipids in neutral fraction were removed on deactivated Florisil column (11g) and the analytes were eluted with 60 mL of hexane, and then 60 mL of dichloromethane/hexane (1:1, v/v). The derivatives of phenolic fractions were purified with concentrated sulfuric acid (H2SO4, 98%), and then passed through a Pasteur pipette column packed with 1:2 H2SO4:silica gel (w/w; 0.5 g) (top) and 0.1 g activated silica gel (bottom). The collected eluates for both neutral and phenolic fractions were concentrated and spiked with recovery standard 13C12 PCB-209 (8 ng).
PBDE analyses were completed by gas chromatography/high resolution mass spectrometry (GC-HRMS, DFS, Thermo-Finnigan, Bremen, Germany) using isotope dilution. The extracts (2 μL) were injected and separated on a DB-5 MS column (15 m × 0.25 mm I.D., 0.10 μm film thickness; J&W Scientific, USA) with helium carrier gas and programmed at 175°C for 2 min; to 280°C at 5°C/min.; to 310°C at 7°C/min; and held for 5 min. OH-PBDEs were determined as methyl derivatives (MeO) using DB-5 MS column (60 m × 0.25 mm I.D., 0.25 μm film thickness; J&W Scientific, USA) with GC programmed at 80°C and held for 2 min followed by a 50°C/min increase to 200°C, 1°C/min to 230°C, and 30°C/min to 300°C and held for 4 min. Injection (2 μL) was made in split/splitless mode at an injector temperature of 250°C. The HRMS was operated in electron impact ionization mode using multiple ion detection, source temperature of 260°C, ionization energy of 42 V, electron current typically at 0.5–0.6 mA, and mass resolution of 10000, with perfluorokerosene (PFK) as mass reference. The temperatures for both the ion source and quadrupole were set to 150°C. Molecular ions were monitored for OH-PBDEs, and tri- to penta-BDEs while M-2Br ions were used to identify hexa- to nona-BDEs.
All glassware was washed, rinsed with acetone and hexane, and baked (500°C for 3 hours). Samples were analyzed using standard laboratory QA/QC protocol; each batch of nine samples were accompanied with a matrix blank (bovine serum; HyClone), a matrix spike control (permatrix-spiked bovine serum), and a standard reference material (NIST SRM 1589a). For the OH-PBDE quantification, we used the five point external calibration curve of MeO-PBDEs and corrected the final concentrations by the surrogate recoveries.
The method detection limit (MDL) was defined as three times the standard deviation (SD) of the blank concentrations except for BDE-209 where the MDL was defined as five times the SD due to the relatively high background contamination for BDE-209. Precision and accuracy of PBDEs from surrogate spikes, reference material, and control samples were within reasonable analytical error ranges while BDE-209 showed lower surrogate recoveries (55%) (Table S2). The mean (± SD) surrogate recovery for 13C12 6-OH-BDE47 was 103 ± 15%.
Data Analysis
We calculated the following statistics for all chemicals with ≥ 50% detection frequency: geometric mean (GM), geometric standard deviation (GSD), median, and 95th percentile estimates. For concentrations below the MDL, we used estimated values provided by the GC-HRMS when available. If estimated values were not available, undetected values were imputed based on a log-normal probability distribution whose parameters were calculated through maximum likelihood estimation [33, 34]. Summary statistics for PBDE congeners are normalized for lipid content (ng/g lipid), and OH-PBDEs are expressed as wet weight (ng/mL).
We log-transformed PBDEs, OH-PBDEs, and TSH prior to regression analysis to account for their non-normal distributions. We used Spearman’s rank correlations to evaluate correlations between PBDE analytes. We used t-tests and analysis of variance (ANOVA) to examine relationships between TH and demographic characteristics.
We ran multiple linear regressions to examine associations between serum concentrations of TH and PBDE analytes with ≥ 50% detection frequency. We modeled PBDE exposure in several ways. First we examined individual analytes. Next, we calculated the sum of lower-brominated PBDEs (BDE- 28, -47, -99, -100, -153) (ΣPBDE5) [13] and the sum of OH-PBDEs (ΣOH-PBDE4). Lastly, to account for differences in structure and activity, we also calculated the sum of chemicals weighted by their relative affinity for binding to transport protein TTR (ΣTTR binders) based on in vitro data [23] and normalized the weighting factors to T4 activity (T4=1). All PBDE models were examined in two ways: 1) PBDEs and OH-PBDEs expressed as ng/g lipid; and 2) PBDEs and OH-PBDEs expressed as ng/ml and then including lipid content as a covariate. The results were virtually the same for both so we only show the models with PBDEs and OH-PBDEs expressed as ng/mL in the main text.
The following covariates were considered for inclusion in our regression models: age (continuous), race/ethnicity (Latina, Black, or White/Asian), parity (nulliparous versus one or more live births), and type of health insurance (public insurance versus private insurance or self-pay) which is a proxy for SES. Due to our limited sample size, we only included covariates that were statistically significant in the final model or that changed the main effect estimate by ≥ 10%. We limited the number of covariates so that no cell size had a sample size below five.
We conducted regression diagnostics on our final models to identify extreme observations that may unduly influence statistical results. Outliers were defined as observations with absolute studentized residual greater than three. There were no statistical outliers identified in the final models presented in the main text. All analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC). A p-value < 0.05 was considered significant and p < 0.10 as marginally significant on two-sided tests.
