Abstract
Rationale
Mechanisms underlying diastolic dysfunction need to be better understood.
Objective
To study the role of titin in diastolic dysfunction using a mouse model of experimental heart failure induced by transverse aortic constriction (TAC).
Methods and Results
Eight weeks post-TAC surgery mice were divided into heart failure (HF) and congestive heart failure (CHF) groups. Mechanical studies on skinned LV myocardium measured total and titin- and extracellular matrix (ECM)-based passive stiffness. Total passive stiffness was increased in both HF and CHF mice and this was due to increases in both ECM-and titin-based passive stiffness, with titin being dominant. Protein expression and titin exon microarray analysis revealed increased expression of the more compliant N2BA isoform at the expense of the stiff N2B isoform in HF and CHF mice. These changes are predicted to lower titin-based stiffness. Because titin’s stiffness is also sensitive to titin phosphorylation by protein kinase A (PKA) and protein kinase C (PKC), back phosphorylation and Western Blot assays with novel phospho-specific antibodies were performed. HF and CHF mice showed hyperphosphorylation of PKA sites and the PEVK S26 PKC sites, but hypophosphorylation of the PEVK S170 PKC site. Protein phosphatase I (PP1) abolished differences in phosphorylation levels and normalized titin-based passive stiffness levels between Ctrl and HF myocardium.
Conclusion
TAC-induced HF results in increased ECM-and titin-based passive stiffness. Changes in titin splicing take place, which lower passive stiffness, but this effect is offset by hyperphosphorylation of residues in titin spring elements, particularly of PEVK S26. Thus complex changes in titin take place that combined are a major factor in the increased passive myocardial stiffness in HF.
Keywords: passive stiffness, titin, diastolic dysfunction
Introduction
Altered hemodynamics, such as elevated blood pressure and aortic valve diseases lead to pathological cardiac hypertrophy and ultimately heart failure (HF), characterized by left ventricular dilation, decreased contractility, and increased myocardial stiffness1. Although the molecular mechanisms that underlie depressed contractility have been investigated extensively, the molecular basis of diastolic dysfunction is less well understood2-4. Understanding the mechanisms that govern diastolic dysfunction is important for understanding a wide range of cardiac diseases including heart failure with preserved ejection fraction (HFpEF), a prevalent disease without effective therapy4-7. The extracellular matrix (ECM) is often considered to dominate passive myocardial stiffness, but work focused on the intracellular protein titin has shown that titin is also important for generating passive muscle stiffness5.
Titin spans from Z-disk to M-line and is extensible in the I-band region of the sarcomere where it functions as a molecular spring that develops passive force in sarcomeres stretched beyond their slack length (~1.9 μm)8. Differential splicing results in two main isoforms: the N2B isoform that is stiff (~3.0 MDa) and the larger N2BA isoform that is more compliant (~3.4 MDa)9. These isoforms are co-expressed in the adult heart with alterations in their expression ratio giving rise to changes in myocardial stiffness10. In addition, titin phosphorylation also alters myocardial stiffness. Both PKA11 and Protein Kinase G (PKG)12 phosphorylate titin (most likely at the same site in the N2B spring element12) and reduce myocardial stiffness (increased compliance). Evidence suggests that hypo-phosphorylation of PKA/PKG sites on titin contribute to reduced compliance in HFpEF patients13. Recently it was found that titin is also a target of Protein Kinase C (PKC)α and that PKCα phosphorylation of two conserved serines in the PEVK region of titin (S26 and S170 in the PEVK of the N2B cardiac titin isoform) increases cardiac stiffness14-16. Here we studied titin in mice with HF induced by transverse aortic constriction (TAC) and focused on myocardial passive stiffness, titin isoform expression, and titin phosphorylation. We found a large increase in myocardial stiffness in HF that can be explained in large part by hyper-phosphorylation of titin spring elements.
Materials and Methods (Details in Supplement)
Transverse Aortic Constriction (TAC)
We used 8 week old male C57BL/6J mice. The aortic banding procedure used a 27 gauge (0.42mm outer diameter) needle to constrict the aorta; details are explained in the Supplement. TAC and sham mice were evaluated 8 weeks after surgery, using echocardiography. Following this, mice were sacrificed, weighed and left ventricular weight (LVW) and lung weights (LW) determined. Hearts were immediately dissected, LV papillary muscles were removed, and the LV was split into bilateral halves that were either frozen or stored in RNAlater. TAC animals were divided into 2 groups based on the left ventricle (LV) weight (LVW)/body weight (BW) ratio (HF 4.25mg/g-6.75mg/g; CHF>6.75mg/g) and lung weight (LW)/BW ratio (HF 4.5mg/g-12mg/g; CHF>12mg/g) (see Figure 1), since LV mass and pleural edema both correlate with degree of heart failure development17. Additionally the two experimental groups had changes in calcium handling proteins SERCA2a, PLB, pPLB, pTnI and MHC expression (See Online Figures I and II) that are similar to reported previously for TAC mice (See Supplement for details). Experiments were approved by the University of Arizona Institutional Animal Care and Use Committee and followed the U.S. National Institutes of Health “Using Animals in Intramural Research” guidelines for animal use.
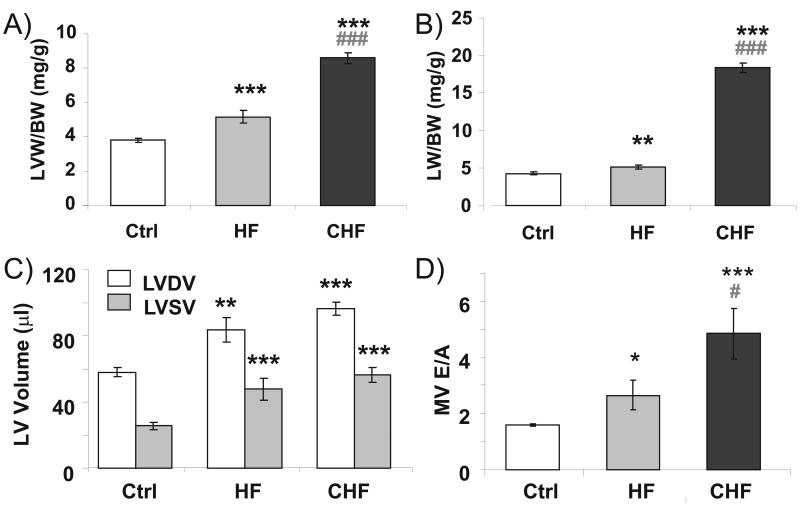
Figure 1. Post-Mortem (A and B) and Echocardiographic (C and D) Analysis.
A) and B) show that HF mice have increased LVW/BW ratios and LW/BW ratios, with the largest changes in the CHF group. Echocardiography revealed increased LV chamber volumes (C), with the largest changes in the CHF group. Doppler echocardiography indicates an increase in MV E/A ratio (D). LV, Left Ventricle; BW, body weight; LW, Lung weight; Ctrl, Control; HF, heart failure; CHF, congestive heart failure; MV, mitral valve. *P<0.05, **P<0.01, ***P<0.001 vs sham-operated controls. #P<0.05, ###P<0.001 vs Heart Failure TAC mice.
