Abstract
MYCN amplification is a major biomarker of poor prognosis, occurring in 25-30% of neuroblastomas. MYCN plays contradictory roles in promoting cell growth and sensitizing cells to apoptosis. We have recently shown that p53 is a direct transcriptional target of MYCN in neuroblastoma and that p53-mediated apoptosis may be an important mechanism of MYCN-induced apoptosis. Although p53 mutations are rare in neuroblastoma at diagnosis, the p53/MDM2/p14ARF pathway is often inactivated through MDM2 amplification or p14ARF inactivation. We hypothesised that reactivation of p53 by inhibition of its negative regulator MDM2, using the MDM2-p53 antagonists Nutlin-3 and MI-63, will result in p53-mediated growth arrest and apoptosis especially in MYCN amplified cells. Using the SHEP Tet21N MYCN regulatable system, MYCN(−) cells were more resistant to both Nutlin-3 and MI-63 mediated growth inhibition and apoptosis compared to MYCN(+) cells and siRNA mediated knockdown of MYCN in 4 MYCN amplified cell lines resulted in decreased p53 expression and activation, as well as decreased levels of apoptosis following treatment with MDM2-p53 antagonists. In a panel of 18 neuroblastoma cell lines treated with Nutlin-3 and MI-63, the sub-set amplified for MYCN had a significantly lower mean GI50 value and increased caspase 3/7 activity compared to the non MYCN amplified group of cell lines, but p53 mutant cell lines were resistant to the antagonists regardless of MYCN status. We conclude that amplification or overexpression of MYCN sensitizes neuroblastoma cell lines with wildtype p53 to MDM2-p53 antagonists and that these compounds may therefore be particularly effective in treating high risk MYCN amplified disease.
Keywords: MYCN, neuroblastoma, MDM2-p53 antagonists, apoptosis, growth inhibition
Introduction
Neuroblastoma is an embryonal malignancy originating from developing neural crest cells which go on to form the sympathetic nervous system. It is the most common solid extracranial malignancy of childhood (Maris et al 2007). Over 50% of cases of neuroblastoma are high risk and difficult to cure, with long term survival rates remaining below 40% despite intense multimodal therapies. MYCN gene amplification is a major marker of adverse prognosis, occurring in 25-30% of neuroblastomas and is strongly associated with progressive disease and treatment failure (Cohn and Tweddle 2004). Infants under 18 months with MYCN amplified tumors have an event-free survival of 26% compared to 83% for infant stage 4 patients without MYCN amplification (Cohn et al 2009).
MYCN belongs to the MYC family of transcription factors that play roles in promoting cell proliferation, differentiation, oncogenesis and apoptosis (Kang et al 2006). These proteins transcriptionally activate target genes by forming heterodimers with MAX, which bind promoters of target genes, typically at E-box sequences (Wenzel et al 1991). MYCN expression alone, targeted to developing neural crest tissue, has been shown to directly result in neuroblastoma tumor formation in transgenic mice (Weiss et al 1997) and there is evidence that MYCN expression sensitizes neuroblastoma cells to apoptosis induced by cytotoxic drugs (Fulda et al 2000, Hogarty 2003). However, since patients with MYCN amplified tumors have such an inferior outcome, acquired aberrations in the apoptotic pathway are thought to be associated with MYCN amplification and to be essential for tumor progression.
p53 is a critical tumor suppressor gene that is mutated in over 50% of adult sporadic cancers. It plays a major role in protecting the cell from genomic instability and tumor development by inducing apoptosis and cell cycle arrest in response to cellular stresses and DNA damage (reviewed in ref. (Michalak et al 2005)). In neuroblastoma, p53 mutations are rare, occurring in <2% of cases at diagnosis and ~15% at relapse (Carr-Wilkinson et al 2010). However, in 1/3 cases in a study of relapsed tumors, p53 was found to be inactivated via other mechanisms that resulted in destabilisation of p53 or disruption of p53 activity (Carr-Wilkinson et al 2010). In neuroblastoma, other mechanisms of p53 inactivation include amplification of the E3 ubiquitin ligase gene MDM2, or impairment of p14ARF, a negative regulator of MDM2 (Carr-Wilkinson et al 2010, Carr et al 2006). MDM2 is the major negative regulator of p53 activity and stability, promoting the ubiquitination and degradation of p53 but also blocking its transcriptional activity by binding to the transactivation domain within the N-terminal of p53. MDM2 is also a transcriptional target of p53, forming a negative feedback loop that allows for tight regulation of p53 under normal circumstances. Non-syntenic co-amplification of MDM2 and MYCN has been reported in neuroblastoma cell lines and tumors, resulting in constant negative regulation of p53 (Carr-Wilkinson et al 2010, Corvi et al 1995). More commonly in tumors, p14ARF function is impaired through methylation or homozygous deletion of the gene (Carr-Wilkinson et al 2010). p14ARF negatively regulates MDM2 and therefore p14ARF inactivation drives cell survival through increased MDM2 activity. MYCN is a central modulator of the p53/MDM2/p14ARF network. There is evidence that both p53 and MDM2 are direct transcriptional targets of MYCN (Chen et al 2010, Slack et al 2005), and that p53 may be important for MYCN induced apoptosis (Chen et al 2010). It has also been reported that p14ARF is activated by c-MYC (Zindy et al 1998), and due to the similarities between MYCN and c-MYC, MYCN may function in a similar way. MDM2 haploinsufficiency in mice has been shown to suppress MYCN-driven neuroblastoma tumorigenesis (Chen et al 2009) and there is evidence that MDM2 may be the critical oncogene by which MYCN amplified neuroblastomas acquire an aggressive phenotype (Slack and Shohet 2005). Since MYCN amplification is thought to be associated with defects in activating or executing apoptotic pathways and that this may be related to overactive MDM2, we hypothesize that MYCN amplified tumors may be more susceptible to compounds that reactivate p53. Several studies have shown that the downstream apoptotic pathway of p53 is generally intact in neuroblastoma (Goldman et al 1996, Hogarty 2003).
The hydrophobic p53-binding pocket of MDM2 is ideal for developing low molecular weight compounds that occupy the pocket and prevent p53 from binding. Compounds have been developed that induce cell cycle arrest and apoptosis in cancer cells, but usually only produce reversible growth arrest in normal cells (Efeyan et al 2007, Vassilev et al 2004). Nutlin-3 is a cis-imidazoline analogue which binds MDM2 at the p53 binding site with a Ki of 36nM (Vassilev et al 2004) and MI-63, a spiro-oxindole, is more potent, binding MDM2 with a Ki of 3nM (Canner et al 2009). In wild-type p53 neuroblastoma cell lines, Nutlin-3 has been reported to induce cell cycle arrest and apoptosis, and surviving cells underwent senescence or neuronal differentiation in cell lines tested, however the relationship to MYCN status was not extensively explored (Van Maerken et al 2006). Although not previously investigated in neuroblastoma or in relation to MYCN, MI-63 has also been shown to induce apoptosis in cell lines with wildtype p53 (Canner et al 2009).
The current study provides evidence that MYCN expression sensitizes neuroblastoma cells to MDM2-p53 antagonists. We show that a) in a MYCN regulatable cell line (Tet21N), MYCN(+) cells are more sensitive to MDM2-p53 antagonist mediated growth inhibition and apoptosis than MYCN(−) cells, b) siRNA-mediated knockdown of MYCN results in a decreased p53 response and decreased induction of apoptosis by MDM2-p53 antagonist treatment, and c) in a panel of 18 cell lines, MYCN amplified cells are more sensitive to Nutlin-3 and MI-63 than non-MYCN amplified cell lines.
Results
MYCN(−) Tet21N SHEP cells are more resistant to Nutlin-3 and MI-63 mediated growth inhibition and apoptosis compared to MYCN(+) cells
Tet21N MYCN regulatable cells were treated with the MDM2-p53 antagonists Nutlin-3 and MI-63 in the presence (MYCN−) or absence (MYCN+) of tetracycline. Growth inhibition assays were performed and as shown in Figure 1A and 1B MYCN(+) cells had significantly lower GI50s compared to MYCN(−) cells (p=0.02 for Nutlin-3, and p=0.0008 for MI-63). GI50 values are shown in Table 1. As a control, growth inhibition assays were performed in Tet21 vector only cells and no difference in GI50 was observed.
