Abstract
Methyl jasmonate is a plant volatile that acts as an important cellular regulator mediating diverse developmental processes and defense responses. We have cloned the novel gene JMT encoding an S-adenosyl-l-methionine:jasmonic acid carboxyl methyltransferase (JMT) from Arabidopsis thaliana. Recombinant JMT protein expressed in Escherichia coli catalyzed the formation of methyl jasmonate from jasmonic acid with Km value of 38.5 μM. JMT RNA was not detected in young seedlings but was detected in rosettes, cauline leaves, and developing flowers. In addition, expression of the gene was induced both locally and systemically by wounding or methyl jasmonate treatment. This result suggests that JMT can perceive and respond to local and systemic signals generated by external stimuli, and that the signals may include methyl jasmonate itself. Transgenic Arabidopsis overexpressing JMT had a 3-fold elevated level of endogenous methyl jasmonate without altering jasmonic acid content. The transgenic plants exhibited constitutive expression of jasmonate-responsive genes, including VSP and PDF1.2. Furthermore, the transgenic plants showed enhanced level of resistance against the virulent fungus Botrytis cinerea. Thus, our data suggest that the jasmonic acid carboxyl methyltransferase is a key enzyme for jasmonate-regulated plant responses. Activation of JMT expression leads to production of methyl jasmonate that could act as an intracellular regulator, a diffusible intercellular signal transducer, and an airborne signal mediating intra- and interplant communications.
Emission of volatiles is among the diverse mechanisms plants use to withstand various environmental stresses. The plant volatiles may be attractive for pollinators and seed-dispersing animals or repellent for a wide range of herbivores. Accumulating evidence suggests that the synthesis of volatiles is also induced by certain external challenges of pathogens and herbivorous animals (1). Not only the attacked plant but also neighboring plants are affected, becoming more attractive to herbivore predators and less susceptible to invaders (2).
A variety of methyl esters of secondary metabolites constitute an important portion of the plant volatiles. For instance, methyl jasmonate (MeJA) is a fragrant compound initially identified from flowers of Jasminum grandiflorum (3), and that is ubiquitously distributed in the plant kingdom (4). MeJA and its free acid jasmonic acid (JA), collectively referred to as jasmonates, are important cellular regulators mediating diverse developmental processes, such as seed germination, flower and fruit development, leaf abscission, and senescence (5). In addition, jasmonates induce plant defense responses against a group of pathogens (6) and mechanical or herbivorous insect-driven wounding (7). In particular, MeJA has become a strong candidate for airborne signals that mediate interplant communication for defense responses (8).
Jasmonates are synthesized in plants via the octadecanoid pathway (9, 10). Briefly, linolenic acid is oxygenated by lipoxigenase (LOX), and then converted to 12-oxo-phytodienoic acid (12-oxo-PDA) by allene oxide synthase (AOS) and allene oxide cyclase. Jasmonic acid is synthesized from the 12-oxo-PDA through reduction and three steps of β-oxidation. Cellular organelles such as plastids (11) or peroxisomes (12) are regarded as the primary site(s) of JA biosynthesis. In addition, a cytoplasmic pathway has also been described (13). JA is then catabolized further to form its volatile counterpart MeJA and numerous conjugates (4).
Because of the similarity of JA to the animal prostaglandin in structure and biogenesis, this compound has been regarded as the primary intracellular transducer. However, it has been observed that a number of biosynthetic intermediates, isomers, derivatives, and metabolites of the octadecanoid pathway are also powerful cellular regulators, depending on biological system (4, 10). For instance, the octadecanoid precursors of JA, including linolenic acid, 13(S)-hydroperoxylinolenic acid and 12-oxo-PDA (14), and a hexadecanoid dinor-oxo-PDA (15) are known to have biological activities in the jasmonate-regulated responses. In addition, a component of flower volatiles cis-jasmone is induced on damage to plant vegetative tissue (16, 17), and has roles as an insect semiochemical and in plant defense (18). The exact role and relative importance of each jasmonate derivative for the cellular responses has not been clearly defined.
Activation of JA biosynthesis is not enough to explain the complexity in biological roles of this family of compounds. It was reported that overexpression of the plastidic flax AOS cDNA caused an increase in JA levels in transgenic potato plants, but this increase did not activate jasmonate-responsive genes (11). Moreover, overexpression of a cytoplasm-localized flax AOS (13) or the Arabidopsis AOS cDNA (19) failed to alter the basal level of jasmonates in the transgenic tobacco and Arabidopsis. Thus, there might be additional key regulatory points for either the accumulation of jasmonates in the cytoplasm or the generation of signal transducers other than JA.
