Abstract
Obesity and associated metabolic and cardiovascular disease risk are correlated with reduced circulating adiponectin (APN) levels. Metabolic and cardiovascular disease risk is also increased following menopause and may be linked to disturbances in estrogen receptor (ER) signaling in adipose. We hypothesized that age-associated estrogen (E2)-deficiency alters the ERα/β ratio in adipose tissue and increases risk for metabolic disease via APN-dependant mechanisms. Visceral adipose was isolated from adult (6 mo) and aged (24 mo) female Fisher344 rats (n=5–6/group) with ovaries intact or removed (OVX) and subjected to western blotting. Notably, weight was greatest in aged OVX (p<0.01) and associated with a two-fold increase in ERβ protein vs adult intact rats (p<0.001). ERα levels were increased in aged OVX vs adult OVX. Intra-adipocyte APN was also increased in aged OVX vs all groups (p<0.01), while circulating APN levels decreased in aged OVX vs adult OVX (p<0.05). Endoplasmic reticulum protein of 44kDa (Erp44) levels remained the same (p=0.09). Adiponectin receptor (AdipoR)1 and peroxisome proliferator-activated receptor (PPAR)α were also unchanged. AdipoR2, PPARγ, and the activated AMP-dependant kinase (pAMPK)/total AMPK ratio all decreased with age (p<0.05). Collectively, these data suggest that age-associated increases in ERβ paired with decreased PPARγ levels may predispose E2-deficent post-menopausal women for increased adiposity, and associated metabolic and cardiovascular disease risk. Reduced circulating APN, and AdipoR2 levels may contribute to age and E2-deficiency linked disease progression.
Keywords: estrogen receptor α, menopause, aging, metabolic syndrome, peroxisome proliferator-activated receptorγ
INTRODUCTION
The prevalence of obesity and associated metabolic risk in women increases significantly after menopause, such that 40–50% of women aged 50–59 develop metabolic complications according to the most recent report from the National Health and Nutrition Examination Survey [1]. However, the signaling alterations by which estrogen (E2)-deficiency increases obesity risk with aging are poorly understood, and development of therapeutic interventions have been stalled by the detrimental effects of hormone replacement on coronary heart disease (CHD) morbidity and mortality in women [2, 3]. Interestingly, estrogen receptor (ER) polymorphisms have been linked to increased metabolic and cardiovascular risk in post-menopausal women, indicating a potential important role for ER isoforms α and/or β in regulating adiposity, metabolic derangements and cardiovascular risk [4–6]. In this regard, both ERα and ERβ are present in adipose tissue and appear to reciprocally regulate adiposity [7].
Increased adiposity associated with the development of metabolic derangements such as insulin resistance are observed in ERα knock out (αERKO) mice [8–11], thus indicating that ERα plays an important role in positively regulating adipose metabolism. In contrast, selective activation of ERβ exacerbates metabolic phenotypes [8], and ovariectomy of αERKO mice ammeliorates increases in adiposity and development of insulin resistance [12]. Thus it is plausible that alterations in ER levels, specifically reductions in ERα and increases in ERβ, not only contribute to increased adiposity with advancing age, but may also play a critical role in increasing metabolic and cardiovascular disease risk in post-menopausal women.
The specific mechanisms by which ERs regulate adiposity and adipocyte metabolism are incompletely understood, however, it is known that ERβ inhibits transcriptional activity of peroxisome proliferator activated receptor gamma (PPARγ a master regulator of insulin sensitivity [13]. PPARγ mediates its effects, in part, by regulating the intracellular processing and secretion of the adipokine, adiponectin (APN). Low plasma levels of APN are not only associated with obesity and metabolic syndrome, but also predictive of CHD risk [14–16]. It is unknown whether and to what extent age-associated E2-deficiency disrupts the balance of ERα and β in adipose tissue and if APN is the causal link between dysregulated ER signaling and disease.