Echocardiography
Echocardiography was performed as described18 using a Vevo 770 System (Visual-Sonics, Toronto, Canada). Details in Supplement.
Gel Electrophoresis
SDS-agarose electrophoresis was performed as previously described19 with additional details in the Supplement.
Phosphorylation Assays
Detection of LV protein phosphorylation and recombinant protein was by protein labeling (32P), phosphoprotein stain (Pro-Q diamond) and Western Blots probed with phospho-specific antibodies (PS26 GL Biochem, Shanghai, and PS170 Genscript, USA) against titin’s pS26 and pS170 found in the PEVK element and against various calcium handling and myofibrillar proteins. See Supplement for details.
Microarray studies
The titin exon microarray experiments were performed as described previously20. Details in Supplement.
Measurement of passive tension
Small muscle strips were attached to a motor and a force transducer via aluminum clips and passive tension was measured in relaxing solution. Sarcomere length (SL) was measured online by laser diffraction. Passive stiffness was determined from the slope of the linear regression line that was fit to the passive tension-SL data in the 1.95-2.05 μm SL range (which encompasses the physiological SL range21). For the protein phosphatase-1 (PP1)-experiments muscles were incubated with PP1 (0.5 U/μl) for 2 hours, and passive stiffness was measured at 20 min intervals. See Supplement for details.
Statistics
Data are presented as mean ± SEM. Group significance was defined using ANOVA followed by Tukey-Kramer multiple comparison test; SL significance was determined using 2-way ANOVA; Student’s t-test was used in Fig. 6A, inset; probability values <0.05 were taken as significant.
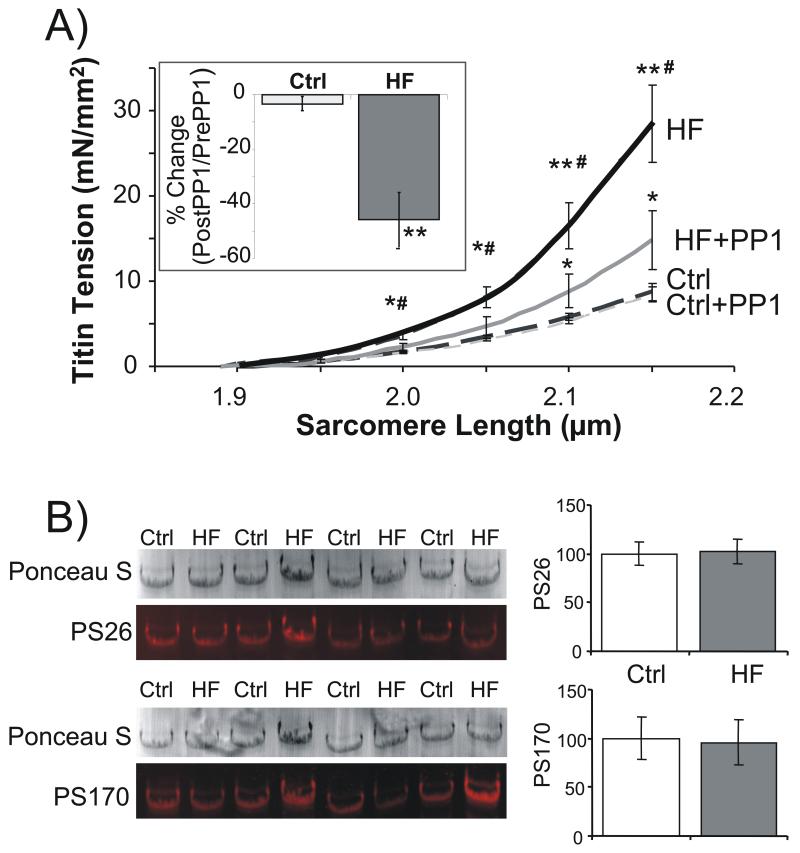
Figure 6. A) Titin-based passive tension and phosphorylation of S26 and S170 following dephosphorylation with PP1.
Before PP1 treatment, passive tension is significantly higher in HF samples (confirming results of Fig. 2). Whereas tensions were unchanged in Ctrl samples following PP1 treatment, HF samples had a significant reduction in their passive tensions. Inset: percent change in titin-based passive stiffness (slope of passive tension -SL relation in 1.95-2.05 μm SL length range) following PP1 treatment. B) WB analysis of titin’s PS26 and PS170 sites in Ctrl and HF samples following PP1 treatment (same samples as in A). WB normalized to Ponceau S based protein levels and results of control samples set to 100%. No differences in phosphorylation levels are detected following PP1 treatment. * significant vs Ctrl (P<0.05); ** significant vs Ctrl (P<0.01); # HF significant vs HF + PP1 (P<0.05).
Results
TAC induced heart failure
Using echocardiography and tissue weight analysis we evaluated hypertrophy, LV chamber dimensions, and LV function in sham operated (Ctrl, n=12), heart failure (HF, n=10), and congestive heart failure (CHF, n=6) mice. The left ventricular weight (LVW) was significantly increased by 34% in HF and 92% in CHF and when normalized to body weight (BW) LVW/BW ratios were significantly increased by 36% and 226%, respectively (see Online Table I and Fig. 1A). Lung weight (LW)/BW was also significantly increased in both HF and CHF with a modest 20% increase in HF and a 346% increase in the CHF group (Online Table I and Fig. 1B). Echocardiography confirmed the hypertrophy and also revealed increased chamber dimensions in both experimental groups with changes that were most severe in the CHF mice (Online Table II and Fig. 1C). Fractional shortening (%FS) and ejection fraction (%EF) were both significantly reduced in the two experimental groups (Online Table 2). We measured expression of β-MHC, an indicator of HF22, and found a trend towards an increase in β-MHC in HF mice and a significant increase in CHF mice (Online Fig. I). We also found trends towards a reduction in SERCA2a and PLB expression and decreased phoshorylation levels of S23/24 of cTnI and S16 of PLB in HF and significant changes in CHF (Online Fig. II A-D). Thus, the combined data from tissue weights, echocardiography, and protein data all reveal that the TAC mice had HF with the most deleterious changes in the CHF group. Doppler echocardiography was used to measure the ratio of peak transmitral early- (E) and late- (A) diastolic velocities, and the deceleration time of the E wave (DT). The E/A ratio was significantly increased by 68% in the HF group and by 207% in the CHF group (Fig. 1D), changes that are due to a modest increase in E and a more severe reduction in the A parameter (Online Table II). These changes are likely to reflect increased ventricular chamber stiffness in both experimental groups with the largest increase in CHF. Consistent with this hypothesis is the reduced DT in CHF (DT is considered to be inversely related to LV chamber stiffness23). Heart rate (HR) during these measurements was not different between the groups (Online Table II), and it is unlikely therefore that measured differences in diastolic function are due to unequal HR.
