Abstract
The Per2 clock gene modulates ethanol consumption, such that mutant mice not expressing functional mPer2 have altered circadian behavior that promotes higher ethanol intake and preference. Experiments were undertaken to characterize circadian-related behavioral effects of mPer2 deletion on ethanol intake and to explore how acamprosate (used to reduce alcohol dependence) alters diurnal patterns of ethanol intake. Male mPer2 mutant and WT (wild-type) mice were entrained to a 12L:12D photocycle and their locomotor and drinking activities were recorded. Circadian locomotor measurements confirmed that mPer2 mutants had an advanced onset of nocturnal activity of about 2 h relative to WTs, and an increased duration of nocturnal activity (p<0.01). Also, mPer2 mutants preferred and consumed more ethanol and had more daily ethanol drinking episodes vs. WTs. Measurements of systemic ethanol using subcutaneous microdialysis confirmed the advanced rise in ethanol intake in the mPer2 mutants, with 24 hr averages being ~60 mM vs. ~25 mM for WTs (p<0.01). A six-day regimen of single i.p. acamprosate injections (300 mg/kg) did not alter the earlier onset of nocturnal ethanol drinking in the mPer2 mutants, but reduced the overall amplitude of drinking and preference (both, p<0.01). Acamprosate also reduced these parameters in WTs. These results suggest that elevated ethanol intake in mPer2 mutants may be a partial consequence of an earlier nighttime activity onset and increase in nocturnal drinking activity. The suppressive action of acamprosate on ethanol intake is not due to an altered diurnal pattern of drinking, but rather, a decrease in the number of daily drinking bouts and drinking per bout.
Keywords: Circadian, Ethanol, Acamprosate, Microdialysis, mPer2 Mutation
INTRODUCTION
There is increasing evidence that the severity of alcohol dependence and craving are modulated by environmental influences acting on the circadian timing system. In humans and in rodent models of alcohol abuse, persistent disruption to the circadian environment, including rotating shift work and housing under constant photic conditions, can affect alcohol intake (Trinkoff and Storr 1998; Gauvin et al. 1997; Clark et al. 2007; Hammer et al. 2010; Rosenwasser et al., 2010; Brager and Glass unpublished results). Genetic factors associated with the circadian system are also linked to alcoholism. In particular, mutations of circadian clock genes, including Per2 and CLOCK, which stabilize endogenous circadian pacemaking, are associated with increased alcohol intake (Spanagel et al. 2005; Ozburn et al. 2010; Sjöholm et al. 2010). In humans, single nucleotide polymorphisms of the Per2 gene are linked to alcoholism that is comorbid with sleep and circadian disturbances (Comasco et al. 2010; Spanagel et al. 2005). Also, mice with mutation of mPer2 have greatly potentiated ethanol intake and preference (Spanagel et al. 2005), and have an earlier onset in nocturnal wheel running activity relative to WTs (Pendergast et al., 2010). Neurochemically, this potentiation in ethanol intake is thought to arise from elevated levels of extracellular glutamate in brain areas controlling alcohol reward and craving, which is considered a neurobiological hallmark of alcohol dependence (Dodd et al. 2000; Gass and Olive-Foster, 2007; Krystal et al. 2003; Spanagel et al. 2005). In line with the glutamatergic hypothesis of alcoholism, acamprosate, a glutamate antagonist and alcohol anti-relapse drug, reduces raised extracellular glutamate levels of mPer2 mutant mice concomitantly with reducing elevated ethanol intake and preference (Spanagel et al. 2005).
Increases in ethanol intake precipitated by environmental and other disruptions like those discussed above can also profoundly and directly impact the circadian timing system. For example, acute and chronic ethanol administration and withdrawal attenuate light-induced phase shifts of behavioral circadian rhythms, impair long-term photic entrainment, and suppress nighttime activity (Brager et al. 2010; Brager et al. 2011; Seggio et al. 2007 2009; Ruby et al. 2009a, b). Ethanol also has been shown to act on extrasynaptic GABA receptors within the circadian clock of the hypothalamic suprachiasmatic nucleus (SCN) and to disrupt glutamatergic (photic) regulation of pacemaker activity (Prosser et al. 2008; McElroy et al. 2009). In addition to affecting photic entrainment processes, ethanol also significantly impairs non-photic circadian phase regulation mediated by serotonergic signaling (Brager et al. 2011; Ruby et al. 2009b).
The mPer2 mutant mouse presents a useful tool for exploring circadian-based mechanisms contributing to the alcoholic phenotype, and also for undertaking studies on drugs like acamprosate used to treat alcoholism. The latter is a critical issue, because although acamprosate is one of the few drugs available for preventing alcohol relapse, its efficacy is limited by several factors, including genetic influences, tolerance, and reduced transport across the gastrointestinal tract and blood-brain barrier (Spanagel and Kiefer 2008; Della Corte et al. 2002). The present study was therefore undertaken in mPer2 mutant mice to: 1) determine the extent to which altered circadian behavior temporally affects ethanol intake; and 2) assess the actions of acamprosate on several interrelated parameters, including ethanol preference, diurnal ethanol drinking patterns, and associated ethanol pharmacokinetics. These results would extend previous work on the combined actions of the circadian system and acamprosate in modulating alcohol intake.
MATERIALS AND METHODS
Animals
Adult, 8 week-old homozygous mPer2 mutant male mice (strain:B6.129S7-Per2tm1Brd/J) and wild-type (WT) controls (strain: B6(Cg)-Tyrc-2J/J) were produced from homozygous mPer2 mutant and wild-type breeding pairs obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Both strains are back-crossed to C57BL/6. Animals were singly housed in polycarbonate cages under a 12:12 light:dark photoperiod (LD) at a light intensity of 270 lux in a temperature-controlled vivarium (23°C) with food (Prolab 3000, PMI Feeds, St. Louis, MO, USA) and water provided ad libitum. The experiments followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and international ethical standards for animal biological rhythm research outlined in Portaluppi (2010), and were approved by the Kent State Institutional Animal Care and Use Committee.