RESULTS
The mean (± SD) age of women was 23 ± 7.3 years, and 84% were younger than 30 years of age. The population was ethnically diverse and the majority was born in the U.S. (92%). Approximately 80% used public health insurance. All participants had TSH levels within the laboratory reference range (0.30 - 4.10 mIU/L second trimester), but two women exceeded the National Academy of Clinical Biochemistry’s recommended upper limit for TSH (2.5 mIU/L) [35]. All participants’ free T4 levels were within the laboratory reference range (0.8 -1.5 ng/dL second trimester) and none of the women had low levels of total T4 (< 7.8 μg/dL) [35]. TH levels were generally similar across race/ethnicity, age, parity, and insurance except for total T4 levels which decreased with age (Spearman rs = −0.52, p < 0.01) (Table 1).
Table 1.
Demographic characteristics of second trimester pregnant women according to their serum concentrations of TH, Northern and Central California, 2008–2009 (N=25)
| % | Measures of thyroid function (Mean (SD))
|
|||
|---|---|---|---|---|
| Free thyroxine (free T4) (ng/dL) | Total thyroxine (total T4) (μg/dL) | Thyroid- stimulating hormone (TSH) (mIU/L) | ||
| All participants | 100 | 1.0 (0.1) | 12.8 (1.6) | 1.29 (0.67) |
| Age (years) | ||||
| 16–19 | 38 | 1.0 (0.1) | 13.5 (1.4) # | 1.44 (0.81) |
| 20–29 | 48 | 1.0 (0.1) | 12.6 (1.6) | 1.15 (0.63) |
| 30–45 | 16 | 1.0 (0.2) | 11.4 (1.9) | 1.35 (0.55) |
| Race/Ethnicity | ||||
| Latina | 36 | 1.0 (0.1) | 13.2 (1.8) | 1.60 (0.88) |
| Black | 40 | 1.0 (0.2) | 12.7 (1.5) | 1.13 (0.47) |
| White or Asian | 24 | 0.9 (0.2) | 12.2 (1.8) | 1.09 (0.52) |
| Parity | ||||
| 0 | 32 | 1.0 (0.1) | 12.4 (2.0) | 1.30 (0.46) |
| ≥ 1 | 68 | 1.0 (0.1) | 12.9 (1.5) | 1.28 (0.77) |
| Method of payment | ||||
| Public insurance (Medi-cal) | 80 | 1.0 (0.1)# | 12.9 (1.6) | 1.18 (0.57) |
| Private insurance or self-pay | 20 | 1.1 (0.2) | 12.3 (2.0) | 1.70 (0.94) |
Abbreviations: SD = standard deviation
Data missing for: total T4 (n=1).
p < 0.10
Of the 37 chemical analytes examined, 17 PBDEs and four OH-PBDEs were detected in at least one participant (Table 2). Five lower-brominated congeners (BDE-28, -47, -99, -100, and -153) were detected in 100% of pregnant women. In half the participants (56%), BDE-47 was the most abundant congener. In the other half, the dominant congener was either BDE-153 or -209. OH-PBDEs were found at lower levels than PBDE congeners (Table S3). Among the OH-PBDEs, 5-OH-BDE47 and 6-OH-BDE-47 were the most abundant.
Table 2.
Descriptive statistics for serum levels of PBDEs and OH-PBDEs in second trimester pregnant women in Northern and Central California, 2008–2009 (N=25)
| ΣPBDE5a | ΣOH- PBDE4b | ΣTTR bindersc,d | MDL | % > MDL | GM(GSD) | 50th | 95th | |
|---|---|---|---|---|---|---|---|---|
| PBDEs (ng/g lipid) | ||||||||
| BDE-028 | X | 0.66 | 100 | 2.32 (1.68) | 2.40 | 6.97 | ||
| BDE-047 | X | X (0.0025) | 11.7 | 100 | 43.1 (1.70) | 42.1 | 123.2 | |
| BDE-066 | 0.61 | 16 | -- | <MDL | 0.71 | |||
| BDE-085 | 0.61 | 72 | 0.90 (1.93) | 0.82 | 2.70 | |||
| BDE-099 | X | 3.63 | 100 | 11.5 (1.82) | 9.80 | 27.2 | ||
| BDE-100 | X | 0.91 | 100 | 9.00 (1.86) | 8.96 | 23.3 | ||
| BDE-153 | X | 0.93 | 100 | 15.5 (1.82) | 16.5 | 38.3 | ||
| BDE-154 | 1.22 | 36 | -- | < MDL | 2.13 | |||
| BDE-183 | 1.22 | 8 | -- | < MDL | 1.36 | |||
| BDE-196 | 1.22 | 4 | -- | < MDL | < MDL | |||
| BDE-197 | 1.22 | 48 | -- | < MDL | 2.95 | |||
| BDE-201 | 1.22 | 4 | -- | < MDL | < MDL | |||
| BDE-203 | 1.22 | 4 | -- | < MDL | < MDL | |||
| BDE-206 | 1.52 | 4 | -- | < MDL | < MDL | |||
| BDE-207 | 1.52 | 52 | 1.63 (1.59) | 1.54 | 3.56 | |||
| BDE-208 | 1.52 | 20 | -- | < MDL | 3.56 | |||
| BDE-209 | 50.9 | 40 | -- | < MDL | 101.2 | |||
| ΣPBDE5a | -- | -- | 85.8 (1.59) | 82.9 | 192.8 | |||
| OH-PBDEs (ng/mL) | ||||||||
| 4′-OH-BDE17 | x | 0.012 | 58 | 0.014 (2.96) | 0.016 | 0.086 | ||
| 5-OH-BDE47 | x | X (3.0) | 0.012 | 83 | 0.028 (3.52) | 0.036 | 0.131 | |
| 6-OH-BDE47 | x | X (0.39) | 0.010 | 92 | 0.025 (2.40) | 0.023 | 0.147 | |
| 4′-OH-BDE49 | x | X (3.5) | 0.010 | 50 | 0.008 (2.01) | 0.010 | 0.020 | |
| ΣOH-PBDE4 | -- | -- | 0.092 (0.75) | 0.084 | 0.349 | |||
| ΣTTR binders | -- | -- | 0.139 (2.44) | 0.151 | 0.500 | |||
Abbreviations: MDL = method detection limit; GM = geometric mean; GSD = geometric standard deviation. GM and GSD only calculated for congeners with detection frequencies ≥ 50%.
