Abstract
The role of the serine protease HtrA2 in neuroprotection was initially identified by the demonstration of neurodegeneration in mice lacking HtrA2 expression or function, and the interesting finding that mutations adjacent to two putative phosphorylation sites (S142 and S400) have been found in Parkinson's disease patients. However, the mechanism of this neuroprotection and the signalling pathways associated with it remain mostly unknown. Here we report that cyclin-dependent kinase-5 (Cdk5), a kinase implicated in the pathogenesis of several neurodegenerative diseases, is responsible for phosphorylating HtrA2 at S400. HtrA2 and Cdk5 interact in human and mouse cell lines and brain, and Cdk5 phosphorylates S400 on HtrA2 in a p38-dependent manner. Phosphorylation of HtrA2 at S400 is involved in maintaining mitochondrial membrane potential under stress conditions and is important for mitochondrial function, conferring cells protection against cellular stress.
Keywords: HtrA2, Cdk5, phosphorylation, mitochondria, Parkinson's disease
The serine protease HtrA2 belongs to the high-temperature-requirement protein family, which contain a trypsin-like protease domain and a regulatory C-terminal PDZ domain.1 HtrA2 is imported into mitochondria by a mitochondrial targeting sequence (MTS) and is then inserted into the inner mitochondrial membrane (IMM). A mature form is generated by proteolysis close to the transmembrane domain (TM) and resides within the inner mitochondrial space (IMS).2 Upon initiation of apoptosis, mature HtrA2 is released into the cytosol, where it exacerbates apoptosis by binding to and neutralising the inhibitor of apoptosis proteins (IAPs). Cytosolic HtrA2 also promotes apoptosis directly by its protease activity.3 Mice lacking HtrA2 protease activity die prematurely and have a parkinsonian, neurodegenerative phenotype.4, 5 Screening of the HtrA2 gene in German Parkinson's disease (PD) patients identified a G399S mutation and an A141S polymorphism.6 An additional R404W mutation was found in a Belgian population,7 but the HtrA2 link to PD has been disputed.8 Functional analyses showed that the G399S mutation and to a lesser extent A141S results in decreased HtrA2 protease activity, mitochondrial dysfunction, altered mitochondrial morphology and increased susceptibility to cellular stress.6 Notably, these mutations are positioned one amino-acid residue downstream from putative kinase phosphorylation sites, serine-142 (S142) and serine-400 (S400), respectively. HtrA2 is phosphorylated close to its TM at S142 upon activation of the p38 signalling pathway and this phosphorylation is dependent on PINK1,9 a mitochondrial putative kinase associated with PD.10 Phosphorylation at S142 increases HtrA2 protease activity, which in turn increases its protective effect.9 Mimicking phosphorylation at S400, which lies in the PDZ domain of HtrA2, dramatically increases HtrA2 protease activity and might be involved in neuroprotection.9 This prompted us to attempt to identify potential kinases that could phosphorylate HtrA2 at S400.
Cyclin-dependent kinase-5 (Cdk5) is a serine/threonine kinase11 and a member of the highly conserved family of cyclin-dependent kinases.12 Cdk5 is unique among its family; first, it is not activated by cyclins but is regulated exclusively by the brain-specific activator p35, its isoform p39 and their respective truncated forms p25/p29 (for a review see Dhavan and Tsai12). Second, Cdk5 does not regulate the cell cycle, rather it has a multi-functional role, with diverse reported substrates.12, 13, 14, 15 Moreover, mice lacking Cdk5 die prematurely and show disruption of neuronal layering.16 Abnormal Cdk5 activity is associated with several neurodegenerative diseases. In particular, Cdk5 accumulates in neurons with early-stage neurofibrillary tangles, in Lewy bodies and in inclusions, principal hallmarks of Alzheimer's disease (AD),17 PD and amyotrophic lateral sclerosis patients, respectively.18, 19 Deregulation of Cdk5 is proposed to be a result of the conversion of p35 to p25; as p25 is less readily turned over than p35, this causes aberrant activation of Cdk5.20 Neurotoxicity causes the cleavage of p35 to p25 by calpain,21 and Cdk5–p25 has been confirmed as a key factor in tau aggregation and tangle formation, two hallmarks of AD.22 Strict control of Cdk5 activity in vital neuronal processes such as mitochondrial function15 may explain why deregulation is associated with neurodegenerative diseases, where dysfunctional mitochondria is implicated.23, 24
We report that Cdk5 is the kinase responsible for the phosphorylation of HtrA2 at S400 upon activation of the p38 signalling pathway. The interaction between HtrA2 and Cdk5 can occur both in the cytosol and in mitochondria, and Cdk5 translocates to mitochondria under stress conditions. Our data suggests that phosphorylation of HtrA2 S400 is important for maintaining mitochondrial membrane potential.
Results
HtrA2 interacts with Cdk5
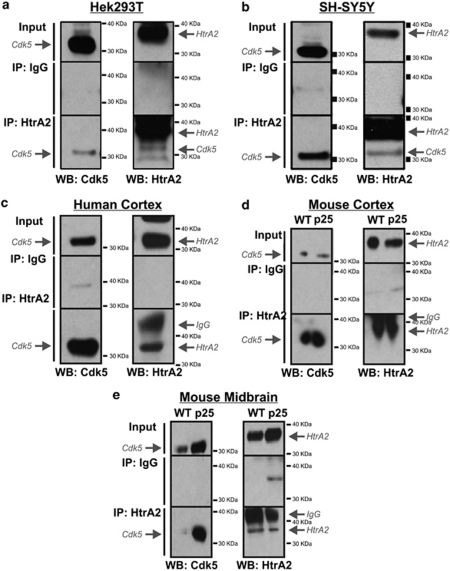
We initially screened for a range of kinases that would phosphorylate recombinant HtrA2 in vitro and identified Cdk5 as one of the candidate kinases responsible for HtrA2 phosphorylation (Supplementary Figure S1A). In two human cell lines, the Hek293T embryonic kidney cells and the SH-SY5Y neuroblastoma cells, endogenous Cdk5 is detected in HtrA2 complexes after immunoprecipitation (IP) using an HtrA2-specific antibody but not an IgG control antibody (Figures 1a and b). These data show that Cdk5 and HtrA2 interact in both neuronal and non-neuronal human cell lines under normal physiological conditions. The interaction was then validated in human brain tissue. We used exon array data generated in our laboratory to check that Cdk5 mRNA was expressed in the human occipital cortex (Supplementary Figure S2A) before we prepared lysates from tissue. We found that Cdk5 and HtrA2 are present in occipital cortex lysates at the protein level and that they interact (Figure 1c). Finally, we investigated whether Cdk5 and HtrA2 interact in the cortex and midbrain of wild-type (WT) mice and mice overexpressing the Cdk5 activator p25. Cdk5 and HtrA2 interact in the cortex of both WT and p25 transgenic mice (Figure 1d). The protein levels of Cdk5 are increased in the midbrains of the p25 transgenic mice as compared with that in the WT animals (Figure 1e). As a result, the interaction is greatly increased in the midbrains of the p25 transgenic mice (Figure 1e). The extents to which Cdk5 and HtrA2 interact under normal physiological conditions vary between the cortex and midbrain in these mice (Figures 1d and e).
Figure 1.