Accordingly, we hypothesized that age-associated E2-deficiency and weight gain alters the ERα/β ratio in favor of ERβ in the adipose tissue which in turn, is linked to reduced protein levels of PPARγ and circulating APN. We also sought to characterize alterations in the downstream ER targets PPARγ, Erp44 and APN, which are likely to influence metabolic and cardiovascular disease risk in this model. γFinally, to gain an appreciation of possible downstream APN derangements in adipocytes in a setting of aging-associated E2-deficiency, we also assessed the adiponectin receptors (AdipoR)s 1 and 2, as well as downstream regulators of lipid metabolism PPARα and AMPK in adipose tissue.
METHODS
Animal Care
Adult (5–6 mo, n=11) and aged (23–24 mo, n=11) female Fischer 344 (F344) rats were obtained from Harlan Sprague Dawley (Indianapolis, IN) and Taconic (Hudson, NY). Rats were exposed to a 12h light/dark cycle and received a standard laboratory rodent diet (LabDiet) and water ad libitum. Rats were anesthetized using a sodium pentobarbital intra-peritoneal injection, sacrificed, and perimetrial and retroperitoneal adipose depots were pooled and collected. Tissue was snap frozen in liquid N2 and stored at −80°C until further analysis. Both animal facilities and protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University. Adequate measures were taken to minimize pain and discomfort of animals.
Surgical Ovariectomy
To model E2 deficiency, adult (n=6) and aged (n=6) F344 female rats underwent surgical ovariectomy (OVX) at 4 and 22 months of age, respectively; surgeries were performed by suppliers. Post-surgery adult and aged OVX animals recovered for four weeks prior to experimental use and thus were age-matched to adult (5 mo) and aged (23 mo) intact animals. Uterine weight was used to confirm E2-deficiency. We have previously documented reduced circulating E2 levels in similarly prepared adult and aged OVX rats through radioimmunoassay [17, 18].
Serum Adiponectin Levels
Serum levels of adiponectin (APN) were assessed using an enzyme-linked immunosorbent assay (ELISA) (EZRADP-62K; Millipore) according to manufacturer’s instructions.
Tissue Homogenization
Adipose tissue (~1 gm) was homogenized with a Polytron (Kinematica) in 3.5 volumes of buffer containing (in mM): 40 HEPES, pH 7.4; 4 EGTA; 100 NaF; 50 KCl; 1 EDTA; 100 β-glycerol phosphate; 2 Benzamidine; 1 Na-orthovanadate; 0.4 Aminoethyl benzenesulfonyl fluoride; 6.5 CHAPS; 1% Triton X-100, and 0.2ug/ml of microcystin. Homogenates were centrifuged at 4°C for 10 min at 10,000 X g and the supernatant was assessed for protein concentration using the method of Bradford [19].
Western Blotting
Protein lysates were subject to separation using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene fluoride membranes and blocked in 6% non-fat dry milk for 2 hours at room temperature as described, by us, previously [20–22]. Membranes were probed overnight at 4°C with primary antibodies purchased from Santa Cruz Biotechnology Inc. at a concentration of 1:1000 for APN (30 kDa, sc-26497), ERα (66 kDa, sc-542), ERβ (56 kDa, sc-8974), PPARγ (67 kDa, sc-7196); and 1:300 for PPARα (55 kDa, sc-9000). Cell Signaling antibodies were used at a concentration of 1:500 for Thr-172 phosphorylated AMPK (pAMPK) (62 kDa, 2531) and 1:1000 for Erp44 (44 kDa, 2886S). A concentration of 1:1000 was used for both AdipoR2 (42 kDa, AdipoR2-1; α-Diagnostics) and AMPK (63 kDa, 07-350; Millipore). AdipoR1 (42 kDa), a gift from R. Ramachandran, was used at a concentration of 1:1000. Membranes were then incubated with either horseradish peroxidase (HRP)-linked anti-rabbit 1:20,000 for ERα, PPARα, PPARγ, Erp44, AMPK, pAMPK, AdipoR1, and AdipoR2 or HRP-linked anti-goat at 1:25,000 for ERβ and APN for 1 h at room temperature and visualized using Enhanced Chemiluminescence (ECL; GE Healthcare). Densitometry was performed using Scion Image (NIH). To correct for potential protein loading errors all membranes were stained with SYPRO Ruby Blot Stain or Ponceau S (100 μg protein/lane) and densitometry performed as previously described [18, 20–22].
