Abstract
Complete surgical resection is the most effective curative treatment for lung cancer. However, many patients with lung cancer also have severe COPD which increases their risk of postoperative complications and their likelihood of being considered “inoperable.” Preoperative Pulmonary Rehabilitation (PR) has been proposed as an intervention to decrease surgical morbidity but there is no established protocol and no randomized study has been published to date.
We tested two preoperative PR interventions in patients undergoing Lung Cancer resection and with moderate-severe COPD in a randomized single blinded design. Outcomes were length of hospital stay and postoperative complications.
The first study tested 4 weeks of guideline-based PR vs.usual care: that study proved to be very difficult to recruit as patients and providers were reluctant to delay surgery. Nine patients were randomized and no differences were found between arms.
The second study tested ten preoperative PR sessions using a customized protocol with nonstandard components (exercise prescription based on self efficacy, inspiratory muscle training, and the practice of slow breathing) (n=10) vs.usual care (N=9). The PR arm had shorter length of hospital stay by 3 days (p=0.058), fewer prolonged chest tubes (11% vs. 63%, p=0.03) and fewer days needing a chest tube (8.8vs.4.3 days p=0.04) compared to the controlled arm.
A ten-session preoperative PR intervention may improve post operative lung reexpansion evidenced by shorter chest tube times and decrease the length of hospital stay, a crude estimator of post operative morbidity and costs. Our results suggest the potential for short term preoperative Pulmonary Rehabilitation interventions in patients with moderate-severe COPD undergoing curative lung resection. 4 weeks of conventional preoperative PR seems non feasible.
Keywords: COPD, Pulmonary Rehabilitation, Lung Cancer, Emphysema, Exercise Outcomes, Thoracic Surgery
Background
Complete surgical resection is currently the only curative treatment for lung cancer. However, many patients with lung cancer also have severe COPD which increases their risk of postoperative complications 1 and their likelihood of being considered “inoperable.” No preoperative intervention to date has been proven to decrease the risk of post operative complications in patients with resectable lung cancer and poor lung function.
Pulmonary Rehabilitation (PR), an intervention that improves exercise capacity in severe COPD6 has been proposed as an adjunctive preoperative therapy to decrease the risk of postoperative pulmonary complications7–13 Despite experts’ recommendations, no randomized study has been published to date.
The present report is the result of two randomized single-blinded exploratory studies (PR vs usual care) funded by the National Cancer Institute aimed to define a preoperative PR intervention that may decrease the operative morbidity of curative lung cancer resections in patients with moderate-severe COPD.
Methods
These exploratory randomized studies were conducted at University of Pittsburgh (IRB#0603002) and Mayo Clinic (IRB# 08-007135). All patients signed an informed consent.
Inclusion criteria
Patients had to have lung cancer resection by open thoracotomy (segment, lobe or pneumonectomy) or by Video Assisted Thoracoscopy (at least lobe) and moderate to severe COPD. Moderate to severe COPD was defined as FEV1/FVC<0.7 and FEV1<61% or FEV1<80% having significant dyspnea defined as grade 3 or higher of the Medical Research Council Dyspnea score (MRC) A previous comprehensive report indicated an FEV1<61% is associated with an increased risk of post operative complications 1.
The first randomized study tested 4 weeks of preoperative PR using the current guidelines for exercise prescription 17 vs usual care.
The second randomized study and tested a ten-session preoperative PR that used the following protocol:
Lower extremity (LE) endurance training had a target time of 20 minutes and was performed on a treadmill or Nu-Step (NuStep, Inc. Ann Arbor, Michigan). Upper extremity (UE) endurance was performed by arm-R-size exercises or the use of an arm ergometer or the Nu-Step.
Strengthening exercises with Thera-band (The Hygenic Corp. Akron OH) were preformed alternating UE/LE every other day. During this program, patients were asked to perform two sets of 10–12 repetitions starting with the lowest Thera-band resistance. If the patient perception was “too easy” or “requires no effort”, resistance was increased. Patients were asked to perform exercises at an intensity that they felt high self efficacy -“very confident”-they could sustain and that was at least light by Borg scale envisioning that in patients with greater self efficacy the likelihood of performing under stress (post-surgery) would increase.