Cdk5 interacts with HtrA2. IP of endogenous HtrA2 together with endogenous Cdk5 from (a) Hek293T cells, (b) SH-SY5Y cells, (c) human brain (occipital cortex) and (d) the cortex of WT or transgenic mice overexpressing p25 (p25), and (e) midbrain. Input lysates, control IP with IgG and HtrA2 IP were run on an SDS-PAGE gel and analysed by western blotting (WB) using antibodies specific to Cdk5 and HtrA2. All experiments were repeated at least three times and representative images are shown
Regulation of Cdk5/HtrA2 interaction
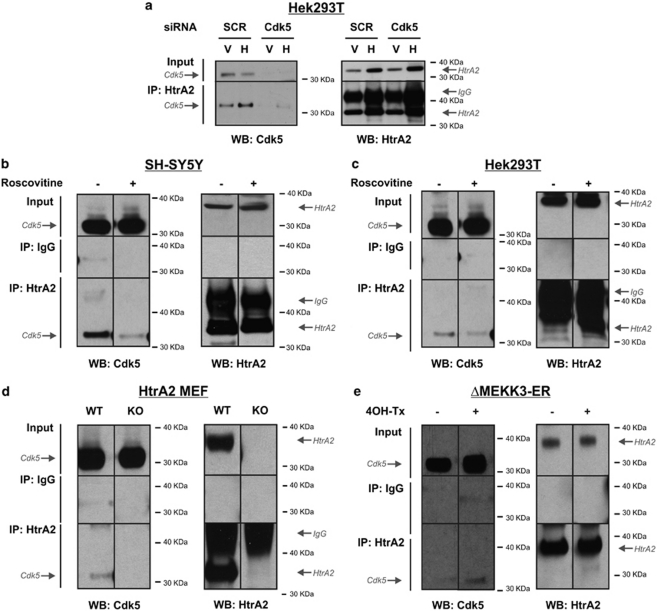
A targeted siRNA against Cdk5 in Hek293T cells knocks down Cdk5 expression by approximately 80% at the protein level as compared with that using a scramble sequence siRNA control (Supplementary Figure S2B). As a result, co-IP from Cdk5-knockdown (KD) cells was undetectable (Figure 2a). The Cdk5 inhibitor Roscovitine also reduced significantly the interaction between Cdk5 and HtrA2 detected by co-IP in SH-SY5Y cells (Figure 2b) and Hek293T cells (Figure 2c). These experiments suggest that the active Cdk5 enzyme preferentially interacts with HtrA2. Consistently, HtrA2 and Cdk5 interact in WT mouse embryonic fibroblasts (MEFs) but not in HtrA2-KO MEFs (Figure 2d). Cdk5 has previously been shown to be activated by a number of stimuli in vitro, including epidermal growth factor (EGF), calcium ionophore and glutamate.25 Accordingly, glutamate increased the interaction between HtrA2 and Cdk5 detected by co-IP in Hek293T cells (Supplementary Figure S2C). Furthermore, we tested whether the interaction between Cdk5 and HtrA2 could be enhanced upon stimulation of the c-jun N-terminal kinase (JNK), p38 stress-activated, mitogen-activated protein kinase (MAPK), ERK MAPK and Akt pathways. For this, we used 4-hydroxytamoxifen (4OH-Tx)-inducible, stable versions of MEKK1, MEKK3, Akt and Raf (Hek293: ΔMEKK1-ER, ΔMEKK3-ER; and NIH3T3: myrAkt-ER and ΔRaf-DD-ER stable cell lines).9, 26 These experiments showed that only activation of the p38 signalling pathway induced significant phosphorylation of HtrA2 (S400) (Supplementary Figure S1B). The interaction between Cdk5 and HtrA2 is increased upon stimulation of the p38 signalling pathway as compared with that under control conditions (Figure 2e). These data indicate that the interaction between HtrA2 and Cdk5 is enhanced following activation of stress signalling pathways.
Figure 2.
Regulation of Cdk5/HtrA2 interaction. Co-IP of endogenous HtrA2 and endogenous Cdk5 in (a) Hek293T cells transfected with a vector control (V) or HtrA2 (H), and with a scramble non-targeting sequence control siRNA (SCR) or siRNA targeting Cdk5 (Cdk5); (b) SH-SY5Y cells or (c) Hek293T cells treated or not with 20 μM Roscovitine for 8 h. (d) WT or HtrA2-KO MEFs and (e) ΔMEKK3-ER cells treated or not with 100 nM 4OH-Tx for 8 h. Input lysates, control IP with IgG and HtrA2 IP were run on an SDS-PAGE gel and analysed by western blotting (WB) using antibodies specific to Cdk5 and HtrA2. All experiments were repeated at least three times and representative images shown
Cdk5 phosphorylates HtrA2 at S400
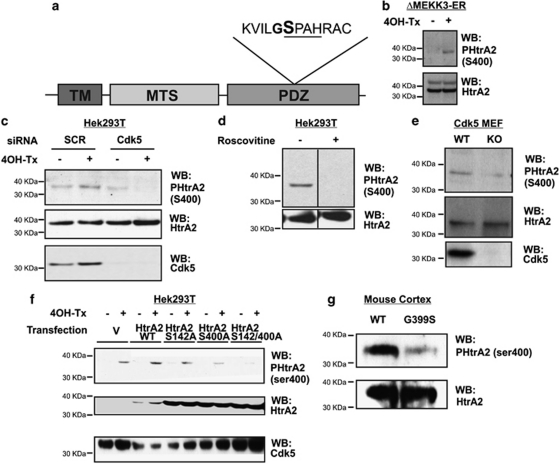
The S400 of HtrA2 forms part of a motif (S/T)PX(K/H/R) identified by Scansite (http://scansite.mit.edu/) to be a consensus sequence for Cdk5 phosphorylation (underlined in Figure 3a). In vitro, Cdk5/p25 preferentially and strongly phosphorylates an S400-containing HtrA2 peptide substrate and not an S142-containing HtrA2 peptide, whereas it is unable to phosphorylate the S400 peptide where the S400 is mutated to an alanine (Supplementary Figure S1C). To further investigate the phosphorylation of HtrA2 by Cdk5, we performed in vitro kinase assays using Cdk5/p25 and recombinant HtrA2. A generic substrate (myelin basic protein, MBP) and a known substrate of Cdk5 (human recombinant Tau) were used as controls. Cdk5 phosphorylates MBP, Tau, WT HtrA2 and HtrA2 S142A. However, HtrA2 S400A and HtrA2 S142/400A are phosphorylated by approximately 60% less than WT HtrA2 (Supplementary Figure S1D), suggesting that Cdk5 preferentially phosphorylates HtrA2 at S400 in vitro. To investigate the phosphorylation of HtrA2 by Cdk5 in situ, we raised an antibody that specifically recognised HtrA2 only when phosphorylated on S400. A phospho-S400 HtrA2 signal was detected in ΔMEKK3-ER Hek293 cells after activation with 4OH-Tx, strongly suggesting that HtrA2 is phosphorylated at this site following activation of the p38 stress pathway (Figure 3b). KD of Cdk5 using a targeted siRNA significantly reduces the phosphorylation of HtrA2 at S400 upon 4OH-Tx stimulation in ΔMEKK3-ER Hek293 cells, indicating that Cdk5 is important for phosphorylation of HtrA2 at this site upon stimulation of the p38 stress pathway (Figure 3c). Consistently, inhibition of Cdk5 activity with Roscovitine significantly reduces the phosphorylation of HtrA2 at S400 in Hek293T cells (Figure 3d), and phosphorylation of HtrA2 S400 in Cdk5-KO MEF cells is decreased as compared with that in WT controls (Figure 3e). These data indicate that Cdk5 is important for the phosphorylation of HtrA2 at the S400 site. This is not to say that Cdk5 is the only kinase responsible for phosphorylating S400, and residual phosphorylation at this site in siRNA- or Roscovitine-treated cells, or in Cdk5-KO MEF cells, may be because of phosphorylation by other kinases such as p38, Cdc2 or GSK3β. Indeed, we have observed some phosphorylation of the S400 peptide by p38 and Cdc2 in vitro (Supplementary Figure S1C), and a weak interaction between HtrA2 and Cdc2 by co-IP (data not shown).