Statistical Analysis
All data are presented as means ± SE and analyzed using the Statistical Analysis System (SAS) general linear model procedure. A two-way ANOVA was used to analyze all western data with age × E2 status as the interaction term. Post hoc analysis was performed on significant interactions using a Tukey test. An α-level of p<0.05 was used for all comparisons and considered statistically significant.
RESULTS
Animal Characteristics
As demonstrated by us previously [22], rat weight was significantly increased with both age and OVX (Table 1; p<0.01). OVX resulted in a significant weight gain in both adult and aged animals (14% and 3%, respectively). Uterine weight decreased significantly with OVX (Table 1; p<0.0001), confirming successful OVX surgery and relative E2-deficiency.
Table 1.
Rat body and uterine weight presented in grams (g); (n=5–6/group).
| Characteristic | Adult | Adult OVX | Aged | Aged OVX |
|---|---|---|---|---|
| N | 5 | 6 | 5 | 6 |
|
| ||||
| Rat Weight (g) | 202.70 | 213.25 | 270.82* | 307.80*† |
| Uterine Weight (g) | 0.60 | 0.12† | 0.59 | 0.32† |
Abbreviations: OVX, ovariectomy.
denotes age effect;
denotes OVX effect (p<0.05).
Estrogen Receptors, PPARγ, and Erp44 Protein Levels
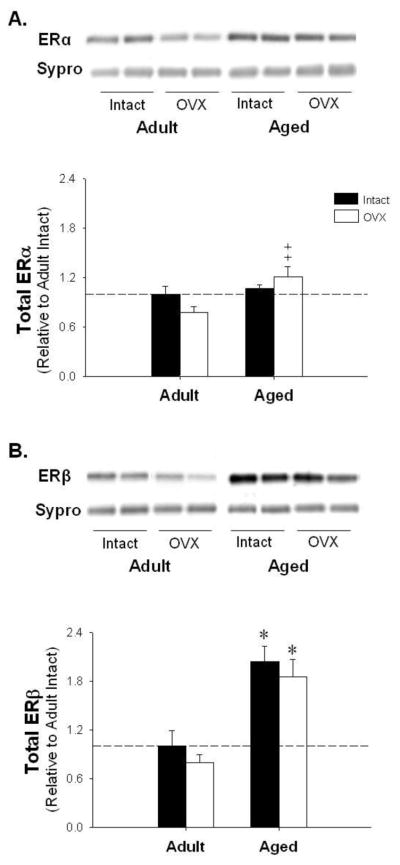
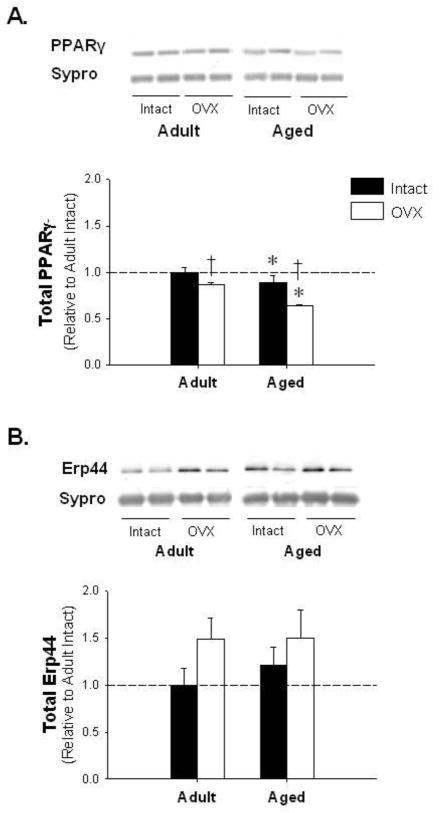
Immunoblotting for ERα, ERβ, PPARγ, and Erp44 was performed in adipose tissue isolated from adult and aged rats and group differences were expressed relative to levels in adult ovary-intact animals. ERα levels showed no significant change with age or E2-deficiency relative to adult control. However, aged OVX animals demonstrated a significant increase in ERα relative to adult OVX (Figure 1A). Furthermore, we observed a two-fold increase in ERβ with age when compared to both adult intact and OVX animals (Figure 1B). Interestingly, PPARγ, which plays a significant role in adipose metabolism, decreased with age and OVX (Figure 2A). Finally, Erp44, a protein downregulated by PPARγ known to directly bind and retain APN intracellularly [23], remained constant with E2-deficiency in adipose tissue isolated from both adult and aged female rats (p=0.09; Figure 2B).
