Abstract
Background
Phlebotomine sand flies are blood-sucking insects that can transmit Leishmania parasites. Hosts bitten by sand flies develop an immune response against sand fly salivary antigens. Specific anti-saliva IgG indicate the exposure to the vector and may also help to estimate the risk of Leishmania spp. transmission. In this study, we examined the canine antibody response against the saliva of Phlebotomus perniciosus, the main vector of Leishmania infantum in the Mediterranean Basin, and characterized salivary antigens of this sand fly species.
Methodology/Principal Findings
Sera of dogs bitten by P. perniciosus under experimental conditions and dogs naturally exposed to sand flies in a L. infantum focus were tested by ELISA for the presence of anti-P. perniciosus antibodies. Antibody levels positively correlated with the number of blood-fed P. perniciosus females. In naturally exposed dogs the increase of specific IgG, IgG1 and IgG2 was observed during sand fly season. Importantly, Leishmania-positive dogs revealed significantly lower anti-P. perniciosus IgG2 compared to Leishmania-negative ones. Major P. perniciosus antigens were identified by western blot and mass spectrometry as yellow proteins, apyrases and antigen 5-related proteins.
Conclusions
Results suggest that monitoring canine antibody response to sand fly saliva in endemic foci could estimate the risk of L. infantum transmission. It may also help to control canine leishmaniasis by evaluating the effectiveness of anti-vector campaigns. Data from the field study where dogs from the Italian focus of L. infantum were naturally exposed to P. perniciosus bites indicates that the levels of anti-P. perniciosus saliva IgG2 negatively correlate with the risk of Leishmania transmission. Thus, specific IgG2 response is suggested as a risk marker of L. infantum transmission for dogs.
Author Summary
Leishmania infantum is the causative agent of zoonotic visceral leishmaniasis in the Mediterranean Basin and Phlebotomus perniciosus serve as the major vector. In the endemic foci, Leishmania parasites are transmitted mostly to dogs, the main reservoir host, and to humans. We studied the canine humoral immune response to Phlebotomus perniciosus saliva and its potential use as a marker of sand fly exposure and consequently as a risk marker for Leishmania transmission. We also characterized major salivary antigens of P. perniciosus. We demonstrated that under laboratory conditions, the levels of anti-P. perniciosus saliva antibodies positively correlated with the number of blood-fed sand flies and therefore, may be used to evaluate the need for, and the effectiveness of, anti-vector campaigns. In parallel, we studied sera of dogs naturally exposed to P. perniciosus in highly active focus of canine leishmaniasis in Southern Italy. Specific antibodies against P. perniciosus saliva were significantly increased according to the ongoing sand fly season. Moreover, the levels of anti-P. perniciosus antibodies in naturally bitten dogs negatively correlated with anti-Leishmania seropositivity. Thus, for dogs living in endemic areas, specific antibody response against saliva of the vector is an important marker for estimating the risk of Leishmania transmission.
Introduction
Leishmania infantum (syn. Leishmania chagasi) is a protozoan parasite that causes zoonotic leishmaniasis, including the life-threatening visceral form, occurring also in the Mediterranean Basin. Parasites are transmitted by the bite of infected phlebotomine sand flies to dogs, the major host and the main domestic reservoir for human visceral leishmaniasis, or to humans. The clinical forms of canine leishmaniasis range from asymptomatic to lethal (reviewed in [1], [2]). Nonetheless, all seropositive infected dogs, including those without any clinical signs, can serve as a source of infection for sand flies in endemic areas [3], [4]. The major vector of canine leishmaniases in Mediterranean countries, including Italy, is Phlebotomus perniciosus [5], [6]. Control programs for human visceral leishmaniasis caused by L. infantum are primarily aimed at preventing sand flies from feeding on dogs to reduce Leishmania transmission among dogs and humans (reviewed in [1], [2]).
Measuring the exposure of dogs to sand fly bites is important for estimating the risk of L. infantum transmission. Recently, it was demonstrated that experimental exposure of dogs to Lutzomyia longipalpis bites elicits the production of specific anti-saliva IgG which positively correlates with the number of blood-fed sand flies [7]. Therefore, monitoring canine IgG levels specific for sand fly saliva could indicate the intensity of exposure to sand fly bites. Such a monitoring technique would be useful for evaluating the need for, and effectiveness of, anti-vector campaigns [7], [8].
Exposure to sand fly bites as well as immunization with sand fly saliva or its compounds elicits in naive hosts protection against Leishmania infection under laboratory conditions (reviewed in [9]). It is widely accepted that the protective effect is mediated by CD4+ Th1 cellular response and characterized by increased production of IFN- γ, which activates macrophages to kill Leishmania parasites (reviewed in [10]). Recently, it was shown that protective effect elicited by inoculation of Lutzomyia longipalpis recombinant proteins in dogs was associated with production of IFN-γ by CD3+ CD4+ T cells and by dominance of IgG2 antibodies [11].
In this study we described the anti-saliva IgG response in dogs experimentally exposed to P. perniciosus under laboratory conditions and those naturally exposed in an endemic focus of L. infantum. We also tested the association between the anti-saliva IgG subclasses and the levels of IFN-γ in Leishmania infantum-seropositive and -seronegative dogs. Additionally, we characterized the major P. perniciosus salivary antigens recognized by sera of experimentally and naturally bitten dogs.