Figure 3.
Cdk5 phosphorylates HtrA2 at S400. (a) A schematic representation of HtrA2 showing major protein domains (TM, MTS and PDZ domain), the S400 phosphorylation site (bold and large print), the Cdk5 consensus sequence (underlined) and the G399S mutation site (bold). (b) HtrA2 is phosphorylated at S400 upon stimulation of the p38 MAPK pathway with 100 nM 4OH-Tx for 8 h in ΔMEKK3-ER cells. Cell lysates were run on an SDS-PAGE gel and analysed by western blotting (WB) using an S400 phospho-specific antibody. Total HtrA2 levels are shown as control. (c) HtrA2 is phosphorylated at S400 (detected by phospho-specific antibody) upon stimulation of the p38 MAPK pathway with 100 nM 4OH-Tx for 8 h in ΔMEKK3-ER cells transfected with a scramble non-targeting siRNA (SCR) but not in those transfected with an siRNA targeting Cdk5 (Cdk5). Total HtrA2 and Cdk5 levels are shown as control. (d) HtrA2 phosphorylation at S400 is inhibited upon treatment with 20 μM Roscovitine for 8 h in Hek293T cells. Total HtrA2 levels are shown as control. (e) HtrA2 phosphorylation at S400 is decreased in Cdk5-KO MEF cells as compared with that in WT cells. Total HtrA2 levels are shown as control. (f) HtrA2 phosphorylation levels at S400 are detected by WB using a phospho-specific antibody. ΔMEKK3-ER cells KD for HtrA2 (using shRNA) and overexpressing WT or phosphomutant HtrA2 were stimulated with 4OH-Tx (top panel). Total levels of HtrA2 and Cdk5 are shown as control. (g) HtrA2 phosphorylation levels at S400 (detected by phospho-specific antibody) are detected by WB in brain lysates from mouse expressing the G399S mutation as compared with that in WT control brain lysates (top panel). Total levels of HtrA2 are shown as control (bottom panel). All experiments were repeated at least three times and representative images shown
Next we overexpressed HtrA2 and the non-phosphorylatable HtrA2 mutants S142A, S400A and S142/400A in HtrA2-KD ΔMEKK3-ER cells with or without stimulation of the p38 pathway. We found that WT and S142A HtrA2 were phosphorylated at S400 upon stimulation of the p38 pathway, whereas the S400A HtrA2 mutant was phosphorylated to a much less extent and the S142/400A HtrA2 mutant was decreased (Figure 3f). These data confirm the specificity of the phospho-S400 HtrA2 antibody. Notably the S142/400A double phosphomutant is the least able to be phosphorylated, suggesting that regulation of HtrA2 by Cdk5 requires a functional serine at the 142 position.
The G399S HtrA2 mutation reported in German PD patients is positioned one amino acid downstream from S400.6, 9 We sought to investigate whether this mutation can affect the phosphorylation of HtrA2 at S400 by Cdk5. We, therefore, assessed HtrA2 phosphorylation at S400 in cortex from transgenic mice expressing the G399S HtrA2 mutation. Interestingly, these brains contain less phospho-HtrA2 S400 as compared with WT mice brains whenever the levels of HtrA2 are equivalent (Figure 3g). This indicates that the G399S HtrA2 mutation reduces its ability to be phosphorylated at the adjacent S400. In addition, we tested the ability of overexpressed G399S HtrA2 to interact with endogenous Cdk5 in Hek293T cells. We found that G399S HtrA2 interacts with endogenous Cdk5 in a similar manner to overexpressed WT HtrA2 (Supplementary Figure S2E), suggesting that the decreased phosphorylation of G399S HtrA2 at S400 is not because of its inability to interact with Cdk5.
Cdk5 and HtrA2 interact in cytosol and mitochondria
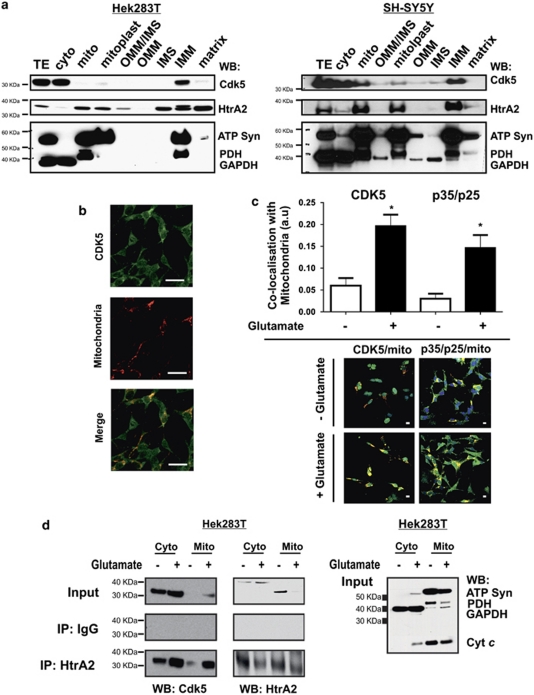
Cdk5 is commonly thought to be distributed throughout the cytoplasm of mammalian cells.12 Cdk5 has also been reported to localise to the nucleus and mitochondria in the central nervous system of WT mice.27 To determine the localisation of Cdk5 in Hek293T and SH-SY5Y cells, cytosolic and mitochondrial subcellular fractionation was performed. Cdk5 was found in both the cytoplasmic and the IMM fractions (Figure 4a). The purity of each fraction was assessed by probing for cytosolic and mitochondrial markers (Figure 4a, lower panels). To confirm these observations, Cdk5 was immunostained in SH-SY5Y cells expressing the dsREDmito plasmid, which labels mitochondria red. Merging the signal for Cdk5 and mitochondria by high-resolution confocal microscopy showed that Cdk5 is indeed localised both in the cytoplasm and in mitochondria (Figure 4b). To investigate whether stress might influence the mitochondrial location of Cdk5, we used immunostaining of Cdk5 in SH-SY5Y cells expressing the dsREDmito plasmid by glutamate treatment. Colocalisation of Cdk5 and mitochondria was determined by high-resolution confocal microscopy, where 3D images were quantified by Pearson's correlation (Zeiss colocalisation software). Interestingly, glutamate treatment significantly increases the mitochondrial pool of Cdk5. This is also the case for the Cdk5 activators p35/p25 (Figure 4c). This suggests that Cdk5 and its activators translocate to mitochondria under stress conditions, and that Cdk5 location may be an important requirement for interaction with mitochondrial substrates such as HtrA2. Consistently, the interaction between HtrA2 and Cdk5 occurs in both the cytosolic and the mitochondrial fractions, and this interaction is increased with glutamate treatment (Figure 4d). These data show that HtrA2 and Cdk5 interact in mitochondria and in the cytosol. Cdk5 translocation to mitochondria may be important for regulating the protease activity of HtrA2 within mitochondria, which is known to be important for maintaining mitochondrial quality control, respiration status and for preventing increased oxidative stress/cell death.28
Figure 4.