Inspiratory muscle training (IMT) was performed using the Threshold Inspiratory Muscle Trainer IMT or the P-Flex valve (Philips Healthcare Andover, MA). Patients were asked to breathe through the device to attain a level of perceived exertion of “Somewhat hard” and sustain that effort as long as they were able with a goal of 15–20 minutes of daily use. Participants were coached to establish a slow, rhythmical pattern of breathing keeping inspiration and expiration times equal while using the IMT. When participants were able to complete 10 minutes of IMT time in a session or noted perceived exertion less than “somewhat hard”, the next IMT setting was increased to achieve the desired effort. Incentive Spirometry training used a protocol that was reported to be associated with decreased postoperative atelectasis after thoracic surgery18.
The Practice of slow breathing (prolonging expiratory time and thereby decreasing respiratory rate to less that 10 breaths per minute) was routinely included. Patients were instructed to “just watching their breath with pursed lips to prolong the expiratory phase” for at least 10 minutes in each PR session. During the practice of slow breathing, we promoted discussions about concerns, fears and expectations regarding surgery. We emphasized the critical importance of the patient’s role in their recovery during the post operative period (ie. Resilience when facing pain and practicing frequent (hourly) posture, shoulder, pulmonary toilet exercises, and particularly early ambulation). Weekend exercise recommendations were given based on their prior performance in PR: individualized goals were set collaboratively. Participants were expected to engage in walking once daily, complete the IMT routine and upper extremity exercises.
Outcome measures
End points of these two studies were hospital length of stay and post operative pulmonary complications (pneumonia (new infiltrate + either fever (>38.5 C) and white cell count >11,000 or fever and purulent secretions), severe atelectasis (requiring bronchoscopy), prolonged chest tubes (>7days), and prolonged mechanical ventilation (>24hours)).
Outcomes were obtained using chart review by a nurse trained in the abstraction of the desired outcomes from the medical records and blinded to the treatment.
Data analysis
Continuous variables were tested between the two treatment arms (PR vs. control) using nonparametric Wilcoxon tests. Categorical variables were tested using Fisher’s exact tests. A linear regression model was used to test for differences in length of stay between arms after adjusting for age and gender. All tests were two-sided tests using 5% type I error rates. SAS (NC) was used for the analyses.
Results
Study #1: 4 weeks of preoperative PR vs. usual care
This study had very poor recruitment, mainly due to the fact that patients or providers were not willing to delay the curative surgery for 4 weeks. Nine patients were randomized to this study in 18 months from a large surgical practice (5 hospitals: academic (three) and community (two)). Patients in each arm had similar baseline characteristics regarding age, lung function, dyspnea score (Medical Research Council Dyspnea score), history of exacerbations and comorbidities. All 5 patients randomized to PR successfully completed 4 weeks of treatment and no adverse effects were observed. This study was finally stopped due to the low likelihood of meaningful accrual during the funding period. No differences were found between the arms in any outcome.
Study #2: Ten sessions of preoperative PR vs usual care
19 patients were randomized to this study in one year from one site (Mayo Clinic). Nine patients were randomized to the control arm and 10 patients to the pulmonary rehabilitation arm. All 10 patients randomized to PR successfully completed 10 face-to-face sessions of treatment in one week (twice a day) and no adverse effects were observed. Table 1 shows patient demographics for these 19 patients. Baseline characteristics were evenly distributed between the two arms. Results of this one-year study needed to be analyzed due to the end of the funding period and report to NIH.
TABLE 1.
Baseline characteristics before ten sessions of Pulmonary Rehabilitation
| Control (N=9) | Pulmonary Rehabilitation (N=10) | p value | |
|---|---|---|---|
| Age, mean (SD) | 72.0 (6.69) | 70.2 (8.61) | 0.71 |
|
| |||
| Female, n (%) | 5 (56%) | 5 (50%) | 0.81 |
|
| |||
| Current Smoker, n (%) | 2 (22%) | 1 (10%) | 0.47 |
| Cigarettes Per Day, mean (SD) | 26 (7.2) | 24 (14.3) | 0.37 |
| Years Smoked Cigarettes, mean (SD) | 46 (6.4) | 43 (10.4) | 0.62 |
| Coronary Artery Disease (%) history | 1 (11%) | 3 (30%) | 0.31 |
| Heart Failure, n (%) history | 0 (0%) | 0 (0%) | -- |
| Diabetes, n (%) | 3 (33%) | 3 (30%) | 0.88 |
| MRC Dyspnea Score | 2.0 (0.82) | 2.3 (1.49) | 0.43 |
| FEV1 % Predicted | 52.1 (13.32) | 43.4 (10.18) | 0.11 |
| DLCO % predicted | 60.3 (18.32) | 48.9 (10.79) | 0.24 |
| Residual Volume % predicted | 160.4 (31.11) | 186.4 (57.97) | 0.60 |
Two patients (one on each arm) were missing length of stay data; because they were considered inoperable once they were in the operating room and were excluded from the outcome analysis (final analyses were based on data from 17 patients). There were no differences in the frequency of open thoracotomies or pneumonectomies between the two groups we handled the analysis to not be affected by any extreme value.