PBDE sum as constructed by Chevrier [13].
One participant missing measurements for OH-PBDEs.
ΣTTR binders is a weighted sum of BDE-47, 5-OH-BDE47, 6-OH-BDE47, and 4′-OH-BDE49. Concentrations are weighted according to their potencies to compete with T4 for binding to transthyretin (TTR) as determined by Hammers et al. [23]. The number in parentheses indicates weighting factors.
All analytes were converted to ng/mL before being weighted and summed.
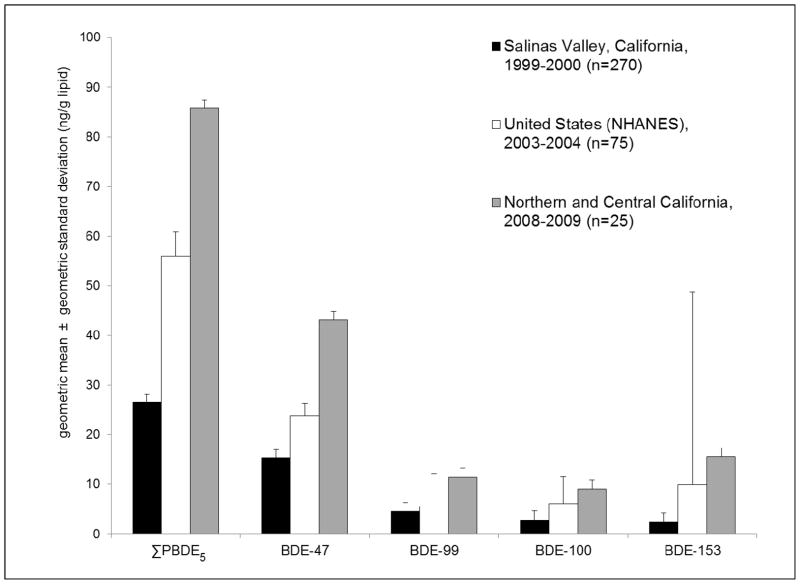
Median concentrations of lower-brominated PBDEs and OH-PBDEs were the highest reported to date in pregnant women worldwide (Figure 1; Table S4). For example, BDE-47 levels (GM = 43.1 ng/g lipid) were twofold higher than levels reported in a U.S. population of pregnant women from NHANES 2003–2004 survey (GM = 23.9 ng/g lipid) [36] and threefold higher than levels reported in a population of Mexican immigrant pregnant women from Salinas Valley in Central California in 1999–2000 (GM = 15.3 ng/g lipid) [13]. Median concentrations of 5-OH-BDE47 and 6-OH-BDE47 were five and 10 times higher than levels measured in pregnant women from Indiana in 2003–2004 [37].
BDE-47 was highly correlated with BDE-28, -99, and -100 (Spearman rs = 0.6 - 0.8, p < 0.001), but not with BDE-153 or -207. The OH-PBDEs were correlated with each other (Spearman rs = 0.5 – 0.7, p < 0.001) but generally not with PBDE congeners (Table S5).
Lower brominated congeners and ΣPBDE5 were positively associated with TSH (Table 3) except for BDE-153. After adjustment for insurance type and lipids, we found positive associations between TSH and ΣPBDE5 (β = 0.40; 95% confidence interval (CI), − 0.07 to 0.87), BDE-47 (β = 0.39; 95% CI, − 0.04 to 0.82), and BDE-85 (β = 0.33; 95% CI, 0.02 to 0.64). Albeit for BDE-47 and Σ PBDE5, the null hypothesis could not be ruled out. In contrast, BDE-207 was associated with reduced TSH (β = − 0.72; 95% CI: − 1.10 to − 0.34). BDE-28 showed a marginally significant inverse association with free T4 although relationships between PBDE levels and free and total T4 were generally weak, inconsistent, and not statistically significant (Table 3).
Table 3.
Associations between PBDEs and TH in second trimester pregnant women in Northern and Central California
| Free T4 (N=25)
|
Total T4 (N=24)
|
TSH (N=25)
|
||||
|---|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | β | 95% CI | |
| PBDE groupings (ng/mL) | ||||||
| ΣPBDE5 | −0.02 | −0.15, 0.12 | 0.50 | −1.04, 2.04 | 0.40 | −0.07, 0.87# |
| Individual congeners (ng/mL) | ||||||
| BDE-28 | −0.11 | −0.24, 0.02# | −0.57 | −2.12, 0.98 | 0.38 | −0.12, 0.87 |
| BDE-47 | −0.04 | −0.16, 0.09 | 0.02 | −1.37, 1.41 | 0.39 | −0.04, 0.82# |
| BDE-85 | −0.01 | −0.10, 0.08 | 0.55 | −0.47, 1.57 | 0.33 | 0.02, 0.64* |
| BDE-99 | 0.01 | −0.09, 0.11 | 0.84 | −0.31, 1.99 | 0.24 | −0.12, 0.60 |
| BDE-100 | −0.02 | −0.12, 0.07 | 0.21 | −1.02, 1.43 | 0.23 | −0.12, 0.59 |
| BDE-153 | 0.04 | −0.06, 0.14 | 0.66 | −0.66, 1.98 | 0.07 | −0.32, 0.45 |
| BDE-207 | 0.06 | −0.07, 0.19 | 0.51 | −0.96, 1.97 | −0.72 | −1.10, −0.34** |
p < 0.05;
p < 0.01;
p < 0.10
Adjusted for lipid content and insurance type; and total T4 models are additionally adjusted for age.