MeJA could be a candidate for such intra- and intercellular signal transducers mediating jasmonate-responsive plant responses, because it can diffuse through the membranes. Characterization of cellular component(s) catalyzing the formation of MeJA would help clarify jasmonate-mediated plant responses.
We have identified an enzyme that catalyzes the methylation of JA to form MeJA. Previously, a floral nectary-specific gene NTR1 was isolated from Brassica campestris (20), which has structural features similar to those of SAMT encoding an S-adenosyl-l-methionine:jasmonic acid carboxyl methyltransferase (21). An Arabidopsis NTR1 homolog, JMT, has been cloned to perform molecular biological studies in more detail. Characteristics of JMT indicated that the gene encodes an S-adenosyl-l-methionine:jasmonic acid carboxyl methyltransferase (JMT), a key enzyme for the jasmonate-regulated plant responses. We propose a possible role of MeJA as an intracellular regulator and a long distance signal mediating intra- and interplant communications.
Materials and Methods
Cloning of JMT.
The gene JMT was isolated from a cDNA (22) and a genomic library of A. thaliana (Columbia ecotype) by screening with the Brassica NTR1 (20). The genomic library was constructed into the XhoI site of partially filled λFixII vector (Stratagene, Heidelberg, Germany). Inserts in the phage DNAs hybridizing with the NTR1 probe were isolated and subcloned into pBluscriptSK(+)II vector. Nucleotide sequences of the overlapping partial clones were determined by using an automated DNA sequencer (Applied Biosystems, model PRISM 377).
Purification of Recombinant JMT Protein.
JMT cDNA was inserted into the pGEX-2T vector (Amersham Pharmacia) at the EcoRI site and fused with glutathione-S-transferase gene (GST). The recombinant was transformed into E. coli BL21. Expressed GST-JMT fusion proteins were purified by using glutathione-agarose beads (Sigma). Further purification was conducted by using a Superdex 300 h 10/30 column on the Superdex 200 FPLC (Amersham Pharmacia) to electrophoretic homogeneity. Fractions exhibiting JMT activity were pooled and dialyzed against the Superdex 200 buffer containing 50 mM Tris⋅HCl (pH 7.5), 100 mM KCl, 5 mM EDTA, and 10 mM β-mercaptoethanol. Purified enzymes were stored at 4°C for 2 months without significant loss of the activity.
Protein concentrations were determined by the method of Bradford (23) by using BSA as a standard.
Assays of JMT Activity and Jasmonate Contents.
JMT activity was determined by measuring the production of MeJA from 1 mM JA and 1 mM S-adenosyl methionine (SAM) including 6.4 μM [14C]SAM. The 50-μl assay buffer contained 50 mM Tris⋅HCl (pH 7.5), 100 mM KCl, and 10 mM β-mercaptoethanol. The reaction mixture was incubated at 20°C for 30 min, and MeJA was extracted with 100 μl of ethyl acetate. Amounts of MeJA synthesized were determined either by counting the radioactivity in the organic phase by using a liquid scintillation counter or by GC-MS.
For GC-MS analyses, total jasmonates were extracted from the enzyme assay mixture or Arabidopsis leaves with 1:2 (vol/vol) mixture of hexane/dichloromethane as described (15). The concentrated samples were separated into MeJA and JA fractions by isocratic silical gel HPLC. MeJA fractions were analyzed directly by GC-MS as described (7), and JA fractions were analyzed after methylation with diazomethane. Total jasmonates were the sum of results obtained from both fractions. Recovery of the standard 9,10-dihydromethyljasmonate added before leaf grinding ranged between 30% and 40%.
In the kinetics studies, appropriate enzyme concentration (20 pmol) and incubation times were taken at which the reaction velocity was linear during the incubation time. Km and Vm values for each substrate were calculated from Lineweaver-Burk plots.
Reverse Transcription (RT)-PCR and Blot Analyses.
For Northern blot analysis, 5 μg of total RNA isolated from leaves of Arabidopsis was electrophoresed on a formaldehyde agarose gel. Alternatively, 2 μg of total RNA was amplified by RT-PCR, and the products were analyzed by Southern blotting.
For genomic Southern blot analysis, 5 μg of Arabidopsis genomic DNA was digested with HindIII and electrophoresed. Blots were probed with the random primer-extended JMT cDNA.
Transgenic Arabidopsis.
Full-length JMT cDNA was digested with Afl3 and inserted into the SmaI site of pBI121 (CLONTECH) in sense orientation under the control of the cauliflower mosaic virus 35S (CaMV 35S) promoter. The construct was transformed into 6-week-old Arabidopsis via Agrobacterium tumefaciens C58C1 after the floral dip transformation procedure (24). The plants were grown at 22°C and 54% relative humidity and exposed to cycles of white light (16 h) and darkness (8 h). Five independent T2 plants were selected and used for further analysis. JMT mRNA expression levels of the transgenic lines were similar to each other. Thus, the line 1 plants were chosen for further experiments.