Methods
Ethical statement
Experiments with dogs exposed to sand fly bites under laboratory conditions
Husbandry of animals in the Animal Center (Germany) complies with the European Commission guidelines for the accommodation of animals used for experimental and other scientific purposes - Commission Recommendation of 18 June 2007 (2007/526/EC). The compliance to aspects of animal welfare law is regularly monitored by the BAH animal welfare commissioner and the state veterinarian. The study design and the experimental procedures were approved by the responsible authorities (LANUV - Regional Authority for Nature, Environment and Consumer protection in North Rhine-Westphalia, Germany).
Experiments with dogs naturally exposed to sand fly bites
All procedures were approved by the Animal Ethics Committee from the Faculty of Veterinary Medicine, University of Bari, Italy and authorized by the Italian Ministry of Health (Authorization number 72/2009C n°69062; 28/11/08). Adverse events were individually registered in accordance to the International Cooperation on Harmonization of Technical Requirements for Registration of Veterinary Medicinal Products (VICH) and Good Clinical Practice (GCP) Guideline (GL9).
Sand flies and salivary gland dissection
A colony of Phlebotomus perniciosus was reared under standard conditions as described in [12]. Salivary glands were dissected from 4–6 day old female sand flies, placed into 20 mM Tris buffer with 150 mM NaCl and stored at −20°C.
Experimental exposure
Twelve laboratory dogs, beagles, were housed and handled in the Bayer Animal Health GmbH animal facility (Leverkusen, Germany). Dogs were sedated and individually exposed to approximately 200 P. perniciosus females as described in [7], [13]. Twenty hours after exposure, sand flies were collected and microscopically examined to assess the ratio of blood-fed females. In two independent experiments, two groups of three dogs each were used. Dogs in groups 2 and 4 wore insecticide-impregnated collars that were administrated 8 days before the first sand fly exposure, for a reduction of sand fly bites. In comparison, dogs in groups 1 and 3 remained without any repellent or insecticide application during the whole study. Therefore, dogs in groups 1 and 3 are hereafter defined as high-exposed (HE) and the dogs in groups 2 and 4 as low-exposed (LE). Dogs were exposed to sand fly bites once a week for five consecutive weeks. For the detailed numbers of blood-fed females see Table 1. Blood samples were collected throughout the study according to the following schedule: before the first exposure (week 0, pre-immune serum), during the sand fly sensitization (weeks 1–5), and weekly after the last exposure for 5 weeks (weeks 6–10).
Table 1. Numbers of blood-fed Phlebotomus perniciosus females per dog.
| Week | Group 1 | Group 2 | Group 3 | Group 4 |
| 1 | 221±5 | 49±15 | 173±8 | 27±4 |
| 2 | 191±47 | 125±69 | 155±18 | 11±6 |
| 3 | 188±7 | 61±20 | 125±6 | 36±15 |
| 4 | 156±4 | 39±11 | 169±12 | 20±3 |
| 5 | 195±9 | 83±36 | 158±11 | 8±1 |
| average | 190±10 | 71±16 | 156±6 | 20±4 |
(average ± standard error; groups 1, 3 – high-exposed dogs; groups 2, 4 – low-exposed dogs).
Field study
Twenty nine mixed-breed young dogs (from 90 to 145 days old) and eleven laboratory reared beagles (120 days old) were enrolled in the trial. All animals were housed in a private open-air shelter in Putignano (Bari province, Apulia, Italy), where P. perniciosus is the most abundant phlebotomine sand fly species [14]. All dogs were vaccinated against common dog pathogens and dewormed as described in [15]. The canine antibody response against P. perniciosus saliva was studied at the beginning (March 2008) and at the end (November 2008) of the sand fly season. In parallel, at four intervals (March, July, November 2008 and March 2009) dogs were tested for L. infantum infection status by serological, cytological and molecular methods. All dogs were L. infantum negative at the beginning of the trial (March 2008), which was proved by all three diagnostic methods used. Leishmania-positive dogs were defined by positive anti-L. infantum serology and, in a subset of seropositive dogs (4 out of 18), the infection was confirmed by PCR or cytology. For details on the diagnostic methods, see [15], [16]. Considering the long incubation period of canine leishmaniasis and the occurrence of sand flies exclusively during the summer season (from June to October) [14], dogs with anti-Leishmania seroconversion in March (2009) are presumed to have become infected during the previous season (2008). Dogs that were seronegative for L. infantum at all four screening intervals were included in the Leishmania-negative group.
Detection of anti – P. perniciosus saliva antibodies
Anti-P. perniciosus IgG, IgG1 and IgG2 were measured by enzyme-linked immunosorbent assay (ELISA) as described in [7] with some modification. Briefly, microtiter plates were incubated with 6% (w/v) low fat dry milk in PBS with 0.05% Tween 20 (PBS-Tw). Canine sera were diluted 1∶200 or 1∶500 in 2% (w/v) low fat dry milk/PBS-Tw. Secondary antibodies (anti-dog IgG, IgG1, or IgG2 from Bethyl laboratories) were diluted and incubated as previously described [7]. Absorbance was measured at 492 nm using a Tecan Infinite M200 microplate reader (Schoeller). The cut-off value (IgG = 0.145; IgG1 = 0.126; IgG2 = 0.165) was determined as less than two times the standard error of the mean of the absorbance of pre-immune serum.