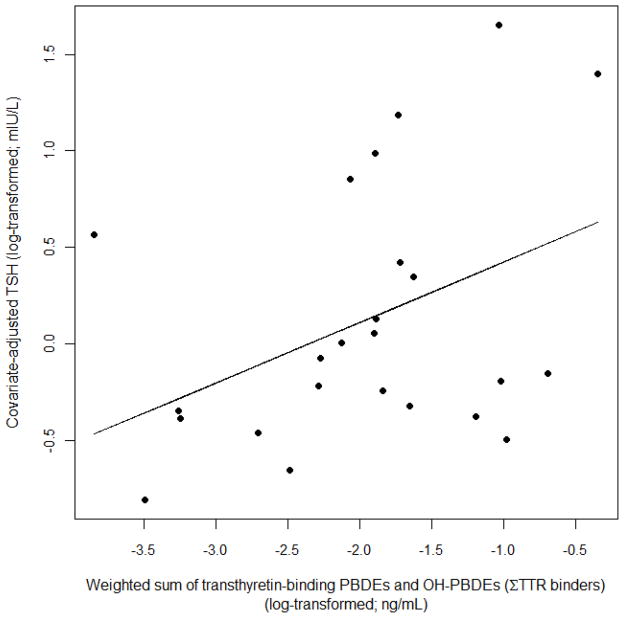
Individual OH-PBDEs and their sum were positively associated with TSH with the strongest associations observed for 4′-OH-BDE-49 (β = 0.50; 95% CI, 0.22 to 0.78) (Table 4). Σ TTR-binders was also associated with increased TSH (Table 4; Figure 2). The effect of Σ TTR-binders on TSH levels (β = 0.31; 95% CI, 0.04 to 0.59) was similar to the effect of Σ OH-PBDEs on TSH (β = 0.29; 95% CI, − 0.05 to 0.64) although the effect estimate for Σ TTR-binders had smaller confidence intervals than Σ OH-PBDEs (Table 4) and a higher adjusted R2 (0.14 versus 0.04, respectively). Relationships between OH-PBDEs and total and free T4 were null except for 6-OH-BDE-47, which was inversely associated with total T4 and marginally significant.
Table 4.
Associations between OH-PBDEs and TH in second trimester pregnant women from Northern and Central California
| Free T4 (N=24)
|
Total T4 (N=23)
|
TSH (N=24)
|
||||
|---|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | β | 95% CI | |
| OH-PBDE groupings (ng/mL) | ||||||
| ΣOH-PBDE4 | −0.04 | −0.13, 0.05 | −0.57 | −1.57, 0.44 | 0.29 | −0.05, 0.64# |
| ΣTTR binders | −0.02 | −0.09, 0.06 | −0.22 | −1.07, 0.63 | 0.31 | 0.04, 0.59* |
| Individual metabolites (ng/mL) | ||||||
| 4′-OH-BDE17 | 0.00 | −0.07, 0.07 | 0.03 | −0.99, 1.05 | 0.16 | −0.13, 0.45 |
| 5-OH-BDE47 | 0.00 | −0.06, 0.05 | −0.08 | −0.70, 0.55 | 0.18 | −0.03, 0.40# |
| 6-OH-BDE47 | −0.03 | −0.10, 0.03 | −0.73 | −1.48, 0.02# | 0.06 | −0.22, 0.34 |
| 4′-OH-BDE49 | 0.00 | −0.09, 0.09 | 0.09 | −0.93, 1.11 | 0.50 | 0.22, 0.78** |
p < 0.05;
p < 0.01;
p < 0.10
Adjusted for lipid content and parity; and total T4 models are additionally adjusted for age.
ΣTTR binders is a weighted sum of BDE-47, 5-OH-BDE47, 6-OH-BDE47, and 4′-OH-BDE49. Concentrations are weighted according to their potencies to compete with T4 for binding to transthyretin (TTR) as determined by Hammers et al. [23].
Figure 2.
Comparison of serum PBDEs levels in pregnant women from three study populations
Discussion
In this initial study of non-smoking, predominately low-income pregnant women from Northern and Central California, we find that they are more highly exposed to lower-brominated PBDEs and OH-PBDEs compared to pregnant women from Salinas Valley, California, a nationally representative sample of U.S. pregnant women, and various populations of pregnant women from Europe and Asia. Moreover, despite the relatively small sample size and the inherent variability of measures of thyroid function, we find positive associations between TSH and serum concentrations of lower-brominated PBDE congeners and OH-PBDEs. These associations were statistically significant for individual chemicals (e.g. BDE-85, 4′-OH-BDE-49) and their weighted sum (e.g. Σ TTR-binders). In contrast, we report an inverse association between BDE-207 and TSH. Associations with total and free T4 were generally weak and inconsistent.
Our study is one of the most extensive characterizations to date of flame retardant exposures in pregnant women. We identified OH-PBDEs as well as congeners characteristic of penta-, octa-, and deca-BDE mixtures. Although California passed a state-wide ban on penta- and octa-BDE in 2003, PBDE exposures remain elevated in our contemporary study population sampled in 2008–2009. These high exposures likely reflect the unintended consequences of California’s furniture flammability standards. For example, the 1975 standard, Technical Bulletin 117, requires that polyurethane foam used in upholstered furniture sold in California must be able to withstand an open flame for 12 seconds. Historically, compliance with this standard was achieved by treating polyurethane foam with penta-BDE [3]. Levels in our population were about three to six times higher than levels measured in a population of Mexican-immigrant women from the agricultural community of Salinas, California who were sampled in 1999–2000 [13]. This difference may be partially explained by year of sample collection since PBDE levels in human tissue have increased over time in the U.S [38]. It may also indicate that PBDEs were more widely used in California than Mexico since most of the women in the Salinas study were born in Mexico and lower-brominated PBDEs have two to 12 year half-lives in human blood [39]. Indeed, a recent study found that California children had seven times higher levels of PBDEs in their serum compared to their Mexican counterparts [39].