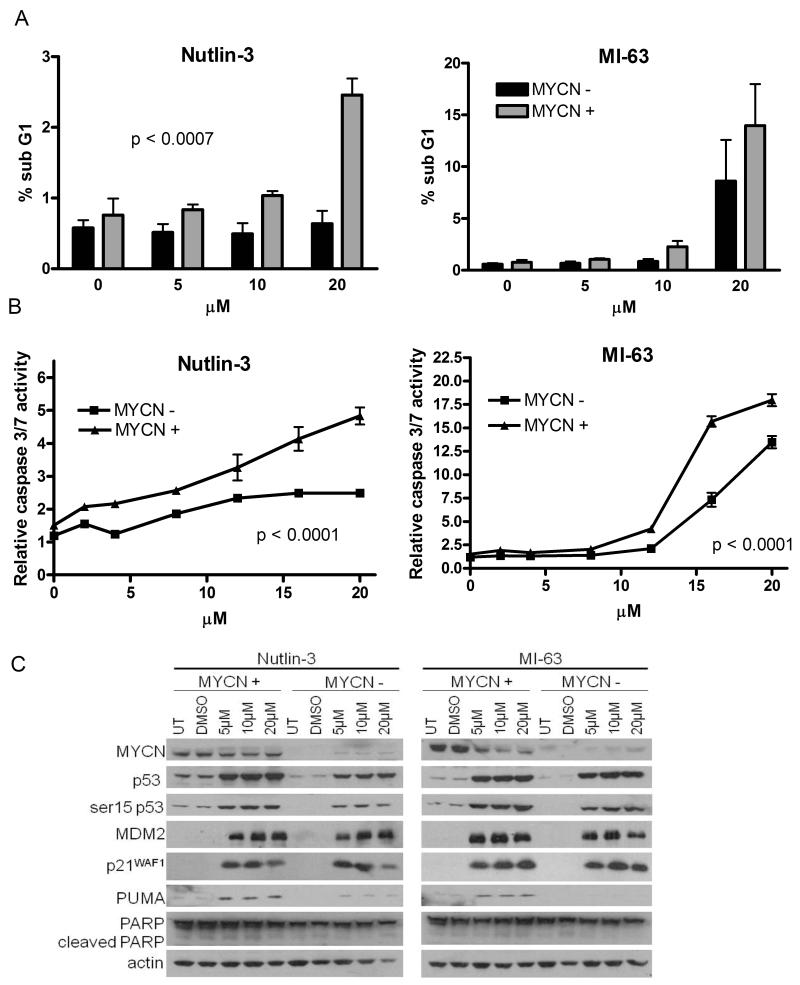
Figure 1. MYCN(+) Tet21N cells are more sensitive to MDM2-p53 antagonist mediated growth inhibition than MYCN(−) Tet21N cells.
Growth inhibition assays for A) Nutlin-3 and B) MI-63 were performed after 72 hour drug exposure. MYCN(+) cells have a significantly lower mean GI50 compared to MYCN(−) cells (p=0.02 for Nutlin-3 and p=0.0008 for MI-63, paired t-test). No difference in GI50s was observed between the control Tet21 cells in the presence and absence of tetracycline. C) Cell cycle analysis after 24 hour drug exposure shows that both MYCN(−) and MYCN(+) cells G1 arrest in response to 2.5μM and 10μM Nutlin-3 or MI-63, and show that MYCN(−) cells have an increased proportion of cells in G1 in control samples compared to MYCN(+) cells.
Table 1.
Summary of GI50 values for Nutlin-3 and MI-63 in 18 neuroblastoma cell lines of varying MYCN and MDM2 amplification status, and the Tet21N conditional MYCN expression system. amp - amplified, non-amp – non-amplified.
| Cell Line | MYCN status | MDM2 status | GI50 (μM) | |
|---|---|---|---|---|
| Nutlin-3 | MI-63 | |||
| NGP | amp | amp | 2.53 ± 0.43 | 1.21 ± 0.04 |
| LS | amp | amp | 2.95 ± 0.12 | 0.98 ± 0.06 |
| NB1691 | amp | amp | 2.80 ± 0.17 | 0.87 ± 0.22 |
| TR14 | amp | amp | 2.91 ± 0.28 | 1.09 ± 0.25 |
| IMR32 | amp | non-amp | 2.53 ± 0.20 | 1.00 ± 0.29 |
| NBLW | amp | non-amp | 0.74 ± 0.07 | 0.85 ± 0.21 |
| SMSKCNR | amp | non-amp | 1.18 ± 0.08 | 0.74 ± 0.05 |
| LAN5 | amp | non-amp | 1.52 ± 0.21 | 0.90 ± 0.16 |
| PER108 | amp | non-amp | 1.64 ± 0.29 | 0.85 ± 0.09 |
| CHLA136 | amp | non-amp | 0.64 ± 0.12 | 0.83 ± 0.03 |
| SHSY5Y | non-amp | non-amp | 3.85 ± 0.98 | 2.01 ± 0.73 |
| GIMEN | non-amp | non-amp | 4.61 ± 0.99 | 1.81 ± 0.45 |
| SJNB1 | non-amp | non-amp | 4.32 ± 0.10 | 1.27 ± 0.20 |
| NB69 | non-amp | non-amp | 1.77 ± 0.12 | 0.72 ± 0.06 |
| LAN6 | non-amp | non-amp | 2.97 ± 0.75 | 2.28 ± 0.62 |
| SKNRA | non-amp | non-amp | 9.85 ± 0.61 | 4.63 ± 0.79 |
| SHEP | non-amp | non-amp | 3.92 ± 0.61 | 0.86 ± 0.14 |
| NBLS | non-amp | non-amp | 3.08 ± 0.68 | 1.25 ± 0.24 |
| MYCN + (Tet21N) |
non-amp | 3.33 ± 0.74 | 1.08 ± 0.14 | |
| MYCN − (Tet21N) | non-amp | 13.76 ± 2.57 | 6.56 ± 1.18 | |
As previously reported MYCN(+) cells have a doubling time of 78.5 hours compared to 90.4 hours for MYCN(−) cells (Bell et al 2006). This difference in growth rate is reflected in the higher proportion of MYCN(−) cells in G1 compared to MYCN(+) cells, which have a greater proportion of cells in S and G2 phases (Figure 1C). Upon 2.5μM Nutlin-3 and MI-63 treatment, both MYCN(+) and MYCN(−) cells G1 arrest, but this effect is more pronounced in MYCN(+) cells due to the lower G1 baseline proportions. Interestingly, there is an increase in the proportion of cells in G2 at 10μM Nutlin-3 and MI-63 in both MYCN(+) and MYCN(−) cells.
Three techniques were used to investigate levels of apoptosis following Nutlin-3 or MI-63 treatment in Tet21N cells. As shown in Figure 2A, there was a significant increase in the percentage of sub G1 DNA following Nutlin-3 treatment (0-20μM) in MYCN(+) cells compared to MYCN(−) cells (p<0.0007), and although not statistically significant (p=0.058), a similar effect was seen after MI-63 treatment. An increase in the induction of caspase 3/7 activity at increasing concentrations of Nutlin-3 (p<0.0001) and MI-63 (p<0.0001) was observed in MYCN(+) compared to MYCN(−) cells (Figure 2B) and Western blotting showed increased expression of the p53 target and apoptotic marker PUMA in MYCN(+) compared to MYCN(−) cells (Figure 2C). Induction of p53 and its transcriptional target gene products was observed in both MYCN(+) and MYCN(−) cells. Higher basal levels of p53 were detected in MYCN(+) cells and after Nutlin-3 treatment, increased p53 induction was observed in MYCN(+) cells compared to MYCN(−) cells. Both compounds induced greater levels of p53 phosphorylation at serine 15 in MYCN(+) cells. Together, this data provide evidence that MYCN(+) cells are more sensitive to MDM2-p53 antagonist mediated growth inhibition and apoptosis.