Western blot analysis
Phlebotomus perniciosus salivary gland homogenate from 5-day-old sand fly females were separated by SDS-PAGE on a 10% gel under non-reducing conditions using the Mini-Protean III apparatus (BioRad). Separated proteins were blotted onto a nitrocellulose (NC) membrane by Semi-Phor equipment (Hoefer Scientific Instruments) and blocked with 5% (w/v) low fat dry milk in Tris-buffered saline with 0.05% Tween 20 (TBS-Tw). Strips of NC membrane were incubated with canine sera diluted 1∶50 (experimentally bitten dogs) or 1∶25 (naturally bitten dogs) in TBS-Tw for 1 hour. The strips were then washed three times with TBS-Tw and incubated with peroxidase-conjugated sheep anti-dog IgG (Bethyl Laboratories) diluted 1∶3000 in TBS-Tw. The chromogenic reaction was developed using a solution containing diaminobenzidine and H2O2.
Mass spectrometry
For mass spectrometric analysis, salivary glands from 5-day-old P. perniciosus females were homogenized by 3 freeze-thaw cycles. Samples were dissolved in non-reducing sample buffer and electrophoretically separated in 10% polyacrylamide SDS gel. Proteins within the gels were visualized by staining with Coomassie Blue G-250 (Bio-Rad). The individual bands were cut and incubated with 10 mM dithiothreitol (DTT) and then treated with 55 mM iodoacetamid. Washed and dried bands were digested with trypsin (5 ng Promega). The alpha-cyano-4-hydroxycinnamic acid was used as a matrix. Samples were measured using a 4800 Plus MALDI TOF/TOF analyzer (AB SCIEX). Peak list from the MS spectra was generated by 4000 Series Explorer V 3.5.3 (AB SCIEX) without smoothing. Peaks with local signal to noise ratio greater than 5 were picked and searched by local Mascot v. 2.1 (Matrix Science) against a database of putative salivary protein sequences derived from a cDNA library [17]. Database search criteria were as follows – enzyme: trypsin, taxonomy: Phlebotomus, fixed modification: carbamidomethylation, variable modification: methionine oxidation, peptide mass tolerance: 80 ppm, one missed cleavage allowed. Only hits that scored as significant (p<0.05) are included.
Statistical analysis
The data from experimentally bitten dogs obtained by ELISA were subjected to GLM ANOVA and Scheffe's Multiple Comparison procedure to analyse differences in kinetics of antibody response between HE and LE dogs at all sampling points. The non-parametric Wilcoxon rank sum test for differences in medians was used for comparison of anti-P. perniciosus IgG, IgG1, IgG2 and IgG1/IgG2 ratios between Leishmania-seropositive and -seronegative dogs. The non-parametric Wilcoxon signed-rank test for differences in medians was used for comparison of antibody increases between March and November blood samples in naturally bitten dogs. For correlation tests we used the non-parametric Spearman rank correlation matrix. For all tests statistical significance was regarded as a p-value less than or equal to 0.05. All statistical analyses were performed using NCSS 6.0.21 software.
Relative risk (the probability of the developing the disease occurring in the group exposed to the risk factor versus a non-exposed group), attributive risk (absolute effect of exposure to the risk factor) and ODDS ratio (odds of an event occurring in the exposed group to the odds of it occurring in non-exposed group) were calculated for dogs from the field study to find out the relationship between the levels of anti-P. perniciosus saliva antibodies and leishmaniasis incidence as described in [18]. Low level of specific antibodies (lower than the cut-off value) was determined as the risk factor and the confidence interval for relative risk was calculated as described in [19].
List of the protein accession numbers
Phlebotomus perniciosus: DQ153102; DQ154099; DQ150622; DQ150621; DQ192490; DQ192491; DQ153100; DQ153101; DQ153104; DQ150624; DQ150623; DQ150620; DQ153105.
Lutzomyia longipalpis: AF132518.
Results
Antibody response in experimentally bitten dogs
To investigate the kinetics of antibody response against anti-P. perniciosus saliva, two groups of experimentally bitten dogs, low-exposed (LE) and high-exposed (HE), were followed for 10 weeks. Five weekly experimental exposures to P. perniciosus bites led to increased levels of anti-saliva specific IgG, IgG1 and IgG2 in both LE and HE groups. No anti-saliva antibodies were detected in any pre-immune dog sera tested.
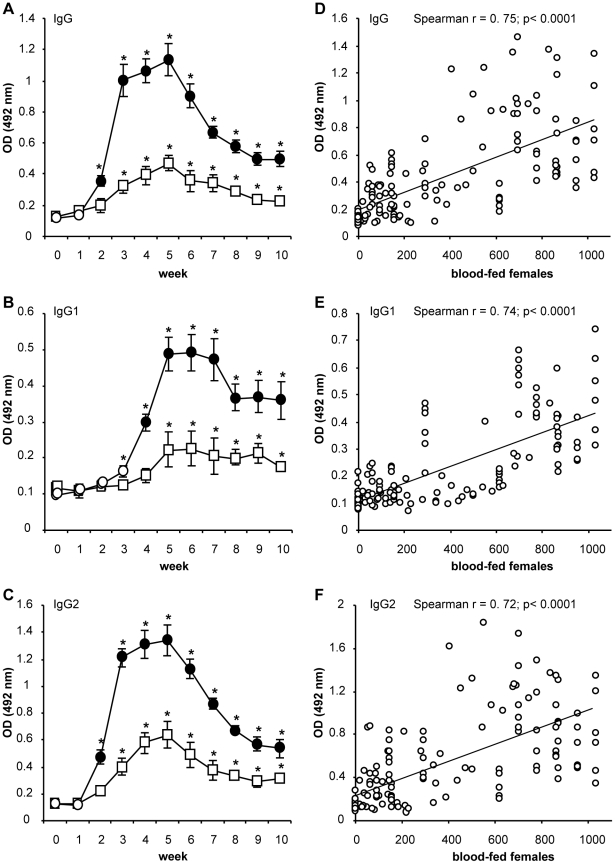
In HE dogs, anti-P. perniciosus antibody levels increased significantly (p<0.05) in comparison to the pre-immune sera after the second (IgG; IgG2) and third exposure (IgG1) (Figure 1A–C). Anti-saliva IgG and IgG2 developed with similar kinetics; rapidly increased after the third exposure, and gradual increase until week five (the last exposure), followed by a steady decrease to the end of the study. Anti-saliva IgG1 increased rapidly between weeks three and five and persisted at elevated levels until the end of the study.