Relatively few published studies of OH-PBDEs or deca-BDE congeners exist to which we can compare our observations. OH-PBDEs in this population were higher than those found in pregnant women from Indiana [37] and Japan [40], and similar to levels found in Nicaraguan children working at a waste disposal site [41]. BDE-209 was more difficult to quantify, but our 95th percentile estimate suggests that some pregnant women may be highly exposed to deca-BDE. Future biomonitoring studies should measure deca-BDE congeners so we can more accurately characterize their potential health risks.
This is the first human study to examine and report an association between OH-PBDEs and TSH. One prior study examined correlations between 6-OH-BDE-47 and total T4 in umbilical cord blood serum and reported a negative but non-significant trend [42].
Hydroxylated PBDEs may be more potent TH disrupters than the parent congeners. OH-PBDEs bind to both TTR and TBG with a higher affinity than do their parent congeners [43, 44]. In addition, some OH-PBDEs bind to TTR with a higher affinity than does T4 itself [23]. The consequences of this binding may be twofold. First, T4 displacement from these serum binding proteins may decrease serum half-life of T4, which is approximately six days in humans [45]. This effect may be enhanced by xenobiotic activation of liver enzymes that conjugate T4 and remove it from serum [8, 46–48]. Second, while TTR only caries about 15% of total T4 in the blood [45], it may be an important mechanism by which T4 crosses the placenta [49]. Thus, OH-PBDEs may gain access to the fetus in part by their ability to bind to TTR. Considering this, it is important that we found that ΣTTR binders was one of the strongest predictor of TSH in our study.
Hydroxylated PBDEs, but not their parent congeners, can also bind to both thyroid hormone receptors TRα and TRβ [50, 51]. Because TRβ mediates the negative feedback action of TH on both the hypothalamus and pituitary gland [5], the combined effects of PBDEs and their hydroxylated metabolites on TH metabolism and on TH negative feedback may be difficult to predict. Likewise, the combined effects of these chemicals on TH action on development and on physiology may be difficult to evaluate.
Our findings also suggest that PBDEs are positively associated with TSH. In principle, this observation should indicate that these contaminants can reduce circulating levels of serum free and total T4, which would be predicted by animal studies [6–8]. However, associations between PBDEs and OH-PBDEs with free and total T4 were generally null. This may indicate that PBDEs affect circulating TSH levels by a mechanism that does not include changes in serum free or total T4. It is not clear what mechanism would induce this pattern because increased serum TSH should either reflect a reduction or increase in serum T4. If neither is the case, then it is possible that the normal relationship between hormones within this system has been altered. Alternatively, these associations could be spurious. However, the limited variability that we observed in the T4 measurements of our study population (CV < 15%) coupled with the endogenous variability of T4 [52], suggests we may require a larger sample size to observe potential associations between PBDEs and T4, if they exist.
Surprisingly, OH-PBDEs were poorly correlated to PBDE congeners. This suggests that metabolism of PBDE congeners is not the only source of OH-PBDEs [53] or that there are inter-individual differences in metabolism of xenobiotics [54]. In either case, measurement of parent PBDEs cannot substitute for OH-PBDEs. Future research should identify influences on human OH-PBDE exposure including both sources and metabolic variability.
Our study results differ from human studies where serum concentrations of PBDEs are considerably lower. Many of these studies either report null associations of PBDEs and TH or effects suggestive of a hyperthyroid-like effect (e.g. a decrease in TSH). For example, Chevrier et al. [13] reported an inverse association between individual PBDE congeners and their sum with serum TSH in 270 pregnant women. Differences in exposure levels and study design may partly explain these discrepancies. PBDEs may exert different effects on the thyroid system at different concentrations. Furthermore, because TH levels fluctuate throughout pregnancy [26], the difference in the timing of TSH measurement may be important. Participants in Chevrier’s study were sampled in the late second and third semesters (~27th week gestation) whereas our study population was sampled between the 19th and 23nd week of pregnancy.
Ours is the first human study to examine and find an inverse association between BDE-207 and TSH. Animal studies on thyroid toxicity of higher-brominated PBDEs are limited. BDE-209 does not bind to TTR [55] nor does it produce hydroxlyated metabolites in human liver cells [21]; therefore it is plausible that thyroid response varies by PBDE structure. Future studies should compare effects of lower- and higher-brominated congeners on TH action in humans.
While the sample size is limited, this study is important because we focused on a vulnerable but understudied population of ethnically diverse and predominately low-income pregnant women in California. We examined a broad range of chemical analytes and used state-of-the-art methods for analysis of PBDEs and TH. We eliminated potential confounding by smoking through our study design and evaluated the potential for confounding by age, race/ethnicity, parity, and SES. However, larger epidemiologic studies with data on more potential confounders and multiple measures of TH during pregnancy are needed to confirm the observed relationships. Future studies should also assess the health effects of moderate TH disruption since the majority of our participants had TH levels within the normal range.
In conclusion, the results of this cross-sectional, pilot study indicate that PBDE exposures are elevated in pregnant women in California, and suggest a relationship with thyroid function. Our results support including measurements of OH-PBDEs in evaluation of PBDE toxicity and weighting PBDEs and OH-PBDEs based on their ability for binding to TTR. This study supports previous research suggesting that brominated flame retardants can influence TH signaling during pregnancy, which requires further investigation as TH are critical to the health of pregnant women and child development. Lastly, this pilot study lays the basis for larger studies to examine the inter-relationships between PBDEs and OH-PBDEs, TH signaling during pregnancy, and adverse maternal and child health outcomes.