Figure 1.
The effects of aging and E2 deficiency on estrogen receptor α (ER)α and ERβ protein levels. Representative blots and adipose protein levels for ERα (Panel A); and ERβ (Panel B). *denotes age effect, ‡ denotes significantly different from adult OVX; (p < 0.05; n=5–6/group). Values are means ± SEM; data is presented relative to adult intact, and corrected with Sypro Ruby Blot Stain.
Figure 2.
Peroxisome proliferator activated receptorγ (PPAR)γ is reduced with age and E2-deficiency while endoplasmic reticulum protein of 44 kDa (Erp44) is unchanged. Representative blots and adipose protein levels for PPARγ (Panel A) and Erp44 (Panel B). * denotes age effect, † denotes OVX effect; (p < 0.05; n=5–6/group). Values are means ± SEM; data is presented relative to adult intact, and corrected with Sypro Ruby Blot Stain.
Adiponectin, Adiponectin Receptors, and Downstream Targets AMPK and PPARα Protein Levels
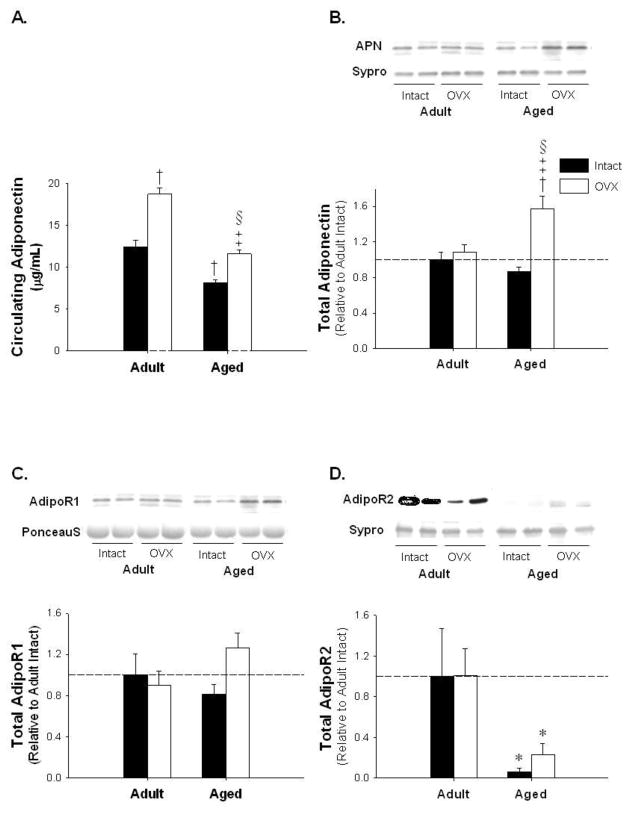
To investigate potential dysregulated autocrine signaling related to ERs in adipose tissue with advancing age and E2-deficiency, we assessed serum APN, intra-adipocyte APN, and its receptors, AdipoR1 and AdipoR2. Circulating APN levels increased by 50 percent with OVX in adult, while reductions of 35 percent and 40 percent were observed in aged and aged OVX, respectively, relative to adult and adult OVX (Figure 3A; p<0.05). In contrast, immunoblotting revealed that APN protein levels were increased in aged OVX animals (Figure 3B; p<0.05). AdipoR1 levels increased in aged OVX (Figure 3C p=0.056). AdipoR2 levels were significantly decreased with age (Figure 3D; p<0.05).
Figure 3.