Figure 1. Anti-sand fly saliva antibody response in dogs experimentally bitten by Phlebotomus perniciosus.
(A–C) Beagle dogs (3 per group) were divided into low-exposed (square) and high-exposed groups (circles) and were exposed to sand fly bites in weeks 1–5. For detailed numbers of blood-fed females see Table 1. Levels of specific IgG (A); IgG1 (B); and IgG2 (C) were measured by ELISA (at 492 nm) in all canine pre-immune and immune sera. Full circles represent significant difference between high- and low-exposed dogs (p<0.05); asterisks indicate significant difference (p<0.05) compared to pre-immune sera. Data are presented as the means ± standard errors of the means from two independent studies. (D–F) Correlation between number of blood-fed sand fly females and the levels of canine anti-P. perniciosus IgG (D); IgG1 (E); and IgG2 (F) was performed using Spearman Rank Correlation Matrix. OD = optical density.
In LE dogs, anti-P. perniciosus antibody levels increased significantly (p<0.05) in comparison to the pre-immune sera after the fourth (IgG; IgG2) and sixth exposure (IgG1) (Figure 1A–C). Similar to HE dogs, kinetics of anti-P. perniciosus IgG and IgG2 in LE dogs was detected at peak levels on week five followed by a rapid decrease. Conversely, IgG1 was measured at peak levels on week six and persisted at elevated quantities to the end of the study (Figure 1A–C).
All HE dogs produced significantly higher levels of anti-P. perniciosus IgG (p = 0.0001), IgG1 (p = 0.0032) and IgG2 (p = 0.0003) compared to LE dogs throughout the study (Figure 1A–C). A positive correlation was detected between number of blood-fed female sand flies and the levels of canine anti-P. perniciosus IgG (r = 0.75, p<0.0001), IgG1 (r = 0.74, p<0.0001) and IgG2 (r = 0.72, p<0.0001) (Figure 1D–F). Overall, sera of experimentally bitten dogs produced higher concentrations of specific IgG2 compared to specific IgG1 (data not shown).
Antibody response in naturally bitten dogs
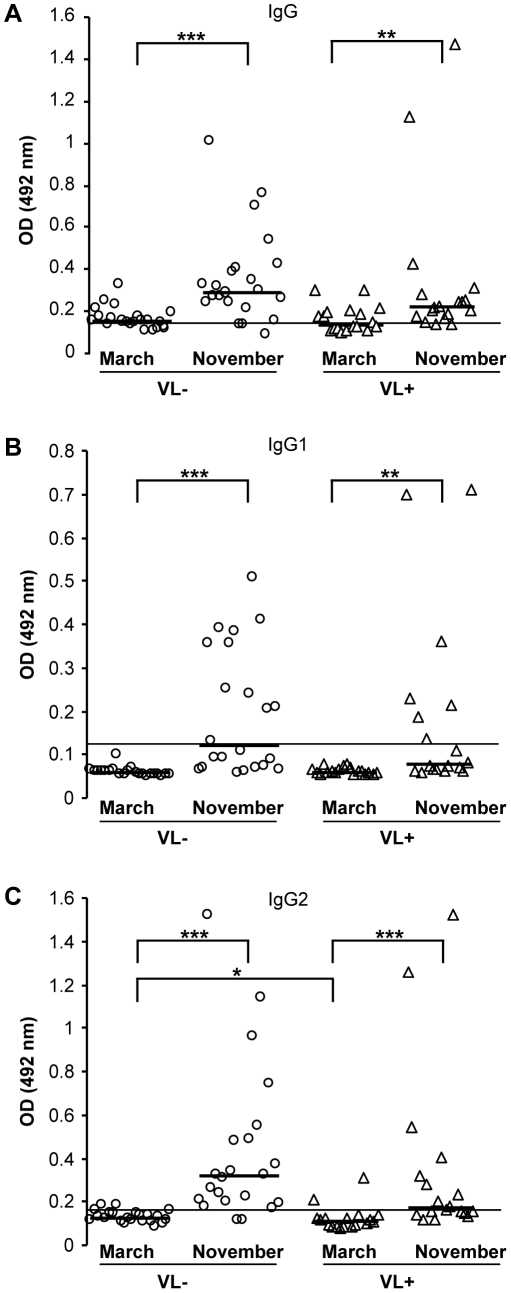
To determine the anti-P. perniciosus saliva antibody levels and the seasonal changes in specific antibody response, canine sera were screened at the beginning and at the end of the sand fly season, March and November, respectively. Incidence of leishmaniasis in dogs naturally exposed to sand flies was high, 18 out of 40 (45%) were found anti-L. infantum seropositive (0/40 in March 2008; 0/40 in July 2008; 5/40 in November 2008; 13/40 in March 2009). In March, higher levels of anti-P. perniciosus IgG and IgG2 (compared to cut-off value) were detected in about 55% and 10% of dog sera, respectively, while IgG1 levels were comparable to pre-immune sera (Table 2). In November, elevated levels of specific IgG were found in 87.5%, IgG2 in 72.5% and IgG1 in 45% of the 40 enrolled dogs (Table 2). In both groups of dogs, Leishmania-positive and Leishmania-negative, specific IgG, IgG1 and IgG2 levels significantly increased during the sand fly season (Figure 2A–C).