Patients in the PR arm had fewer days needing a chest tube (mean days of 4.7 vs. 9.0, p=0.03) and a lower incidence of prolonged chest tubes (>7days) compared to the controlled arm (11% vs. 63%, p=0.03). Also, patients in the PR arm had on average over fewer days in the hospital compared to patients in the control arm, a difference that was very close to being significant (mean days of 6.4 vs. 11.1, p=0.058). Patients did not improve the shuttle walk test (baseline test was 31 shuttles or 310 meters) after the short term PR (p=NS).
Discussion
These exploratory studies represent the first randomized trials of preoperative PR in lung cancer resection in patients with poor lung function and may serve as a guide for future research in the field, particularly in the design of large confirmatory studies.
We believe these exploratory studies have aspects that deserve communication at this stage:
Our finding about the non feasibility of 4 weeks of preoperative PR is reflected the literature: the scarcity of publications, no one definitive, even when PR before lung resection has been proposed for more than a decade eloquently speaks about that. In 1997 a publication from Sloan Kettering Cancer reported an ongoing study of 3 weeks of preoperative PR that never translated in a full manuscript. After that only a few small non controlled studies (from large centers) suggested the benefit11–13,19,20. Our experience reflects that patients (and many times providers) facing a potentially curative resection want the cancer out NOW. While doing preoperative PR is appropriate and recommended by experts 9,8,7,19, the lack of evidence using the current methods suggest the need of new protocols adjusted to the perioperative setting.
The development of a short and feasible preoperative PR protocol was the natural consequence of the failure of the longer one. Our 10-session protocol showed a high likelihood of decreasing hospital length of stay, a very meaningful outcome that is crude estimation of post operative morbidity and costs. We believe we observed a true and meaningful difference in length of stay favoring the PR group (Figure 1) that barely missed the classic and rigid standards of significance (our p value for the difference of length of stay was 0.058). It is important to clarify again that we handled the analysis to not be affected by any extreme value and that both groups were balanced in procedures (pneumonectomies, thoracotomies, VATS). We found a significant and meaningful decrease in the time of chest tube drainage and less prolonged chest tubes, a well defined pulmonary complication of lung resection in severe COPD. Our results in all outcomes favored the PR group (table two), something that reassured this group on the possible effectiveness of the short intervention, but did not attain statistical significance due to our sample size.
Figure 1.
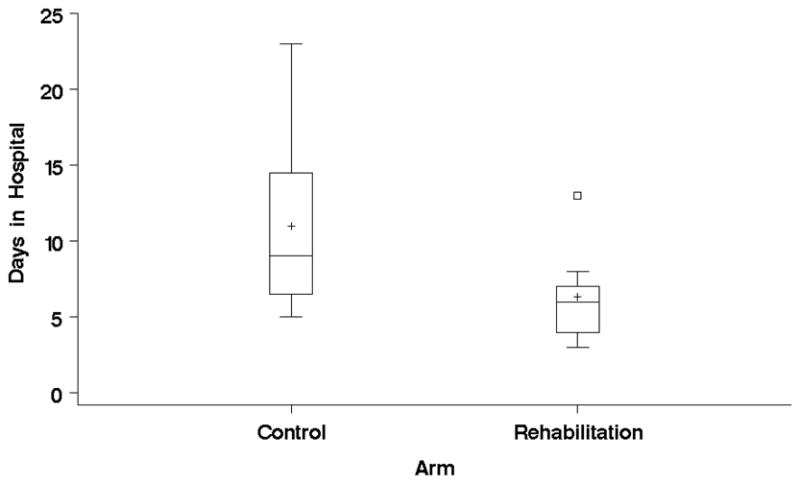
Box plot of Days in the Hospital by Treatment Arm
The goal when designing the second exploratory study was to test a feasible intervention: the one that patients and provider would agree, including evidence based components not routinely recommended in PR. Those components were: self efficacy-based exercise prescription, the routine use of inspiratory muscle training (IMT), and a dedicated practice of slow breathing (considered “the relaxation part” by patients).