Tissue mechanics and passive stiffness in HF and CHF TAC mice
Mechanical experiments were performed on skinned myocardial tissue. Maximal active tension was significantly reduced in both HF (n=5) and CHF (n=4) heart failure groups as compared to controls (n=4) (34.2±1.5mN/mm2 and 30.3±2.4mN/mm2, respectively, vs. 41.8±2.6mN/mm2 in Ctrl; Table 1). This active tension reduction is consistent with other studies on rodents with experimental CHF (30% reduction in tension in CHF rats24 vs. 28% in our work in CHF mice) and might be due to increased PKCα signaling that causes hyperphosphorylation of myofilament proteins24. Passive tension was measured by stretching the skinned tissue in relaxing solution from the slack sarcomere length (SL) to a maximal SL of 2.15 μm. In pilot studies, the maximal SL at the end of the stretch was 2.3 μm but this resulted in irreversible damage in both the HF and CHF tissues, as revealed by reduced passive tension in subsequent stretches. This phenomenon was avoided by limiting the maximal SL to 2.15 μm. Passive tension (Fig. 2A, left and right panels) and passive stiffness (slope of passive tension-SL relation in the physiological SL range21, Table 1) were significantly increased in HF and CHF mice with higher values in HF. To establish whether this increase involves residual actomyosin interaction we performed experiments in the presence of 20 mM BDM (2,3 butanedione monoxime), a known inhibitor of actomyosin interaction. BDM had no effect on passive tension (results not shown), suggesting that the increased passive tension in HF and CHF is due to only passive material properties of the myocardium. It is also important to highlight that although differences in calcium handling proteins exist, in both expression level and phosphorylation status (Online Fig. II), these changes are unable to explain our findings considering that the experiments were carried out on muscle that had been demembranated with detergent (Triton X-100). To evaluate whether elevated passive tension might be due to the oxidizing conditions that are known to exist in HF25, and which are know to also affect titin26 we also performed experiments in which we increased the level of the reducing agent dithiothreitol (DTT) in the relaxing solution (from 1 mM to 10 mM) but again found no effects on passive tension (Online Fig. III).
Table 1. Mechanical Analysis.
| CTRL | HF | CHF | |
|---|---|---|---|
| Maximal Active tension, mN/mm2 | 41.8±2.6 | 34.2±1.5* | 30.3±2.4* |
| Total Passive Stiffness(1), mN/(mm2 μm/SL) | 33.0±5.9 | 80.4±4.2*** | 68.6±9.4** |
| ECM-based Passive Stiffness, mN/(mm2 μm/SL) | 7.4±2.1 | 22.4±4.0** | 27.0±8.6* |
| Titin Stiffness, mN/(mm2 μm/SL) | 25.6±6.4 | 64.8±5.3*** | 39.9±8.2*# |
Maximal active tension: pCa 4.0; activated at SL 2.0.
Stiffness is defined as the slope of the linear fits of the tension- sarcomere length (SL) relation in the physiological SL range of 1.95-2.05 μm.
P<0.05
P<0.01
P<0.001 vs sham-operated controls.
P<0.05 vs Heart Failure TAC mice.
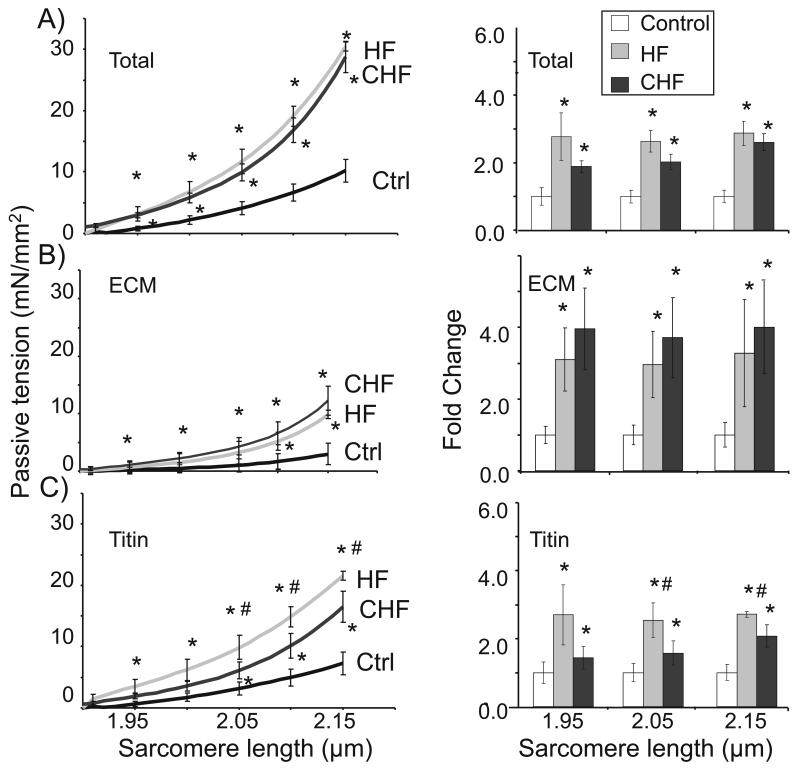
Figure 2. Effect of TAC-induced heart failure on passive tension in skinned myocardium.
Total passive tension (A) is significantly higher in HF and CHF groups, due to both an increase in ECM (B) and titin (C) based passive tension. Right panels show the fold change in passive tensions at SL 1.95, 2.05, and 2.15 μm. Results indicate a significant increase in both HF and CHF at all three sarcomere lengths. Titin-based passive tensions in CHF were slightly reduced relative to HF.
In order to determine the specific contributions of titin vs. collagen to passive stiffness, muscles were treated with high concentrations of KCl and KI (Methods) to extract thick and thin filaments and therefore remove the sarcomeric anchors of titin. This abolishes titin’s ability to develop passive tension but leaves the extracellular matrix (ECM)-based passive tension intact (see also 27-28). KCl/KI extraction revealed that ECM-based passive tension (Figure 2B, left and right panels) and stiffness (Table 1) in HF and CHF mice are significantly increased, consistent with the known presence of fibrosis in TAC-induced HF29. Subtracting ECM-based tension from total tension shows that both HF and CHF mice had significantly greater titin-based passive tension (Figure 2C, left and right panels) and stiffness (Table 1), compared to controls. Interestingly, titin-based passive tension and stiffness in CHF were reduced relative to HF. Thus, increased collagen-based and titin-based passive tensions are responsible for increased passive tension in TAC-induced HF and CHF. Online Fig. IV compares the relative contribution of titin and collagen to overall passive tension. In all three groups (Ctrl, HF and CHF) titin is the main contributor to passive tension. Titin contributes ~75% and ECM ~25% in both Ctrl and HF whereas titin’s contributions falls in CHF to ~60% and ECM-based tension increases to ~40% of total.