Table 2. Numbers of dogs positive for anti-Phlebotomus perniciosus antibodies in Leishmania infantum-seropositive and -seronegative dogs.
| Leishmania negative dogs (n = 22) | Leishmania positive dogs (n = 18) | |||||
| March | November | Increase(%) | March | November | Increase (%) | |
| IgG | 14 | 19 | 144*** | 8 | 15 | 104** |
| IgG1 | 0 | 11 | 235*** | 0 | 7 | 220** |
| IgG2 | 2 | 20 | 249*** | 2 | 9 | 205*** |
| IgG1/IgG2a | 0.47* | 0.54 | 15 | 0.57* | 0.73 | 28 |
(a – significant difference in IgG1/IgG2 ratio between Leishmania-seropositive and -seronegative groups; *** p<0.001; ** p<0.01; * p<0.05).
Figure 2. Anti-sand fly saliva antibody response in dogs naturally bitten by Phlebotomus perniciosus.
Anti-P. perniciosus IgG (A); IgG1 (B) and IgG2 (C) response was measured in sera of naturally bitten dogs from endemic area of visceral leishmaniasis. All dogs were Leishmania infantum seronegative at the beginning of the trial. ELISA was performed against P. perniciosus salivary gland homogenate using canine sera from Leishmania infantum-seropositive dogs (open triangle, n = 18) and Leishmania-seronegative dogs (open circles, n = 22). Serum samples were taken at the beginning (March) and at the end of the sand fly season (November). The symbols indicate results of each serum tested, bars represent median values of the groups. Lines represent cut-off values (two times the standard error of the mean of the absorbance of experimentally bitten dog pre-immune sera). Asterisks indicate statistical significance between Leishmania-seropositive and -seronegative dogs and significant increase of antibodies during the sand fly season within the group (* p<0.05; ** p<0.01; *** p<0.001). OD = optical density.
Leishmania-positive and Leishmania-negative dogs did not statistically differ in IgG and IgG1 production (Figure 2A, B); however, a significant difference was found in IgG2 levels (Figure 2C). Indeed, Leishmania-positive dogs revealed significantly lower anti-P. perniciosus IgG2 at the beginning (p = 0.047) and at the end (p = 0.05) of sand fly season (Figure 2C). Negative correlation was found between the levels of anti-P. perniciosus saliva IgG2 and the risk of Leishmania transmission, supported well by epidemiological parameters: relative risk = 2.6 (95% confidence interval: 0.66; 10.63); attributive risk = 1.6; and ODDS ratio = 10. Sera of all naturally bitten dogs showed significantly higher levels of specific IgG2 compared to specific IgG1 (data not shown). Moreover, the IgG1/IgG2 ratio differed between Leishmania-positive and -negative dogs; Leishmania-positive dogs revealed higher IgG1/IgG2 ratio, although the difference was statistically significant only at the beginning of sand fly season (p = 0.039) (Table 2). Furthermore, higher levels of IFN-γ were detected in sera of Leishmania-negative dogs throughout the study but with no statistically significant difference (Figure S1).
Identification and characterization of P. perniciosus salivary antigens
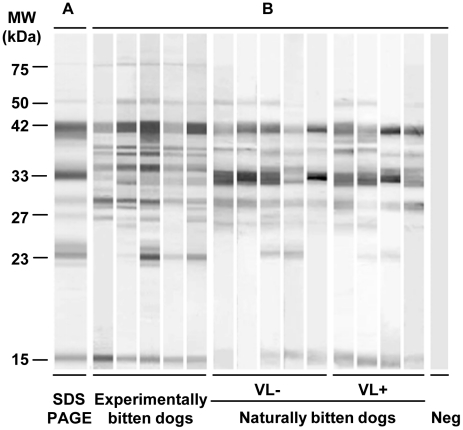
Phlebotomus perniciosus salivary antigens were studied using sera of naturally and experimentally bitten dogs. Pre-immune sera of experimentally bitten dogs did not recognize any of the salivary proteins by Western blot analysis (Figure 3).
Figure 3. Anti-sand fly saliva antibody response in dogs experimentally and naturally bitten by Phlebotomus perniciosus.
(A) Total protein profile, Commassie blue-stained SDS-PAGE gel after electrophoresis of P. perniciosus salivary gland homogenate. (B) Western blot of P. perniciosus salivary proteins recognized by sera of repeatedly bitten dogs. Western blot analysis was performed by sera of experimentally and naturally bitten dogs: Leishmania infantum-seronegative (VL−) and L. infantum-seropositive (VL+). Pre-immune serum of experimentally bitten dog was used as negative control (Neg).
Sera of experimentally exposed dogs produced 11 bands on a salivary gland Western blot with approximate molecular weights of 75, 50, 42, 40, 38, 34, 33, 29, 27, 23 and 14 kDa (Figure 3). The molecular weights of salivary antigens recognized by canine sera were similar in all dogs tested with the exception of the 23 and 27 kDa protein bands (recognized only by some sera). The salivary gland antigens most intensely recognized by the sera of all experimentally bitten dogs had molecular weights of 42, 38, 33 and 29 kDa.