We believe that by targeting self efficacy more than intensity as the primary focus of all exercise training we introduced an innovative behavioral aspect in this short PR intervention. We envisioned that patients that felt very confident in performing certain key activities (ambulation, upper extremity mobility, stretching), they would have a higher likelihood of doing them in the postoperative setting: and we communicated that exact message to the patients. To achieve this goal of greater self efficacy, we empowered patients to do what they were really confident to do, intending to foster their autonomy in their rehabilitation process and preparation for surgery. Self efficacy has been identified as an important mediator for behavioral changes in patients with COPD 21 and cancer 22–24, and very recent reports emphasized the importance of promoting self efficacy in PR25,26. The self efficacy theory 27,28 is the foundation of many disease self management programs 29–31. We speculate that greater self efficacy (for dealing with symptoms and for starting early post operative ambulation even in the context of pain and dyspnea) mediated, at least in part, the benefits observed. We could not attribute the benefits of the intervention to increased exercise capacity - as there was a lack of change in the shuttle walk test pre/post rehabilitation-.
Our finding of shorter time of chest tubes in place may indicate a better lung reexpansion, a result that may be associated with the routine use of preoperative Inspiratory Muscle Training (IMT). The positive impact of preoperative physiotherapy involving IMT on lung reexpansion is not a new discovery: in a recent study that included 263 patients undergoing thoracic surgery, patients received 1 day of preoperative PR (incentive spirometry, deep breathing exercises, coughing and promotion of early ambulation) and the incidence of postoperative atelectasis was significantly decreased by 50% 18. The impact of preoperative IMT on the outcomes of thoracic surgery has been addressed by a randomized study published in JAMA by Hulzebos et al that randomized of 279 patients to 2 weeks of preoperative IMT vs. usual care that reported a reduction of post operative complications in patient undergoing thoracotomy. The latter being the strongest evidence to date for the use of IMT in the preoperative setting32. In Moreover, another recent randomized study showed that inspiratory muscle strength was a determinant of functional capacity after thoracic surgery33.
The practice slow breathing (decreasing the respiratory rate by prolonging the expiratory phase ), has been one of the initial strategies of PR (while practicing pursed-lips breathing) and is still an open area for therapeutic target in COPD as recently suggested by Macklem34. Slow breathing has been shown beneficial in COPD by decreasing sympathetic tone 35, in thoracic surgery improving postoperative pain management 36 and invariable brought a sense of relaxation in our group, a factor associated also with improved post operative outcomes 37 (and for that reason we strongly recommended in the post operative period).
Why a 2-week intervention?
Three studies reported improved postoperative outcomes with short interventions: the study by Hulzebos et al (2 weeks of IMT) mentioned before32, a study by Sekine et al reported that 2 weeks of preoperative PR decreased length of hospital stay 20 and a study reported that 1 day of preoperative physiotherapy significantly decreased post operative atelectasis18.
Our results require confirmation by a larger and likely multicenter study. Even in awareness of the preliminary nature of our results, we believe our findings have practical implications: a short feasible preoperative PR protocol may have potential to improve surgical and may easily translate to practice. In addition, our work may serve for a start in filling the knowledge gap on effective preoperative care including aspects that are not routinely used: IMT, the practice of slow breathing and self efficacy based exercise prescription, all aspect of PR that can make a difference in outcomes. The use of preoperative PR is a challenge in rehabilitation science (duration and components) and our results may represent a possible answer.
TABLE 2.