Circadian activity measurements
General circadian locomotor activity was measured using overhead passive infrared motion detectors interfaced with a computerized data acquisition system (Clocklab, Coulbourn Instruments, Whitehall, PA, USA). Data were collected in 1 min bins, and activity onset associated with lights-off (designated as zeitgeber time [ZT] 12) was defined by the initial 6 min period that: 1) coincided with an intensity of activity that exceeded 10% of the maximum rate for the day; 2) preceded by at least 4 hr of inactivity; and 3) followed by at least 60 min of sustained activity. Activity offset was defined as a final period of activity that: 1) was immediately preceded by at least 60 min of activity and; 2) was followed by at least 4 hr of inactivity. Nocturnal activity duration (alpha) represented the time difference between activity onset and offset. The initial nocturnal activity period represented the total duration of activity for up to five hours after activity onset. Diurnal ethanol drinking patterns were measured using a drinkometer consisting of a recessed sipper tube with a light beam sensor system triggered by head insertion during a drinking episode (Coulbourn Instruments). Drinking bouts were measured across the light and dark phases of LD, with each bout representing the sum of the drinking events collected in each 1 min bin from the Clocklab data acquisition system.
Microdialysis and Ethanol Measurement
The microdialysis procedures used here were similar to those described in our previous studies on systemic ethanol measurement (Brager et al. 2010). Two days prior to experimentation, the animals were anesthetized with pentobarbital (Nembutal: 35.0 mg/kg) and a microdialysis probe (CMA Microdialysis, North Chelmsford, MA, USA) was inserted subcutaneously in the subscapular region. The implant was secured with a suture. After the 2-day recovery period, probes of freely-behaving mice were connected to a calibrated syringe pump (CMA/100; Bioanalytical Systems Inc. West Lafayette, IN, USA), and continuously perfused over 24 hr with filtered artificial cerebrospinal fluid (ACSF) at a flow rate of 1.0 μl/min and sampling interval of 30 min. Probe efficiency for ethanol was assessed in dialysate samples collected from probes (n=4) submerged in a known ethanol standard (50 mM) at a perfusion flow rate of 1 μl/min. Sampling was undertaken at 35°C, 37°C and 39°C to determine if fluctuations in body temperature within this range would significantly affect probe efficiency. At each temperature, probe efficiency was 40% (p<0.05), which was used to estimate systemic ethanol concentrations. Ethanol in microdialysate was measured with an Analox AM-1 Alcohol Analyzer (Lunenburg, MA, USA) in a sample volume of 5 μl.
Experimental Protocols
Experiment 1. Circadian drinking profiles of mPer2 mutant and wild-type mice
All mice received free-choice of a 15% (v/v) ethanol solution (Sigma, St. Louis, MO, USA) or water throughout this experiment. Behavioral and drinking rhythms of individually-caged mPer2 mutant and WT mice (n=7, for each) that had previously entrained to LD were monitored for 4 wks and 1 wk, respectively. Bottle positions were alternated daily during the 3 wks of behavioral monitoring, but not during the 1 wk of concomitant behavioral and drinking monitoring due to drinkometer constraints. The length of alpha, the circadian time of activity onset, the number of normalized daily ethanol drinking bouts, and ethanol intake and preference (daily ethanol/total fluid intake measured to the nearest 0.25 ml 2 h before lights-off from plastic 50 ml graduated tubes [Fisher Scientific, Pittsburgh, PA, USA]) were analyzed.
Experiment 2. Effects of mPer2 mutation and acamprosate on ethanol intake and pharmacokinetics
Systemic ethanol pharmacokinetics under free-choice of a 15% ethanol solution or water were characterized in mPer2 mutant and WT mice (n=4, for both groups) using subcutaneous in vivo microdialysis. Pharmacokinetic measurements included peak ethanol concentration and circadian time of peak ethanol concentration. Samples were collected every 30 min and stored at −20°C for analysis. Under the same experimental conditions, separate groups of mPer2 mutant and WT mice received a daily, i.p. injection of acamprosate (300 mg/kg; Merck, Lyon, France) in physiological saline or saline alone (volume: 0.5 ml) delivered at ZT 10 over a six day period. The daily timing of drug administration was based on the averaged onset of ethanol drinking in the mPer2 mutants. On the last day of acamprosate treatment, 24 hr systemic microdialysis sampling was undertaken to assess the effects of the acamprosate on the ethanol pharmacokinetic profile established in the prior trial. Additional separate groups of mPer2 mutant and WT mice received a daily, i.p. injection of acamprosate (300 mg/kg) in physiological saline or saline alone delivered at ZT 6 over a six day period (n=4, for both groups) to assess the potential of acamprosate to act as a non-photic phase-advancing stimulus of behavioral circadian rhythms.