Plant Treatments and Inoculation.
Wound treatment was carried out on leaves of 5-week-old Arabidopsis by cutting with scissors and pinching with forceps. MeJA, ethylene or 12-oxo-PDA was applied at a concentration of 50 μM. Samples were collected 2 h after wounding, or 48 h after the chemical treatments. During the treatments, plants were kept in an open area, but the treated leaves were enclosed in a plastic bag to separate from distal leaves.
For the pathogen-resistance tests, 7-week-old Arabidopsis plants were inoculated with the virulent pathogen B. cinerea. Conidial spores (1 × 105 per ml) suspended in potato dextrose broth were sprayed onto the plants until droplets ran off. Inoculated plants were incubated at 100% relative humidity for 1 day, transferred into growth chamber maintaining 90% relative humidity, and grown further for 2 days.
Results
Structural Features of JMT.
The JMT cDNA and genomic DNA sequence revealed a putative 45-kDa polypeptide consisting of 389-aa residues spanning 4 exons (Fig. 1). The JMT protein contains motifs that are conserved among O-methyltransferases (25, 26), including the binding sites (the motifs I and III) of SAM, a well-known methyl donor in plant cells. In particular, the JMT protein showed 85, 43, and 40% amino acid sequence similarities with B. campestris NTR1, an orthologue of JMT (20), Clarkia breweri SAMT, a SAM:salicylic acid carboxyl methyltransferase (21), and Antirrhinum majus BAMT, a SAM:benzoic acid carboxyl methyltransferase (27), respectively. The SAM-binding motifs of JMT and NTR1 occur in the N-terminal portion of the protein, instead of C-terminal side as observed in other O-methyltransferases. In addition, the number of amino acid residues between the two motifs is also beyond the range that has been observed in other SAM-using O-methyltransferases. JMT lacks an organelle-specific transit signal peptide and hydrophobic regions long enough to be integrated in membrane, and thus appears to be a cytoplasmic enzyme.
Figure 1.
Comparison of the JMT amino acid sequence and related methyl transferases. JMT, Brassica NTR1 (20), C. breweri SAMT (21), and A. majus BAMT (27) were aligned with the clustal x. Shaded boxes represent amino acid residues that are identical among the three proteins. Gaps (as hyphens) are introduced to maximize the alignment. Amino acid residues identical to those of the motifs I and III that are conserved in O-methyltransferases for the binding of methyl donor are indicated by asterisks.
Enzyme Activity of JMT Protein.
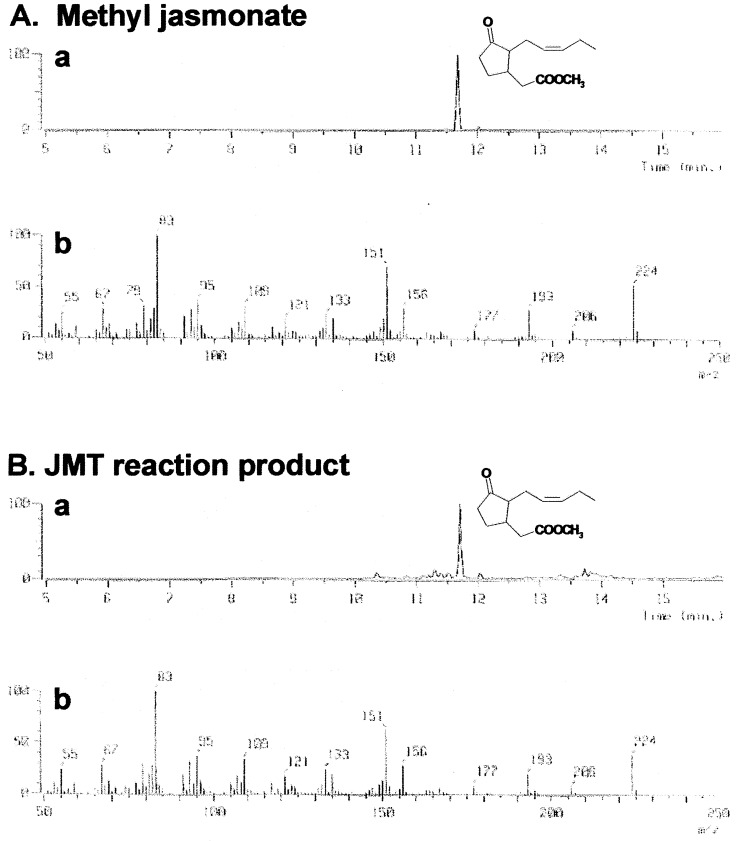
To test the enzyme's activity, affinity-purified recombinant JMT was incubated with various putative substrates in the presence of SAM (Table 1). JA reacted with JMT to produce its methyl ester, MeJA. GC-MS analysis demonstrated that the reaction product from JA was authentic MeJA (Fig. 2). In contrast, 9,10-dihydrojasmonic acid was a poor substrate for JMT, and JMT did not convert 12-oxo-PDA, salicylic acid (SA), benzoic acid, linolenic acid, and cinnamic acid to the corresponding methyl esters (Table 1).