Supplementary Material
Figure 3.
Association between serum levels of TSH and the weighted sum of transthyretin-binding PBDEs and OH-PBDEs (ΣTTR binders) after adjustment for parity and lipid content in second trimester pregnant women in Northern and Central California (Adjusted R2 = 0.14; p = 0.03). ΣTTR binders is a weighted sum of BDE-47, 5-OH-BDE47, 6-OH-BDE47, and 4′-OH-BDE49. Concentrations are weighted according to their potencies to compete with T4 for binding to transthyretin (TTR) as determined by Hammers et al. [23].
Acknowledgments
We thank Jackie Schwartz, Tanya Pasternak, and Dr. Jody Steinauer for their assistance with recruitment and sample collection; Janet Pan for her input in the manuscript; Dr. Jonathan Chevrier for additional information on serum PBDE levels from the CHAMACOS study; and Dr. Robert Letcher of Environment Canada for sharing his methoxy PBDE standards. This work was supported by a grant from the Passport Foundation Science Innovation Fund; a generous gift from Mrs. Audrey McMahon of the Learning Disabilities Association of America; National Institute of Environmental Health Sciences (NIEHS) K99ES019881; and NIEHS ViCTER supplement to 5R01ES010026. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
Footnotes
The supplemental information includes: percent recoveries of internal standards; descriptive statistics for PBDEs in ng/mL; comparison of PBDE serum concentrations in pregnant women across study locations; and correlation matrix of PBDEs and OH-PBDEs. This material is available free of charge at http://pubs.acs.org.
Literature Cited
- 1.Harrad S, Diamond M. New directions: exposure to polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs): current and future scenarios. Atmos Environ. 2006;40(6):1187–1188. [Google Scholar]
- 2.Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE. Human internal and external exposure to PBDEs - A review of levels and sources. Int J Hyg Environ Health. 2009;212(2):109–134. doi: 10.1016/j.ijheh.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Zota A, Rudel RA, Morello-Frosch RA, Brody JB. Elevated house dust and serum concentrations of PBDEs in California: unintended consequences of furniture flammability standards? Environ Sci Technol. 2008;42(21):8158–8164. doi: 10.1021/es801792z. [DOI] [PubMed] [Google Scholar]
- 4.Zota AR, Adamkiewicz G, Morello-Frosch RA. Are PBDEs an environmental equity concern? Exposure disparities by socioeconomic status. Environ Sci Technol. 2010;44(15):5691–92. doi: 10.1021/es101723d. [DOI] [PubMed] [Google Scholar]
- 5.Zoeller RT, Tan SW, Tyl RW. General background on the hypothalamic-pituitary-thyroid (HPT) axis. Crit Rev Toxicol. 2007;37(1–2):11–53. doi: 10.1080/10408440601123446. [DOI] [PubMed] [Google Scholar]
- 6.Hallgren S, Darnerud PO. Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and chlorinated paraffins (CPs) in rats - testing interactions and mechanisms for thyroid hormone effects. Toxicology. 2002;177(2–3):227–243. doi: 10.1016/s0300-483x(02)00222-6. [DOI] [PubMed] [Google Scholar]
- 7.Hallgren S, Sinjari T, Hakansson H, Darnerud PO. Effects of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) on thyroid hormone and vitamin A levels in rats and mice. Arch Toxicol. 2001;75(4):200–208. doi: 10.1007/s002040000208. [DOI] [PubMed] [Google Scholar]
- 8.Szabo DT, Richardson VM, Ross DG, Diliberto JJ, Kodavanti PRS, Birnbaum LS. Effects of perinatal PBDE exposure on hepatic Phase I, Phase II, Phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups. Toxicol Sci. 2009;107(1):27–39. doi: 10.1093/toxsci/kfn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan J, Chen L, Chen DH, Guo H, Bi XH, Ju Y, Jiang P, Shi JB, Yu ZQ, Yang J, Li LP, Jiang Q, Sheng GY, Fu JM, Wu TC, Chen XM. Elevated serum polybrominated diphenyl ethers and thyroid-stimulating hormone associated with lymphocytic micronuclei in Chinese workers from an e-waste dismantling site. Environ Sci Technol. 2008;42(6):2195–2200. doi: 10.1021/es702295f. [DOI] [PubMed] [Google Scholar]
- 10.Meeker JD, Johnson PI, Camann D, Hauser R. Polybrominated diphenyl ether (PBDE) concentrations in house dust are related to hormone levels in men. Sci Total Environ. 2009;407(10):3425–3429. doi: 10.1016/j.scitotenv.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turyk ME, Persky VW, Imm P, Knobeloch L, Chatterton R, Anderson HA. Hormone disruption by PBDEs in adult male sport fish consumers. Environ Health Perspect. 2008;116(12):1635–1641. doi: 10.1289/ehp.11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dallaire R, Dewailly E, Pereg D, Dery S, Ayotte P. Thyroid function and plasma concentrations of polyhalogenated compounds in Inuit adults. Environ Health Perspect. 2009;117(9):1380–1386. doi: 10.1289/ehp.0900633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chevrier J, Harley KG, Bradman A, Gharbi M, Sjodin A, Eskenazi B. Polybrominated diphenyl ether (PBDE) flame retardants and thyroid hormone during pregnancy. Environ Health Perspect. 2010;118(10):1444–9. doi: 10.1289/ehp.1001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim TH, Lee YJ, Lee E, Patra N, Lee J, Kwack SJ, Kim KB, Chung KK, Han SY, Han JY, Lee BM, Kim HS. Exposure assessment of polybrominated diphenyl ethers (PBDE) in umbilical cord blood of Korean infants. J Toxicol Env Health Part A. 2009;72(21–22):1318–1326. doi: 10.1080/15287390903212436. [DOI] [PubMed] [Google Scholar]