Intra-adipocyte adiponectin (APN) is significantly increased with age-associated E2-deficiency while circulating APN is significantly increased with OVX and reduced with age. Adiponectin Receptor 1 (AdipoR) 1 is unchanged with age or E2-deficiency while AdipoR2 is decreased with age. Circulating APN (Panel A), representative blots and adipose protein levels for APN (Panel B), AdipoR1 (Panel C), and AdipoR2 (Panel D). * denotes age effect, † denotes significantly different from adult intact, ‡ denotes significantly different from adult OVX, § denotes significantly different from aged intact; (p < 0.05; n=5–6/group). Values are means ± SEM; data is presented relative to adult intact. APN and AdipoR2 are corrected with Sypro Ruby Blot Stain and AdipoR1 is corrected with Ponceau S.
While AMPK and pAMPK levels did not change with age-associated E2-deficiency (Figure 4A and B; p=0.39 and p=0.29, respectively), the ratio of active pAMPK to AMPK was significantly decreased with age (Figure 4C; p<0.05). PPARα, a downstream target of AdipoR2 known to regulate lipid metabolism [24], was unchanged with age or E2-deficiency (Figure 5).
Figure 4.
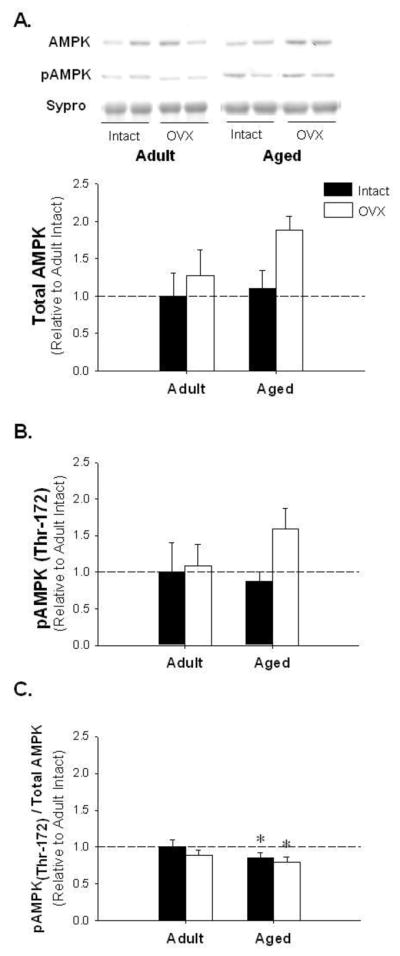
Adenosine monophosphate-dependent protein kinase (AMPK) and activated AMPK (pAMPK) protein levels are unchanged, yet the pAMPK to total AMPK ratio is decreased with age-associated E2 deficiency. Representative blots for AMPK and pAMPK and adipose protein levels for AMPK (Panel A), pAMPK (Panel B), pAMPK to AMPK ratio (Panel C). * denotes age effect; (p < 0.05; n=5–6/group). Values are means ± SEM; data is presented relative to adult intact, AMPK and pAMPK are corrected with Sypro Ruby Blot Stain.
Figure 5.
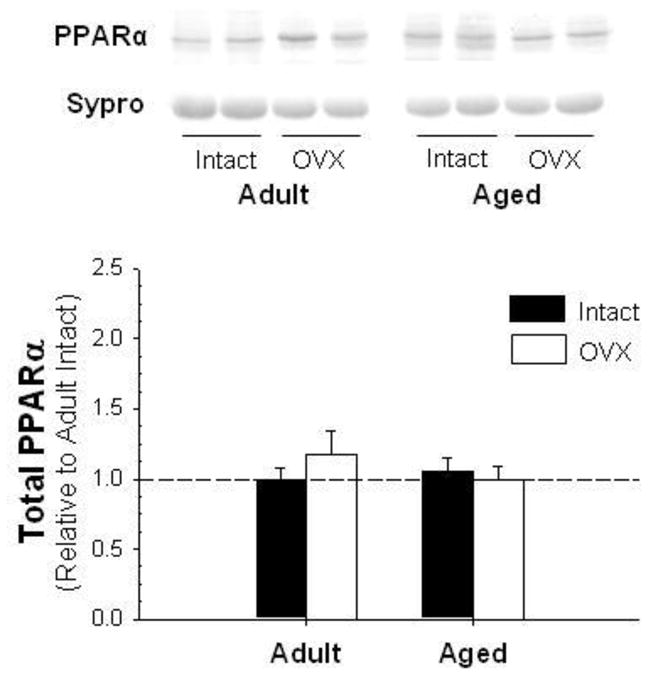
Representative blots for PPARα. Values are means ± SEM; data is presented relative to adult intact and corrected with Ponceau S stain.