Sera of naturally bitten dogs with both negative and positive anti-L. infantum serology reacted with up to 9 protein bands of 50, 42, 38, 34, 33, 29, 27, 23 and 14 kDa. All naturally exposed dogs tested in both groups recognized similar salivary antigens and the most intensive reactions were detected with the 42 and 33 kDa salivary antigens.
Mass spectrometry revealed that the main antigens recognized by sera of bitten dogs were salivary endonuclease (50 kDa - DQ154099), yellow proteins (42 kDa - DQ150622; 40 kDa - DQ150621), apyrases (38 kDa - DQ192490; 38 kDa - DQ192491; 33 kDa - DQ192491), antigen-5 protein (29 kDa - DQ153101), D7 proteins (27 kDa - DQ153104; 23 kDa - DQ150624; 23 kDa - DQ150623, and proteins of the SP-15 like protein family (14 kDa - DQ150620; 14 kDa - DQ153105) (Table 3).
Table 3. Phlebotomus perniciosus salivary proteins recognized by sera of bitten dogs.
| MW (kDa) | NCBI acc. number | Best match to NR protein database | ||
| Sequence name | E-value | Comments | ||
| 75 | DQ153102 | 29 kDa salivary protein (PpeSP08) | 2.2e-6 | unknown |
| 50 | DQ154099 | 41 kDa salivary protein (PpeSP32) | 3.5e-9 | endonuclease |
| 42 | DQ150622 | 43 kDa yellow-related salivary protein (PpeSP03B) | 1.1e-68 | yellow protein |
| 40 | DQ150621 | 42 kDa yellow-related salivary protein (PpeSP03) | 4.5e-54 | yellow protein |
| 38 | DQ192490 | 35.5 kDa salivary protein (PpeSP01) | 5.6e-54 | apyrase |
| 38 | DQ192491 | 35.3 kDa salivary protein (PpeSP01B) | 0.035 | apyrase |
| 34 | DQ153100 | 33 kDa salivary protein (PpeSP06) | 2.2e-24 | unknown |
| 33 | DQ192491 | 35.3 kDa salivary protein (PpeSP01B) | 2.8e-72 | apyrase |
| 33 | DQ153102 | 29 kDa salivary protein (PpeSP08) | 0.0019 | unknown |
| 29 | DQ153101 | 30 kDa antigen 5-related salivary protein (PpeSP07) | 1.4e-12 | Ag 5 protein |
| 27 | DQ153104 | 27 kDa D7-related salivary protein (PpeSP10) | 0.0012 | D7 protein |
| 23 | DQ150624 | 27 kDa D7-related salivary protein (PpeSP04B) | 1.8e-16 | D7 protein |
| 23 | DQ150623 | 24.5 kDa D7-related salivary protein (PpeSP04) | 0.0069 | D7 protein |
| 14 | DQ150620 | 14.8 kDa salivary protein (PpeSP02) | 2.2e-13 | SP15 like protein |
| 14 | DQ153105 | 13 kDa salivary protein (PpeSP11) | 4.5e-15 | SP15 like protein |
Discussion
Canine antibody response against P. perniciosus saliva was studied in dogs bitten by sand flies under well-defined laboratory conditions as well as in dogs from an endemic focus of visceral leishmaniasis in Italy.
In experimentally bitten dogs we observed a significant increase in production of specific IgG, IgG1 and IgG2 in the course of 10 weeks and a positive correlation was found between the levels of specific antibodies and the number of blood-fed females P. perniciosus. Anti-saliva specific IgG and IgG2 developed with similar kinetics and correspond well with previous results [7] in dogs experimentally bitten by Lutzomyia longipalpis. While in sera of healthy dogs, IgG1 and IgG2 usually occur in comparable concentrations [20], IgG2 prevailed in sera of bitten dogs in our study as well as in dogs experimentally bitten by L. longipalpis [7], [11].
In our field trial, we detected the increase in number of anti-P. perniciosus saliva seropositive dogs as well as in the amount of specific antibodies in dog sera as the sand fly season progressed. Statistically significant increases in production of specific IgG, IgG1 and IgG2 were observed in both Leishmania-positive and Leishmania-negative dogs at the end of sand fly season. Interestingly, Leishmania-positive dogs revealed significantly lower anti-P. perniciosus saliva IgG2 compared to Leishmania-negative dogs and the IgG1/IgG2 ratio was significantly higher in Leishmania-positive dogs. These data may suggest either that dogs with low IgG2 levels were at the higher risk of becoming Leishmania-infected or that Leishmania infection decreases the production of IgG2 in bitten dogs. Considering the IFN-γ levels in canine sera, that were shown to positively correlate with the protective Th1 immune response [11], it seems that the first hypothesis is more feasible. Although, the difference in IFN- γ production between Leishmania-negative and Leishmania–positive dogs was not statistically significant.
Published data from field studies suggests that humoral immune responses against sand fly saliva vary between hosts with cutaneous and visceral forms of leishmaniases (reviewed in [9], [21]). In foci of cutaneous leishmaniases caused by L. tropica and L. braziliensis, the levels of specific anti-sand fly saliva antibodies in humans positively correlated with the risk of Leishmania transmission [22], [23]. In contrast, in foci of visceral leishmaniasis caused by L. infantum, levels of human anti-sand fly saliva antibodies positively correlated with anti-Leishmania DTH (delayed-type hypersensitivity) and thus with protection against potential infection [24], [25]. So far, those studies have been performed only in humans. In canids, several studies showed presence of anti-sand fly saliva antibodies in sera from endemic areas in Brazil [8], [26], [27], however our study is the first describing the association with canine leishmaniasis.