Table 1.
Relative methyltransferase activity of Arabidopsis JMT on jasmonic acid and related substrates
| Substrates | Relative activity, % |
|---|---|
| (±) Jasmonic acid | 100 |
| Dihydrojasmonic acid | 18 |
| Linolenic acid | <1 |
| 12-oxo-phytodienoic acid | <1 |
| Salicylic acid | <1 |
| Benzoic acid | <1 |
| Cinnamic acid | <1 |
All substrates were tested at a 1 mM concentration, and methyltransferase activity was determined by measuring the radioactivity of transferred [14C]methyl group from SAM. The relative activity of JMT with jasmonic acid was set arbitrarily at 100%. Values are the averages of three independent measurements.
Figure 2.
Assay of JMT activity by GC-MS analysis. (A) MeJA standard. (B) Reaction product from JA by JMT (10 pmol) under the standard assay condition (Materials and Methods). The reaction products were extracted with ethyl acetate and analyzed by GC-MS. (a) Mass chromatograms; (b) mass spectrums. The mass of MeJA is 224 Da.
Optimal conditions for MeJA production by JMT were determined (data not shown). Highest enzyme activity was observed at between pH 7.0–8.0, at 20°C, and in the presence of 100 mM KCl. Presence of divalent ions such as Ca2+, Cu2+, Fe2+, Mg2+, Mn2+, and Zn2+ in the reaction mixture was inhibitory for the JMT enzyme activity. Approximately 80% of the enzyme activity was abolished by divalent ions at 5 mM concentration. At the same concentration, monovalent ions such as Na+ and NH4+ also exhibited inhibitory effect, reducing the MeJA production by 50–60%.
Under the standard enzyme reaction conditions described in Materials and Methods, kinetic parameters for the two substrates, JA and methyl donor SAM, were calculated from Lineweaver-Burk plots. Km values of JMT for JA and SAM were 38.5 μM and 6.3 μM, respectively (Table 2). Turnover number kcat values for the two substrates were 25 S−1 and 70 S−1, respectively.
Table 2.
Kinetic parameters of JMT
| Substrate | Km, μM | Vm, nmol/ min | kcat, s−1 | kcat/Km, μM−1⋅s−1 |
|---|---|---|---|---|
| S-adenosyl methionine | 6.3 ± 0.7 | 84 ± 8.2 | 70 ± 6.8 | 11.1 ± 0.9 |
| (±) Jasmonic acid | 38.5 ± 5.8 | 30 ± 3.6 | 25 ± 3.0 | 0.7 ± 0.05 |
The values are the average of three independent measurements. Each value was obtained by Lineweaver-Burk plots, which were linear within experimental error.
Expression Pattern of JMT in Arabidopsis.
RT-PCR analysis revealed that JMT was expressed differentially in different organs, developmental stages, and in response to various stimuli. JMT RNA was not detected in young seedlings but was expressed in most parts of mature plants, particularly in rosettes, cauline leaves, and flowers (Fig. 3A). Expression of JMT in the flower was temporally regulated with the highest expression during the opening of flower at the stage 13 (28); expression was reduced abruptly on flower opening (Fig. 3B). JMT expression was induced by wounding or MeJA treatment but not by ethylene (Fig. 3C), exhibiting similar to the expression pattern of the JA-inducible gene JR2 (29). JMT was not induced when plants were treated with both MeJA and ethylene, whereas PDF1.2 was synergistically induced by this treatment. JMT was also induced in distal leaves systemically by wounding or MeJA treatment, but not by 12-oxo-PDA (Fig. 3D).
Figure 3.

Analysis of JMT gene expression by RT-PCR. Two micrograms of total RNA isolated was amplified by RT-PCR, and the products were analyzed by using a JMT gene-specific probe. (A) Organ- and developmental stage-specific expression. (B) Stage-specific expression during the flower development. Flower stages are designated according to Bowman (28). Stage 13 is at the anthesis. (C) Local expression induced by various treatments. Expression of PDF1.2 and JR2 genes are also analyzed as references. (D) Systemic expression induced by wounding or MeJA. The treated leaves (local) were enclosed in a plastic bag to be separated from distal leaves (systemic) and the plants were kept in an open area.