Differential splicing and phosphorylation of titin
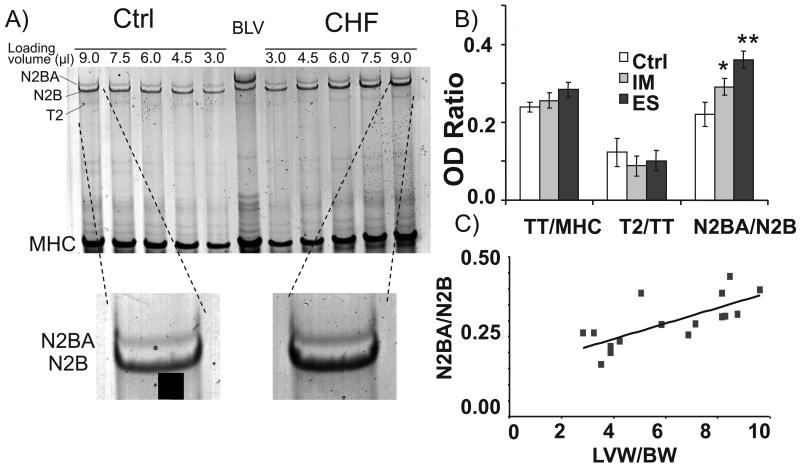
Differential splicing results in the expression of two main cardiac titin isoforms, the stiff N2B and the larger and more compliant N2BA isoforms10. Using high-resolution gel-electrophoresis we evaluated the titin isoform expression ratio in TAC mice with the expectation that upregulation of the stiff N2B isoform might explain the increased titin-based passive tension of the HF and CHF tissues. Consistent with earlier studies30 the control animals have a N2BA:N2B expression ratio of ~0.2 (Figure 3A and B), confirming that the shorter, stiffer N2B isoform is dominant in the mouse heart. The HF and CHF mice showed an increase in the more compliant N2BA isoform (Fig. 3B, Online Table III) that correlates with the LVW/BW ratio (P<0.001) (Figure 3C). The increased expression of the compliant N2BA isoform is opposite of what was expected from the measured increase in titin-based passive stiffness. To test whether this discrepancy might be due to changes in expression levels of titin we determined the relative amount of titin per mg of tissue and the amount of titin relative to the expression level of myosin heavy chain (MHC). Neither HF nor CHF groups were significantly different in their level of titin expression (Online Table III). Finally, we also evaluated the titin degradation product, T2 and found no significant change following aortic constriction (Online Table III). Thus the only significant change that was found is the upregulation of the compliant N2BA isoform at the expense of the stiff N2B isoform. Because this finding was unexpected we also evaluated titin expression at the transcript level by performing a titin exon expression analysis using a homemade microarray on which all 358 exons found in the mouse are represented. We found significant upregulation of a large group of PEVK and Ig exons in CHF tissues compared to Ctrl (Online Table IV) confirming that CHF mice express a larger titin than Ctrl heart tissue.
Figure 3. Titin isoform expression.
(A) Top panel: Representative 1% agarose gel image in which control LV and a CHF LV protein samples were loaded at a range of loading volumes. For analysis, the slope of the linear range of the relationship between integrated optical density and loaded volume was obtained for each protein. Bottom panel: Enlarged region of gel showing titin in Ctrl and CHF samples. (B) Analysis revealed Ctrl N2BA:N2B ratios of ~0.2. However, HF and CHF samples both showed an increase in the more compliant N2BA isoform. (C) N2BA:N2B ratios correlate with the LVW/BW ratio. * significant vs Ctrl (P<0.05); ** (P<0.01).
Because titin’s stiffness can also be varied through phosphorylation, with PKA and PKG phosphorylation decreasing stiffness11-12,31 and PKC phosphorylation increasing stiffness14-15 we also evaluated titin’s phosphorylation levels. Back phosphorylation assays revealed reduction in 32P incorporation in response to phosphorylation by PKA (Figure 4) that indicate hyper-phosphorylation of HF and CHF mice, changes that are expected to lower passive stiffness11-12,31. Recently, PKCα was shown to phosphorylate titin specifically at S11878 and S12022 (S26 and S170, respectively, in the PEVK region of the N2B cardiac isoform) with evidence that suggests that phosphorylation of S26 induces a large increase in passive stiffness14-15. Therefore, we developed phospho-specific antibodies (pS26 and pS170) that positively recognize PKCα phosphorylation of both recombinant PEVK protein and mouse myocardium. Validation of these new antibodies with recombinant proteins in which S26 and S170 were mutated showed that the antibodies are specific to the phospho-S26 or phospho-S170, and that protein phosphatase-I (PP1) dephosphorylates these sites (Online Fig. V). We also showed that the popular phospho-protein stain Pro-Q diamond does not specifically detect the phosphorylated PEVK sites but that Pro-Q diamond can be used to detect PKA phosphorylation of the N2B element (Online Figure VI). Thus, phospho-specific antibodies are required to study PKC phosphorylation. The phospho-specific antibodies were used in a Western Blot analysis of LV proteins and this showed in both HF and CHF tissues a large reduction in phosphorylation of PEVK’s S170 site (Fig. 5A); interestingly we found increased PKCα phosphorylation of PEVK’s S26 site (Fig. 5B).
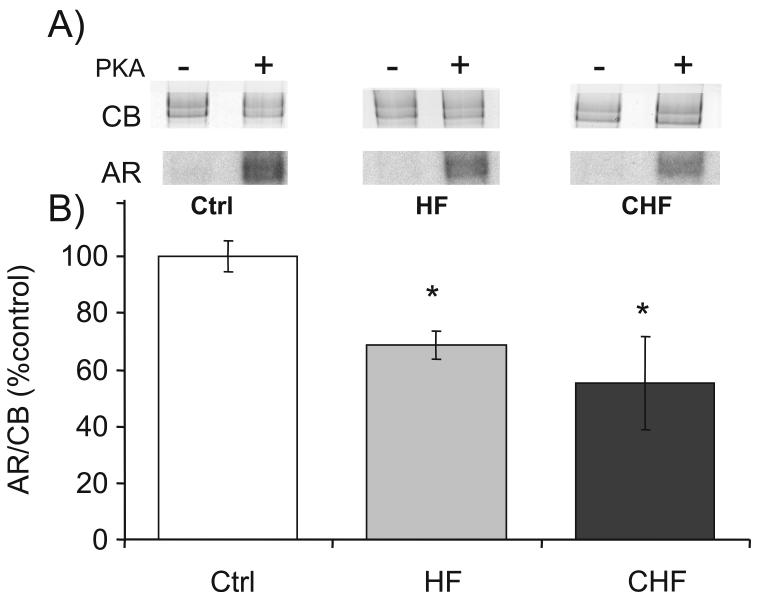
Figure 4. Determination of PKA phosphorylation status.
(A) Representative results on mouse skinned LV incubated in [γ-32P]ATP without (-) or with (+) preincubation with PKA. Top panels: Coomassie blue (CB) stained gel and bottom panels: corresponding autoradiograph (AR). Note: AR detects only available phosphorylation; hence, less [γ-32P] incorporation is equivalent to less available phosphorylation sites. (B) Both HF and CHF samples have reduced [γ-32P] incorporation, indicating hyperphosphorylation of their PKA sites. * significant vs Ctrl (P<0.05).
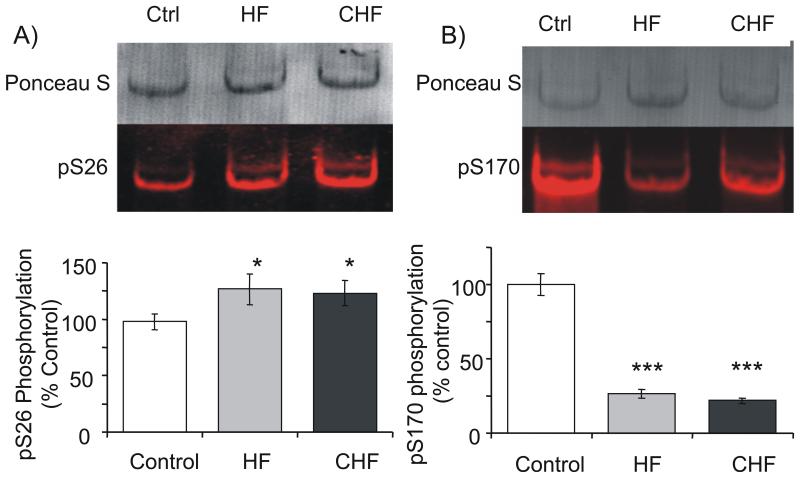
Figure 5. Identification of Titin’s PKCα phosphorylation levels.