Localisation of the Cdk5/HtrA2 interaction. (a) Total extract (TE), cytosol (cyto), crude mitochondria (mito), mitoplast consisting of IMM and matrix, OMM, IMS, IMM and matrix fractions were prepared from Hek293T cells (left panels) and SH-SY5Y cells (right panels), and subjected to SDS-PAGE. Western blots (WBs) were probed for total Cdk5, HtrA2 and markers of IMM (ATP Syn), matrix (PDH) and cytosol (GAPDH). (b) Immunofluorescence in fixed SH-SY5Y cells expressing dsREDmito, showing mitochondria (red) and showing Cdk5 linked to an Alexa-Fluor-488 secondary antibody (green), with the merge showing colocalisation (yellow). Representative images are shown where n≥20. Images were visualised by Z-stack confocal microscopy. Magnification: × 63 oil immersion. Scale bar=20 μm. (c) Colocalisation of Cdk5 and p35/p25 with mitochondria in SH-SY5Y cells expressing dsREDmito treated or not with 5 mM glutamate for 4 h, and fixed and probed for Cdk5 or p35/p25 linked to an Alexa-Fluor-488 secondary antibody (upper panel). Colocalisation of Cdk5 or p35/p25 with mitochondria was quantified by using a Zeiss colocalisation analysis software on Z-stack confocal microscopy images. Mean colocalisation score±S.D. are shown, where n=4. Statistical analysis was performed by Student's t-test, untreated versus glutamate treated, where *P<0.05. Representative confocal images of immunofluorescence in fixed SH-SY5Y cells expressing dsREDmito, showing mitochondria (red) and showing Cdk5 or p35/p25 linked to an Alexa-Fluor-488 secondary antibody (green), with the merge showing co-localisation (yellow). Magnification: × 40. Scale bar=20 μM (lower panels). (d) Co-IP of endogenous HtrA2 and endogenous Cdk5 in the cytosolic (cyto) and mitochondrial fractions (mito) of Hek293T cells treated or not with 5 mM glutamate for 4 h. WB of co-IP input lysates, control IP with IgG and HtrA2 IP were probed with antibodies specific to Cdk5 and HtrA2. (e) WB of co-IP input lysates were probed with an IMM marker (ATP Syn), matrix marker (PDH), cytosolic marker (GAPDH) and IMS/apoptosis marker (cyt-c). All experiments were repeated at least three times and representative images shown
HtrA2 phosphorylation at S400 by Cdk5 is important for mitochondrial function
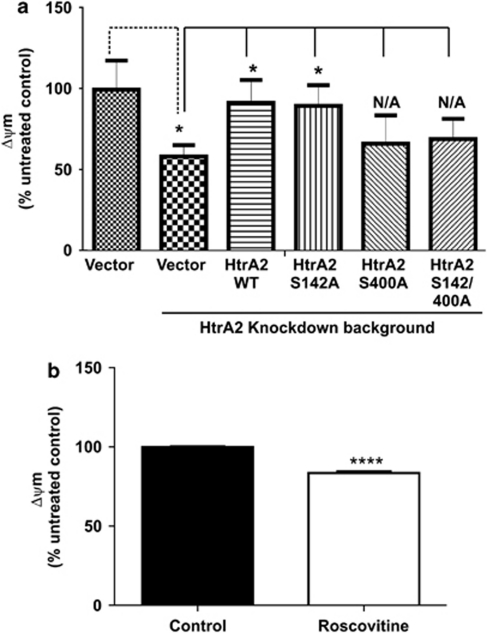
The neuroprotective function of HtrA2 has been attributed to its mitochondrial role, but little is understood about the exact mechanism. We, therefore, investigated the relevance of HtrA2 phosphorylation at S400 to mitochondrial membrane potential (Δψm), a marker of mitochondrial health, using TMRM, a cationic, mitochondrial-selective probe, by live-cell microscopy. We overexpressed HtrA2 and a non-phosphorylatable HtrA2 carrying S-to-A mutations in an HtrA2 KD. We then measured the Δψm in these cells under normal conditions and following activation of the p38 signalling pathway. HtrA2 KD was confirmed by western blotting (Supplementary Figure S2B). Silencing HtrA2 results in a significantly lower (∼50%) Δψm in cells where the p38 pathway is activated (Figure 5a), whereas HtrA2 silencing in non-stressed cells does not have any significant effect on the Δψm. Loss of Δψm in HtrA2-silenced cells where the p38 pathway is activated is restored by overexpression of WT and S142A HtrA2 but not by S400A or S142/400A HtrA2 overexpression (Figure 5a). Furthermore, we measured Δψm in Hek293T cells in the presence of the Cdk5 inhibitor Roscovitine. We found a subtle, but consistently lower Δψm in cells where Cdk5 activity was inhibited (15% reduction; Figure 5b). These data suggest that Cdk5 when activated under stress conditions is likely to phosphorylate HtrA2 at S400 in order to maintain mitochondrial health. We have previously reported that mutations mimicking phosphorylation of HtrA2 at S400 are important for regulating the protease activity of this enzyme.9 According to our current data, phosphorylation of S400 of HtrA2 appears to be instrumental to mitochondrial health, and phosphorylation within the PDZ domain of HtrA2 at S400 by Cdk5 may be an important mechanism to modulate HtrA2 activity under stress conditions.
Figure 5.
Relevance of HtrA2 phosphorylation by Cdk5. (a) Mitochondrial membrane potential (Δψm) determined by TMRM fluorescence in live ΔMEKK3-ER cells with an HtrA2 KD background, overexpressing WT HtrA2 or HtrA2 phosphomutants, stimulated or not with 4OH-Tx for 8 h. Mean maximum TMRM fluorescence was determined for approximately 80 individual mitochondria in a field of view for each condition, for each independent experiment (n=3), from Z-stack images using the Andor IQ analysis software. The data are expressed as mean maximum TMRM fluorescence in 4OH-Tx-stimulated cells as a percentage of that in untreated controls. Statistical analyses: Two-way ANOVA where treatment is significant (P≤0.01) and HtrA2 expression is significant (P<0.001), and one-way ANOVA where HtrA2 is insignificant in untreated cells but significant (P<0.001) in 4OH-Tx-treated cells, where n=3. Student's t-test compared the effect of the treatment in untransfected cells and 4OH-Tx-treated, untransfected cells to that in 4OH-Tx-treated cells transfected with HtrA2 and HtrA2 phosphomutants (shown on graph), where *P≤0.05 (n=3). (b) Δψm is determined by TMRM fluorescence in live Hek293T cells treated or not with 20 μM Roscovitine for 8 h. Mean maximum TMRM fluorescence was determined for approximately 80 individual mitochondria in a field of view for each condition, for each independent experiment (n=3), from Z-stack images using the Andor IQ analysis software. Statistical analyses: Student's t-test compared the effect of the Roscovitine treatment with the control where ****P≤0.0001
Discussion
We have shown previously that phosphomimetic mutants of HtrA2 at S400 result in increased proteolytic activity and contribute to enhanced resistance to mitochondrial stress.9 Here we show that one of the kinases responsible for phosphorylation of HtrA2 at S400 is Cdk5. The interaction between HtrA2 and Cdk5 occurs in both mitochondria and cytosol. Activation of Cdk5 activity increases its interaction with HtrA2, the phosphorylation of HtrA2 at S400 and translocation of Cdk5 to mitochondria. Δψm is compromised in cells where the p38 pathway is activated and HtrA2 is silenced. Overexpression of WT HtrA2 but not the non-phosphorylatable S400A HtrA2 mutant rescues this phenotype, suggesting that phosphorylation of HtrA2 at S400 by Cdk5 is important for mitochondrial health.
HtrA2 is phosphorylated at S400 upon stimulation of the p38 stress signalling pathway. We have shown previously that activation of this pathway induced the phosphorylation of HtrA2 at another site, S142, also adjacent to an amino acid found mutated in patients with PD.9 Taken together, these findings suggest that HtrA2 phosphorylation at both of these sites is important for the cellular stress response. HtrA2 is also known to be phosphorylated at S212 by Akt, which reduces its serine protease activity and proapoptotic function, thereby promoting cell survival.29 By phosphorylating S400, Cdk5 could also regulate the proapoptotic function of HtrA2 once it is released into the cytosol from mitochondria. Phosphorylation at S400 was shown previously to increase HtrA2 serine protease activity in vitro.9 HtrA2 promotes cell death by its ability to bind to IAPs but also through its serine protease activity1, 3 and might, therefore, also modulate its proapoptotic function, especially as the interaction between Cdk5 and HtrA2 is partly detected in the cytosolic fractions.
