Abstract
Aging is a non modifiable risk factor for stroke. Since not all strokes can be prevented, a major emerging area of research is the development of effective strategies to enhance functional recovery after stroke. However, in the vast majority of pre-clinical stroke studies, the behavioral tests used to assess functional recovery have only been validated for use in young animals, or are designed for rats. Mice are increasingly utilized in stroke models but well validated behavioral tests designed for rats are not necessarily reproducible in mice. We examined a battery of behavioral tests to evaluate functional recovery in an aging murine model of stroke. We found that the vertical pole, hanging wire and open field can accurately assess acute behavioral impairments after stroke in both young and aging male mice, but animals recover rapidly on these tasks. The corner test can accurately and repeatedly differentiate stroke from sham animals up to 30 days post stroke and can be performed reliably in aging mice. Aging male mice had significantly worse behavioral impairment compared to young male mice in the first two weeks after stroke but eventually recovered to the same degree as young mice. In contrast, chronic infarct size, as measured by ipsilateral cerebral atrophy, was significantly lower in aging male mice compared to young male mice. Reactive gliosis, formation of glial scar, and an enhanced innate immune response was seen in the aging brain and may contribute to the delayed behavioral recovery seen in aged animals.
Keywords: Cerebral ischemia, Aging, Functional recovery, Cerebral blood flow (CBF), Cerebral atrophy, Microglia, Glial scar, Astrocytes, GFAP (glial fibrillary acid protein), Iba1
1. INTRODUCTION
Ischemic stroke is the third leading cause of death in the United States and the number one cause of adult disability (Mackay J, Mensch G, 2004). Since 75–89% of strokes occur in individuals over the age of 65 (Feigin et al., 2003), the aging population bears the major brunt of stroke related mortality and disability. Despite this, most preclinical studies utilize only young animals, in which rates of recovery, even from large strokes, are quite dramatic (Liu and McCullough). The avoidance of aged animals in experimental studies is due in part to technical difficulties, frailty, high mortality and the high cost of aging animals. However, it is becoming increasingly clear that the aged brain responds differently to experimental stroke than the young (Howard et al., 1987, Nakayama et al., 1994). Aging is a complex process that leads to an altered innate immune environment in the brain (Lucin and Wyss-Coray, 2009). As many therapeutic strategies to treat stroke have been developed in young animals, concerns exist regarding the translation of these therapies to aging animals, and more importantly, to patients at the highest risk for stroke, the elderly.
It is also increasingly recognized that assessment of stroke-induced behavioral deficits and functional recovery is as important as histological infarct quantification as an outcome measure. Traditionally, acute histological assessment using quantification of lesion volume has been used as the primary outcome measure in experimental stroke studies. The goal of clinicians is to enhance functional recovery and improve quality of life for stroke patients, therefore there is increasing recognition of the importance of assessment of long term behavioral outcomes as endpoints in pre-clinical studies. This is not only important to reduce the economic burden of stroke related disability, but also vital for overcoming the “translational road block” to the development of stroke therapies (Endres et al., 2008). To address these questions, the goals of this study were; 1. To design a battery of simple, inexpensive and reliable tests to follow long term behavioral improvement in aging mice after experimental stroke; 2. To assess differences in recovery in young vs. aging mice; 3. To compare histological and functional outcomes in the aging mice and; 4. To assess the immunological response to stroke in the aging brain.
2. METHODS
Experimental animals and housing
Adult 2 month (young, equivalent to young humans around 18 years old) and 15–16 month (aging, equivalent to aging human around 55 years old) C57BL/6 male mice (Flurkey, Harrison et al, 2007) were kept in cages of two animals per cage both before and after surgery on sawdust bedding (light cycle 12/12 h light/dark). The average weight of the young mice (n=14 for stroke, n=4 for sham) was 25.8+/− 0.5 grams and that of aging mice (n=14 for stroke, n=3 for sham) was 34.67+/−1.2 grams before surgery. All animals had access to chow and water ad libitium. All mice were handled three days prior to surgery and were selected for sham or stroke (MCAO) surgery in a randomized manner and a blinded observer performed the behavioral testing. All animals were fed with wet mash up to one week after surgery to ensure adequate nutrition for chronic endpoints. Mash and water is left in containers on the cage floor so animals that are relatively immobile do not have to rear. Animals are monitored daily (including weekends) to ensure that they are adequately hydrated. There was no mortality 24 hours after MCAO surgery in either the young and aging mice. One aging mouse died within one week after MCAO surgery and was excluded from the analysis.