RESULTS
Experiment 1. Circadian drinking profiles of mPer2 mutant and wild-type mice
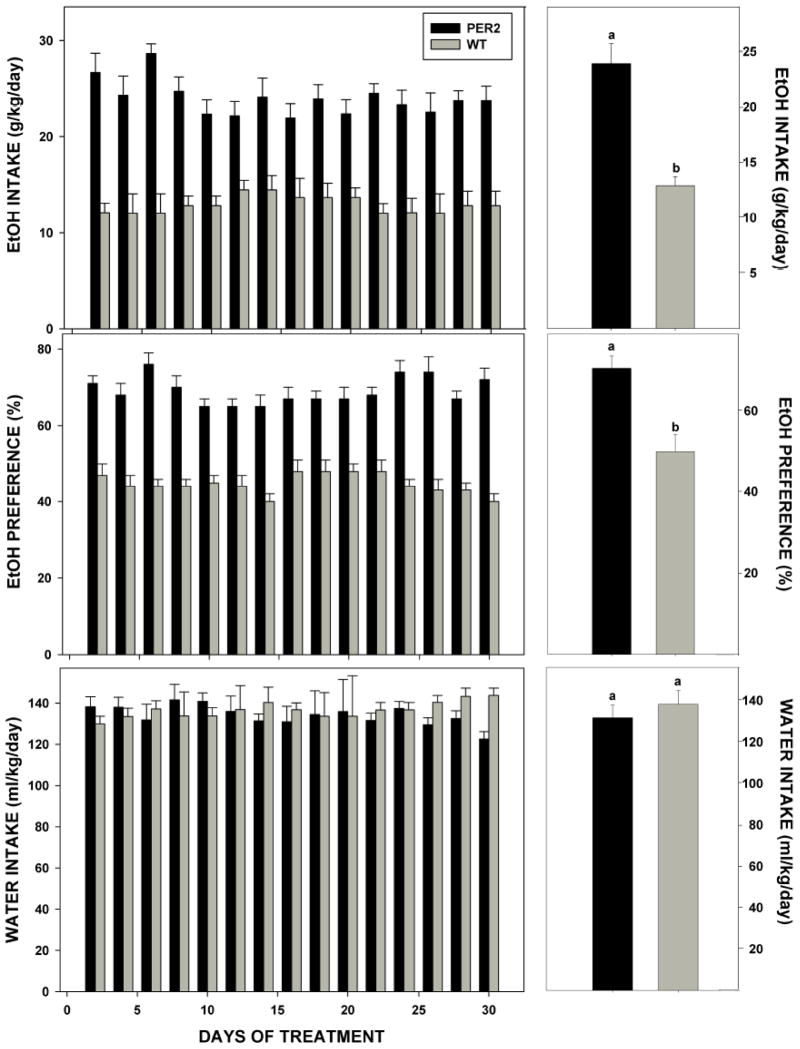
Under LD, the nocturnal activity period began 2.2±0.2 h earlier in mPer2 mutants compared to WTs (ZT 10.0±0.4 vs. ZT 12.2±0.2, respectively; F1,10=34.5; p<0.01). Nocturnal activity duration of the mPer2 mutants was extended by a similar amount (2.0±0.2 h; F1,10=17.1; p<0.01). Values for total daily ethanol intake, ethanol preference, and total fluid intake were greater for the mPer2 mutants vs. WTs (Intake: 23.9±1.5 g/kg/day vs. 12.9±1.4 g/kg/day, respectively; F1,10=104.2; p<0.01; Preference: 70.4±3.1% vs. 44.7±2.4%, respectively; F1,10=92.9; p<0.01; Total Fluid: 309.6±11.7 ml/kg/day vs. 242.6±12.0 ml/kg/day, respectively; F1,10=13.6; p<0.01). However, daily pure water intake did not differ between the two groups (mPer2 mutants: 131.6±6.1 ml/kg/day; WTs: 138.2±6.6 ml/kg/day; F1,10=0.4; p>0.05). The drinkometer measurements revealed that mPer2 mutants had more ethanol drinking bouts across the entire 24 hr circadian day, including the dark-phase vs. WTs (24 hr: 14±2 bouts vs. 8±1, bouts respectively; F1,10=108.0; p<0.01; Dark-phase: 11±2 bouts vs. 6±1 bouts, respectively; F1,10=74.0; p<0.01). These results are summarized in Figs. 1 & 2.
FIGURE 1.
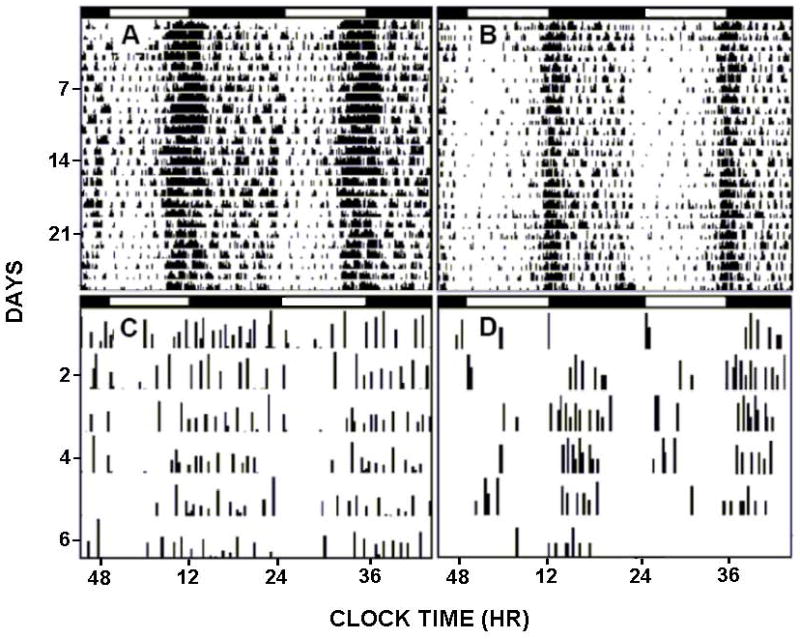
Representative double-plotted actograms of general circadian locomotor activity (top) and ethanol drinking (bottom) of individual mPer2 mutant (A,C) and wild-type (WT) (B,D) mice. Horizontal filled and empty bars represent the dark and light phases of the 24 hr LD cycle, respectively.
FIGURE 2.