In mitochondria, HtrA2 is thought to function in a similar manner to that of its bacterial homologues DegS and DegP, which are involved in protection against cell stress.5 Loss of HtrA2 results in the accumulation of unfolded proteins in mitochondria, defective mitochondrial respiration and increased oxidative stress.28 Increase in HtrA2 protease activity by Cdk5 phosphorylation might be important in removing unfolded/damaged proteins in mitochondria. Indeed, both HtrA2 and Cdk5 have been linked recently to autophagy and mitochondrial fission,30, 31, 32 two processes that can be initiated by damage to mitochondrial proteins and which are critical for mitochondrial health. Whether HtrA2 phosphorylation at S400 occurs in the cytosol and/or in mitochondria remains to be determined. However, we show that Cdk5 partially localises with mitochondria in human SH-SY5Y neuroblastoma cells, and that the mitochondrial pool of Cdk5 and its activators p35/p25 is increased following glutamate treatment, suggesting that Cdk5 is available at the mitochondria for phosphorylating HtrA2 under stress conditions where increased protease activity is required.
Certain kinases have an increasingly recognised role in mitochondrial processes,24 and some kinases that are required in mitochondria, including Cdk5, do not contain an MTS. How they gain entry into the mitochondria remains unclear. It is possible that they could associate with scaffold proteins imported to the mitochondria through an MTS. In the case of Cdk5, it is possible that the distribution of the active protein is dictated by its association with p35. p35 contains an N-terminal myristoylation signal motif,20 which targets the protein complex to cellular membranes, where many substrates of Cdk5 reside.
Mitochondrial dysfunction is increasingly recognised as having a crucial role in neurodegenerative diseases; in particular PD. The PD-associated proteins PINK1, parkin and HtrA2 all have important roles at the mitochondria. Contrary to PINK1 and HtrA2, parkin does not include an MTS,33 yet parkin was shown recently to be recruited by PINK1 to depolarised mitochondria for initiating their clearance from the cell by autophagy.34, 35 PINK1, parkin and HtrA2 have been proposed to be components of inter-connected molecular pathways that ultimately lead to mitochondrial dysfunction in PD,6, 10 but the necessity for HtrA2 in these pathways has been disputed, in particular in Drosophila.36, 37 Similarly, in humans, whether or not HtrA2 contributes to PD pathogenesis is an matter of debate.8 We show here that the PD-associated kinase PINK1, previously shown to bind to HtrA2,9 is not required for the interaction between HtrA2 and Cdk5 (Supplementary Figure S2D). Although importantly, Cdk5 also phosphorylates the PD-associated protein parkin and regulates its E3 ubiquitin ligase activity,38 emphasising cellular cross-talk between the pathways associated with neurodegeneration.
Strikingly, we show that HtrA2 phosphorylation at S400 is significantly reduced in the brain of mice carrying the G399S HtrA2 mutation, although total levels of HtrA2 protein remain unchanged. This could indicate that HtrA2 phosphorylation (and therefore, increase of its proteolytic activity) is important for mitochondrial function, known to be deregulated in neurodegenerative disorders. We also found that mice overexpressing the Cdk5 activator associated with neurodegeneration, p25, have increased interaction between Cdk5 and HtrA2 in the midbrain, the brain region preferably destroyed in PD. These mice have a well-characterised neurodegenerative phenotype with age-related deficits in learning and memory.39 Our data suggest that active Cdk5 interacts with HtrA2 and that this is important for HtrA2 protective function in mitochondria. This modulation of HtrA2 may reflect the fine tuning necessary to respond to cellular signals accordingly. Although we suggest that activation of Cdk5 under stress conditions increases the interaction between HtrA2 and Cdk5, this appears to promote mitochondrial health as a stress response. One could hypothesise that the interaction of HtrA2 and Cdk5 could also be proapoptotic, in particular if Cdk5 and HtrA2 interact in the cytosol. Deregulation of Cdk5 is widely reported to promote neurodegeneration; however, it is the constitutive activation of Cdk5 rather than activation of Cdk5 that is thought to promote neurodegeneration (Patrick et al.20; for a review see Dhavan and Tsai12). Therefore, we propose that transient phosphorylation of HtrA2 by Cdk5 under normal conditions does not contribute to neurodegeneration directly; rather it is a key process responsible for modulating HtrA2 function to fine tune the correct response to the cellular environment.
The exact mechanism of HtrA2 function in mitochondria awaits further characterisation. HtrA2 may be a key player in maintaining mitochondrial protein quality control through its ability to clear damaged or misfolded proteins. We have shown that loss of Δψm in HtrA2-silenced cells under stress induced by the activation of the p38 pathway was rescued by expressing WT and S142A HtrA2 but not the non-phosphorylatable S400A or S142/400A HtrA2 mutants. These data suggest that HtrA2 phosphorylation at S400, and likely subsequent increase of its protease activity, may be important for HtrA2 mitochondrial function. Indeed, previous work indicated that phosphorylation of HtrA2 S400 conferred protection from cell death.9 As mitochondria do not have a ubiquitin proteasome system, clearance of damaged mitochondrial proteins or regulation of the levels of proteins involved in mitochondrial dynamics is an essential process, which in mitochondrial compartments is thought to be heavily reliant on mitochondrial proteases such as HtrA2. Indeed, modulation of the levels of the intra-mitochondrial fusion protein OPA1 by HtrA2 protease activity has been shown recently.40 Mitochondrial function is of critical importance in neurons and mitochondrial dysfunction is well documented to be a major factor underlying the pathogenesis of neurodegenerative diseases.31, 32 We have defined a mitochondrial stress response pathway involving Cdk5 and HtrA2, two proteins associated with neurodegeneration. These events may be relevant for neurodegenerative diseases and further characterisation of the pathway involving HtrA2 and Cdk5 that lead to mitochondrial dysfunction may allow better understanding of disease pathogenesis.
Materials and Methods
Antibodies
Anti-HtrA2 rabbit polyclonal and phospho-Cdk5 rabbit polyclonal (Y15) antibodies were obtained from R&D Systems (Minneapolis, MN, USA). The anti-Cdk5 mouse monoclonal antibody (clone DC17) was from Millipore (Billerica, MA, USA). The anti-p35/p25 rabbit polyclonal antibody was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The anti-Apotrack cocktail (anti-ATP synthase CVα, anti-pyruvate dehydrogenase-E1α, anti-glyceraldehyde-3-phosphate dehydrogenase and anti-cytochrome-c monoclonal antibodies) was from Mitosciences (Eugene, OR, USA). The anti-cdc2 mouse monoclonal antibody was from Cell Signaling Technology (Beverley, MA, USA). The anti-S400 HtrA2 phospho-specific rabbit polyclonal antibody was generated against peptide [C]-Ahx-KVILG-pS-PAHRAG-amide, on the basis of the sequence surrounding the S400 residue on human HtrA2 by Cambridge Research Biochemicals (Cambridge, UK). The anti-mouse and anti-rabbit antibodies coupled to horseradish peroxidase and bovine immunoglobulins (IgG) were from Sigma Aldrich (Poole, UK). The anti-mouse and anti-rabbit antibodies coupled to Alexa-Fluor-488 and TMRM were from Molecular Probes, Invitrogen, Life Technologies (Carlsbad, CA, USA).