Figure 2. MYCN(+) Tet21N cells are more sensitive to MDM2-p53 antagonist mediated apoptosis than MYCN(−) Tet21N cells, following 24 hours treatment.
A) The sub G1 DNA fraction was significantly lower in MYCN(−) compared to MYCN(+) cells following Nutlin-3 treatment (p=0.0007, 2-way ANOVA) and although of borderline significance, a decrease in the sub G1 fraction is observed in MYCN(−) cells compared to MYCN(+) cells following MI-63 treatment. (p=0.058 for MI-63, 2-Way ANOVA), B) Caspase 3/7 activity was significantly reduced in MYCN(−) compared to MYCN(+) cells following Nutlin-3 and MI-63 treatment (p<0.0001 for both Nutlin-3, and MI-63, 2-way ANOVA). C) Western blots showed no difference in induction of p53, MDM2 and p21WAF1 in MYCN(−) and MYCN(+) cells following Nutlin-3 and MI-63 treatment but there were increased levels of phosphorylated p53 and PUMA in MYCN(+) compared to MYCN(−) cells. Actin was used as a loading control.
Knockdown of MYCN increases resistance of MYCN amplified neuroblastoma cell lines to Nutlin-3 and MI-63 mediated induction of p53 and apoptosis
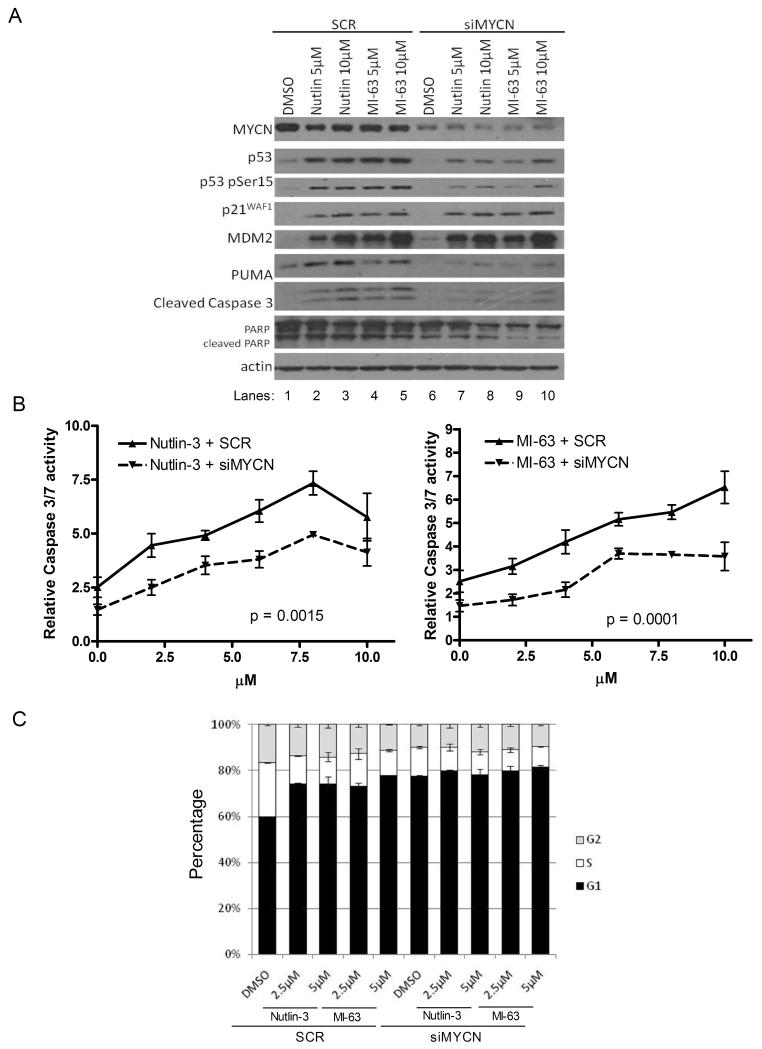
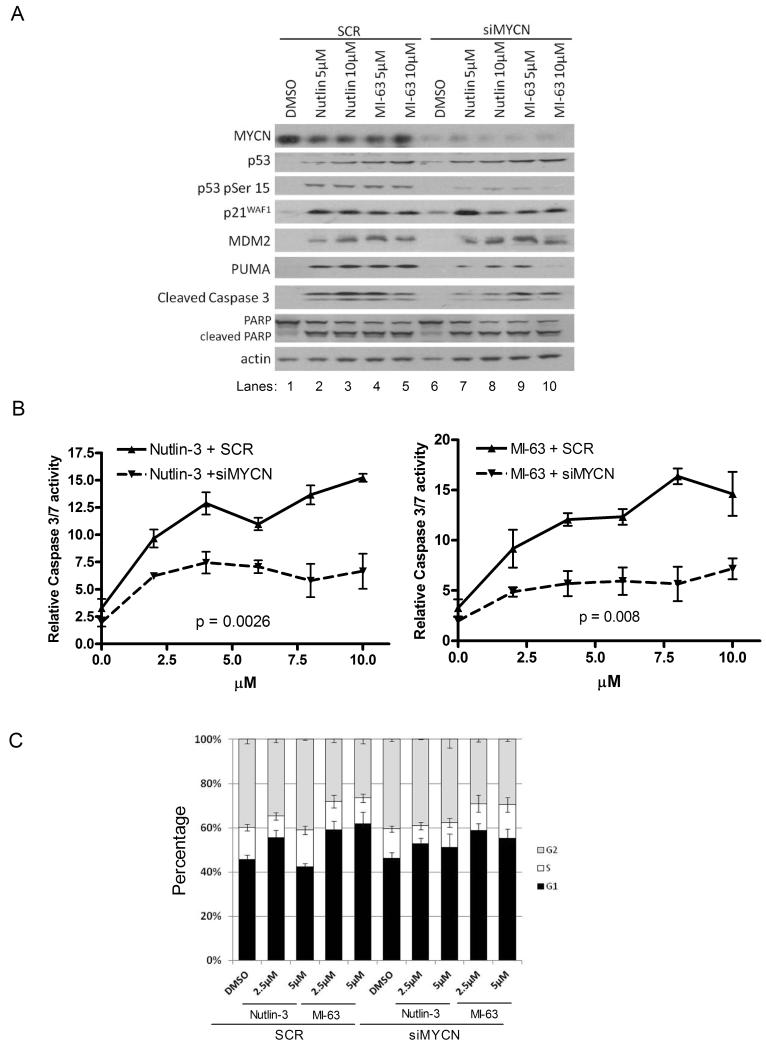
To further investigate the role of MYCN on the sensitivity of neuroblastoma cell lines to MDM2-p53 antagonists, MYCN was knocked down by siRNA treatment in two MYCN and MDM2 co-amplified cell lines TR14 and LS, and 2 MYCN amplified but non-MDM2 amplified cell lines LAN5 and IMR-32. MYCN siRNA or SCR siRNA (30-50nmol/L) was added to cells for 24 hours then removed and replaced with 0, 5 or 10 μM Nutlin-3 or MI-63 for a further 24 hours. In all four cell lines, TR14 (Figure 3A), LAN5 (Figure 4A), LS (Figure S1A) and IMR32 (Figure S2) treatment with Nutlin-3 or MI-63, resulted in a clear increase in p53 induction, p53 phosphorylation (serine 15), induction of p53 target genes (p21WAF1, MDM2 and PUMA) and induction of the apoptotic markers PUMA, cleaved caspase 3 and cleaved PARP (Lanes 1-5). In all cell lines, high levels of MYCN knockdown were achieved as shown by comparison of DMSO controls with SCR and siMYCN (lane 1 vs. lane 6, Figures 3A, 4A, S1A and S2). MYCN knockdown alone had little effect on p53 or p53 targets but with MDM2-p53 antagonist treatment MYCN knockdown decreased the p53 and apoptotic response. p53 levels decreased in 3/4 cell lines, and levels of phosphorylated p53 decreased in all cell lines (Figures 3A, 4A, S1A and S2, lanes 7-10 compared to lanes 2-5) indicating reduced p53 activation. p21WAF1 or MDM2 levels either decreased or didn’t change and in all cases, a decrease in at least 2 of the apoptotic markers PUMA, cleaved caspase 3 and cleaved PARP was observed.