Left panels: Ethanol (EtOH) intake, ethanol preference, and water intake in mPer2 mutant (PER2) and wild-type (WT) mice provided free-choice ethanol/water plotted every other day for 30 days (n=7/group). Right panels: Same respective values averaged over the 30 day treatment period. Bars represent means+SE. Bars with different letters are significantly different, p<0.05.
Experiment 2. Effects of mPer2 mutation and acamprosate on ethanol intake and pharmacokinetics
Baseline values for daily ethanol intake and preference, pure water intake, and total fluid intake in the mPer2 mutants and WTs were comparable to those in Experiment 1 (Ethanol Intake: 24.9±1.8 g/kg vs. 12.5±1.8 g/kg respectively; F1,14=119.3; p<0.01; Fig. 3; Preference: 68.5±2.0% vs. 45.5±1.0%, respectively; F1,14=136.2; p<0.01; Fig. 3; Water Intake: 128.0±7.9 g/kg vs. 132.4±8.4 g/kg, respectively; F1,14=0.5; p>0.05; Total Fluid: 305.4±11.0 g/kg vs. 235.8±10.8 g/kg respectively; F1,14=22.8; p<0.01). Following the regimen of daily i.p acamprosate injections, ethanol intake and preference were reduced in mPer2 mutants by 73.9±4.1% and 65.0±2.1% and in WTs by 77.4±1.9% and 72.1±1.3%, respectively (all, p<0.01). Higher values for ethanol intake and preference were maintained in the mPer2 mutants over WTs at the end of the acamprosate treatment regimen (Intake: F1,6=16.8; Preference: F1,6=51.4; both, p<0.01). In the saline injection controls the values for ethanol intake and preference were unchanged from baseline levels in both genotypes (mPer2 mutant; Intake: 27.9±2.0 g/kg; Preference: 72.3±1.2%; WT; Intake: 12.0±1.8 g/kg; Preference: 44.0±1.0%; all, p>0.05; Fig. 3). Higher values for ethanol intake and preference were also maintained in the mPer2 mutants over WTs at the end of the saline treatment regimen (intake: F1,6=462.3; preference; F1,6=152.9; both p<0.01).
FIGURE 3.

Ethanol (EtOH) intake (left) and preference (right) in mPer2 mutant (PER2) and wild-type (WT) mice during the daily regimen of i.p. acamprosate (ACAMP; 300 mg/kg/day) or saline injection (n=4/group). Bars represent means±SE. For each parameter, bars with different letters are significantly different (p<0.05). Asterisks denote strain difference (p<0.05).
Acamprosate reduces systemic ethanol levels and the number of ethanol drinking bouts across circadian phases
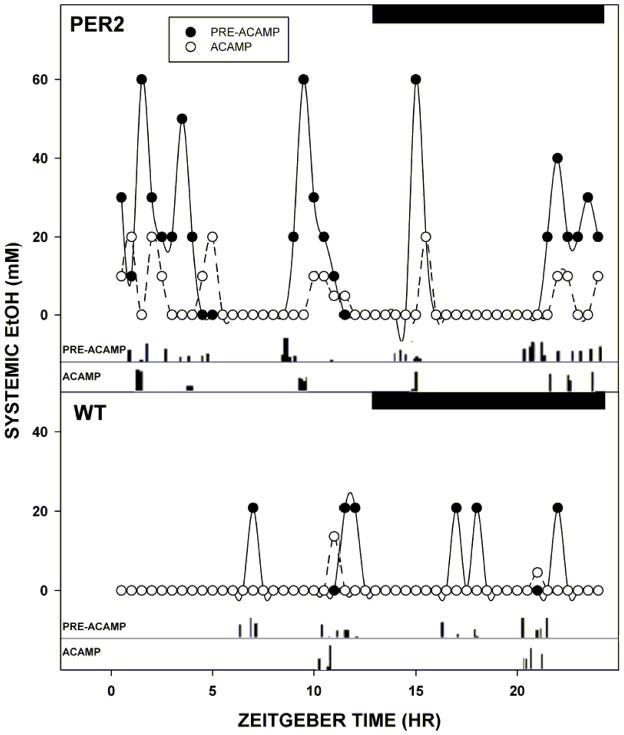
The baseline frequency of daily total ethanol drinking bouts in mPer2 mutants averaged twice that of WTs (16±2 bouts vs. 8±1 bouts, respectively; F1,10=43.9; p<0.01). Subcutaneous ethanol concentrations following drinking bouts were also significantly greater in mPer2 mutants vs. WTs (~60 mM vs. ~25 mM, respectively; F1,10=38.2; p<0.01; Fig. 4). A drinking bout preceded the appearance of systemic ethanol by 20–40 min. In both genotypes, daily acamprosate injections reduced the frequency of daily ethanol drinking bouts and subsequent systemic ethanol peaks (mPer2 mutants: 9±1 bouts and ~20 mM, respectively; WTs: 4±2 bouts and ~15 mM, respectively; all, p<0.05). Higher values for daily ethanol drinking bouts and subcutaneous ethanol peaks were maintained in the mPer2 mutants over WTs at the end of the acamprosate treatment regimen (Bouts: F1,10=6.5; Ethanol: F1,10=23.2; both, p<0.01). In mPer2 mutants, acamprosate reduced the frequency of daily ethanol drinking bouts and subsequent systemic ethanol peaks across the nocturnal activity period (Pre-acamprosate: 13±2 bouts and ~60 mM, respectively; Post-acamprosate: 7±1 bouts and ~20 mM, respectively; both, p<0.01; Fig 4). In WTs, acamprosate also reduced systemic ethanol peaks across their nocturnal activity period (Pre-acamprosate: ~25 mM; Post-acamprosate: ~15 mM; p<0.01), and completely suppressed ethanol drinking and subsequent systemic ethanol peaks across the daytime (Pre-acamprosate: 2±1 bouts and ~10 mM, respectively; Post-acamprosate: 0±0 bouts and ~0 mM, respectively; both, p<0.05; Fig. 4). In the saline controls, the frequency of daily ethanol drinking bouts and subcutaneous ethanol concentrations in mPer2 mutants and WTs did not differ from baseline measures (all, p>0.05). Higher values for daily total ethanol drinking bouts and subcutaneous ethanol concentrations were maintained in the mPer2 mutants over WTs at the end of the saline treatment regimen (Bouts: F1,10=31.5; Ethanol: F1,10=46.5; both, p<0.01).