Newly made phospho-specific antibodies to titin’s PCKα phosphorylation target PEVK sites, (A) pS26 and (B) pS170, were evaluated in HF and CHF samples as compared to Ctrl. Top panels: Representative WB images. Bottom panels: Analysis shows (A) hyperphosphorylation of PEVK’s S26 and (B) a large reduction of S170 phosphorylation. * Significant vs WT (P<0.05); *** Significant vs WT (P<0.001).
In order to elucidate the contribution of PKCα phosphorylation to the increased titin-based passive stiffness in heart failure LV tissue, Ctrl and HF samples were incubated in PP1 (0.5 U/μl) for 2 hours with tensions measured both before and after PP1 treatment. Baseline titin-based passive tension (Fig. 6A) was increased in HF samples, consistent with results of Fig. 2. Following PP1 treatment, titin-based passive tension was unchanged in Ctrl LV. However, incubation of HF LV in PP1 resulted in a significant decrease in passive tension (Fig 6A) and stiffness (Fig. 6A, inset). We performed a phosphorylation analysis of S26 and S170 using the samples from the mechanical studies and found following PP1 treatment no differences between Ctrl and HF samples (see Fig. 6B). Thus, PP1 treatment normalizes both differences in phosphorylation of S26 and S170 and in titin-based passive stiffness.
Discussion
There is heightened awareness that it is critically important to fully understand the underlying mechanisms of diastolic dysfunction, because of its clinical importance and absence of effective therapies6-7,32. In this work we studied a TAC-induced HF mouse model, we segregated mice in HF and CHF groups, and performed a myocardial passive stiffness analysis with a focus on titin. A large increase in passive myocardial stiffness was found in both the HF and CHF groups that is most severe in HF and somewhat less in CHF mice. The data further indicates that heart failure-induced fibrosis results in an increase in ECM-based passive stiffness, and importantly, that titin plays a major role in increased myocardial passive stiffness. The contribution of titin to passive myocardial stiffness is greatly increased in HF mice, relative to that of sham mice, and this might be due to hyperphosphorylation of a PKC site in titin’s PEVK region. In CHF mice titin-based stiffness is increased as well but not as much as in HF mice, which can be explained by the increased expression of the compliant N2BA titin that we found. Below we discuss our findings in detail.
Total passive myocardial stiffness was increased ~2.5 fold in the HF mice and ~2-fold in the CHF mice (Schematically shown in Fig. 7A). The total stiffness increase in the HF group is slightly higher than recently reported by Bradshaw et al33 in papillary muscle of TAC mice (~1.6-fold increase), which might be explained by the more severe hypertrophy in our study. ECM-based stiffness was found to be ~3-fold higher in the HF group and ~4-fold higher in the CHF group than in the sham mice (Table 1). These increases can be explained by the increased ECM collagen-content and increased collagen cross-linking that are known to take place in the TAC-mouse model33. The increase in titin-based stiffness is consistent with the recently reported increased passive tension of skinned cardiac myocytes isolated from mice with severe TAC34. The increase in passive material properties of the myocardium will increase diastolic stiffness of the LV wall in HF and CHF. Diastolic stiffness can also be increased by incomplete relaxation due to for example the well known reduction in SERCA2a levels in CHF (see also Online Fig. IIA) and the increased calcium sensitivity of the myofilaments that some34 (but not others24) have reported. Increased calcium sensitivity is unlikely to explain the increased stiffness that we found in skinned muscle because measurements were made in relaxing solution, and, furthermore, the actomyosin inhibitor BDM had no effect on passive stiffness. In summary, passive myocardial stiffness of HF and CHF mice is increased due to changes in both ECM and titin and this increase is expected to contribute to elevated diastolic stiffness.
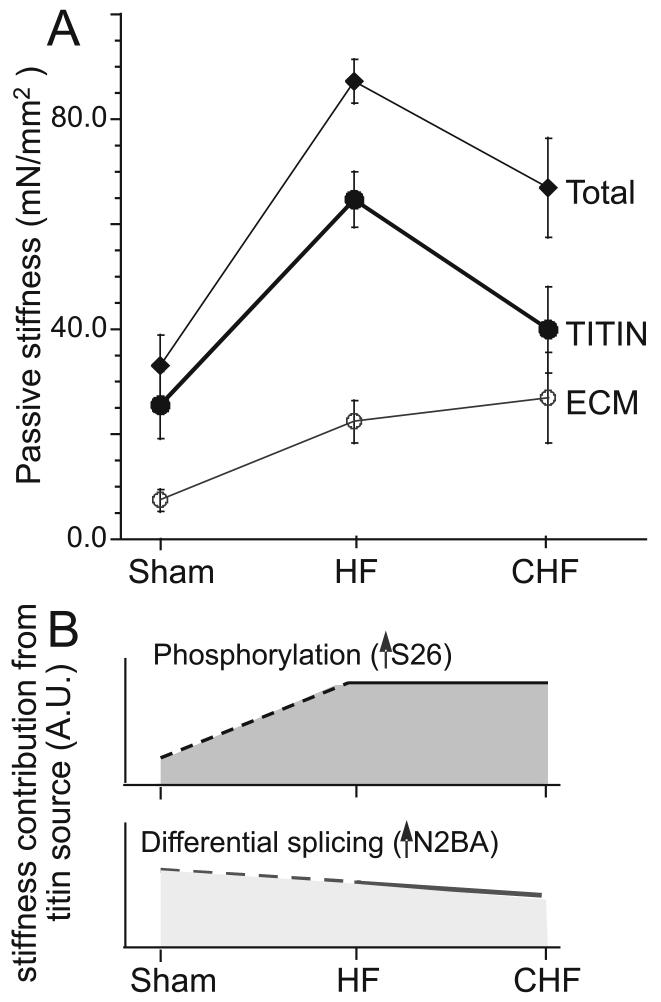
Figure 7. Passive stiffness in HF and CHF myocardium.
A) Comparison of total, ECM, and titin passive stiffness (from Table 1). B) Schematic showing effects on passive stiffness of increase in S26 phosphorylation (top) and upregulation of N2BA titin (bottom).