Canine sera recognized more than eleven P. perniciosus antigenic bands by Western blot and the most intense reaction was often observed against a 42 kDa band. Mass spectrometry identified the 42 kDa band as a single protein belonging to the Yellow protein family (DQ150622). Previously, another Yellow protein of 47.3 kDa (AF132518) was reported as the major antigen recognized by sera of dogs bitten by L. longipalpis in the field [26]. The recombinant L. longipalpis Yellow proteins (rLJM11 and rLJM17) prepared in mammalian expression system kept their antigenicity and were successfully used to screen dog sera from Brazil [27], predicting similar features for Yellow protein of P. perniciosus. All canine sera tested recognized additional three major antigens of the 38, 33 and 29 kDa; the 38 and 33 kDa proteins are apyrases and the 29 kDa antigen represents the antigen 5-related protein family. These four antigens (42, 38, 33 and 29 kDa) are promising candidates as markers of sand fly exposure.
In conclusion, we confirmed that levels of antibodies against sand fly saliva positively correlate with the number of blood-fed sand flies and therefore, monitoring canine antibody response to specific sand fly salivary proteins may evaluate the need for, and effectiveness of, anti-vector campaigns. Moreover, this is the first study demonstrating relationship between the anti-sand fly saliva antibodies and the status of L. infantum infection in dogs. The levels of anti-P. perniciosus IgG2 in dogs naturally bitten by this sand fly species negatively correlate with the anti-Leishmania seropositivity. Thus, for dogs living in endemic area specific IgG2 response against saliva of the vector is suggested as a risk marker of L. infantum transmission.
Supporting Information
IFN-γ in the sera of Leishmania infantum -seropositive and -seronegative dogs naturally bitten by Phlebotomus perniciosus during the sand fly season. Concentrations of IFN-γ were measured by ELISA using the Quantikine canine IFN-γ immunoassay (R&D Systems) following the manufacturer's guidelines. Serum samples, standards and controls were added without any dilutions. Absorbance was measured at 450 nm using a Tecan Infinite M200 microplate reader (Schoeller). Data were transformed and assessed as described in manufacturer's instructions (R&D Systems).
(TIF)
Acknowledgments
We are grateful to Dr. Helena Kulikova and Stepanka Hlavova for excellent technical and administrative assistance. We thank Dr. Ryan C. Jochim for critical reading of the manuscript.
Footnotes
The authors have declared that no competing interests exist.
This project was funded by EU grant 2011-261504 EDENEXT and the paper is catalogued by the EDENEXT Steering Committee as EDENEXT001. The research was supported by Ministry of Education of the Czech Republic (MSM 0021620828 and LC 06009), by Czech Science Foundation (206/09/0777; 206/09/H026; 206/09/0822) and by Charles University (GAUK – 13009/2009). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gramiccia M, Gradoni L. The current status of zoonotic leishmaniases and approaches to disease control. Int J Parasitol. 2005;35:1169–1180. doi: 10.1016/j.ijpara.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Miró G, Cardoso L, Pennisi MG, Oliva G, Baneth G. Canine leishmaniosis – new concepts and insights on an expanding zoonosis: part two. Trends Parasitol. 2008;24:371–377. doi: 10.1016/j.pt.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Molina R, Amela C, Nieto J, Sanandres M, Gonzalez F, et al. Infectivity of dogs naturally infected with Leishmania infantum to colonized Phlebotomus perniciosus. Trans R Soc Trop Med Hyg. 1994;88:491–493. doi: 10.1016/0035-9203(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 4.Otranto D, Paradies P, de Caprariis D, Stanneck D, Testini G, et al. Toward diagnosing Leishmania infantum infection in asymptomatic dogs in an area where leishmaniasis is endemic. Clin Vaccine Immunol. 2009;16:337–343. doi: 10.1128/CVI.00268-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maroli M, Bigliocchi F, Khoury C. Sandflies in Italy: observations on their distribution and methods for control. Parassitologia. 1994;36:251–264. [PubMed] [Google Scholar]
- 6.Kilick-Kendrick R. The biology and control of Phlebotomine sand flies. Clin Dermatol. 1999;17:279–289. doi: 10.1016/s0738-081x(99)00046-2. [DOI] [PubMed] [Google Scholar]
- 7.Hostomska J, Rohousova I, Volfova V, Stanneck D, Mencke N, et al. Kinetics of canine antibody response to saliva of the sand fly Lutzomyia longipalpis. Vector Borne Zoonotic Dis. 2008;8:443–450. doi: 10.1089/vbz.2007.0214. [DOI] [PubMed] [Google Scholar]