FIGURE 4.
Twenty-four hour pharmacokinetic profiles of systemic ethanol (EtOH) levels measured using subcutaneous microdialysis in an individual mPer2 mutant (PER2) and a wild-type (WT) mouse superimposed with their ethanol drinking rhythms under free-choice ethanol/water conditions before (PRE-ACAMP) and during (ACAMP) the daily regimen of i.p. acamprosate injection (300 mg/kg/day). Black bars represent the dark-phase of the 24 hr LD cycle, with lights-off at zeitgeber time 12.
General Circadian Activity
Similar to the experiment above, the nocturnal activity period began 2.2±0.2 h earlier in mPer2 mutants compared to WTs F(1,12)=34.8; p<0.01). In both genotypes, daily acamprosate or saline treatments did not alter the circadian time of nocturnal activity onset (p>0.05) or alter the duration of initial nocturnal activity (Baseline: 4.2 ± 1.2 h [mPer2 mutant] and 3.0±0.8 h [WT]; Treatment: 4.3 ± 1.6 h [mPer2 mutant] and 4.1 ± 1.0 h [WT]; p>0.05; Fig. 5). Also, in both genotypes, a daily regimen of systemic acamprosate or saline at ZT 6 did not phase-advance behavioral circadian rhythms (F(1,12)=0.3; p>0.05).
FIGURE 5.
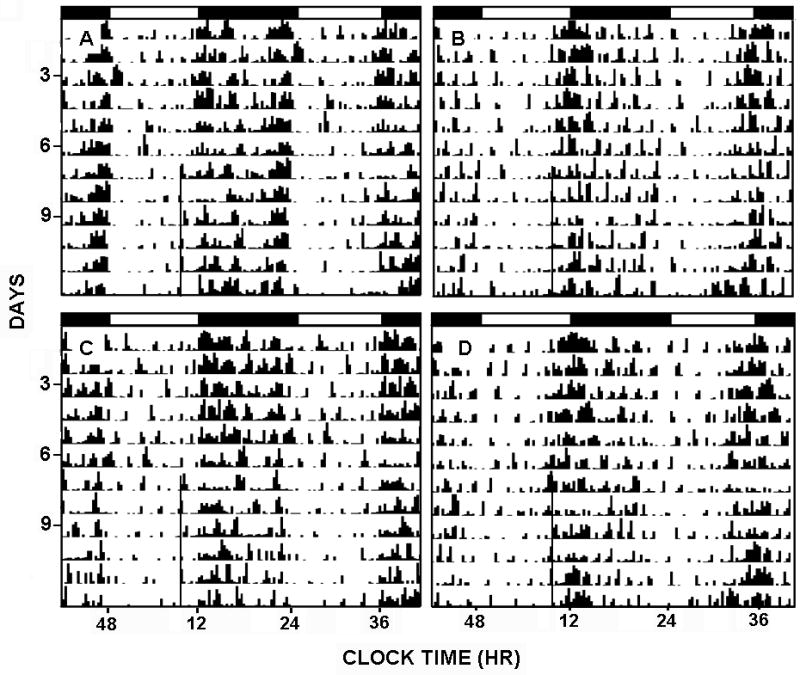
Acamprosate treatment towards the end of the light-phase of a 24 hr LD cycle [ZT 10] does not affect circadian clock phase. Shown are representative, double-plotted actograms of general circadian locomotor activity prior to and during (black line) a daily regimen of i.p acamprosate treatment (300 mg/kg; A, acamprosate in WT; B, acamprosate in Per2 mutant; C, saline in WT; D, saline in Per2 mutant). Black and white bars represent the 12 hrs of lights-off and lights-on periods, respectively.
DISCUSSION
There is growing evidence in humans and in rodent models of alcoholism that the circadian clock gene, mPer2, has substantial modulatory influence over ethanol intake and preference (Spanagel et al. 2005; Comasco et al. 2010; reviewed in Rosenwasser, 2010). For this reason, the mPer2 mutant mouse presents an important animal model for studying factors contributing to alcohol dependence, as well as the effects of disrupted circadian behavioral rhythms on ethanol intake (reviewed in Rosenwasser, 2010). This highly alcohol-preferring mouse also offers a propitious model for researching pharmacologic (acamprosate) interventions for the treatment of alcoholism. Results from these experiments confirmed that mPer2 mutants have potentiated ethanol intake relative to WTs, which was associated with increased numbers of drinking bouts over the 24 h day, and notably with a ~2 h advance in activity onset associated with the mPer2 phenotype. It was also found that a daily regimen of i.p. acamprosate injections substantially suppressed ethanol intake by reducing the number of daily ethanol drinking bouts in mPer2 mutant and WT mice without altering the diurnal pattern of ethanol drinking.