Figure 3. siRNA knockdown of MYCN (30nmol/L for 24 hours) decreases the sensitivity of MYCN and MDM2 co-amplified TR14 cells to Nutlin-3 and MI-63 treatment (24 hours).
A) Western blot showing induction of p53 responsive genes and levels of apoptotic markers. Actin was used as a loading control. B) Caspase 3/7 activity was significantly reduced compared to SCR siRNA following MYCN knockdown (p=0.0015 for Nutlin-3, p=0.0001 for MI-63, 2-way ANOVA). C) Cell cycle analysis showed a slight G1 arrest following Nutlin-3 or MI-63 treatment and no further effect upon MYCN knockdown despite MYCN knockdown alone inducing a slight G1 arrest.
Figure 4. siRNA knockdown of MYCN (50nmol/L for 24 hours) decreases the sensitivity of MYCN amplified LAN5 cells to MDM2-p53 antagonist treatment (24 hours).
A) Western blot showing the effect on the p53 response and levels of apoptotic markers. Actin was used as a loading control. B) Caspase 3/7 activity was significantly reduced following MYCN knockdown (p<0.0026 for Nutlin-3, p<0.008 for MI-63, 2-way ANOVA). C) Cell cycle analysis showed no G1 arrest following Nutlin-3 or MI-63 treatment and no further effect upon MYCN knockdown.
Apoptosis following MYCN knockdown and MDM2-p53 antagonist treatment was further investigated in TR14, LAN5 and LS cells. Caspase 3/7 activity assays were performed for TR14 and LAN5 cells (Figure 3B and 4B). After Nutlin-3 or MI-63 treatment, a dose dependent increase in caspase 3/7 activity was seen in both cell lines with both compounds (Figure 3B and 4B), and an increase in sub G1 DNA % in LS cells (Figure S1B). Following MYCN knockdown, there was a reduction in caspase 3/7 activity in TR14 and LAN5 cells (Figures 3B and 4B at 0μM) compared to SCR control, and a significant reduction in caspase activity was observed in both TR14 and LAN5 cell lines following MYCN knockdown and Nutlin-3 or MI-63 treatment, (TR14 p=0.0015 Nutlin-3, p=0.0001 MI63, and LAN5 p=0.0026 Nutlin-3 and p=0.008 MI-63). Levels of sub G1 DNA in LS cells (Figure S1B) reduced after MYCN knockdown following Nutlin-3 (p=0.08) and MI-63 (p<0.05) treatment compared to SCR. These data are consistent with the reduction in levels of apoptotic markers shown by Western blot showing that removal of MYCN by siRNA knockdown results in a decreased apoptotic response to MDM2-p53 antagonists.
Knockdown of MYCN does not alter the cell cycle response to MDM2-p53 antagonists
The effect of MDM2-p53 antagonists on the cell cycle was investigated in TR14, LAN5 and LS cells. TR14 cells underwent a slight G1 arrest upon Nutlin-3 or MI-63 treatment (Figure 3C), whereas LAN5 (Figure 4C) and LS (Figure S1C) cells did not arrest. Knockdown of MYCN induced a G1 arrest in both TR14 and LS cells, but the effect of MDM2-p53 antagonists was not influenced by MYCN knockdown. This data suggests that upon MDM2-p53 antagonist treatment, some neuroblastoma cell lines G1 arrest and that the effect is cell line dependent, but the response is not altered upon MYCN knockdown.
MYCN amplified neuroblastoma cell lines are more sensitive to MDM2-p53 antagonist mediated growth inhibition than non MYCN amplified neuroblastoma cell lines
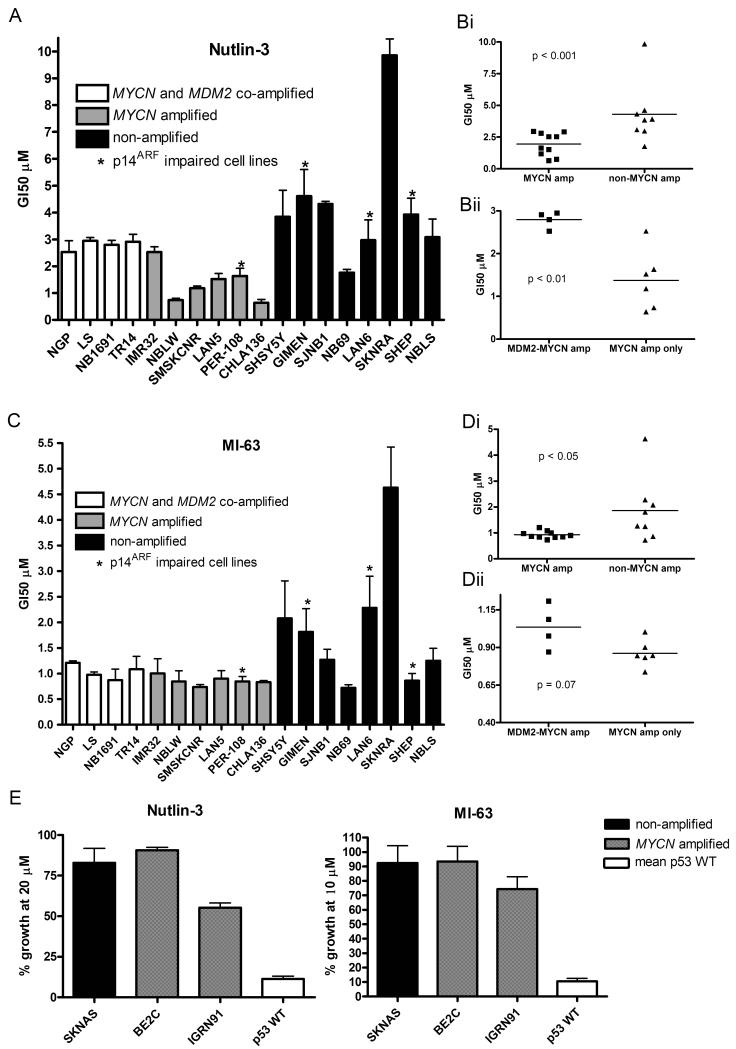
Induction of a p53 response from MDM2-p53 antagonists can initiate a number of effects, including 2 important antitumor responses, cell cycle arrest and apoptosis. A panel of 12 MYCN amplified (including 4 MYCN and MDM2 co-amplified) and 8 non-MYCN amplified neuroblastoma cell lines were investigated for their sensitivity to Nutlin-3 and MI-63 mediated growth inhibition, which takes into account both growth arrest and cell death. GI50 values for all p53 wildtype cell lines are shown in Table 1. In response to Nutlin-3 (Figure 5A, 5Bi) and MI-63 (Figure 5C, 5Di), MYCN amplified neuroblastoma cell lines underwent more growth inhibition compared to non-amplified cell lines with a significant difference in mean GI50s (p<0.001 for Nutlin-3 and p<0.05 for MI-63). Overall a more varied response to the antagonists was seen in the non-amplified cell lines compared to MYCN amplified cell lines. Interestingly, the p53 wildtype SKNRA cell line was most resistant to both Nutlin-3 and MI-63. MYCN-only amplified cell lines were also more sensitive to Nutlin-3 mediated growth inhibition compared to MYCN and MDM2 co-amplified neuroblastoma cell lines (Figure 5Bii) (p<0.01) and although this difference did not reach statistical significance, the same trend was observed for MI-63 (Figure 5Dii). A number of cell lines in this panel had impaired p14ARF function; PER-108 (methylated) and GIMEN (methylated), SHEP (homozygous deletion) and LAN-6 (homozygous deletion) (Carr et al 2006). There appeared to be a varied response to MDM2-p53 antagonist mediated growth inhibition with no evidence that p14ARF status affects the response to these compounds. Two MYCN amplified p53 mutant cell lines (Be2C and IGRN91) and 1 non-MYCN amplified p53 mutant cell line (SKNAS) were investigated for their sensitivity to Nutlin-3 and MI-63. As shown in Figure 5E, p53 mutant cell lines were highly resistant to these compounds regardless of MYCN status. 50% growth inhibition was not achieved in these cell lines with the highest concentrations of Nutlin-3 (20μM) and MI-63 (10μM) used to generate GI50 values in the other p53 wildtype cell lines tested.