- 15.Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ Health Perspect. 2003;111(9):1249–1252. doi: 10.1289/ehp.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbstman JB, Sjodin A, Apelberg BJ, Witter FR, Halden RU, Patterson DG, Panny SR, Needham LL, Goldman LR. Birth delivery mode modifies the associations between prenatal polychlorinated biphenyl (PCB) and polybrominated diphenyl ether (PBDE) and neonatal thyroid hormone levels. Environ Health Perspect. 2008;116(10):1376–82. doi: 10.1289/ehp.11379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brent GA. Maternal thyroid function: interpretation of thyroid function tests in pregnancy. Clinical obstetrics and gynecology. 1997;40(1):3–15. doi: 10.1097/00003081-199703000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Smallridge RC, Glinoer D, Hollowell JG, Brent G. Thyroid function inside and outside of pregnancy: what do we know and what don’t we know? Thyroid. 2005;15(1):54–59. doi: 10.1089/thy.2005.15.54. [DOI] [PubMed] [Google Scholar]
- 19.LaFranchi SH, Haddow JE, Hollowell JG. Is thyroid inadequacy during gestation a risk factor for adverse pregnancy and developmental outcomes? Thyroid. 2005;15(1):60–71. doi: 10.1089/thy.2005.15.60. [DOI] [PubMed] [Google Scholar]
- 20.Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O’Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341(8):549–55. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 21.Stapleton HM, Kelly SM, Pei R, Letcher RJ, Gunsch C. Metabolism of polybrominated diphenyl ethers (PBDEs) by human hepatocytes in vitro. Environ Health Perspect. 2009;117(2):197–202. doi: 10.1289/ehp.11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meerts I, van Zanden JJ, Luijks EAC, van Leeuwen-Bol I, Marsh G, Jakobsson E, Bergman A, Brouwer A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol Sci. 2000;56(1):95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- 23.Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Visser TJ, Van Velzen MJM, Brouwer A, Bergman A. Biotransformation of brominated flame retardants into potentially endocrine-disrupting metabolites, with special attention to 2,2′,4,4 ′-tetrabromodiphenyl ether (BDE-47) Mol Nutr Food Res. 2008;52(2):284–298. doi: 10.1002/mnfr.200700104. [DOI] [PubMed] [Google Scholar]
- 24.Freitas J, Cano P, Craig-Veit C, Goodson ML, Furlow JD, Murk AJ. Detection of thyroid hormone receptor disruptors by a novel stable in vitro reporter gene assay. Toxicol In Vitro. 2011;25(1):257–66. doi: 10.1016/j.tiv.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Li F, Xie Q, Li X, Li N, Chi P, Chen J, Wang Z, Hao C. Hormone Activity of Hydroxylated Polybrominated Diphenyl Ethers on Human Thyroid Receptor-beta: In Vitro and In Silico Investigations. Environ Health Perspect. 2010;118(5):602–6. doi: 10.1289/ehp.0901457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soldin OP, Tractenberg RE, Hollowell JG, Jonklaas J, Janicic N, Soldin SJ. Trimester-specific changes in maternal thyroid hormone, thyrotropin, and thyroglobulin concentrations during gestation: trends and associations across trimesters in iodine sufficiency. Thyroid. 2004;14(12):1084–1090. doi: 10.1089/thy.2004.14.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang R, Nelson JC, Weiss RM, Wilcox RB. Accuracy of free thyroxine measurements across natural ranges of thyroxine binding to serum proteins. Thyroid. 2000;10(1):31–39. doi: 10.1089/thy.2000.10.31. [DOI] [PubMed] [Google Scholar]
- 28.Phillips DL, Pirkle JL, Burse VW, Bernert JT, Henderson LO, Needham LL. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18(4):495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- 29.La Guardia MJ, Hale RC, Harvey E. Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flame-retardant mixtures. Environ Sci Technol. 2006;40(20):6247–6254. doi: 10.1021/es060630m. [DOI] [PubMed] [Google Scholar]
- 30.Park JS, Linderholm L, Charles MJ, Athanasiadou M, Petrik J, Kocan A, Drobna B, Trnovec T, Bergman A, Hertz-Picciotto I. Polychlorinated biphenyls and their hydroxylated metabolites (OH-PCBs) in pregnant women from eastern Slovakia. Environ Health Perspect. 2007;115(1):20–27. doi: 10.1289/ehp.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers E, Petreas M, Park JS, Zhao GM, Charles MJ. Evaluation of four capillary columns for the analysis of organochlorine pesticides, polychlorinated biphenyls, and polybrominated diphenyl ethers in human serum for epidemiologic studies. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences. 2004;813(1–2):269–285. doi: 10.1016/j.jchromb.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 32.Sandau CD. Analytical chemistry of hydroxylated metabolites of PCBS and other halogenated phenolic compounds in blood and their relationship to thyroid hormone and retinol homeostasis in humans and polar bears. Carleton University; Ottawa, Ontario: 2000. [Google Scholar]
- 33.Baccarelli A, Pfeiffer R, Consonni D, Pesatori AC, Bonzini M, Patterson DG, Bertazzi PA, Landi MT. Handling of dioxin measurement data in the presence of non-detectable values: overview of available methods and their application in the Seveso chloracne study. Chemosphere. 2005;60(7):898–906. doi: 10.1016/j.chemosphere.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 34.Helsel DR. Less than obvious - statistical treatment of data below the detection limit. Environ Sci Technol. 1990;24(12):1766–1774. [Google Scholar]