Plasmids and reagents
Expression constructs encoding HtrA2 were generated by PCR and sub-cloning the HtrA2 cDNA into the pcDNA3 vector (Invitrogen). The S306A, S142A, S400A and S142A/S400A HtrA2 mutants were generated by site-directed mutagenesis by using the QuickChange kit (Stratagene, La Jolla, CA, USA) and confirmed by DNA sequencing. The Cdk5-HA pCMV vector was a gift from F Plattner. The p35-myc and p25-GFP vectors were purchased from Addgene (Cambridge, MA, USA). The pBP3-hbER* vector encoding human ΔMEKK1-ER* and the ΔMEKK3-ER stable cell line was a gift from S Cook. The myrAkt-ER and ΔRaf-DD-ER stable cell lines were a gift from A Schulze (CR-UK, London). The ER stable cell lines were serum-starved 16 h before treatment with 100 nM 4OH-Tx for 8 h. 4OH-Tx was from Sigma Aldrich. The dsREDmito expression vector was purchased from Clontech (Mountain View, CA, USA). The Effectene transfection reagent was from Qiagen (Hilden, Germany). The non-targeting scramble siRNA and the targeted siRNA (siGenome SMARTpool) against human Cdk5 and PINK1, and the Dharmafect transfection reagent were from Dharmacon, Thermo Fisher Scientific (Waltham, MA, USA). The targeted siRNA directed against a site at the 3′-UTR of human HtrA2 was purchased from Sigma Genosys (Dorset, UK; #Hs01_00010310). Recombinant ERK1, ERK2, Cdk5, p38α, p38β, p38γ and p38δ were obtained from Millipore. Recombinant Cdc2 was from New England Biolabs (Ipswich, MA, USA). Recombinant MBP and Human Tau were from Sigma Aldrich.
Cell culture
Human neuroblastoma (SH-SY5Y) cells were a gift from EE Billett (NTU, UK) and Hek293T cells were purchased from ECCAC. Stable cell lines expressing ΔMEKK1-ER (Hek293) and dsREDmito (SH-SY5Y) were cloned in our laboratory under initial antibiotic selection. For experiments, all cells were seeded at a density of approximately 4 × 104 cells/cm2 on plastic culture plates, coverslips or flasks, and grown to 75–80% confluence in Dulbecco's modified Eagle's medium (DMEM) medium containing 10% (v/v) foetal bovine serum (FBS) (or 10% (v/v) foetal calf serum (FCS) for MEFs) and 2 mM -glutamine at 37°C in a 5% CO2 humidified atmosphere. Overexpression of HtrA2 was achieved by using the Effectene transfection reagent from Qiagen according to the manufacturer's instructions. KD of Cdk5, PINK1, HtrA2 or scramble sequence control KD using siRNA were achieved by using the Dharmafect 1 transfection reagent from Dharmacon, Thermo Fisher Scientific according to the manufacturer's instructions.
Protein biochemistry
Cells were lysed in 10 mM Tris–HCl (pH 7.6), 5 mM EDTA, 50 mM NaCl, 30 mM sodium pyrophosphate, 50 mM sodium fluoride, 1 mM sodium orthovanadate and 2% (v/v) CHAPS ((3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxy-1-propanesulphonicacid)) and the insoluble pellet was removed before denaturation by boiling in SDS gel sample buffer and being resolved by SDS–PAGE. For IP, cells were lysed in the same buffer. After the insoluble material had been pelleted, the supernatants were divided into two equal parts; one was immunoprecipitated overnight with an antibody raised against HtrA2 (2.5 μg) and the other was immunoprecipitated overnight with a bovine IgG antibody (1 μg) as control. Both HtrA2 and control IgG-complexes were then isolated by using protein-A–sepharose beads (30 μl washed in lysis buffer) and subjected to SDS–PAGE. All gels were transferred to Immobilon PVDF membranes (Millipore). Membranes to be incubated with anti-HtrA2 phospho S400 antibody were autoclaved between glass plates containing a sandwich of paper towels and chromatography paper soaked in PBS-Tween. All membranes were subsequently incubated with the indicated primary antibody before being incubated with the appropriate secondary antibody.
Preparation of cytosolic and mitochondrial fractions for IP
Cells were washed and resuspended in 200 μl TES buffer (70 mM Tris, 1 mM EDTA and 0.25 M sucrose, pH 7.4). An equal volume of digitonin (2 mg/ml dissolved in MES buffer: 19.8 mM EGTA, 19.8 mM EDTA, 0.25 M -mannitol and 19.8 mM MES, pH 7.4) was added to the samples until ∼90% of the cells were permeabilised, as determined by Trypan blue uptake. The lysates were centrifuged at 900 × g for 2 min at 4°C, and the insoluble pellet was discarded and the supernatant was centrifuged once more at 900 × g for 2 min to remove any remaining insoluble material. The supernatant was further centrifuged at 20 000 × g for 5 min at 4°C to obtain the cytosolic fraction and a crude mitochondrial pellet. The mitochondrial pellet was resuspended in 400 μl of TES/digitonin/MES as described above. Both mitochondrial fractions and cytosolic fractions were made up to 1 ml with TES buffer for IP.
Subcellular fractionation
Briefly, cell pellets (washed with PBS) were resuspended in 1 ml of TES buffer (see above) and homogenised in a hand held homogeniser by 10 passes with a loose-fitting pestle and then 10 passes with a tight-fitting pestle. The homogenised cells were centrifuged at 1000 × g for 10 min at 4°C. The insoluble/nuclear pellet was washed in TES buffer and centrifuged at 1000 × g. The supernatant was centrifuged at 10 000 × g for 15 min at 4°C. The resulting cytosolic fraction was further centrifuged at 100 000 × g to remove any contamination. The mitochondrial pellet was washed in TES buffer and centrifuged at 10 000 × g for 10 min at 4°C. The mitochondrial fraction was agitated with digitonin (0.2 mg/mg mitochondrial protein) for 15 min at 4°C. The permeabilised mitochondria were centrifuged at 9800 × g for 10 min at 4°C. The mitoplast pellet was resuspended in TES buffer and both mitoplast and outer mitochondrial membrane (OMM)/IMS fractions were sonicated every 15 s for 1 min (14 μA amplitude). Following sonication the fractions were centrifuged at 100 000 × g for 1 h at 4°C to separate the OMM from the IMS and the IMM from the matrix space.
Human and mouse brain tissue
Human brain tissue was obtained from the Queen Square Brain Bank (London) (where it had been donated with informed consent from the next of kin) with approval from the National Hospital for Neurology and Neurosurgery–Institute of Neurology Joint Research Ethics Committee. Four flash-frozen brains with idiopathic PD, and four control brains, were used as age-matched and pH-matched controls. Frozen sections were obtained from the occipital cortex and white matter was obtained from four unaffected individuals. Tissue was homogenised in 10 mM Tris–HCl (pH 7.6), 5 mM EDTA, 50 mM NaCl, 30 mM sodium pyrophosphate, 50 mM sodium fluoride, 1 mM sodium orthovanadate and 2% CHAPS; incubated on ice for 45 min and centrifuged at 13 000 × g for 15 min at 4°C. Flash-frozen whole mouse brains from p25-overexpressing transgenic mice and corresponding age- and sex-matched WT controls were a gift from F Plattner. Animal husbandry and experimental procedures were performed in full compliance with the United Kingdom Animal (Scientific Procedures) Act of 1986. Mouse tissue of equal weight was homogenised and prepared for SDS-PAGE and/or IP as described above for human tissue. Tissue supernatant lysates from the cortex of mice overexpressing G399S HtrA2 under the control of the murine prion promoter were provided by RK, as cortex was among the brain regions showing highest expression levels of the transgene (unpublished data).