Circadian drinking profiles of mPer2 mutant and wild-type mice
Studies focusing on circadian-related influences on alcohol dependence have revealed that circadian gene mutations profoundly affect ethanol intake. For example, in mice, mutation of the circadian gene CLOCK enhances ethanol intake and preference (Ozburn et al. 2010). Likewise, mutation of the mPer2 gene causes marked increases in these parameters, which are linked to alterations in glutamatergic transmission (Spanagel et al. 2005). In drosophila, mutations for Per and Tim each reduce tolerance to repeated application of ethanol (Pohl et al. 2010). Notably from a clinical perspective, single-nucleotide polymorphisms of Per2 and CLOCK in humans are associated with increased alcohol consumption (Spanagel et al. 2005; Sjöholm et al. 2010). Conversely, manipulating the circadian clock by bright light therapy (which aids in the re-alignment of endogenous circadian pacemaker activity) has positive, therapeutic effects on relapse risk (Landolt et al. 2001; Schmitz et al. 1997). From these studies, it is apparent that a normally functioning circadian timing system limits alcohol use and dependence, and that perturbations of this system can promote alcohol dependence. The present demonstrations of potentiated ethanol intake and preference in mPer2 mutant mice that led to greater total fluid intake in the absence of a compensatory increase in pure water intake, lend credence to this idea, and support the findings of Spanagel (2005), who initially reported increased levels of ethanol drinking (and craving) in mPer2 mutants vs. WT controls. The present circadian behavioral analyses also extend these findings through the demonstration that the enhanced ethanol intake in mPer2 mutant mice is associated, in part, with disturbed circadian behavior: namely a ~2 h advance in the onset of nocturnal locomotor activity (positive phase-angle of circadian entrainment) and a corresponding increase in the duration of the activity/wakefulness period (alpha). These data suggest that the advanced phase of behavioral activity in mPer2 mutant mice is associated with an elongated phase of nocturnal ethanol intake not exhibited by WTs, thus possibly contributing to increased daily ethanol intake. There also was an increased number of drinking bouts during the dark-phase in the mPer2 mutants, which is the predominate circadian phase of ethanol intake in nocturnal rodents (Brager et al. 2010; Ruby et al. 2009b; Dole and Gentry 1984; reviewed in Hiller-Sturmhöfel and Kulkosky 2001). In addition, the mPer2 mutants exhibited more drinking episodes throughout the light phase compared to WTs, whose ethanol drinking mostly occurred near light-dark transitions. One limitation of this study, however, is that it does not differentiate between entrained (diurnal) vs. free-running (circadian) patterns of ethanol intake. Previous assessments of circadian patterns of ethanol intake under constant darkness in SP (spontaneously hypertensive) rats have observed that circadian patterns of ethanol intake mirror behavioral rhythms (Pasley et al. 1987). To date, assessments of circadian patterns of ethanol intake have not been undertaken in mPer2 mutant mice, which have a shorter endogenous period of wheel-running activity compared to wild-type controls (Pendergast et al. 2010). Hence, it will be imperative in future experiments to determine the extent to which elevated ethanol intake in mPer2 mutant mice is related to strain-dependent relationships in circadian behavioral and ethanol drinking rhythms and also to understand possible downstream (pleiotropic) effects of the mPer2 gene mutation on ethanol intake.
Effects of mPer2 mutation and acamprosate on ethanol pharmacokinetics
This study represents the first characterization of the 24 h profile of systemic ethanol assessed using subcutaneous microdialysis concomitantly with 24 h drinkometer measurements in mPer2 mutant mice. Subcutaneous, rather than blood sampling of ethanol was used as it is less invasive, and follows a similar time-course (Engleman et al. 2008). Several points are evident from this experiment. First, ethanol drinking bouts preceded systemic ethanol peaks by ~30min. This relationship is similar to that reported previously in mice and hamsters (Brager et al. 2010; Ruby et al. 2009b; Dole and Gentry, 1984). Second, the effect of multiple closely-spaced drinking bouts is cumulative, resulting in prolonged elevations of peripheral levels of ethanol such as that found near the dark-light transition of LD. Third, the profiles of drinking bouts and systemic ethanol peaks differ greatly between mPer2 mutants and WTs, with the mutants exhibiting a 2-fold greater frequency of drinking and markedly higher systemic peak levels of ethanol. Both parameters are reflective of the mPer2 mutants’ avidity for ethanol.
It is important to note the relatively low levels of free-choice ethanol intake in the mPer2 mutant study of Spanagel (2005) vs. those reported in the present and other studies. In that study, WT mice on 12% ethanol consumed 4 g/kg/day. This is considerably lower than that observed by Logan (2011; personal communication), where mice of a similar strain on 10% ethanol consumed 8 g/kg/day. Their mice were backcrossed to a C57BL/6 line, which when on 10% ethanol, consume 11 g/kg/day (Belknap et al. 1993). These values are comparable to ours, where WT mice on 15% EtOH consumed 13 g/kg/day. The reason for these disparities in ethanol intake is unknown; however, it is unlikely that the 2–3% difference in ethanol concentration between studies is the causative factor. Rather, it may be due to differences in the genetic backgrounds of the mPer2 mutant and wild-type mice, which were obtained from intercrosses between heterozygous Per2Brdm1 mice on a 129SvEvBrd/C57BL/6-Tyrc-Brd background, or the drinking procedure, which included a regimen of ethanol administration involving a ramping up of ethanol concentrations over several days that were not used in the other studies. Daily ethanol intake of the mPer2 mutants in the Spanagel (2005) study was also relatively low (7.5 g/kg/day @ 12%) compared to the studies of Logan (15 g/kg/day @ 10%) and ourselves (24 g/kg/day @ 15% using the same mutation).