DISCUSSION
The primary focus of the current investigation was to examine, for the first time, links between increased adiposity known to occur with age-associated E2-deficiency, ER protein levels and APN processing and secretion signaling in visceral adipose tissue of female rats. We also investigated potential APN autocrine signaling derangements in aged and E2-deficient adipose tissue, including downstream regulators of lipid metabolism PPARα and AMPK. Collectively, the results presented herein suggest increased ERβ protein levels are associated with compromised APN secretion, as well as reduced AdipoR2 levels thus providing potentially important observations regarding increased metabolic risk in aged women.
Evidence from αERKO models indicate that unopposed ERβ signaling promotes weight gain and a metabolic phenotype in adult mice [10–12]. Previous data also show that ERα is reduced with OVX induced E2-deficiency in adult rats, and we observed a similar phenomenon in the current study (Figure 1A) [25]. Interestingly, we also observed a significant increase in ERα in aged OVX vs adult OVX animals which underscores the importance of using an aging model to elucidate the combined effects of age and E2-deficiency to study metabolic phenotypes. The most striking finding we observed, however, was a two-fold increase in ERβ in both aged and aged OVX rats. That we observe greatest weight gain in aged E2-deficient animals co-incident with a robust increase in ERβ suggests that ERβ alone, or perhaps the shift in the ERα/β ratio favoring ERβ, may play a role in the development of age-associated increases in adiposity.
Because ERβ is known to negatively regulate PPARγ transcriptional activity in 3T3-L1 cells and PPARγ activity is also enhanced in βERKO mice [13], we assessed PPARγ levels as a potential indicator of aberrant ERβ signaling. Indeed, we observed significant reductions in PPARγ protein levels with age-associated E2-deficiency, which may limit the capacity of PPARγ to upregulate APN, and in turn, negatively affect glucose and lipid metabolism in insulin sensitive tissues [26–29].
Regarding circulating APN, we observed increased levels in OVX rats which are similar to findings observed in adult mice [30]. Previous findings also indicate reduced circulating APN levels are reduced with aging in association with metabolic and cardiovascular disease status [14–16]. Here, reduced circulating APN levels in aged OVX rats were associated with elevated intra-adipocyte APN protein levels. It is also interesting to note that Erp44, a protein which directly acts to retain and prevent secretion of APN during post-translational processing [23], was maintained in adult and aged animals (p=0.09; Figure 2B). Collectively, these findings suggest the possibility of APN retention in adipocytes of aged E2-deficient animals, thereby providing a mechanism to reduce circulating APN and increase metabolic disease risk. Our results further suggest that this mechanism may be driven by ERβ, although additional studies are indicated to investigate this important issue.
Finally, reduced autocrine signaling in adipose may also play a role in further exacerbating adiposity and metabolic disease in aged E2-deficient animals. Thus we examined protein levels for both AdipoR1 and 2 in adipose tissue. While no group differences were observed for AdipoR1, AdipoR2 was significantly decreased with age. Reduced AdipoR2, with unchanged R1 levels has also been observed in diabetic rats indicating, as expected, aged E2-deficient animals are likely progressing to a metabolic phenotype [31]. Metabolic dysregulation associated with age and E2-deficency in our model is further evidenced by the significant decrease in the pAMPK/AMPK ratio in aged and aged OVX animals, and while speculative, may result in impaired pAMPK-driven processes like glucose uptake and fatty acid mobilization in adipocytes [24, 32].
CONCLUSIONS
We have demonstrated that the unique state of E2-deficiency in aged rats is associated with an altered ERα/β ratio in favor of ERβ. With increased ERβ protein levels we also observe significantly decreased PPARγ, circulating APN, and AdipoR2 levels which set the stage for increased adiposity and subsequent metabolic dysregulation specifically in aged E2-deficient animals. Further studies within the context of aging and E2-deficiency are required to determine if postmenopausal weight gain, metabolic syndrome, and CHD risk are linked through ERβ-driven derangements in adipose tissue linked to reduced circulating APN and AdipoR2 levels.