Postoperative Outcomes after ten sessions of Pulmonary Rehabilitation
| STUDY #2 | Control (N=8) | Pulmonary Rehabilitation (N=9) | p value |
|---|---|---|---|
| Days in Hospital, mean (SD) | 11.0 (6.3) | 6.3 (3.0) | 0.058 |
| ICU Hours, mean (SD) | 40.5 (75.2) | 14.9 (44.7) | 0.39 |
| Number of patient that had Post-Op Pulmonary Complications, n (%) | 5 (63%) | 3 (33%) | 0.23 |
| Ventilation Hours, mean (SD) | 33.3 (61.9) | 6.0 (18.0) | 0.39 |
| Number of patient that had prolonged Chest Tube (>7days), n (%) | 5 (63%) | 1 (11%) | 0.03 |
| Average number of days with Chest Tubes, mean (SD) | 8.8 (5.3) | 4.3 (2.1) | 0.04 |
| Respiratory Failure, n (%) | 2 (25%) | 1 (11%) | 0.45 |
| Pneumonia, n (%) | 2 (25%) | 1 (11%) | 0.45 |
| Number of patients requiring bronchoscopy for atelectasis (%) | 2 (25%) | 1 (11%) | 0.45 |
Acknowledgments
Funding Source: This research was funded by Grant # K23CA106544-05-06 from the National Cancer Institute.
We sincerely thank Dr. Frank Sciurba and Dr. James Jett, mentors for Dr. Benzo’s grant from the National Institutes of Health.
Footnotes
“Conflict of interest statement”
None declared by any of the authors
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Licker MJ, Widikker I, Robert J, et al. Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg. 2006;81:1830–1837. doi: 10.1016/j.athoracsur.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 2.Bolliger CT, Jordan P, Soler M, et al. Exercise capacity as a predictor of postoperative complications in lung resection candidates. Am J Respir Crit Care Med. 1995;151:1472–1480. doi: 10.1164/ajrccm.151.5.7735602. [DOI] [PubMed] [Google Scholar]
- 3.Win T, Jackson A, Groves AM, et al. Relationship of shuttle walk test and lung cancer surgical outcome. Eur J Cardiothorac Surg. 2004;26:1216–1219. doi: 10.1016/j.ejcts.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 4.Benzo R, Kelley GA, Recchi L, et al. Complications of lung resection and exercise capacity: A meta-analysis. Respir Med. 2007 doi: 10.1016/j.rmed.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax. 2001;56:89–108. doi: 10.1136/thorax.56.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ries AL, Make BJ, Lee SM, et al. The effects of pulmonary rehabilitation in the national emphysema treatment trial. Chest. 2005;128:3799–3809. doi: 10.1378/chest.128.6.3799. [DOI] [PubMed] [Google Scholar]
- 7.Nici L. Preoperative and postoperative pulmonary rehabilitation in lung cancer patients. Thorac Surg Clin. 2008;18:39–43. doi: 10.1016/j.thorsurg.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Shannon VR. Role of pulmonary rehabilitation in the management of patients with lung cancer. Curr Opin Pulm Med. 16:334–339. doi: 10.1097/MCP.0b013e32833a897d. [DOI] [PubMed] [Google Scholar]
- 9.Wilson DJ. Pulmonary rehabilitation exercise program for high-risk thoracic surgical patients. Chest Surg Clin N Am. 1997;7:697–706. [PubMed] [Google Scholar]
- 10.Benzo RP. Pulmonary rehabilitation in lung cancer: a scientific opportunity. J Cardiopulm Rehabil Prev. 2007;27:61–64. doi: 10.1097/01.HCR.0000265030.02521.f1. [DOI] [PubMed] [Google Scholar]
- 11.Jones LW, Peddle CJ, Eves ND, et al. Effects of presurgical exercise training on cardiorespiratory fitness among patients undergoing thoracic surgery for malignant lung lesions. Cancer. 2007;110:590–598. doi: 10.1002/cncr.22830. [DOI] [PubMed] [Google Scholar]
- 12.Cesario A, Ferri L, Galetta D, et al. Pre-operative pulmonary rehabilitation and surgery for lung cancer. Lung Cancer. 2007;57:118–119. doi: 10.1016/j.lungcan.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Bobbio A, Chetta A, Ampollini L, et al. Preoperative pulmonary rehabilitation in patients undergoing lung resection for non-small cell lung cancer. Eur J Cardiothorac Surg. 2008;33:95–98. doi: 10.1016/j.ejcts.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 16.Puhan MA, Garcia-Aymerich J, Frey M, et al. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet. 2009;374:704–711. doi: 10.1016/S0140-6736(09)61301-5. [DOI] [PubMed] [Google Scholar]
- 17.Nici L, Donner C, Wouters E, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173:1390–1413. doi: 10.1164/rccm.200508-1211ST. [DOI] [PubMed] [Google Scholar]