In vitro kinase assay
Recombinant HtrA2 (5 μg) was incubated with 100 ng of the indicated kinase in a buffer containing 20 μCi of [γ-33P]ATP, 50 mM Tris–HCl (pH 7.6), 10 mM MgCl2 and 10 μM for 1 h at 30°C in a final volume of 12 μl. The assays with recombinant HtrA2, MBP or Tau as substrate were resolved on a NuPAGE 4–12% Bis–Tris gel (Invitrogen) and the assays, using a peptide as substrate, were separated on 16% Tricine gels (Invitrogen). After electrophoresis, proteins were transferred to an Immobilon PVDF membrane and exposed to a phosphoscreen. The incorporated radioactivity was detected by autoradiography.
Microscopy–immunocytochemistry–colocalisation
SH-SY5Y cells expressing dsREDmito were plated on glass coverslips and treated or not with 5 mM glutamate for 4 h. Cells were fixed using 90% (v/v) methanol in PBS for 30 min at −20°C. Fixed cells were then permeabilised using 0.5% (v/v) Triton X-100 in PBS for 5 min at room temperature (RT). The detergent was washed out with three changes of PBS before blocking with 1% (w/v) bovine serum albumin (BSA) in PBS for 30 min at RT. The primary antibody was diluted according to the manufacturer's instructions in 1% (w/v) BSA in PBS and incubated in a humidity chamber for 1–2 h at RT. Unbound antibody was washed out with three changes of PBS before incubation with an anti-mouse or rabbit secondary antibody conjugated to Alexa-Fluor-488 for 0.5–1 h at RT. Unbound antibody was washed out with three changes of PBS. The fixed cells were stained with 1 μM DAPI for 5 min at RT before being washed and mounted on glass slides using Vectashield mounting medium. To determine colocalisation between antibody (green) and mitochondria (bright red), confocal images were obtained using a Zeiss 510 uv-vis CLSM equipped with a META detection system and a × 63 oil-immersion objective. The 488 nm argon laser was used to excite the dye bound to antibody and a 580 nm laser was used to excite the dsREDmito. Laser power was kept to a minimum, and confocal images were collected in the y and z plane and a 3D image was generated. The colocalisation score was quantified by using the Zeiss software to analyse the overlap of red and green on each 3D image of a field of view. At least three fields of view were analysed for each independent experiment and n=3. Laser power was kept constant throughout each image in each experiment.
Live microscopy-mitochondrial membrane potential
Cells were loaded with TMRM (50 nM) in Hank's Buffered Salt Solution with calcium and magnesium ions for 40 min at 37°C in a 5% CO2 humidified atmosphere. TMRM was excited using a 543-nm laser line and fluorescence was measured using a 560 nm long-pass filter. Confocal images were obtained using a Zeiss 510 uv-vis CLSM equipped with a META detection system and a × 40 oil-immersion objective. Illumination intensity was kept to a minimum (at 0.1–0.2% of laser output) to avoid phototoxicity, and the pinhole was set to give an optical slice of 2 mm. Mean maximum TMRM fluorescence was determined for approximately 80–100 individual mitochondria in a field of view for each condition, for each independent experiment (n=3), from Z-stack images by using the Andor IQ (Belfast, UK) analysis software.
Statistical analyses
All measurements were performed in triplicate and data are expressed as the means±S.E.M or mean % control±% control S.D. Statistical significance for multiple comparisons was performed by one-way ANOVA followed by LSD correction, and statistical significance for comparison of two variables was performed by Student's t-test. In all cases, P<0.05 was considered significant.
Acknowledgments
We thank Dr. Mina Ryten (UCL, Institute of Neurology) and the MRC Sudden Death Brain Bank, Edinburgh, for providing the control human brain tissue for co-IP experiments; Dr. Florian Plattner (UCL, Institute of Neurology) for providing the p25-overexpressing mouse brains and the WT age-matched controls; Dr. Emma Deas (UCL, Institute of Neurology) for providing the SH-SY5Y cells stably expressing dsREDmito. This work was supported by a career development award from the MRC (G0700183). This work was also supported in part by the Wellcome Trust/MRC Joint Call in Neurodegeneration award (WT089698) to the UK Parkinson's Disease Consortium (UKPDC) whose members are from the UCL/Institute of Neurology, the University of Sheffield and the MRC Protein Phosphorylation Unit at the University of Dundee. The generation and characterisation of HtrA2 transgenic mice were supported by grants from the Faculty of Medicine, University of Tübingen (Fortuene 1517-0-0) and the German Research Council (DFG, KR2119/3-2) to RK.
Glossary
- OMM
outer mitochondrial membrane
- IMS
inner mitochondrial space
- IMM
inner mitochondrial membrane
- IAPs
inhibitor of apoptosis proteins
- IP
immunoprecipitation
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by M Deshmukh
Supplementary Material
References
- Li W, Srinivasula SM, Chai J, Li P, Wu JW, Zhang Z, et al. Structural insights into the proapoptotic function of mitochondrial serine protease HtrA2/Omi. Nat Struct Biol. 2002;9:436–441. doi: 10.1038/nsb795. [DOI] [PubMed] [Google Scholar]
- Vande Walle L, Lamkanfi M, Vandenabeele P. The mitochondrial serine protease HtrA2/Omi: an overview. Cell Death Differ. 2008;15:453–460. doi: 10.1038/sj.cdd.4402291. [DOI] [PubMed] [Google Scholar]
- Van Loo G, van Gurp M, Depuydt B, Srinivasula SM, Rodriguez I, Alnemri ES, et al. The serine protease Omi/HtrA2 is released from mitochondria during apoptosis. Omi interacts with caspase-inhibitor XIAP and induces enhanced caspase activity. Cell Death Differ. 2002;9:20–26. doi: 10.1038/sj.cdd.4400970. [DOI] [PubMed] [Google Scholar]
- Jones JM, Datta P, Srinivasula SM, Ji W, Gupta S, Zhang Z, et al. Loss of Omi mitochondrial protease activity causes the neuromuscular disorder of mnd2 mutant mice. Nature. 2003;425:721–727. doi: 10.1038/nature02052. [DOI] [PubMed] [Google Scholar]
- Martins LM, Morrison A, Klupsch K, Fedele V, Moisoi N, Teismann P, et al. Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice. Mol Cell Biol. 2004;24:9848–9862. doi: 10.1128/MCB.24.22.9848-9862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss KM, Martins LM, Plun-Favreau H, Marx FP, Kautzmann S, Berg D, et al. Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson's disease. Hum Mol Genet. 2005;14:2099–2111. doi: 10.1093/hmg/ddi215. [DOI] [PubMed] [Google Scholar]
- Bogaerts V, Nuytemans K, Reumers J, Pals P, Engelborghs S, Pickut B, et al. Genetic variability in the mitochondrial serine protease HTRA2 contributes to risk for Parkinson disease. Hum Mutat. 2008;29:832–840. doi: 10.1002/humu.20713. [DOI] [PubMed] [Google Scholar]