Transgenic Arabidopsis Overexpressing JMT.
Full-length JMT cDNA (1.5 kb) was fused with the CaMV 35S promoter, and transformed into Arabidopsis to generate transgenic plants overproducing the JMT. Genomic Southern blot analysis confirmed successful integration of the transgene in the genome of the transgenic plants (1.2-kb HindIII fragment in the line 1) where the endogenous gene (6.5-kb fragment) is present as a single copy (Fig. 4A). In Northern blot analysis, JMT cDNA probe detected 1.5-kb transcripts overexpressed in leaves of the transgenic plants (Fig. 4B). We have analyzed five independent T2 plants. Phenotypic characteristics such as mRNA expression levels, MeJA concentrations, and responses to the various treatments were similar in all lines.
Figure 4.
Southern and Northern blot analyses of transgenic plants overexpressing JMT. (A) Five micrograms of Arabidopsis genomic DNA isolated from leaves of wild type (W) or transgenic JMT (T) plants (line 1) was digested with HindIII and electrophoresed. Sizes of the markers in kilobase pairs are indicated on the left side of the gel. (B) Five micrograms of total RNA isolated from wild-type (W) or transgenic (T) plants (line 1) was electrophoresed on a formaldehyde agarose gel. Location of 25S and 18S rRNAs are shown on the right. Blots were probed with a random primer-extended cDNA. JMT mRNA expression levels of other transgenic lines (2 through 5) were similar to that of line 1 plants.
GC-MS analysis revealed that the transgenic plants contained about 3-fold higher level of MeJA, whereas JA content was not altered (Table 3). This result is consistent with the enzymatic activity of JMT observed in vitro (see Fig. 2).
Table 3.
Levels of JA and MeJA in wild and transgenic Arabidopsis plants, ng/g fresh wt
| Compound | Wild | Transgenic |
|---|---|---|
| Jasmonic acid | 26.53 ± 8.24 | 23.72 ± 7.82 |
| Methyl jasmonate | 19.37 ± 4.87 | 58.50 ± 9.65 |
| Total jasmonate | 45.90 ± 13.11 | 82.22 ± 17.47 |
JA and MeJA in rosette leaves of wild-type and transgenic (line 1) Arabidopsis were separated by HPLC and quantified with GC-MS (7). Total JA is the sum of JA and MeJA. Values represent the mean ± SD of three independent experiments. JA and MeJA levels in other transgenic lines (2 through 5) were similar to that of line 1 plants within experimental errors.
The transgenic Arabidopsis exhibited phenotypes visually indistinguishable from those of nontransformed plants. In the transgenic plants, however, expression of jasmonate-responsive genes (6, 29) such as LoxII, AOS, JR2, JR3, DAH1, PDF1.2, and VSP was elevated in the absence of wounding or jasmonate treatment (Fig. 5). In contrast, SA-responsive genes (6) such as PR1, PR5, and ST were not induced in these plants. This result is reminiscent of the gene expression pattern induced by externally applied jasmonates.
Figure 5.
Expression analysis of jasmonate-responsive genes in transgenic Arabidopsis-overexpressing JMT gene. Five micrograms of total RNA was isolated from rosette leaves of wild-type (W) and transgenic (T) Arabisdopsis line 1, respectively. Northern blots were hybridized with each of the random primer-extended cDNA clones as indicated. LOXII, lipoxygenase II; AOS, allene oxide synthase; JR2, jasmonate response protein 2; JR3, jasmonate responsive aminohydrolase; DHS1, 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase; PDF1.2, plant defensin 1.2; VSP, vegetative storage protein, PR1, pathogen related protein 1; PR5, acidic thaumatin-like protein; ST, sulfonyl transferase; 25S, 25S ribosomal RNA. Expression patterns of the genes in other transgenic lines (2 through 5) were similar to that of line 1 plants.
The transgenic plants exhibited elevated resistance to the virulent pathogen B. cinerea (Fig. 6). After 3 days of inoculation, wild-type Arabidopsis plants exhibited severe symptom of disease, whereas the transgenic plants did not.
Figure 6.
Resistance of the transgenic Arabidopsis against a fungal pathogen, B. cinerea. Seven-week-old Arabidopsis plants were inoculated with the virulent pathogen B. cinerea. Conidial spores (1 × 105 per ml) suspended in potato dextrose broth were sprayed on plants until droplets ran off. Inoculated plants were grown further for 3 days. Left, wild-type Arabidopsis; right, transgenic Arabidopsis (line 1) constitutively expressing JMT gene. Degree of resistance of other transgenic lines (2 through 5) to the fungal infection was similar to that of line 1 plants.