- 18.Yanez-Brage I, Pita-Fernandez S, Juffe-Stein A, et al. Respiratory physiotherapy and incidence of pulmonary complications in off-pump coronary artery bypass graft surgery: an observational follow-up study. BMC Pulm Med. 2009;9:36. doi: 10.1186/1471-2466-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spruit MA, Janssen PP, Willemsen SC, et al. Exercise capacity before and after an 8-week multidisciplinary inpatient rehabilitation program in lung cancer patients: a pilot study. Lung Cancer. 2006;52:257–260. doi: 10.1016/j.lungcan.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Sekine Y, Chiyo M, Iwata T, et al. Perioperative rehabilitation and physiotherapy for lung cancer patients with chronic obstructive pulmonary disease. Jpn J Thorac Cardiovasc Surg. 2005;53:237–243. doi: 10.1007/s11748-005-0032-8. [DOI] [PubMed] [Google Scholar]
- 21.Lemmens KM, Nieboer AP, Huijsman R. Designing patient-related interventions in COPD care: empirical test of a theoretical model. Patient Educ Couns. 2008;72:223–231. doi: 10.1016/j.pec.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Jerant A, Franks P, Kravitz RL. Associations between pain control self-efficacy, self-efficacy for communicating with physicians, and subsequent pain severity among cancer patients. Patient Educ Couns. doi: 10.1016/j.pec.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Porter LS, Keefe FJ, Garst J, et al. Caregiver-Assisted Coping Skills Training for Lung Cancer: Results of a Randomized Clinical Trial. J Pain Symptom Manage. doi: 10.1016/j.jpainsymman.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Weert E, Hoekstra-Weebers JE, May AM, et al. The development of an evidence-based physical self-management rehabilitation programme for cancer survivors. Patient Educ Couns. 2008;71:169–190. doi: 10.1016/j.pec.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 25.Janssen DJ, Engelberg RA, Curtis JR. Toward patient-tailored education in COPD. Patient Educ Couns. 81:1–2. doi: 10.1016/j.pec.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 26.Bentsen SB, Wentzel-Larsen T, Henriksen AH, et al. Self-efficacy as a predictor of improvement in health status and overall quality of life in pulmonary rehabilitation--an exploratory study. Patient Educ Couns. 81:5–13. doi: 10.1016/j.pec.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 27.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 28.Bandura A. Swimming against the mainstream: the early years from chilly tributary to transformative mainstream. Behav Res Ther. 2004;42:613–630. doi: 10.1016/j.brat.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Heisler M, Vijan S, Makki F, et al. Diabetes control with reciprocal peer support versus nurse care management: a randomized trial. Ann Intern Med. 153:507–515. doi: 10.7326/0003-4819-153-8-201010190-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bodenheimer T, Lorig K, Holman H, et al. Patient self-management of chronic disease in primary care. Jama. 2002;288:2469–2475. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- 31.Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26:1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- 32.Hulzebos EH, Helders PJ, Favie NJ, et al. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. Jama. 2006;296:1851–1857. doi: 10.1001/jama.296.15.1851. [DOI] [PubMed] [Google Scholar]
- 33.Stein R, Maia CP, Silveira AD, et al. Inspiratory muscle strength as a determinant of functional capacity early after coronary artery bypass graft surgery. Arch Phys Med Rehabil. 2009;90:1685–1691. doi: 10.1016/j.apmr.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Macklem PT. Therapeutic implications of the pathophysiology of COPD. Eur Respir J. 35:676–680. doi: 10.1183/09031936.00120609. [DOI] [PubMed] [Google Scholar]
- 35.Raupach T, Bahr F, Herrmann P, et al. Slow breathing reduces sympathoexcitation in COPD. Eur Respir J. 2008;32:387–392. doi: 10.1183/09031936.00109607. [DOI] [PubMed] [Google Scholar]
- 36.Friesner SA, Curry DM, Moddeman GR. Comparison of two pain-management strategies during chest tube removal: relaxation exercise with opioids and opioids alone. Heart Lung. 2006;35:269–276. doi: 10.1016/j.hrtlng.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Kshettry VR, Carole LF, Henly SJ, et al. Complementary alternative medical therapies for heart surgery patients: feasibility, safety, and impact. Ann Thorac Surg. 2006;81:201–205. doi: 10.1016/j.athoracsur.2005.06.016. [DOI] [PubMed] [Google Scholar]