Acknowledgments
The authors would like to thank Dr. R. Ramachandran for his generous gift of the AdipoR1 antibody, Dr. K. Saupe for his valuable insight on AMPK, Richard Ball for adiponectin ELISA studies, and Jennifer Novotny-Buchanan, Amy M. Simpson, and Sarah J. Jefferson for assistance with animal care. This project was supported by: NIH RO1 HL091097 (dhk), NIH RO1 HL091097-01A2S1 (dhk), NIH RC2 AA019403 (dhk).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002 Jan 16;287(3):356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 2.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004 Apr 14;291(14):1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 3.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003 Aug 7;349(6):523–34. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 4.Rexrode KM, Ridker PM, Hegener HH, Buring JE, Manson JE, Zee RY. Polymorphisms and haplotypes of the estrogen receptor-beta gene (ESR2) and cardiovascular disease in men and women. Clin Chem. 2007 Oct;53(10):1749–56. doi: 10.1373/clinchem.2007.091454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo JC, Zhao X, Scuteri A, Brockwell S, Sowers MR. The association of genetic polymorphisms in sex hormone biosynthesis and action with insulin sensitivity and diabetes mellitus in women at midlife. Am J Med. 2006 Sep;119(9 Suppl 1):S69–78. doi: 10.1016/j.amjmed.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Schuit SC, Oei HH, Witteman JC, Geurts van Kessel CH, van Meurs JB, Nijhuis RL, et al. Estrogen receptor alpha gene polymorphisms and risk of myocardial infarction. JAMA. 2004 Jun 23;291(24):2969–77. doi: 10.1001/jama.291.24.2969. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Xu A, Knight C, Xu LY, Cooper GJ. Hydroxylation and glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin. Potential role in the modulation of its insulin-sensitizing activity. J Biol Chem. 2002 May 31;277(22):19521–9. doi: 10.1074/jbc.M200601200. [DOI] [PubMed] [Google Scholar]
- 8.Barros R, Machando U, Warner M, Gustafsson J-A. Muscle GLUT4 regulation by estrogen receptors ERβ and ERα PNAS. 2006;103(5):1605–8. doi: 10.1073/pnas.0510391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, et al. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia. 2006 Mar;49(3):588–97. doi: 10.1007/s00125-005-0105-3. [DOI] [PubMed] [Google Scholar]
- 10.Heine P, Taylor J, Iwamoto G, Lubahn D, Cooke P. Increased adipose tissue in male and female estrogen receptor-a knockout mice. PNAS. 2000;97(23):12729–34. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribas V, Nguyen MT, Henstridge DC, Nguyen AK, Beaven SW, Watt MJ, et al. Impaired Oxidative Metabolism and Inflammation are Associated with Insulin Resistance in ER{alpha} Deficient Mice. Am J Physiol Endocrinol Metab. 2009 Nov 17; doi: 10.1152/ajpendo.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naaz A, Zakroczymski M, Heine P, Taylor J, Saunders P, Lubahn D, et al. Effect of ovariectomy on adipose tissue of mice in the absence of estrogen receptor alpha (ERalpha): a potential role for estrogen receptor beta (ERbeta) Horm Metab Res. 2002;34:758–63. doi: 10.1055/s-2002-38259. [DOI] [PubMed] [Google Scholar]
- 13.Foryst-Ludwig A, Clemenz M, Hohmann S, Hartge M, Sprang C, Frost N, et al. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS Genet. 2008 Jun;4(6):e1000108. doi: 10.1371/journal.pgen.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999 Apr 2;257(1):79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 15.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000 Jun;20(6):1595–9. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 16.Shibata R, Numaguchi Y, Matsushita K, Sone T, Kubota R, Ohashi T, et al. Usefulness of adiponectin to predict myocardial salvage following successful reperfusion in patients with acute myocardial infarction. Am J Cardiol. 2008 Jun 15;101(12):1712–5. doi: 10.1016/j.amjcard.2008.02.057. [DOI] [PubMed] [Google Scholar]