Discussion
An enzyme catalyzing the methylation of JA to form MeJA has been identified in this study. The Arabidopsis gene JMT encodes a protein homologous to a new class of carboxyl methyltransferases, such as the B. campestris floral nectary-specific NTR1 (20), the C. breweri SAMT (21), and the A. majus BAMT (27) (Fig. 1). The recombinant JMT specifically converts JA into MeJA in vitro (Table 1 and Fig. 2). The kinetic parameters of JMT (Table 2) were comparable to those of SAMT (21) and BAMT (30). Increased level of MeJA in the transgenic plants overexpressing JMT (Table 3) indicate that the protein encoded by JMT is indeed a SAM:jasmonic acid carboxyl methyltransferase.
It was found in this study that substrate specificity and kinetic characteristics of the recombinant NTR1 protein of B. campestris (20) were almost identical to those of JMT (data not shown), suggesting that these two enzymes are orthologues. It appears that JMT and NTR1 catalyze MeJA production in the cytoplasm, because these proteins lack apparent organ-specific transit signal peptide and hydrophobic regions long enough to be integrated in membranes (Fig. 1). In fact, an immuno-localization experiment demonstrated that NTR1 was located in the cytoplasm of Brassica cells (20).
JMT was expressed differentially in different organs at particular developmental stages and induced by wounding (Fig. 3). The expression pattern of JMT is consistent with previous observations of jasmonate distribution among developing tissues and of jasmonate-responsive defense responses (5). JMT was activated when Arabidopsis tissues were treated with exogenous MeJA (Fig. 3C). Thus, MeJA can amplify JMT expression induced at the developmental stages and by external stimuli including wounding. Ethylene inhibited JMT expression. In contrast, PDF1.2 encoding a defense protein defensin (31–33) was synergistically induced by MeJA and ethylene treatments, as was reported for a number of plant defense genes (34, 35). These results suggest that the synergistic effect exerted by ethylene on the activation of PDF1.2 is not by the enhancement of MeJA synthesis but rather, as proposed (31), by a cross-talk between the independent signaling pathways derived from them.
JMT was also induced systemically in distal leaves after wounding or MeJA treatment (Fig. 3D). JMT might be activated in the distal leaves by a long distance signal. Systemin (36, 37) and electrical signals (38) have been proposed to mediate the systemic induction of jasmonate synthesis in response to wounding. In addition, MeJA itself may act as such a systemic signal. It was suggested that systemic accumulation as well as movement among plants of jasmonates occur via the vapor phase in the form of MeJA (8, 39). Alternatively, MeJA may diffuse to distal parts of the plant through phloem to act as a long distance intercellular transducer, as observed in the study of systemin transport (36). Recently, a putative systemin receptor has been identified (40, 41). The systemin-receptor binding increases severalfold in response to MeJA (41). Thus, it is possible that MeJA and systemin migrate through phloem together exerting a synergistic mode of action.
It has been assumed that JA is the primary intracellular signal transducer and that exogenously applied or airborne MeJA is converted to JA in plant tissue. As described, however, MeJA may have its own role in developmental processes and defense responses. We tested this hypothesis by generating transgenic Arabidopsis that constitutively express the JMT gene to provide elevated levels of MeJA in the cytoplasm. The transgenic plants contained elevated level of MeJA without altering JA content (Table 3). In the transgenic plants, expression of various jasmonate-responsive genes was elevated in the absence of wounding or jasmonate treatment (Fig. 5). Expression of PDF1.2 is known to be responsible for plant defense against a group of pathogenic fungi (31–33). Indeed, the transgenic plants exhibited enhanced resistance to the virulent pathogen B. cinerea (Fig. 6).
It has been suggested that AOS is a major control point in the octadecanoid signaling pathway (42, 43). However, overexpression of the plastidic flax AOS cDNA in transgenic potato plants was not enough to trigger a constitutive expression of wound- or jasmonate-responsive genes, even though the plants contained 6- to 12-fold increased levels of JA (11). The transgenic plants responded to wounding by activating the expression of the genes responsible for the stresses. It was thus proposed that mechanical damage or water stress facilitates the movement of JA sequestered in the chloroplast to the cytoplasm where its receptors are presumably present. However, free acid JA might not be able to move across the cellular membrane without a carrier because of its acidic nature, as observed in a study of intracellular distribution of abscisic acid (44). It was also proposed that such external stimuli might activate an alternative JA biosynthetic pathway located in the cytoplasm. However, JA levels in the transgenic plants overexpressing cytoplasm-localized AOS were not increased unless the tissues were damaged (13, 19). Thus, even though AOS is a control point in the biosynthesis of jasmonates, there might be other regulatory enzymes or mechanisms for the activation of jasmonate-responsive genes. As shown in the present study with the transgenic Arabidopsis, expression of JMT can induce genes such as LOXII and AOS (Fig. 5). This induction may provide more substrate for MeJA synthesis and further induce jasmonate-responsive genes.