A possible explanation for the increase in titin-based stiffness is the recently discovered disulfide crosslinking (S-S) that occurs in the N2B element under oxidative stress26, a state that is common during heart failure25. Because such crosslinking increases titin-based passive stiffness26, we tested the possibility that the increase in stiffness in HF mice was due to increased S-S bonding by performing experiments under reducing conditions (10 mM DTT). Results indicate that oxidation of titin was not the cause of the increase in passive stiffness in heart failure. Titin-based passive stiffness can be regulated by differential splicing of titin; titin molecules with a longer extensible region increase sarcomere compliance. Two cardiac titin isoforms are coexpressed within the sarcomere, the N2B and N2BA isoforms with short (stiff) and long (compliant) extensible regions, respectively, and their expression ratio is an important determinant in titin-based passive stiffness10. Furthermore, it has been demonstrated in both a canine tachycardia-induced model of dilated cardiomyopathy35 and in spontaneously hypertensive rats36 that a decrease in the expression of N2BA titin is accompanied by an increase in myocardial passive stiffness. Therefore, we hypothesized that the increase in titin-based myocardial stiffness might be due to increases in the stiffer N2B isoform, resulting in more passive tension when stretched to a specific sarcomere length. In contrast to the expected increase in expression level of the stiffer N2B isoform, both protein (Fig. 3) and transcript (Online Table IV) data showed that titin’s compliant N2BA isoform actually increases its expression level (Fig. 3B). Although this data is opposite from what we expected, the result does correspond to isoform changes previously seen in human patients with ischemic human heart disease37, dilated cardiomyopathy38-39, HFpEF13, and aortic stenosis13. Thus although differential splicing is a prominent mechanism for altering passive stiffness, the change in splicing that occurs in HF can not explain the increased titin-based stiffness that we found, and in fact it is excepted to lower passive stiffness instead.
Titin’s stiffness can also be altered through phosphorylation, with disparate effects via the PKA/PKG and PKC pathways. Both PKA and PKG have been found to phosphorylate the cardiac specific N2B element and reduce titin’s stiffness11-12. On the other hand, PKCα phosphorylates the PEVK region of titin and this increases passive stiffness 15-16. Recent studies have shown that the PKA/PKG pathway is important in explaining the increased passive stiffness of myocytes in HFpEF patients where a reduction has been found in the phosphorylation level of PKA/PKG sites in titin and where treating the skinned cardiac myocytes from HFpEF patients with PKA lowers passive stiffness towards that of the control cells13. Therefore, we thought that the observed shift from the stiffer N2B isoform to a more compliant N2BA isoform in our study could be thought of as a compensatory mechanism for increased myocardial stiffness, and that a reduction in the basal phosphorylation level of PKA accounts for the increased stiffness. Yet again, in contrast to our hypothesized results, PKA phosphorylation analysis showed hyper-phosphorylation of PKA sites (Fig. 4). Similar to the increased compliant isoforms that we found, these changes are predicted to decrease passive tension. One hypothesis for why these results are different from those in the human study discussed above is as follows. Recently, Hamdani et. al 40 reported differences in phosphorylation levels of regulatory proteins in a variety of animals ranging from mice to man. Data revealed that the basal phosphorylation level is species-dependent and that reduced PKA-mediated phosphorylation was found only in end-stage failing human myocardium. We hypothesize therefore that mice with heart failure are less sensitive to defects in β-adrenergic signaling than humans and speculate that this explains why titin’s PKA sites are not hypophosphorylated in our study. Hyperphosphorylation of PKA sites might potentially be explained by the recent finding that titin’s PKA sites in the N2B region of titin can also be phosphorylated by PKG12 and the elevated PKG activity in TAC mice that has been reported41. Clearly myofilament protein phosphorylation is a complex process with alternative pathways that can have similar or disparate results.
Recently, our laboratory has found that titin is also a target of PKCα and that PKCα phosphorylation of titin increases cardiac stiffness15-16. Two sites were found in titin’s PEVK region (S26 and S170 in the PEVK region of the N2B isoform) that are present in all known cardiac and skeletal muscle titin isoforms. We produced phospho-specific antibodies against the two sites (Online Fig. V) and used them to assay site-specific phosphorylation events within titin. The results showed hypo-phosphorylation of the S170 PKCα site and hyper-phosphorylation of S26 (Fig. 5). Because we have shown that PKC phosphorylation increases passive stiffness both at the single molecule14 and single cell levels15-16, it is possible that the hyperphosphorylation of S26 plays an important role in the increased titin-based stiffness. The hyperphosphorylation of PKC sites is consistent with the increased expression levels of PKCα that have been found in mice subjected to TAC41. That hyperphosphorylation of titin is indeed a mechanism for increased passive stiffness is supported by the de-phosphorylation studies in which passive stiffness was measured before and after PP1 treatment (Fig 6) and that showed that PP1 significantly lowered the high passive stiffness of HF myocardium.
It is interesting that two phosphorylation sites 144 residues apart (PEVK S26 and S170) and phosphorylated by the same kinase have such different phosphorylation levels in HF myocardium. Previous studies on PKC phosphorylation sites showed that PKCα prefers basic residues N-and C-terminal to the phosphorylation site42. These studies also indicate that PKC preferentially phosphorylates serines with basic residues within 3 amino acids of both the C-terminal and N-terminal sides of it. Based on these studies PKC would have a stronger affinity towards S26 than S170 due to the closer proximity of the neighboring basic amino acids (lysine (K) and arginine (R)) (see Online Fig. VII), which is consistent with the experimental PKC phosphorylation data on recombinant PEVK proteins15. Other possibilities exist as well, such as protein binding at or near S170.
The PP1 experiments lowered passive stiffness (Fig. 6), and because PKA/PKG and PKC sites have been shown to be dephosphorylated by PP112,15 either one or both might explain the passive stiffness reduction. However, dephosphorylation of PKA sites increases passive stiffness12 and the S170 PKC site already has a low phosphorylation level prior to PP1 treatment (Fig. 5B) and, thus, they are unlikely candidates. On the other hand S26 is more promising because single molecule studies have shown that it is more important than S170 in determining passive stiffness14, and S26 is hyper-phosphorylated in HF tissue where stiffness is high and its dephosphorylation (PP1 treatment) is expected to cause a reduction of stiffness, as we found. Thus, phosphorylation of S26 is likely to be crucial in determining the contribution of titin to myocardial stiffness. The significant increase in phosphorylation of S26 in HF is consistent with the increased PKCα activity that has been reported to occur in CHF rats24. Considering that the PKCα-based passive stiffness modulation pathway was only discovered recently it is unknown whether our findings in the HF mouse model extrapolate to different disease models. It is interesting to note, however, that a recent HFpEF study reported that cellular passive stiffness following PKA treatment reduced passive tension but remained higher than in control cells (in fact N2BA titin was upregulated, similar to what we found, and this upregulation predicts lower tension than in the control cells)13. This discrepancy can be explained by an elevated phosphorylation level of the PKCα-site S26. Thus, the role of S26 in the increased passive stiffness that was revealed in our study of the TAC-induced HF mouse model might be relevant in other diseases as well.
In summary, we found a significant increase in passive myocardial stiffness in HF and that titin plays an important role in this increase. Phosphorylation analysis and PP1 studies identified hyperphosphorylation of titin’s S26 as a possible candidate for explaining part of the increased passive stiffness in HF. Lower titin-based stiffness was found in CHF than HF, which might be due to the upregulated N2BA titin in CHF compared to HF mice. Thus, altered hemodynamics trigger complex changes in titin’s spring, with hyperphosphorylation of S26 initially causing an increase in passive stiffness, in addition to titin-splicing changes during progression to CHF that lower passive stiffness (See schematic of Fig. 7B). Considering that hyperphosphorylation of S26 appears to be a dominant effect in passive stiffness changes during the progression to HF, the PKC pathway warrants further investigation as it is a potential target for therapeutics aimed at lowering diastolic stiffness.