- Simon-Sanchez J, Singleton AB. Sequencing analysis of OMI/HTRA2 shows previously reported pathogenic mutations in neurologically normal controls. Hum Mol Genet. 2008;17:1988–1993. doi: 10.1093/hmg/ddn096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plun-Favreau H, Klupsch K, Moisoi N, Gandhi S, Kjaer S, Frith D, et al. The mitochondrial protease HtrA2 is regulated by Parkinson's disease-associated kinase PINK1. Nat Cell Biol. 2007;9:1243–1252. doi: 10.1038/ncb1644. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JC, Plun-Favreau H. Emerging pathways in genetic Parkinson's disease: autosomal-recessive genes in Parkinson's disease – a common pathway. FEBS J. 2008;275:5758–5766. doi: 10.1111/j.1742-4658.2008.06708.x. [DOI] [PubMed] [Google Scholar]
- Beaudette KN, Lew J, Wang JH. Substrate specificity characterization of a cdc2-like protein kinase purified from bovine brain. J Biol Chem. 1993;268:20825–20830. [PubMed] [Google Scholar]
- Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- Zukerberg LR, Patrick GN, Nikolic M, Humbert S, Wu CL, Lanier LM, et al. Cables links Cdk5 and c-Abl and facilitates Cdk5 tyrosine phosphorylation, kinase upregulation, and neurite outgrowth. Neuron. 2000;26:633–646. doi: 10.1016/s0896-6273(00)81200-3. [DOI] [PubMed] [Google Scholar]
- Niethammer M, Smith DS, Ayala R, Peng J, Ko J, Lee MS, et al. NUDEL is a novel Cdk5 substrate that associates with LIS1 and cytoplasmic dynein. Neuron. 2000;28:697–711. doi: 10.1016/s0896-6273(00)00147-1. [DOI] [PubMed] [Google Scholar]
- Qu D, Rashidian J, Mount MP, Aleyasin H, Parsanejad M, Lira A, et al. Role of Cdk5-mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson's disease. Neuron. 2007;55:37–52. doi: 10.1016/j.neuron.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna, Pant HC, et al. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei JJ, Grundke-Iqbal I, Iqbal K, Bogdanovic N, Winblad B, Cowburn RF. Accumulation of cyclin-dependent kinase 5 (cdk5) in neurons with early stages of Alzheimer's disease neurofibrillary degeneration. Brain Res. 1998;797:267–277. doi: 10.1016/s0006-8993(98)00296-0. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Kawamoto Y, Nakano S, Akiguchi I, Kimura J. p35(nck5a) and cyclin-dependent kinase 5 colocalize in Lewy bodies of brains with Parkinson's disease. Acta Neuropathol. 1997;94:153–157. doi: 10.1007/s004010050687. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Kawamoto Y, Nakano S, Ikemoto A, Akiguchi I, Kimura J. Cyclin-dependent kinase 5 in Lewy body-like inclusions in anterior horn cells of a patient with sporadic amyotrophic lateral sclerosis. Neurology. 1997;48:267–270. doi: 10.1212/wnl.48.1.267. [DOI] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kwon YT, Li MW, Peng JM, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- Noble W, Olm V, Takata K, Casey E, Mary O, Meyerson J, et al. Cdk5 is a key factor in tau aggregation and tangle formation invivo. Neuron. 2003;38:555–565. doi: 10.1016/s0896-6273(03)00259-9. [DOI] [PubMed] [Google Scholar]
- Burchell VS, Gandhi S, Deas E, Wood NW, Abramov AY, Plun-Favreau H. Targeting mitochondrial dysfunction in neurodegenerative disease: Part I. Expert Opin Ther Targets. 2010;14:369–385. doi: 10.1517/14728221003652489. [DOI] [PubMed] [Google Scholar]
- Burchell VS, Gandhi S, Deas E, Wood NW, Abramov AY, Plun-Favreau H. Targeting mitochondrial dysfunction in neurodegenerative disease: Part II. Expert Opin Ther Targets. 2010;14:497–511. doi: 10.1517/14728221003730434. [DOI] [PubMed] [Google Scholar]
- Cheung ZH, Fu AKY, Ip NY. Synaptic roles of Cdk5: implications in higher cognitive functions and neurodegenerative diseases. Neuron. 2006;50:13–18. doi: 10.1016/j.neuron.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Garner AP, Weston CR, Todd DE, Balmanno K, Cook SJ. Delta MEKK3:ER* activation induces a p38 alpha/beta 2-dependent cell cycle arrest at the G2 checkpoint. Oncogene. 2002;21:8089–8104. doi: 10.1038/sj.onc.1206000. [DOI] [PubMed] [Google Scholar]
- Xie Z, Sanada K, Samuels BA, Shih H, Tsai LH. Serine 732 phosphorylation of FAK by Cdk5 is important for microtubule organization, nuclear movement, and neuronal migration. Cell. 2003;114:469–482. doi: 10.1016/s0092-8674(03)00605-6. [DOI] [PubMed] [Google Scholar]
- Moisoi N, Klupsch K, Fedele V, East P, Sharma S, Renton A, et al. Mitochondrial dysfunction triggered by loss of HtrA2 results in the activation of a brain-specific transcriptional stress response. Cell Death Differ. 2009;16:449–464. doi: 10.1038/cdd.2008.166. [DOI] [PubMed] [Google Scholar]
- Yang L, Sun M, Sun XM, Cheng GZ, Nicosia SV, Cheng JQ. Akt attenuation of the serine protease activity of HtrA2/Omi through phosphorylation of serine 212. J Biol Chem. 2007;282:10981–10987. doi: 10.1074/jbc.M700445200. [DOI] [PubMed] [Google Scholar]
- Wong ASL, Lee RHK, Cheung AY, Yeung PK, Chung SK, Cheung ZH, et al. Cdk5-mediated phosphorylation of endophilin B1 is required for induced autophagy in models of Parkinson's disease. Nat Cell Biol. 2011;13:568–579. doi: 10.1038/ncb2217. [DOI] [PubMed] [Google Scholar]
- Meuer K, Suppanz IE, Lingor P, Planchamp V, Goricke B, Fichtner L, et al. Cyclin-dependent kinase 5 is an upstream regulator of mitochondrial fission during neuronal apoptosis. Cell Death Differ. 2007;14:651–661. doi: 10.1038/sj.cdd.4402087. [DOI] [PubMed] [Google Scholar]
- Li B, Hu Q, Wang H, Man N, Ren H, Wen L, et al. Omi/HtrA2 is a positive regulator of autophagy that facilitates the degradation of mutant proteins involved in neurodegenerative diseases. Cell Death Differ. 2010;17:1773–1784. doi: 10.1038/cdd.2010.55. [DOI] [PubMed] [Google Scholar]
- Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, Minoshima S, et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25:302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin-induced mitophagy in the pathogenesis of Parkinson disease. Autophagy. 2009;5:706–708. doi: 10.4161/auto.5.5.8505. [DOI] [PubMed] [Google Scholar]
- Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci USA. 2010;107:378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth AJ, Lee JR, Ho VM, Flick R, Chowdhury R, McQuibban GA. Rhomboid-7 and HtrA2/Omi act in a common pathway with the Parkinson's disease factors Pink1 and Parkin. Dis Model Mech. 2008;1:168–174. doi: 10.1242/dmm.000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J, Cao JH, Dodson MW, Clark IE, Kapahi P, Chowdhury RB, et al. Loss-of-function analysis suggests that Omi/HtrA2 is not an essential component of the PINK1/PARKIN pathway invivo. J Neurosci. 2008;28:14500–14510. doi: 10.1523/JNEUROSCI.5141-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham E, Rott R, Liani E, Szargel R, Engelender S. Phosphorylation of Parkin by the cyclin-dependent kinase 5 at the linker region modulates its ubiquitin-ligase activity and aggregation. J Biol Chem. 2007;282:12842–12850. doi: 10.1074/jbc.M608243200. [DOI] [PubMed] [Google Scholar]
- Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40:471–483. doi: 10.1016/s0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- Kieper N, Holmstrom KM, Ciceri D, Fiesel FC, Wolburg H, Ziviani E, et al. Modulation of mitochondrial function and morphology by interaction of Omi/HtrA2 with the mitochondrial fusion factor OPA1. Exp Cell Res. 2010;316:1213–1224. doi: 10.1016/j.yexcr.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.