Figure 5. MYCN amplified neuroblastoma cell lines are more sensitive to MDM2-p53 antagonists than non-MYCN amplified neuroblastoma cell lines.
A panel of neuroblastoma cell lines were treated with A), B) Nutlin-3 and C), D) MI-63 with doses ranging from 0-20μM and 0-10μM respectively to obtain GI50s. A significant difference was observed between GI50s for both Bi) Nutlin-3 (p<0.001) and Di) MI-63 (p<0.05). There was an increase in GI50s in MYCN and MDM2 co-amplified cell lines compared to MYCN only amplified cell lines for Bii) Nutlin-3 (p<0.01) but not Dii) MI-63 (p=0.07). Mann-Whitney tests were used to calculate p values. E) p53 mutant cell lines including one non-amplified and 2 MYCN amplified were treated with up to 20μM Nutlin-3 and 10μM MI-63. All three cell lines were resistant to Nutlin-3 and MI-63 compared to wildtype p53 cell lines. Cell lines marked * are p14ARF impaired (methylation or homozygous deletion).
MYCN amplified neuroblastoma cell lines are more sensitive to MDM2-p53 antagonist mediated apoptosis compared to non MYCN amplified neuroblastoma cell lines
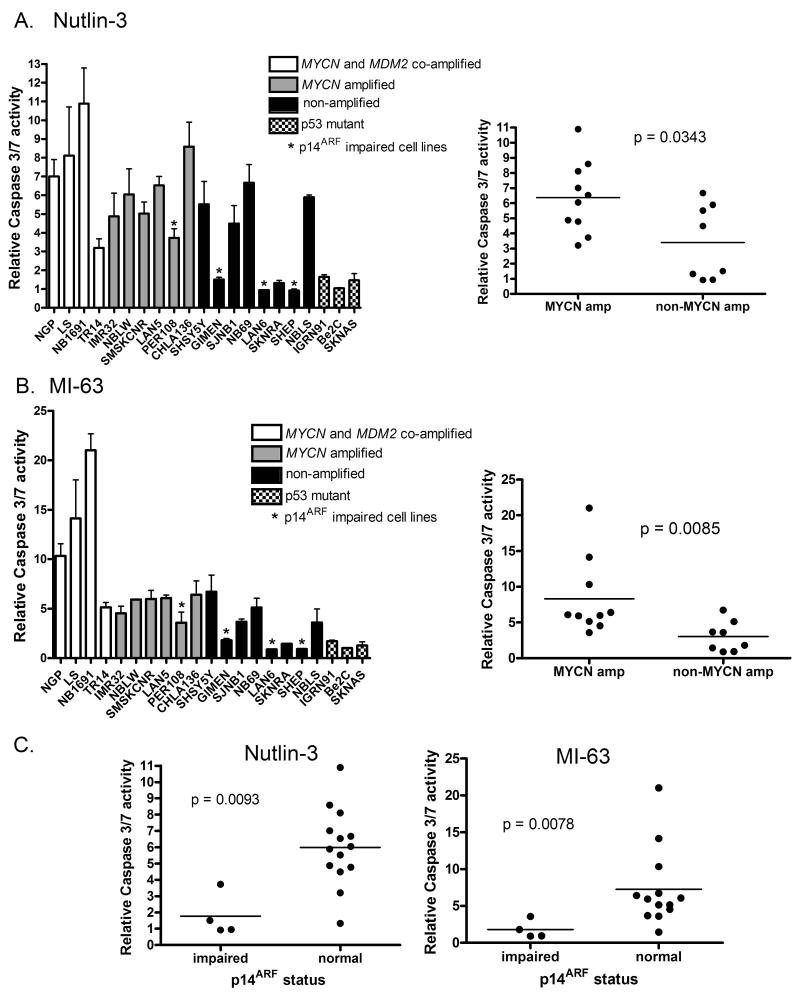
As a marker of apoptosis, caspase 3/7 activity following Nutlin-3 or MI-63 treatment was determined in the same panel of neuroblastoma cell lines that were assessed for growth inhibition. In response to 5μM Nutlin-3 (Figure 6A) and 2.5μM MI-63 (Figure 6B), MYCN amplified neuroblastoma cell lines showed higher mean caspase 3/7 activity compared to non MYCN amplified cell lines (p=0.0343 for Nutlin-3 and p=0.0085 for MI-63). Interestingly, despite no obvious resistance to growth inhibition, p14ARF impaired cell lines, particularly those that are not MYCN amplified, appear to be especially resistant to MDM2-p53 antagonist mediated activation of caspase 3/7 activity, which was comparable to that of p53 mutant cell lines (Figure 6C). In addition the MYCN and MDM2 co-amplified cell lines that display increased caspase 3/7 activity have been previously reported to express high levels of p14ARF mRNA and protein (Carr et al 2006).
Figure 6. MYCN amplified neuroblastoma cell lines are more sensitive to MDM2-p53 antagonists mediated apoptosis than non-MYCN amplified neuroblastoma cell lines.
A panel of neuroblastoma cell lines were treated with A) 5μM Nutlin-3 and B) 2.5μM MI-63 and relative caspase 3/7 activity determined. The difference in caspase 3/7 activity was significantly higher in MYCN amplified compared to non-MYCN amplified cell lines (p=0.0343 for Nutlin-3, and p=0.0085 for MI-63). p53 mutant cell lines had low levels of caspase activity. Cell lines marked * are p14ARF impaired (methylation or homozygous deletion). C) p14ARF impaired cell lines have significantly lower levels of caspase 3/7 activity compared to cell lines with normal p14ARF (p=0.0093 for Nutlin-3, p=0.0078 for MI-63, Mann-Whitney test).
Discussion
Neuroblastoma accounts for 15% of childhood cancer related deaths (Maris and Matthay 1999). MYCN amplification is a powerful and reliable biomarker of poor prognosis in neuroblastoma and is used to stratify patients into a high risk group requiring intensive treatment. However, current therapies are insufficient for these patients, resulting in high mortality rates, a high incidence of relapse and treatment related toxicity (Matthay et al 1999).
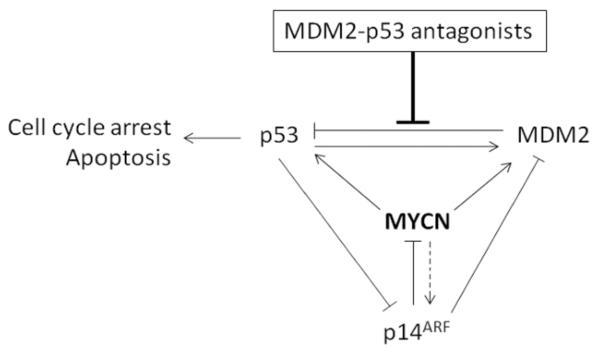
MYCN plays roles in the contradictory pathways of promoting both cell survival (Lutz et al 1996) and sensitizing cells to apoptosis (Fulda et al 2000, van Noesel et al 2003), but in neuroblastomas abnormalities within the apoptotic pathways can occur in association with MYCN amplification (Hogarty 2003). p53, the major tumor suppressor in the cell, is generally wild-type and active at diagnosis and patients respond well to initial therapy. However, high risk patients often relapse and although p53 mutations are still relatively rare (~15% of cases), p53 function is often inactivated through disruption of the p53/MDM2/p14ARF network, of which MYCN is a central modulator (Figure 7), resulting in chemoresistant disease (Carr-Wilkinson et al 2010). A number of studies have shown that pathways downstream of p53 are intact in neuroblastoma and that p53 can induce apoptotic responses (Hogarty 2003, Hosoi et al 1994, Tweddle et al 2003, Vogan et al 1993). However, in response to DNA damage, MYCN amplified cell lines fail to undergo G1 arrest compared to non-amplified cell lines (Bell et al 2006). In response to Nutlin-3 and MI-63 we found that changes in cell cycle distribution were cell line dependent. It has been previously reported that the negative regulator of p53, MDM2, is the critical oncogene product by which MYCN amplified neuroblastomas acquire a more aggressive behaviour (Slack and Shohet 2005), and that MYCN and MDM2 work together to inhibit apoptosis (Alt et al 2003, Wang et al 2006). There are a number of mechanisms by which MDM2 might contribute to the aggressive phenotype of MYCN amplified neuroblastoma. MDM2 has been reported to be a direct transcriptional target of MYCN and therefore MYCN-driven expression of MDM2 may contribute to p53 inactivation in neuroblastoma (Slack et al 2005). MDM2 is co-amplified with MYCN in about 6% of neuroblastomas but more frequently the negative regulator of MDM2, p14ARF is inactivated through methylation or deletion, and has been reported to occur in 29% of tumors, resulting in hyperactive MDM2 (Carr-Wilkinson et al 2010, Corvi et al 1995). In addition, we have recently reported that p53 is a direct transcriptional target of MYCN, and that p53 induction by MYCN may be an important contributory mechanism by which MYCN sensitizes cells to apoptosis (Chen et al 2010).