Supplementary Material
What was known
Diastolic dysfunction is a prominent aspect of heart failure (HF).
Mechanisms that underlie diastolic dysfunction are not well understood
What new information does this article contribute
We focused on passive stiffness of the left ventricle (LV) using a mouse model of HF and studied the extracellular matrix (ECM) and titin.
HF results in increased ECM- and titin-based passive stiffness. Changes in titin include isoform switching and hyperphosphorylation of titin’s molecular spring region.
Complex changes in titin take place that in combination are a major factor in the increased passive myocardial stiffness in HF.
Elucidation of the mechanisms that govern diastolic dysfunction is important for understanding a wide range of cardiac diseases, including heart failure with preserved ejection fraction. The ECM is often considered to dominate passive myocardial stiffness, but work focused on the intracellular protein titin has shown that titin is also important. We studied diastolic dysfunction and the role of titin in a mouse model of experimental HF. Mechanical studies on skinned LV myocardium showed that total passive stiffness was increased in HF and this was due to increases in both ECM-and titin-based passive stiffness, with titin being dominant. Although the expression level of titin isoforms were affected, these changes cannot account for the increase in titin stiffness in HF. Investigations into the role of several kinases (PKA and PKC) and phosphatases (PP1) suggest that the increase in titin-based stiffness couldbe explained in large part by hyper-phosphorylation of titin spring elements. Considering that hyperphosphorylation of ser-26 in the pro-glu-val-lys spring element appears to be an important determinant of changes in passive stiffness during progression to HF, the PKC pathway warrants further investigation as it is a potential target for therapeutics aimed at lowering diastolic stiffness.
Acknowledgements
We thank the Granzier lab members for assistance and feedback on the manuscript.
Source of funding
Supported by NIH grant HL062881 (HG), T-31 HL07249(BH), and generous support from the Allan and Alfie Norville Endowed Chair (HG)
Non-standard Abbreviations and Acronyms
- TAC
Transverse aortic constriction (TAC).
- HF
Heart failure
- CHF
congestive heart failure
- LV
Left ventricle
- LVW
Left ventricle
- BW
Body weight
- LW
Lung weight
- ECM
extracellular matrix
- PKA
Protein Kinase A
- PKG
Protein Kinase G
- PKC
Protein kinase C
- PP1
Protein phosphatase I
- PEVK
Proline (P) Glutamate (E) Valine (V) Lysine (K)
- SL
Sarcomere length
- HFpEF
Heart failure with preserved ejection fraction ()
- BDM
(2,3 butanedione monoxime),
- DTT
Dithiothreitol
- WT
Wild type
- LVID
LV internal diastolic diameter
- IVS
Inter ventricular septum
- LVPW
LV posterior wall
- LVPW
LV posterior wall
- EF
Ejection fraction
- FS
Fractional shortening
- MV E
Mitral valve E velocity
- MV A
Mitral valve A velocity
- DT
Deceleration time of the E wave
Footnotes
Disclosures
None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mann DL. Heart Failure: A Companion to Braunwald’s Heart Disease. 2004. [Google Scholar]
- 2.Katz AM, Zile MR. New molecular mechanism in diastolic heart failure. Circulation. 2006;113:1922–5. doi: 10.1161/CIRCULATIONAHA.106.620765. [DOI] [PubMed] [Google Scholar]
- 3.Zile MR, Baicu CF, Bonnema DD. Diastolic heart failure: definitions and terminology. Prog Cardiovasc Dis. 2005;47:307–13. doi: 10.1016/j.pcad.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. Jama. 2006;296:2209–16. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 5.Kass DA, Bronzwaer JG, Paulus WJ. What mechanisms underlie diastolic dysfunction in heart failure? Circ Res. 2004;94:1533–42. doi: 10.1161/01.RES.0000129254.25507.d6. [DOI] [PubMed] [Google Scholar]
- 6.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–9. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 7.Paulus WJ, van Ballegoij JJ. Treatment of heart failure with normal ejection fraction: an inconvenient truth! J Am Coll Cardiol. 2010;55:526–37. doi: 10.1016/j.jacc.2009.06.067. [DOI] [PubMed] [Google Scholar]
- 8.LeWinter MM, Granzier H. Cardiac titin: a multifunctional giant. Circulation. 2010;121:2137–45. doi: 10.1161/CIRCULATIONAHA.109.860171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trombitas K, Redkar A, Centner T, Wu Y, Labeit S, Granzier H. Extensibility of isoforms of cardiac titin: variation in contour length of molecular subsegments provides a basis for cellular passive stiffness diversity. Biophys J. 2000;79:3226–34. doi: 10.1016/S0006-3495(00)76555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trombitas K, Wu Y, Labeit D, Labeit S, Granzier H. Cardiac titin isoforms are coexpressed in the half-sarcomere and extend independently. Am J Physiol Heart Circ Physiol. 2001;281:H1793–9. doi: 10.1152/ajpheart.2001.281.4.H1793. [DOI] [PubMed] [Google Scholar]
- 11.Yamasaki R, Wu Y, McNabb M, Greaser M, Labeit S, Granzier H. Protein kinase A phosphorylates titin’s cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ Res. 2002;90:1181–8. doi: 10.1161/01.res.0000021115.24712.99. [DOI] [PubMed] [Google Scholar]
- 12.Kruger M, Kotter S, Grutzner A, Lang P, Andresen C, Redfield MM, Butt E, dos Remedios CG, Linke WA. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res. 2009;104:87–94. doi: 10.1161/CIRCRESAHA.108.184408. [DOI] [PubMed] [Google Scholar]
- 13.Borbely A, Falcao-Pires I, van Heerebeek L, Hamdani N, Edes I, Gavina C, Leite-Moreira AF, Bronzwaer JG, Papp Z, van der Velden J, Stienen GJ, Paulus WJ. Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009;104:780–6. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 14.Anderson BR, Bogomolovas J, Labeit S, Granzier H. The Effects of PKCalpha Phosphorylation on the Extensibility of Titin’s PEVK Element. J Struct Biol. 2010 doi: 10.1016/j.jsb.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hidalgo C, Hudson B, Bogomolovas J, Zhu Y, Anderson B, Greaser M, Labeit S, Granzier H. PKC phosphorylation of titin’s PEVK element: a novel and conserved pathway for modulating myocardial stiffness. Circ Res. 2009;105:631–8. doi: 10.1161/CIRCRESAHA.109.198465. 17 p following 638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hudson BD, Hidalgo CG, Gotthardt M, Granzier HL. Excision of titin’s cardiac PEVK spring element abolishes PKCalpha-induced increases in myocardial stiffness. J Mol Cell Cardiol. 2009 doi: 10.1016/j.yjmcc.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao Y, Ishikura F, Beppu S, Asakura M, Takashima S, Asanuma H, Sanada S, Kim J, Ogita H, Kuzuya T, Node K, Kitakaze M, Hori M. Echocardiographic assessment of LV hypertrophy and function in aortic-banded mice: necropsy validation. Am J Physiol Heart Circ Physiol. 2002;282:H1703–8. doi: 10.1152/ajpheart.00238.2001. [DOI] [PubMed] [Google Scholar]