- 8.Gomes RB, Mendonça IL, Silva VC, Ruas J, Silva MB, et al. Antibodies against Lutzomyia longipalpis saliva in the fox Cerdocyon thous and the sylvatic cycle of Leishmania chagasi. Trans R Soc Trop Med Hyg. 2007;101:127–133. doi: 10.1016/j.trstmh.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira F, Jochim RC, Valenzuela JG, Kamhawi S. Sand flies, Leishmania, and transcriptome-borne solutions. Parasitol Int. 2009;58:1–5. doi: 10.1016/j.parint.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baneth G, Koutinas AF, Solano-Gallego L, Bourdeau P, Ferrer L. Canine leishmaniosis-new concepts and insights on an expanding zoonosis:part one. Trends Parasitol. 2008;24:324–330. doi: 10.1016/j.pt.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Collin N, Gomes R, Teixeira C, Cheng L, Laughinghouse A, et al. Sand fly salivary proteins induce strong cellular immunity in a natural reservoir of visceral leishmaniasis with adverse consequences for Leishmania. PLoS Pathog. 2009;5:e1000441. doi: 10.1371/journal.ppat.1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volf P, Volfova V. Establishment and maintenance of sand fly colonies. J Vector Ecol. 2011;36:S1–S9. doi: 10.1111/j.1948-7134.2011.00106.x. [DOI] [PubMed] [Google Scholar]
- 13.Mencke N, Volf P, Volfova V, Stanneck D. Repellent efficacy of a combination containing imidacloprid and permethrin against sand flies (Phlebotomus papatasi) on dogs. Parasitol Res. 2003;90:S108–S111. doi: 10.1007/s00436-003-0905-7. [DOI] [PubMed] [Google Scholar]
- 14.Tarallo VD, Dantas-Torres F, Lia RP, Otranto D. Phlebotomine sand fly population dynamics in a leishmaniasis endemic peri-urban area in southern Italy. Acta Trop. 2010;116:227–234. doi: 10.1016/j.actatropica.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Otranto D, de Caprariis D, Lia RP, Tarallo V, Lorusso V, et al. Prevention of endemic canine vector-borne diseases using imidacloprid 10% and permethrin 50% in young dogs: a longitudinal field study. Vet Parasitol. 2010;172:323–332. doi: 10.1016/j.vetpar.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Otranto D, Paradies P, de Caprariis D, Stanneck D, Testini G, et al. Toward diagnosing Leishmania infantum infection in asymptomatic dogs in an area where leishmaniasis is endemic. Clin Vaccine Immunol. 2009;16:337–343. doi: 10.1128/CVI.00268-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson JM, Oliveira F, Kamhawi S, Mans BJ, Reynoso D, et al. Comparative salivary gland transcriptomics of sandfly vectors of visceral leishmaniasis. BMC Genomics. 2006;7:a.n. 52. doi: 10.1186/1471-2164-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerstman BB. Epidemiology kept simple: an introduction to traditional and modern epidemiology. Willey-Liss, Hoboken, New Yersey; 2003. 417 [Google Scholar]
- 19.Katz J, Baptista J, Azen SP, Pike MC. Obtaining confidence intervals for the risk ratio in cohort studies. Biometrics. 1978;34:469–474. [Google Scholar]
- 20.Mazza G, Whiting AH, Day MJ, Duffus WPH. Development of an enzyme-linked immunosorbent assay for the detection of IgG subclasses in the serum of normal and diseased dogs. Res Vet Sci. 1994;57:133–139. doi: 10.1016/0034-5288(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 21.Rohousova I, Volf P. Sand fly saliva: effects on host immune response and Leishmania transmission. Folia Parasitol. 2006;53:161–171. [PubMed] [Google Scholar]
- 22.Rohousova I, Ozensoy S, Ozbel Y, Volf P. Detection of species-specific antibody response of humans and mice bitten by sand flies. Parasitology. 2005;130:493–499. doi: 10.1017/s003118200400681x. [DOI] [PubMed] [Google Scholar]
- 23.de Moura TR, Oliveira F, Novais FO, Miranda JC, Clarencio J, et al. Enhanced Leishmania braziliensis Infection Following Pre-Exposure to Sandfly Saliva. PloS Negl Trop Dis. 2007;1:e84. doi: 10.1371/journal.pntd.0000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barral A, Honda E, Caldas A, Costa J, Vinhas V, et al. Human immune response to sand fly salivary gland antigens: a useful epidemiological marker? Am J Trop Med Hyg. 2000;62:740–745. doi: 10.4269/ajtmh.2000.62.740. [DOI] [PubMed] [Google Scholar]
- 25.Gomes RB, Brodskyn C, de Oliveira CI, Costa J, Miranda JC, et al. Seroconversion against Lutzomyia longipalpis saliva concurrent with the development of anti-Leishmania chagasi delayed-type hypersensitivity. J Infect Dis. 2002;186:1530–1534. doi: 10.1086/344733. [DOI] [PubMed] [Google Scholar]
- 26.Bahia D, Gontijo NF, Leon IR, Perales J, Pereira MH, et al. Antibodies from dogs with canine visceral leishmaniosis recognise two proteins from the saliva of Lutzomyia longipalpis. Parasitol Res. 2007;100:449–454. doi: 10.1007/s00436-006-0307-8. [DOI] [PubMed] [Google Scholar]
- 27.Teixeira C, Gomes R, Collin N, Reynoso D, Jochim R, et al. Discovery of markers of exposure specific to bites of Lutzomyia longipalpis, the vector of Leishmania infantum chagasi in Latin America. PLoS Negl Trop Dis. 2010;4:e638. doi: 10.1371/journal.pntd.0000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IFN-γ in the sera of Leishmania infantum -seropositive and -seronegative dogs naturally bitten by Phlebotomus perniciosus during the sand fly season. Concentrations of IFN-γ were measured by ELISA using the Quantikine canine IFN-γ immunoassay (R&D Systems) following the manufacturer's guidelines. Serum samples, standards and controls were added without any dilutions. Absorbance was measured at 450 nm using a Tecan Infinite M200 microplate reader (Schoeller). Data were transformed and assessed as described in manufacturer's instructions (R&D Systems).
(TIF)