The six-day daily acamprosate treatment regimen significantly reduced overall daily ethanol intake in mPer2 mutants and WTs, with saline treatment having no effect. Similar to previous findings, acamprosate treatment reduced mPer2 mutant ethanol intake to that of untreated WT mice. Acamprosate also reduces enhanced ethanol intake in C57BL mice subjected to the drinking-in-the-dark protocol (Gupta et al. 2008). Our combined drinkometer and microdialysis assessments revealed that acamprosate reduced daily total drinking bouts and systemic ethanol peaks without altering the general diurnal pattern of ethanol drinking. The lack of an effect of systemic acamprosate on the circadian phase of ethanol intake was expected given that acamprosate, when administered at midday, does not alter circadian clock phase. In agreement with other studies (see Spanagel et al. 2005), we also found that systemic acamprosate treatment suppressed ethanol intake and preference in mPer2 mutants, although higher values of ethanol intake and preference were evident in the mPer2 mutants vs. WTs at the end of the regimen of acamprosate treatment. Possibly this is related, in part, to the advanced activity onset that was unaffected by acamprosate. Here, we additionally found that systemic acamprosate treatment (300 mg/kg) significantly suppressed ethanol intake and preference in WTs, despite the observation that systemic acamprosate (200 mg/kg) had no effect on WT intake and preference levels in Spanagel et al. 2005. A study in C57BL/6J mice with elevated ethanol intake induced by a drinking-in-the-dark protocol showed that systemic acamprosate treatment at 200 mg/kg suppressed ethanol intake (Gupta et al. 2008). Hence, discrepancies between our findings and those of Spanagel et al. 2005 may be due to differences in the genetic backgrounds of the mPer2 mutant and wild-type mice or the drinking procedure described earlier in the discussion. the It is thought that acamprosate’s attenuation of ethanol intake is due to its suppressive action on hyper-glutamatergic signaling associated with heightened alcohol drive during withdrawal (reviewed in Spanagel and Kiefer, 2008; Krystal et al. 2003; Dodd et al. 2000). This is likely reflected in acamprosate’s reduction of ethanol drive observed here. The hyper-glutamatergic state observed in mPer2 mutants is attributable to reduced glutamate reuptake by its transporter (EAAT1; Beaulé et al. 2009). Notably, microdialysis measurements of glutamate levels in the striatum have revealed that a twice daily regimen of i.p. acamprosate injection reduces glutamate levels and subsequent ethanol craving in mPer2 mutant mice to WT levels (Spanagel et al. 2005).
Conclusions and Significance
These experiments represent the first assessments of the behavioral/drinking chronotype of mPer2 mutant mice, extending the work of Spanagel et al. (2005). These experiments point to a causative relationship between mPer2 behavioral disruption and elevated ethanol intake. Affirmation of this relationship (possibly involving pleiotropic effects of the mPer2 mutation) will depend on studies investigating changes in ethanol intake following circadian disruptions in WTs and the rescuing of perturbed circadian behavior in mPer2 mutants. Our work also extends previous studies on acamprosate effects in mPer2 mutant mice by showing that acamprosate decreases ethanol intake through the reduction of the number of daily ethanol drinking bouts and subsequent systemic ethanol peaks, without altering the diurnal pattern of ethanol drinking.
Acknowledgments
This work was supported by National Institute on Alcohol Abuse and Alcoholism grants AA-017898 to R.A. Prosser and J.D. Glass
References
- Beaulé C, Swanstrom A, Leone MJ, Herzog ED. Circadian modulation of gene expression, but not glutamate uptake in mouse and rat cortical astrocytes. PLoS One. 2009;4:e7476. doi: 10.1371/journal.pone.0007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Brager AJ, Ruby CL, Prosser RA, Glass JD. Chronic ethanol disrupts circadian photic entrainment and impairs daily locomotor activity in the mouse. Alcohol Clin Exp Res. 2010;34:1266–1273. doi: 10.1111/j.1530-0277.2010.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager AJ, Ruby CL, Prosser RA, Glass JD. Acute ethanol impairs photic and serotonergic phase-resetting in the mouse. Alcohol Clin Exp Res. 2011;35:1–9. doi: 10.1111/j.1530-0277.2011.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JW, Fixaris MC, Belanger GV, Rosenwasser AM. Repeated light–dark phase shifts modulate voluntary ethanol intake in male and female high alcohol-drinking (HAD1) rats. Alcohol Clin Exp Res. 2007;31:1699–1706. doi: 10.1111/j.1530-0277.2007.00476.x. [DOI] [PubMed] [Google Scholar]
- Comasco E, Nordquist N, Gokturk C, Aslund C, Hallman J, Oreland L, Nilsson KW. The clock gene PER2 and sleep problems: association with alcohol consumption among Swedish adolescents. Upsala J of Med Sci. 2010;115:41–48. doi: 10.3109/03009731003597127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Corte L, Crichton RR, Duburs G, Nolan K, Tipton F, Tirzitis G, Ward RJ. The use of taurine analogues to investigate taurine function and their potential therapeutic applications. Amino Acids. 2002;23:367–379. doi: 10.1007/s00726-002-0210-2. [DOI] [PubMed] [Google Scholar]
- Dodd PR, Beckmann AM, Davidson MS, Wilce PA. Glutamate-mediated transmission, alcohol, and alcoholism. Neurochem Int. 2000;37:509–533. doi: 10.1016/s0197-0186(00)00061-9. [DOI] [PubMed] [Google Scholar]
- Dole VP, Gentry RT. Toward an analogue of alcoholism in mice: scale factors in the model. Proc Natl Acad Sci USA. 1984;81:3543–6. doi: 10.1073/pnas.81.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman EA, Ingraham CM, Franklin KM, Keith CM, McClaren JA, Schultz JA, Morzorati SL, O’Connor S, Thielen RJ, Murphy JM, McBride WJ. In vivo time-course changes in ethanol levels sampled with subcutaneous microdialysis. Alcohol Clin Exp Res. 2008;32:435–442. doi: 10.1111/j.1530-0277.2007.00587.x. [DOI] [PubMed] [Google Scholar]