- 18.Radke MH, Peng J, Wu Y, McNabb M, Nelson OL, Granzier H, Gotthardt M. Targeted deletion of titin N2B region leads to diastolic dysfunction and cardiac atrophy. Proc Natl Acad Sci U S A. 2007;104:3444–9. doi: 10.1073/pnas.0608543104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lahmers S, Wu Y, Call DR, Labeit S, Granzier H. Developmental control of titin isoform expression and passive stiffness in fetal and neonatal myocardium. Circ Res. 2004;94:505–13. doi: 10.1161/01.RES.0000115522.52554.86. [DOI] [PubMed] [Google Scholar]
- 20.Buck D, Hudson BD, Ottenheijm CA, Labeit S, Granzier H. Differential splicing of the large sarcomeric protein nebulin during skeletal muscle development. J Struct Biol. 2010 doi: 10.1016/j.jsb.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung C, Granzier H. Dissection of determinants of passive pressure and measurement of sarcomere length in the mouse left ventricle. Journal of Molecular and Cellular Cardiology. 2011;50:731–9. doi: 10.1016/j.yjmcc.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krenz M, Robbins J. Impact of beta-myosin heavy chain expression on cardiac function during stress. J Am Coll Cardiol. 2004;44:2390–7. doi: 10.1016/j.jacc.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 23.Little WC, Ohno M, Kitzman DW, Thomas JD, Cheng CP. Determination of left ventricular chamber stiffness from the time for deceleration of early left ventricular filling. Circulation. 1995;92:1933–9. doi: 10.1161/01.cir.92.7.1933. [DOI] [PubMed] [Google Scholar]
- 24.Belin RJ, Sumandea MP, Allen EJ, Schoenfelt K, Wang H, Solaro RJ, de Tombe PP. Augmented protein kinase C-alpha-induced myofilament protein phosphorylation contributes to myofilament dysfunction in experimental congestive heart failure. Circ Res. 2007;101:195–204. doi: 10.1161/CIRCRESAHA.107.148288. [DOI] [PubMed] [Google Scholar]
- 25.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–8. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grutzner A, Garcia-Manyes S, Kotter S, Badilla CL, Fernandez JM, Linke WA. Modulation of titin-based stiffness by disulfide bonding in the cardiac titin N2-B unique sequence. Biophys J. 2009;97:825–34. doi: 10.1016/j.bpj.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granzier HL, Irving TC. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J. 1995;68:1027–44. doi: 10.1016/S0006-3495(95)80278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Y, Cazorla O, Labeit D, Labeit S, Granzier H. Changes in titin and collagen underlie diastolic stiffness diversity of cardiac muscle. J Mol Cell Cardiol. 2000;32:2151–62. doi: 10.1006/jmcc.2000.1281. [DOI] [PubMed] [Google Scholar]
- 29.Xia Y, Lee K, Li N, Corbett D, Mendoza L, Frangogiannis NG. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem Cell Biol. 2009;131:471–81. doi: 10.1007/s00418-008-0541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Granzier HL, Radke MH, Peng J, Westermann D, Nelson OL, Rost K, King NM, Yu Q, Tschope C, McNabb M, Larson DF, Labeit S, Gotthardt M. Truncation of titin’s elastic PEVK region leads to cardiomyopathy with diastolic dysfunction. Circ Res. 2009;105:557–64. doi: 10.1161/CIRCRESAHA.109.200964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukuda N, Wu Y, Nair P, Granzier HL. Phosphorylation of titin modulates passive stiffness of cardiac muscle in a titin isoform-dependent manner. J Gen Physiol. 2005;125:257–71. doi: 10.1085/jgp.200409177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 33.Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Boggs J, Lacy JM, Zile MR. Pressure overload-induced alterations in fibrillar collagen content and myocardial diastolic function: role of secreted protein acidic and rich in cysteine (SPARC) in post-synthetic procollagen processing. Circulation. 2009;119:269–80. doi: 10.1161/CIRCULATIONAHA.108.773424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Deel ED, de Boer M, Kuster DW, Boontje NM, Holemans P, Sipido KR, van der Velden J, Duncker DJ. Exercise training does not improve cardiac function in compensated or decompensated left ventricular hypertrophy induced by aortic stenosis. J Mol Cell Cardiol. 2011;50:1017–25. doi: 10.1016/j.yjmcc.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Wu Y, Bell SP, Trombitas K, Witt CC, Labeit S, LeWinter MM, Granzier H. Changes in titin isoform expression in pacing-induced cardiac failure give rise to increased passive muscle stiffness. Circulation. 2002;106:1384–9. doi: 10.1161/01.cir.0000029804.61510.02. [DOI] [PubMed] [Google Scholar]
- 36.Warren CM, Jordan MC, Roos KP, Krzesinski PR, Greaser ML. Titin isoform expression in normal and hypertensive myocardium. Cardiovasc Res. 2003;59:86–94. doi: 10.1016/s0008-6363(03)00328-6. [DOI] [PubMed] [Google Scholar]
- 37.Neagoe C, Kulke M, del Monte F, Gwathmey JK, de Tombe PP, Hajjar RJ, Linke WA. Titin isoform switch in ischemic human heart disease. Circulation. 2002;106:1333–41. doi: 10.1161/01.cir.0000029803.93022.93. [DOI] [PubMed] [Google Scholar]
- 38.Nagueh SF, Shah G, Wu Y, Torre-Amione G, King NM, Lahmers S, Witt CC, Becker K, Labeit S, Granzier HL. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation. 2004;110:155–62. doi: 10.1161/01.CIR.0000135591.37759.AF. [DOI] [PubMed] [Google Scholar]
- 39.Makarenko I, Opitz CA, Leake MC, Neagoe C, Kulke M, Gwathmey JK, del Monte F, Hajjar RJ, Linke WA. Passive stiffness changes caused by upregulation of compliant titin isoforms in human dilated cardiomyopathy hearts. Circ Res. 2004;95:708–16. doi: 10.1161/01.RES.0000143901.37063.2f. [DOI] [PubMed] [Google Scholar]
- 40.Hamdani N, de Waard M, Messer AE, Boontje NM, Kooij V, van Dijk S, Versteilen A, Lamberts R, Merkus D, Dos Remedios C, Duncker DJ, Borbely A, Papp Z, Paulus W, Stienen GJ, Marston SB, van der Velden J. Myofilament dysfunction in cardiac disease from mice to men. J Muscle Res Cell Motil. 2008;29:189–201. doi: 10.1007/s10974-008-9160-y. [DOI] [PubMed] [Google Scholar]
- 41.Nagayama T, Hsu S, Zhang M, Koitabashi N, Bedja D, Gabrielson KL, Takimoto E, Kass DA. Sildenafil stops progressive chamber, cellular, and molecular remodeling and improves calcium handling and function in hearts with pre-existing advanced hypertrophy caused by pressure overload. J Am Coll Cardiol. 2009;53:207–15. doi: 10.1016/j.jacc.2008.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.House C, Wettenhall RE, Kemp BE. The influence of basic residues on the substrate specificity of protein kinase C. J Biol Chem. 1987;262:772–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.