Figure 7. MYCN is a central modulator of the p53/MDM2/p14ARF negative feedback loop.
MDM2-p53 antagonists block the MDM2-p53 interaction.
Here we investigate the effect of MYCN on the response to the MDM2-p53 antagonist Nutlin-3 and the more potent MI-63, not previously investigated in neuroblastoma. We show by three independent methods that MYCN amplification and expression sensitizes neuroblastoma cell lines to MDM2-p53 antagonists. Firstly, using the SHEP Tet21N MYCN regulatable cell line, both Nutlin-3 and MI-63 are more effective at inducing growth inhibition and apoptosis in MYCN(+) compared to MYCN(−) cells, as shown by growth inhibition assays, caspase 3/7 activity, the sub G1 DNA content on FACs analysis and induction of the p53 regulated apoptotic marker PUMA. Secondly, we show that following siRNA knockdown of MYCN in two MYCN amplified and two MYCN and MDM2 co-amplified neuroblastoma cell lines, Nutlin-3 and MI-63 treatment resulted in a decreased p53 response and decreased levels of apoptosis. Thirdly, we assessed growth inhibition and caspase activity in a panel of 18 cell lines and found that both Nutlin-3 and MI-63 were more effective at inducing growth inhibition and apoptosis in MYCN amplified cells compared to non MYCN amplified cells. We also show that the effect is dependent on wildtype p53 in both MYCN amplified and non-amplified cell lines, consistent with a number of reports showing that these compounds require wild-type p53, as expected if their action is target-specific (Van Maerken et al 2006, Vassilev et al 2004). Whereas all MYCN amplified cell lines investigated were responsive to MDM2-p53 antagonists, the response of non-amplified cell lines was more variable. This is unsurprising as MYCN amplification is directly responsible for transformation; non amplified cases are likely to have a variety of other genetic abnormalities or defects in apoptotic pathways that may not be present in MYCN amplified tumors.
Our data differs from previous findings that report no correlation between MYCN status and response to Nutlin-3 (Barbieri et al 2006, Van Maerken et al 2006). Van Maerken et al. tested a panel of 7 p53 wildtype cell lines (3 MYCN amplified), and found no significant difference in the cell viability response or apoptotic response to Nutlin-3 in MYCN amplified compared to non-MYCN amplified cell lines (Van Maerken et al 2006). In another study IC50 values for Nutlin-3 in 2 MYCN regulatable cell lines were determined and whilst in agreement with our extensive observations that there was increased sensitivity in MYCN(+) compared to MYCN(−) Tet21 cells, it was reported that there was no difference in IC50s for MYCN(+/−) MYCN3 cells (Barbieri et al 2006). To test the effect of MYCN on sensitivity to MDM2-p53 antagonists we used several methods and a total of 22 cell lines to investigate the influence on cell growth and cell death, and found similar results using two structurally unrelated MDM2-p53 antagonists. Furthermore, previous studies with Tet21N cells have reported that MYCN(+) cells were more sensitive to apoptosis from cytotoxic drugs than MYCN(−) cells (Fulda et al 2000), and a recent study reports that MYCN sensitizes neuroblastoma cell lines to the apoptotic effects of bleomycin (Petroni et al 2011), in agreement with our data that MYCN is sensitizing cells to apoptosis. p14ARF is often inactivated in neuroblastoma (Carr-Wilkinson et al 2010), is expressed at higher levels in MYCN and MDM2 co-amplified neuroblastoma cell lines than MYCN amplified non-MDM2 amplified cell lines (Carr et al 2006), and may inhibit MYCN transcriptional activity (Amente et al 2007). There is also evidence that p14ARF is induced by c-MYC, and therefore MYCN may function in a similar way (Zindy et al 1998). Interestingly the 3 non MYCN amplified p14ARF impaired cell lines were especially resistant to Nutlin-3 and MI-63 mediated apoptosis, supporting recent findings showing that silencing of p14ARF results in decreased susceptibility to undergo apoptosis and overexpression of p14ARF results in a stronger caspase 3/7 response (Van Maerken et al 2011).
We found that MYCN and MDM2 co-amplified neuroblastomas had reduced sensitivity to Nutlin-3 mediated growth inhibition, and a similar trend with MI-63, compared to MYCN only amplified cell lines. However, we saw increased levels of caspase 3/7 activity in these cell lines compared to MYCN amplified and non-amplified cell lines, consistent with previous reports that increased levels or amplification of MDM2 increases Nutlin-3 induced apoptosis as shown in liposarcomas and AML (Kojima et al 2005, Muller et al 2007). However, MYCN and MDM2 co-amplified cell lines have previously been reported to have increased p14ARF mRNA and protein expression (Carr et al 2006), and it may be this increase in p14ARF that is sensitizing these cell lines to increased caspase activity. Our data indicates that MDM2 co-amplification may increase the resistance of MYCN amplified neuroblastomas to Nutlin-3 mediated growth inhibition but may sensitise to apoptosis, and therefore the effect of MDM2 warrants further investigation.
Our observations support studies that both MDM2 and p53 are induced by MYCN, that MDM2 is a critical oncogene product by which MYCN amplified neuroblastomas acquire a more aggressive phenotype and that MYCN sensitizes cells to p53-mediated apoptosis. Under normal circumstances, both p53 and MYCN induce MDM2, but upon MDM2 inhibition MYCN-mediated transcription of p53 allows p53 to accumulate and increases activity. In addition there is evidence that both p53 and c-MYC inhibit anti-apoptotic factors such as the Bcl-2 and Bcl-x, another mechanism by which amplification of MYCN may promote apoptosis together with p53 if as is likely this is also true of MYCN (Chipuk and Green 2006, van Noesel and Versteeg 2004).
Interestingly, p53 has been shown to inhibit the mTOR pathway which is involved in senescence (Demidenko et al 2010), and there is evidence that p53 induced cell cycle arrest is reversible as long as mTOR is also inhibited, suggesting that induction of senescence, reversible cell cycle arrest and cell death in response to Nutlin-3 may be determined by the status of mTOR (Korotchkina et al 2010). The activation status of mTOR may therefore be important in determining the response to MDM2-p53 antagonists in neuroblastoma.
In conclusion, we have several lines of evidence that MYCN sensitizes neuroblastoma cell lines to MDM2-p53 antagonists through p53-dependent growth inhibition and apoptosis, and may provide a promising therapeutic approach for patients with high-risk MYCN amplified neuroblastoma with wild-type p53. Previous studies show that MYCN amplification sensitizes cells to chemotherapeutic drugs and that Nutlin-3 induces senescence in normal cells that might actually protect the cell against cytotoxic drugs (Efeyan et al 2007, Fulda et al 2000). Furthermore Nutlin-3 synergises with chemotherapy in neuroblastoma cells (Barbieri et al 2006) suggesting that patients with MYCN amplified tumors may be particularly responsive to MDM2- p53 antagonists in combination with chemotherapeutic drugs.