We propose here that JMT is a key enzyme for the jasmonate-regulated plant responses and that MeJA can act as an intracellular regulator, a diffusible intercellular signal transducer, or an airborne signal mediating intra- and interplant communications. Some signals generated during an early event of developmental processes or defense responses may activate JMT that can self-amplify, stimulate, or regulate its own expression, propagating the MeJA-mediated cellular responses throughout whole plants.
Acknowledgments
We thank Dr. John Mullet of the Texas A&M University and Dr. Minkyun Kim of Seoul National University for critical reviews of the manuscript. We thank Dr. José Sánchez-Serrano of the Centro National de Biotecnologiá for providing cDNA clones for JR2 and JR3. This work was supported by grants from the Korea Research Foundation and in part from the ScigenHarvest Co., Korea. H.S.S. and J.-J.C. are recipients of fellowships from the Ministry of Education through the Brain Korea 21 Project.
Abbreviations
- JMT
S-adenosyl-l-methionine:jasmonic acid carboxyl methyltransferase
- MeJA
methyl jasmonate
- JA
jasmonic acid
- SAM
S-adenosyl-l-methionine
- AOS
allene oxide synthase
- 12-oxo-PDA
12-oxo-phytodienoic acid
- RT-PCR
reverse transcription–PCR
Footnotes
References
- 1.Paré P W, Tumlinson J H. Plant Physiol. 1999;121:325–331. [PMC free article] [PubMed] [Google Scholar]
- 2.Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J. Nature (London) 2000;406:512–525. doi: 10.1038/35020072. [DOI] [PubMed] [Google Scholar]
- 3.Demole E, Lederer E, Mercier D. Helv Chim Acta. 1962;45:675–685. [Google Scholar]
- 4.Hamberg M, Gardner H W. Biochim Biophys Acta. 1992;1165:1–18. doi: 10.1016/0005-2760(92)90069-8. [DOI] [PubMed] [Google Scholar]
- 5.Creelman R A, Mullet J E. Proc Natl Acad Sci USA. 1995;92:4114–4119. doi: 10.1073/pnas.92.10.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reymond P, Farmer E E. Curr Opin Plant Biol. 1998;1:404. doi: 10.1016/s1369-5266(98)80264-1. [DOI] [PubMed] [Google Scholar]
- 7.Creelman R A, Tierney M L, Mullet J E. Proc Natl Acad Sci USA. 1992;89:4938–4941. doi: 10.1073/pnas.89.11.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farmer E E, Ryan C A. Proc Natl Acad Sci USA. 1990;87:7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Creelman R A, Mullet J E. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- 10.Beale M H, Ward J L. Nat Prod Rep. 1998;15:533–548. doi: 10.1039/a815533y. [DOI] [PubMed] [Google Scholar]
- 11.Harms K, Atzorn R, Brash A, Kühn H, Wasternack C, Willmitzer L, Peña-Cortés H. Plant Cell. 1995;7:1645–1654. doi: 10.1105/tpc.7.10.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stintzi A, Browse J. Proc Natl Acad Sci USA. 2000;97:10625–10630. doi: 10.1073/pnas.190264497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, Avdiushko S, Hildebrand D F. Plant Mol Biol. 1999;40:783–793. doi: 10.1023/a:1006253927431. [DOI] [PubMed] [Google Scholar]
- 14.Farmer E E, Ryan C A. Plant Cell. 1992;4:129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber H, Vick B A, Farmer E E. Proc Natl Acad Sci USA. 1997;94:10473–10478. doi: 10.1073/pnas.94.19.10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loughrin J H, Manukian A, Heath R R, Tumlinson J H. J Chem Ecol. 1995;21:1217–1227. doi: 10.1007/BF02228321. [DOI] [PubMed] [Google Scholar]
- 17.Paré P W, Tumlinson J H. Plant Physiol. 1997;114:1161–1167. doi: 10.1104/pp.114.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birkett M A, Campbell C A M, Chamberlain K, Guerrieri E, Hick A J, Martin J L, Matthes M, Napier J A, Pettersson J, Pickett J A, et al. Proc Natl Acad Sci USA. 2000;97:9329–9334. doi: 10.1073/pnas.160241697. . (First Published July 18, 2000; 10.1073/pnas.160241697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laudert D, Schaller F, Weiler E W. Planta. 2000;211:163–165. doi: 10.1007/s004250000316. [DOI] [PubMed] [Google Scholar]