- 17.Hunter JC, Korzick DH. Age- and sex-dependent alterations in protein kinase C (PKC) and extracellular regulated kinase 1/2 (ERK1/2) in rat myocardium. Mech Ageing Dev. 2005 May;126(5):535–50. doi: 10.1016/j.mad.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Hunter JC, Kostyak JC, Novotny JL, Simpson AM, Korzick DH. Estrogen deficiency decreases ischemic tolerance in the aged rat heart: Roles of PKCdelta, PKCepsilon, Akt, and GSK3beta. Am J Physiol Regul Integr Comp Physiol. 2007 Feb;292(2):R800–9. doi: 10.1152/ajpregu.00374.2006. [DOI] [PubMed] [Google Scholar]
- 19.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 20.Kostyak JC, Hunter JC, Korzick DH. Acute PKCdelta inhibition limits ischaemia-reperfusion injury in the aged rat heart: role of GSK-3beta. Cardiovasc Res. 2006 May 1;70(2):325–34. doi: 10.1016/j.cardiores.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Korzick DH, Kostyak JC, Hunter JC, Saupe KW. Local delivery of PKCepsilon-activating peptide mimics ischemic preconditioning in aged hearts through GSK-3beta but not F1-ATPase inactivation. Am J Physiol Heart Circ Physiol. 2007 Oct;293(4):H2056–63. doi: 10.1152/ajpheart.00403.2007. [DOI] [PubMed] [Google Scholar]
- 22.Novotny JL, Simpson AM, Tomicek NJ, Lancaster TS, Korzick DH. Rapid estrogen receptor-alpha activation improves ischemic tolerance in aged female rats through a novel protein kinase C epsilon-dependent mechanism. Endocrinology. 2009 Feb;150(2):889–96. doi: 10.1210/en.2008-0708. [DOI] [PubMed] [Google Scholar]
- 23.Wang ZV, Schraw TD, Kim JY, Khan T, Rajala MW, Follenzi A, et al. Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Biol. 2007 May;27(10):3716–31. doi: 10.1128/MCB.00931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007 Mar;13(3):332–9. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 25.Gorres BK, Bomhoff GL, Gupte AA, Geiger PC. Altered estrogen receptor expression in skeletal muscle and adipose tissue of female rats fed a high-fat diet. J Appl Physiol. Jan 13; doi: 10.1152/japplphysiol.00541.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gustafson B, Jack MM, Cushman SW, Smith U. Adiponectin gene activation by thiazolidinediones requires PPAR[gamma]2, but not C/EBP[alpha]--evidence for differential regulation of the aP2 and adiponectin genes. Biochemical and Biophysical Research Communications. 2003;308(4):933. doi: 10.1016/s0006-291x(03)01518-3. [DOI] [PubMed] [Google Scholar]
- 27.Combs TP, Wagner JA, Berger J, Doebber T, Wang W-J, Zhang BB, et al. Induction of Adipocyte Complement-Related Protein of 30 Kilodaltons by PPAR{gamma} Agonists: A Potential Mechanism of Insulin Sensitization. Endocrinology. 2002 March 1;143(3):998–1007. doi: 10.1210/endo.143.3.8662. [DOI] [PubMed] [Google Scholar]
- 28.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002 Nov;8(11):1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 29.Vidal-Puig A, Jimenez-Linan M, Lowell BB, Hamann A, Hu E, Spiegelman B, et al. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest. 1996 Jun 1;97(11):2553–61. doi: 10.1172/JCI118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Combs TP, Berg AH, Rajala MW, Klebanov S, Iyengar P, Jimenez-Chillaron JC, et al. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. 2003 Feb;52(2):268–76. doi: 10.2337/diabetes.52.2.268. [DOI] [PubMed] [Google Scholar]
- 31.Bauer S, Weigert J, Neumeier M, Wanninger J, Schaffler A, Luchner A, et al. Low-abundant adiponectin receptors in visceral adipose tissue of humans and rats are further reduced in diabetic animals. Arch Med Res. Feb;41(2):75–82. doi: 10.1016/j.arcmed.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes. 2003 Jun;52(6):1355–63. doi: 10.2337/diabetes.52.6.1355. [DOI] [PubMed] [Google Scholar]