Materials and Methods
Cell Lines and reagents
MYCN amplified neuroblastoma cell lines used in this study were NGP, LS, TR14, NB1691, IMR-32, NBLW, SMSKCNR, LAN5, PER-108, CHLA136, and the p53 mutant cell lines SKNBe2C and IGRN91. Non-MYCN amplified cell lines used were SHSY5Y, GIMEN, SJNB1, NB69, LAN6, SKNRA, SHEP, NBLS and the p53 mutant cell line SKNAS. The conditional MYCN-expressing SHEP Tet21N cell line was used and cells cultured for at least 48 hours in 1μg/ml of tetracycline (Sigma, Dorset, UK) to switch off MYCN. Tet21 vector only cells were used as a control (Lutz et al 1996). All cell lines were cultured in RPMI medium (Sigma, Dorset, UK) supplemented with 10% FCS, and 200μg/ml of G-418 antibiotic was added to Tet21N and Tet21 media. Cell lines were validated upon receipt using cytogenetic analysis courtesy of Nick Bown, Institute of Human Genetics, Newcastle University, U.K. Nutlin-3 was purchased from Enzo Life Sciences (Exeter, UK) and MI-63 was kindly provided by Siena Biotech (Siena, Italy) as part of a Framework Programe 6 DePPICT consortium collaboration.
Growth inhibition assays
Cells were plated in 96-well plates (Corning, UK) for 24 hours before treatment with 0- 20μM Nutlin-3 or 0-10μM MI-63 for 72 hours. Cells were then fixed in 50% trichloroacetic acid and sulphorhodamine B assays performed as previously described (Skehan et al 1990). A SpectroMax 250 (Molecular Devices, Berkshire, UK) was used for densitometric scanning of the plates and GI50 values calculated using Prism Version 4.0 software.
RNA interference
Cells were seeded at 4×105 cells/well in 6-well plates (Corning, Amsterdam, Netherlands). At 30-50% confluency, 40nM (NGP, LS, IMR32) or 50nM (LAN5) of siRNA duplexes against MYCN were transfected into cells with Lipofectamine™ reagent (Invitrogen, Paisley, UK) using previously described sequences (Bell et al 2006), for 24 hours. All experiments were performed in triplicate.
Western analysis
Previously described methods were used with 25μg of protein (Tweddle et al 2001). Primary antibodies used were MYCN (Calbiochem, Nottingham, UK; OP13), MDM2 (Calbiochem; OP40), p21WAF1 (Calbiochem; OP68), PARP-1/2 (Santa Cruz, CA, USA; H-250), at 1:100, p53 (Novocastra, Newcastle, UK; DO-7) at 1:200, Phospho-p53(Ser15) (Cell signalling, Danvers, MA, USA; 9284) and Cleaved Caspase-3 (Cell Signalling; Asp175) at 1:1000, and β-actin (Sigma-Aldrich, Dorset, UK; AC-40) at 1:500. Secondary goat anti-mouse/rabbit (Dako, Glostrup, Denmark) antibodies conjugated to horseradish peroxidase were used at 1:1000.
Flow cytometry
For cell cycle and sub-G1 analysis, adherent and non-adherent cells were harvested, washed in PBS and fixed in 4:1 Methanol:Acetone. FACs analysis was performed using previously described methods (Bell et al 2006). The Becton Dickinson FACscan was used and data analysed using CellQuest software.
Caspase activity
5000 cells/well were seeded in 96 well plates. After treatment, a 1:1 volume of Caspase-Glo® 3/7 Reagent (Promega, Southhampton, UK) was added to wells for 1 hour, transferred to a white plate and then analysed on a microplate Luminometer (Berthold Technologies, Herefordshire, UK).
Statistical Analyses
All statistical tests were performed in GraphPad Prism (GraphPad Software, Inc.). Tests were performed using log values and all t-tests were two-sided.
Supplementary Material
Acknowledgements
We thank the following for cell lines: Sue Cohn (NBLW and NBLS), Linda Harris (SJNB1), Penny Lovat (SHSY5Y, SHEP and IMR32), John Maris (NB69), Patrick Reynolds (SKNRA, SMSKCNR, LAN5, LAN6 and CHLA136), Manfred Schwab (LS and SHEP Tet21N) Rogier Versteeg (NGP), Barbara Spengler (SKNBe2C), Micro Ponzoni (GIMEN), Ursula Kees (PER108), Maria Lastowska (TR14), Clinton Stewart (NB1691) and Jean Bénard (SKNAS, IGRN91). We are grateful to Mike Cole (Newcastle Cancer Centre, Newcastle University) for his statistical advice and to Cancer Research UK for funding this work.
Sources of Support: Cancer Research UK
Footnotes
Conflict of Interest The authors declare no conflict of interest.
Supplementary information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Contributor Information
Laura D. Gamble, Newcastle Cancer Centre at the Northern Institute for Cancer Research,, Newcastle University, Newcastle upon Tyne, United Kingdom.
Ursula R. Kees, University of Western Australia, Perth, Australia.
Deborah A. Tweddle, Newcastle Cancer Centre at the Northern Institute for Cancer Research, Newcastle University, Newcastle upon Tyne, United Kingdom.
John Lunec, Newcastle Cancer Centre at the Northern Institute for Cancer Research, Newcastle University, Newcastle upon Tyne, United Kingdom.
References
- Alt JR, Greiner TC, Cleveland JL, Eischen CM. Mdm2 haplo-insufficiency profoundly inhibits Myc-induced lymphomagenesis. Embo J. 2003;22:1442–1450. doi: 10.1093/emboj/cdg133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amente S, Gargano B, Diolaiti D, Della Valle G, Lania L, Majello B. p14ARF interacts with N-Myc and inhibits its transcriptional activity. FEBS Lett. 2007;581:821–825. doi: 10.1016/j.febslet.2007.01.062. [DOI] [PubMed] [Google Scholar]
- Barbieri E, Mehta P, Chen Z, Zhang L, Slack A, Berg S, et al. MDM2 inhibition sensitizes neuroblastoma to chemotherapy-induced apoptotic cell death. Mol Cancer Ther. 2006;5:2358–2365. doi: 10.1158/1535-7163.MCT-06-0305. [DOI] [PubMed] [Google Scholar]
- Bell E, Premkumar R, Carr J, Lu X, Lovat PE, Kees UR, et al. The role of MYCN in the failure of MYCN amplified neuroblastoma cell lines to G1 arrest after DNA damage. Cell Cycle. 2006;5:2639–2647. doi: 10.4161/cc.5.22.3443. [DOI] [PubMed] [Google Scholar]
- Canner JA, Sobo M, Ball S, Hutzen B, DeAngelis S, Willis W, et al. MI-63: a novel small-molecule inhibitor targets MDM2 and induces apoptosis in embryonal and alveolar rhabdomyosarcoma cells with wild-type p53. Br J Cancer. 2009;101:774–781. doi: 10.1038/sj.bjc.6605199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr-Wilkinson J, O’Toole K, Wood KM, Challen CC, Baker AG, Board JR, et al. High Frequency of p53/MDM2/p14ARF Pathway Abnormalities in Relapsed Neuroblastoma. Clin Cancer Res. 2010;16:1108–1118. doi: 10.1158/1078-0432.CCR-09-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr J, Bell E, Pearson AD, Kees UR, Beris H, Lunec J, et al. Increased frequency of aberrations in the p53/MDM2/p14(ARF) pathway in neuroblastoma cell lines established at relapse. Cancer Res. 2006;66:2138–2145. doi: 10.1158/0008-5472.CAN-05-2623. [DOI] [PubMed] [Google Scholar]
- Chen L, Iraci N, Gherardi S, Gamble LD, Wood KM, Perini G, et al. p53 is a direct transcriptional target of MYCN in neuroblastoma. Cancer Res. 2010;70:1377–1388. doi: 10.1158/0008-5472.CAN-09-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Lin Y, Barbieri E, Burlingame S, Hicks J, Ludwig A, et al. Mdm2 deficiency suppresses MYCN-Driven neuroblastoma tumorigenesis in vivo. Neoplasia. 2009;11:753–762. doi: 10.1593/neo.09466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Green DR. Dissecting p53-dependent apoptosis. Cell Death Differ. 2006;13:994–1002. doi: 10.1038/sj.cdd.4401908. [DOI] [PubMed] [Google Scholar]