- 35.Mandel SJ, Spencer CA, Hollowell JG. Are detection and treatment of thyroid insufficiency in pregnancy feasible? Thyroid. 2005;15(1):44–53. doi: 10.1089/thy.2005.15.44. [DOI] [PubMed] [Google Scholar]
- 36.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the US: NHANES 2003–2004. Environ Health Perspect. 2011;119(6):878–885. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiu XH, Bigsby RM, Hites RA. Hydroxylated metabolites of polybrominated fiphenyl rthers in human blood samples from the United States. Environ Health Perspect. 2009;117(1):93–98. doi: 10.1289/ehp.11660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ Sci Technol. 2004;38(4):945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- 39.Eskenazi B, Fenster L, Castorina R, Marks AR, Sjödin A, Rosas LG, Holland N, Guerra AG, López-Carrillo L, Bradman A. A comparison of PBDE serum concentrations in Mexican and Mexican-American children living in California. Environ Health Perspect. 2011 doi: 10.1289/ehp.1002874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawashiro Y, Fukata H, Inoue MO, Kubonoya K, Jotaki T, Takigami H, Sakai SI, Mori C. Perinatal exposure to brominated flame retardants and polychlorinated biphenyls in Japan. Endocr J. 2008;55(6):1071–1084. doi: 10.1507/endocrj.k08e-155. [DOI] [PubMed] [Google Scholar]
- 41.Athanasiadou M, Cuadra SN, Marsh G, Bergman A, Jakobsson K. Polybrominated diphenyl ethers (PBDEs) and bioaccumulative hydroxylated PBDE metabolites in young humans from Managua, Nicaragua. Environ Health Perspect. 2008;116(3):400–408. doi: 10.1289/ehp.10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wan Y, Choi K, Kim S, Ji K, Chang H, Wiseman S, Jones PD, Khim JS, Park S, Park J, Lam MHW, Giesy JP. Hydroxylated polybrominated diphenyl ethers and bisphenol A in pregnant women and their matching fetuses: placental transfer and potential risks. Environ Sci Technol. 2010;44(13):5233–5239. doi: 10.1021/es1002764. [DOI] [PubMed] [Google Scholar]
- 43.Cao J, Lin YA, Guo LH, Zhang AQ, Wei Y, Yang Y. Structure-based investigation on the binding interaction of hydroxylated polybrominated diphenyl ethers with thyroxine transport proteins. Toxicology. 2010;277(1–3):20–28. doi: 10.1016/j.tox.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 44.Marchesini GR, Meimaridou A, Haasnoot W, Meulenberg E, Albertus F, Mizuguchi M, Takeuchi M, Irth H, Murk AJ. Biosensor discovery of thyroxine transport disrupting chemicals. Toxicol Appl Pharmacol. 2008;232(1):150–160. doi: 10.1016/j.taap.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 45.Schussler GC. The thyroxine-binding proteins. Thyroid. 2000;10(2):141–9. doi: 10.1089/thy.2000.10.141. [DOI] [PubMed] [Google Scholar]
- 46.Wu SY, Green WL, Huang WS, Hays MT, Chopra IJ. Alternate pathways of thyroid hormone metabolism. Thyroid. 2005;15(8):943–958. doi: 10.1089/thy.2005.15.943. [DOI] [PubMed] [Google Scholar]
- 47.Richardson VM, Staskal DF, Ross DG, Diliberto JJ, DeVito MJ, Bimbaum LS. Possible mechanisms of thyroid hormone disruption in mice by BDE 47, a major polybrominated diphenyl ether congener. Toxicol Appl Pharmacol. 2008;226(3):244–250. doi: 10.1016/j.taap.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 48.Qatanani M, Zhang J, Moore DD. Role of the constitutive androstane receptor in xenobiotic-induced thyroid hormone metabolism. Endocrinology. 2005;146(3):995–1002. doi: 10.1210/en.2004-1350. [DOI] [PubMed] [Google Scholar]
- 49.Landers KA, McKinnon BD, Li H, Subramaniam VN, Mortimer RH, Richard K. Carrier-mediated thyroid hormone transport into placenta by placental transthyretin. J Clin Endocrinol Metab. 2009;94(7):2610–6. doi: 10.1210/jc.2009-0048. [DOI] [PubMed] [Google Scholar]
- 50.Kojima H, Takeuchi S, Uramaru N, Sugihara K, Yoshida T, Kitamura S. Nuclear hormone receptor activity of polybrominated diphenyl ethers and their hydroxylated and methoxylated metabolites in transactivation assays using Chinese hamster ovary cells. Environ Health Perspect. 2009;117(8):1210–1218. doi: 10.1289/ehp.0900753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li F, Xie Q, Li XH, Li N, Chi P, Chen JW, Wang ZJ, Hao C. Hormone activity of hydroxylated polybrominated diphenyl ethers on human thyroid receptor-beta: in vitro and in silico investigations. Environ Health Perspect. 2010;118(5):602–606. doi: 10.1289/ehp.0901457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab. 2002;87(3):1068–72. doi: 10.1210/jcem.87.3.8165. [DOI] [PubMed] [Google Scholar]
- 53.Teuten EL, Xu L, Reddy CM. Two abundant bioaccumulated halogenated compounds are natural products. Science. 2005;307(5711):917–920. doi: 10.1126/science.1106882. [DOI] [PubMed] [Google Scholar]
- 54.Dorne J, Walton K, Renwick AG. Human variability in xenobiotic metabolism and pathway-related uncertainty factors for chemical risk assessment: a review. Food Chem Toxicol. 2005;43(2):203–216. doi: 10.1016/j.fct.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 55.Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MHA, Andersson PL, Legler J, Brouwer A. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci. 2006;92(1):157–173. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.