- Gass JT, Foster-Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvin DV, Baird TJ, Vanecek SA, Briscoe RJ, Vallett M, Holloway FA. Effects of time-of-day and photoperiod phase shifts on voluntary ethanol consumption in rats. Alcohol Clin Exp Res. 1997;21:817–825. [PubMed] [Google Scholar]
- Gupta T, Syed YM, Revis AA, Miller SA, Martinez M, Cohn KA, Demeyer MR, Patel KY, Brzezinska WJ, Rhodes JS. Acute effects of acamprosate and MPEP on ethanol drinking-in-the-dark in male C57BL/6J mice. Alcohol Clin Exp Res. 2008;32:1992–1998. doi: 10.1111/j.1530-0277.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- Hammer SB, Ruby CL, Brager AJ, Prosser RA, Glass JD. Environmental modulation of alcohol intake in hamsters: effects of wheel running and constant light exposure. Alcohol Clin Exp Res. 2010;34:1651–1658. doi: 10.1111/j.1530-0277.2010.01251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller-Sturmhöfel S, Kulkosky P. Chronobiological regulation of alcohol intake. Alcohol Res Health. 2001;25:141–8. [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D’Souza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther. 2003;99:79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- McElroy B, Zakaria A, Glass JD, Prosser RA. Ethanol modulates mammalian circadian clock phase resetting through extrasynaptic gaba receptor activation. Neuroscience. 2009;164:842–848. doi: 10.1016/j.neuroscience.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt HP, Gillin JC. Sleep abnormalities during abstinence in alcohol-dependent patients: aetiology and management. CNS Drugs. 2001;15:413–425. doi: 10.2165/00023210-200115050-00006. [DOI] [PubMed] [Google Scholar]
- Ozburn AR, Gordon EA, McClung CA. ClockΔ19 mutants exhibit increased ethanol preference and consumption. Alcohol Clin Exp Res. 2010;34:116A. [Google Scholar]
- Pasley JN, Powell EW, Halberg F. Strain differences in circadian drinking behaviors of ethanol and water in rats. Prog Clin Biol Res. 1987;227B:467–71. [PubMed] [Google Scholar]
- Pendergast JS, Friday RC, Yamazaki S. Distinct functions of Period2 and Period3 in the mouse circadian system revealed by in vitro analysis. PLoS ONE. 2010;5:e8552. doi: 10.1371/journal.pone.0008552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl JB, Sherazee N, Ghezzi A, Atkinson NS. Circadian genes are involved in alcohol related behaviors in Drosophila melanogaster. Alcohol Clin Exp Res. 2010;34:477A. [Google Scholar]
- Portaluppi F, Smolensky MH, Touitou Y. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int. 2010;25:1911–1929. doi: 10.3109/07420528.2010.516381. [DOI] [PubMed] [Google Scholar]
- Prosser RA, Mangrum CA, Glass JD. Acute ethanol modulates glutamatergic and serotonergic phase shifts of the mouse circadian clock in vitro. Neuroscience. 2008;152:837–848. doi: 10.1016/j.neuroscience.2007.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser AM, Clark JW, Fixaris MC, Belanger GV, Foster JA. Effects of repeated light-dark phase shifts on voluntary ethanol and water intake in male and female Fischer and Lewis rats. Alcohol. 2010:229–237. doi: 10.1016/j.alcohol.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Ruby CL, Brager AJ, DePaul MA, Prosser RA, Glass JD. Chronic ethanol disrupts circadian behavior and photic phase-resetting in the hamster. Am J Physiol. 2009b;297:R729–R737. doi: 10.1152/ajpregu.00268.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby CL, Prosser RA, DePaul MA, Roberts RJ, Glass JD. Acute ethanol impairs photic and nonphotic circadian phase resetting in the Syrian hamster. Am J Physiol. 2009a;296:R411–R418. doi: 10.1152/ajpregu.90782.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack RL, Auckley D, Auger RR, Carskadon MA, Wright KP, Vitiello MV, Zhdanova IV. Circadian rhythm sleep disorders: part I, basic principles, shift work, and jet lag disorders. Sleep. 2007;30:1460–1483. doi: 10.1093/sleep/30.11.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz M, Frey R, Pichler P, Ropke H, Anderer P, Saletu B, Rudas S. Sleep quality during alcohol withdrawal with bright light therapy. Prog Neuropsychopharmacol Bio Psychiatry. 1997;21:965–77. doi: 10.1016/s0278-5846(97)00092-4. [DOI] [PubMed] [Google Scholar]
- Seggio JA, Fixaris MC, Reed JD, Logan RW, Rosenwasser AM. Chronic ethanol intake alters circadian phase shifting and free-running period in mice. J Biol Rhythms. 2009;24:304–312. doi: 10.1177/0748730409338449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seggio JA, Logan RW, Rossenwasser AM. Chronic ethanol intake modulates photic and non-photic circadian phase responses in the Syrian hamster. Pharmacol Biochem Behav. 2007;87:297–305. doi: 10.1016/j.pbb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöholm LK, Kovanen L, Sarrikoski ST, Schalling M, Lavebratt C, Partonen T. CLOCK is suggested to associate with comorbid alcohol use and depressive disorders. J Circadian Rhythms. 2010;8:1–9. doi: 10.1186/1740-3391-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Kiefer F. Drugs for relapse prevention of alcoholism: ten years of progress. Trends Pharmacol Sci. 2008;29:109–115. doi: 10.1016/j.tips.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nature Med. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- Trinkoff AM, Storr CL. Work schedule characteristics and substance use in nurses. Am J Ind Med. 1998;34:266–27. doi: 10.1002/(sici)1097-0274(199809)34:3<266::aid-ajim9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Zornoza T, Cano M, Polache A, Granero L. Pharmacology of acamprosate: an overview. CNS Drug Rev. 2003;9:359–374. doi: 10.1111/j.1527-3458.2003.tb00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]