- Cohn SL, Tweddle DA. MYCN amplification remains prognostically strong 20 years after its “clinical debut”. Eur J Cancer. 2004;40:2639–2642. doi: 10.1016/j.ejca.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol. 2009;27:289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvi R, Savelyeva L, Breit S, Wenzel A, Handgretinger R, Barak J, et al. Non-syntenic amplification of MDM2 and MYCN in human neuroblastoma. Oncogene. 1995;10:1081–1086. [PubMed] [Google Scholar]
- Demidenko ZN, Korotchkina LG, Gudkov AV, Blagosklonny MV. Paradoxical suppression of cellular senescence by p53. Proc Natl Acad Sci U S A. 2010;107:9660–9664. doi: 10.1073/pnas.1002298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efeyan A, Ortega-Molina A, Velasco-Miguel S, Herranz D, Vassilev LT, Serrano M. Induction of p53-dependent senescence by the MDM2 antagonist nutlin-3a in mouse cells of fibroblast origin. Cancer Res. 2007;67:7350–7357. doi: 10.1158/0008-5472.CAN-07-0200. [DOI] [PubMed] [Google Scholar]
- Fulda S, Lutz W, Schwab M, Debatin KM. MycN sensitizes neuroblastoma cells for drug-triggered apoptosis. Med Pediatr Oncol. 2000;35:582–584. doi: 10.1002/1096-911x(20001201)35:6<582::aid-mpo19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Goldman SC, Chen CY, Lansing TJ, Gilmer TM, Kastan MB. The p53 signal transduction pathway is intact in human neuroblastoma despite cytoplasmic localization. Am J Pathol. 1996;148:1381–1385. [PMC free article] [PubMed] [Google Scholar]
- Hogarty MD. The requirement for evasion of programmed cell death in neuroblastomas with MYCN amplification. Cancer Lett. 2003;197:173–179. doi: 10.1016/s0304-3835(03)00103-4. [DOI] [PubMed] [Google Scholar]
- Hosoi G, Hara J, Okamura T, Osugi Y, Ishihara S, Fukuzawa M, et al. Low frequency of the p53 gene mutations in neuroblastoma. Cancer. 1994;73:3087–3093. doi: 10.1002/1097-0142(19940615)73:12<3087::aid-cncr2820731230>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Kang JH, Rychahou PG, Ishola TA, Qiao J, Evers BM, Chung DH. MYCN silencing induces differentiation and apoptosis in human neuroblastoma cells. Biochem Biophys Res Commun. 2006;351:192–197. doi: 10.1016/j.bbrc.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K, Konopleva M, Samudio IJ, Shikami M, Cabreira-Hansen M, McQueen T, et al. MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy. Blood. 2005;106:3150–3159. doi: 10.1182/blood-2005-02-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotchkina LG, Leontieva OV, Bukreeva EI, Demidenko ZN, Gudkov AV, Blagosklonny MV. The choice between p53-induced senescence and quiescence is determined in part by the mTOR pathway. Aging (Albany NY) 2010;2:344–352. doi: 10.18632/aging.100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz W, Stohr M, Schurmann J, Wenzel A, Lohr A, Schwab M. Conditional expression of N-myc in human neuroblastoma cells increases expression of alpha-prothymosin and ornithine decarboxylase and accelerates progression into S-phase early after mitogenic stimulation of quiescent cells. Oncogene. 1996;13:803–812. [PubMed] [Google Scholar]
- Maris JM, Matthay KK. Molecular biology of neuroblastoma. J Clin Oncol. 1999;17:2264–2279. doi: 10.1200/JCO.1999.17.7.2264. [DOI] [PubMed] [Google Scholar]
- Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- Michalak E, Villunger A, Erlacher M, Strasser A. Death squads enlisted by the tumour suppressor p53. Biochem Biophys Res Commun. 2005;331:786–798. doi: 10.1016/j.bbrc.2005.03.183. [DOI] [PubMed] [Google Scholar]
- Muller CR, Paulsen EB, Noordhuis P, Pedeutour F, Saeter G, Myklebost O. Potential for treatment of liposarcomas with the MDM2 antagonist Nutlin-3A. Int J Cancer. 2007;121:199–205. doi: 10.1002/ijc.22643. [DOI] [PubMed] [Google Scholar]
- Petroni M, Veschi V, Prodosmo A, Rinaldo C, Massimi I, Carbonari M, et al. MYCN sensitizes human neuroblastoma to apoptosis by HIPK2 activation through a DNA damage response. Mol Cancer Res. 2011;9:67–77. doi: 10.1158/1541-7786.MCR-10-0227. [DOI] [PubMed] [Google Scholar]
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Slack A, Chen Z, Tonelli R, Pule M, Hunt L, Pession A, et al. The p53 regulatory gene MDM2 is a direct transcriptional target of MYCN in neuroblastoma. Proc Natl Acad Sci U S A. 2005;102:731–736. doi: 10.1073/pnas.0405495102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack A, Shohet JM. MDM2 as a critical effector of the MYCN oncogene in tumorigenesis. Cell Cycle. 2005;4:857–860. doi: 10.4161/cc.4.7.1790. [DOI] [PubMed] [Google Scholar]
- Tweddle DA, Malcolm AJ, Cole M, Pearson AD, Lunec J. p53 cellular localization and function in neuroblastoma: evidence for defective G(1) arrest despite WAF1 induction in MYCN-amplified cells. Am J Pathol. 2001;158:2067–2077. doi: 10.1016/S0002-9440(10)64678-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweddle DA, Pearson AD, Haber M, Norris MD, Xue C, Flemming C, et al. The p53 pathway and its inactivation in neuroblastoma. Cancer Lett. 2003;197:93–98. doi: 10.1016/s0304-3835(03)00088-0. [DOI] [PubMed] [Google Scholar]
- Van Maerken T, Speleman F, Vermeulen J, Lambertz I, De Clercq S, De Smet E, et al. Small-molecule MDM2 antagonists as a new therapy concept for neuroblastoma. Cancer Res. 2006;66:9646–9655. doi: 10.1158/0008-5472.CAN-06-0792. [DOI] [PubMed] [Google Scholar]
- Van Maerken T, Rihani A, Dreidax D, De Clercq S, Yigit N, Marine JC, et al. Functional analysis of the p53 pathway in neuroblastoma cells using the small-molecule MDM2 antagonist nutlin-3. Mol Cancer Ther. 2011 doi: 10.1158/1535-7163.MCT-10-1090. [DOI] [PubMed] [Google Scholar]
- van Noesel MM, Pieters R, Voute PA, Versteeg R. The N-myc paradox: N-myc overexpression in neuroblastomas is associated with sensitivity as well as resistance to apoptosis. Cancer Lett. 2003;197:165–172. doi: 10.1016/s0304-3835(03)00101-0. [DOI] [PubMed] [Google Scholar]
- van Noesel MM, Versteeg R. Pediatric neuroblastomas: genetic and epigenetic ‘danse macabre’. Gene. 2004;325:1–15. doi: 10.1016/j.gene.2003.09.042. [DOI] [PubMed] [Google Scholar]
- Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- Vogan K, Bernstein M, Leclerc JM, Brisson L, Brossard J, Brodeur GM, et al. Absence of p53 gene mutations in primary neuroblastomas. Cancer Res. 1993;53:5269–5273. [PubMed] [Google Scholar]
- Wang P, Greiner TC, Lushnikova T, Eischen CM. Decreased Mdm2 expression inhibits tumor development induced by loss of ARF. Oncogene. 2006;25:3708–3718. doi: 10.1038/sj.onc.1209411. [DOI] [PubMed] [Google Scholar]
- Weiss WA, Aldape K, Mohapatra G, Feuerstein BG, Bishop JM. Targeted expression of MYCN causes neuroblastoma in transgenic mice. Embo J. 1997;16:2985–2995. doi: 10.1093/emboj/16.11.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel A, Cziepluch C, Hamann U, Schurmann J, Schwab M. The N-Myc oncoprotein is associated in vivo with the phosphoprotein Max(p20/22) in human neuroblastoma cells. Embo J. 1991;10:3703–3712. doi: 10.1002/j.1460-2075.1991.tb04938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindy F, Eischen CM, Randle DH, Kamijo T, Cleveland JL, Sherr CJ, et al. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.