- 20.Song J T, Seo H S, Song S I, Lee J S, Choi Y D. Plant Mol Biol. 2000;42:647–655. doi: 10.1023/a:1006381625421. [DOI] [PubMed] [Google Scholar]
- 21.Ross J R, Nam K H, D'Auria J C, Pichersky E. Arch Biochem Biophys. 1999;367:9–16. doi: 10.1006/abbi.1999.1255. [DOI] [PubMed] [Google Scholar]
- 22.Newman T, de Bruijn F J, Green P, Keegstra K, Kende H, McIntosh L, Ohlrogge J, Raikhel N, Somerville S, Thomashow M, Retzel E, Somerville C. Plant Physiol. 1994;106:1241–1255. doi: 10.1104/pp.106.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 24.Clough S J, Bent A F. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 25.Kagan R M, Clarke S. Arch Biochem Biophys. 1994;310:417–427. doi: 10.1006/abbi.1994.1187. [DOI] [PubMed] [Google Scholar]
- 26.Joshi C P, Chiang V L. Plant Mol Biol. 1998;37:663–674. doi: 10.1023/a:1006035210889. [DOI] [PubMed] [Google Scholar]
- 27.Dudareva N, Murfitt L M, Mann C J, Gorenstein N, Kolosova N, Kish C M, Wood K. Plant Cell. 2000;12:949–961. doi: 10.1105/tpc.12.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowman J L. Arabidopsis: An Atlas of Morphology and Development. New York: Springer; 1994. [Google Scholar]
- 29.Rojo E, León J, Sánchez-Serrano J J. Plant J. 1999;20:135–142. doi: 10.1046/j.1365-313x.1999.00570.x. [DOI] [PubMed] [Google Scholar]
- 30.Murfitt L M, Kolosova N, Mann C J, Dudareva N. Arch Biochem Biophys. 2000;382:145–151. doi: 10.1006/abbi.2000.2008. [DOI] [PubMed] [Google Scholar]
- 31.Penninckx I A M A, Thomma B P H J, Buchala A, Métraux J-P, Broekaert W F. Plant Cell. 1998;10:2103–2113. doi: 10.1105/tpc.10.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomma B P H J, Eggermont K, Penninckx I A M A, Mauch-Mani B, Vogelsang R, Cammue B P A, Broekaert W F. Proc Natl Acad Sci USA. 1998;95:15107–15111. doi: 10.1073/pnas.95.25.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomma B P H J, Eggermont K, Tierens K F, Broekaert W F. Plant Physiol. 1999;121:1093–1102. doi: 10.1104/pp.121.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, Chang P-F L, Liu D, Narasimhan M L, Raghothama K G, Hasegawa P M, Bressan R A. Plant Cell. 1994;6:1077–1085. doi: 10.1105/tpc.6.8.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Donnell P J, Calvert C, Atzorn R, Wasternack C, Leyser H M O, Bowles D J. Science. 1996;274:1914–1917. doi: 10.1126/science.274.5294.1914. [DOI] [PubMed] [Google Scholar]
- 36.Pearce G, Strydom S, Johnson S, Ryan C A. Science. 1991;253:895–898. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- 37.Ryan C A. Biochim Biophys Acta. 2000;1477:112–121. doi: 10.1016/s0167-4838(99)00269-1. [DOI] [PubMed] [Google Scholar]
- 38.Wildon D C, Thain J F, Minchin P E H, Gubb I R, Reilly A J, Skipper Y D, Doherty H M, O'Donnell P J, Bowles D J. Nature (London) 1992;360:62–65. [Google Scholar]
- 39.Franceschi V R, Grimes H D. Proc Natl Acad Sci USA. 1991;83:6745–6749. doi: 10.1073/pnas.88.15.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meindl T, Boller T, Felix G. Plant Cell. 1998;10:1561–1570. doi: 10.1105/tpc.10.9.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheer J M, Ryan C A. Plant Cell. 1999;11:1525–1535. doi: 10.1105/tpc.11.8.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laudert D, Weiler E W. Plant J. 1998;15:675–684. doi: 10.1046/j.1365-313x.1998.00245.x. [DOI] [PubMed] [Google Scholar]
- 43.Sivasankar S, Scheldrick B, Rothstein S J. Plant Physiol. 2000;122:1335–1342. doi: 10.1104/pp.122.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heilmann B, Hartung W, Gimmler H. Z Pflanzenphysiol Biol. 1980;97. S.:67–78. [Google Scholar]