The present study was conducted in accordance with the National Institute of Health guidelines for the care and use of animals in research and under protocols approved by the Center for Lab Animal Care at the University of Connecticut Health Center.
Ischemic model
Transient cerebral ischemia was induced by 60 minutes of middle cerebral artery occlusion (MCAO) (0.21 mm silicone coated suture for young and 0.23 mm for aging) under Isoflurane anesthesia, as previously described (McCullough et al., 2005) and (Liu et al., 2009). Sham animals were subjected to the same procedure but the suture was not advanced into the middle cerebral artery. A mono-therm system was used to monitor and maintain rectal temperature at approximately 37°C during surgery through an automated temperature control feedback system. Cerebral blood flow (CBF) was measured by laser Doppler flowmetry (LDF, Moor Instruments Ltd, England) in all animals as previously described (McCullough et al., 2005). Only mice in which CBF dropped to 85% of control immediately after MCAO were included in the stroke cohorts. Neurological deficit scores (NDS) were recorded immediately after stroke. The scoring system was as follows: 0, no deficit; 1, forelimb weakness and torso turning to the ipsilateral side when held by tail; 2, circling to affected side; 3, unable to bear weight on affected side; and 4, no spontaneous locomotor activity or barrel rolling.
Behavioral tests
All the animals were tested on each behavioral task twice, one day prior to surgery to assess for baseline paw preference or abnormalities in limb usage and were then randomized into stroke and sham groups. The mice enrolled for surgeries did not demonstrate any behavioral deficits (prior to surgery) and no behavioral deficits were observed due to aging. Mice which had baseline asymmetries in limb usage or did not show a drop in CBF by 85% during MCAO were not included in the behavioral analysis. All animals were subjected to a battery of behavior tasks at specific time points over a period of four weeks after stroke or sham surgery. The testing was done at a fixed time in the morning. All behavioral testing equipment and surfaces were cleaned with 70% ethanol before and after testing for each animal. All the mice were tested on hanging wire, vertical pole and corner every alternate day after stroke for four weeks. Open field test was performed every seven days.
Hanging wire
The hanging wire tests both limb strength and balance after stroke but has not been validated in older mice (Li et al., 2004, Abe et al., 2009). This test was performed as described by (Hattori et al., 2000), with slight modification. A wire cage top, dimensions, 18 inch × 9 inch with its edges taped off was used for this experiment. The mouse was placed on the center of the wire lid and the lid was slowly inverted and placed on top of cage. The wire lid was 9 inch above the cage bedding. Latency to fall from the wire was recorded and scored. The time out period was 60 seconds. The scoring was as follows: 0, no attempt to hold and balance, falls off instantaneously; 1, attempts to balance on wire but falls in <20 seconds; 2, attempts to balance on wire but falls in ≥ 20 and <40 seconds; 3, attempts to balance on wire but falls in ≥40 and < 60 seconds and 4, able to steadily balance itself on the wire for all 60 seconds.
Vertical Pole
The Vertical Pole test is a motor task that can assess forelimb strength, ability to grasp and balance in mice (Bouet et al., 2007). This test was carried out as described by (Paylor et al., 1998) with modifications in scoring. Briefly, a pole 15 inches in length and 0.75 inches in diameter was uniformly wrapped with tape to increase traction. The pole was held in a horizontal position and the mouse was placed on its center. The pole was slowly lifted to a vertical or 90 degrees position and the mouse held in the vertical position for 60 seconds or until it fell. The total time the mouse stayed on the pole was recorded and converted to a score as follows: 0, unable to balance and falls before the pole reaches 45 or 90 degrees angle; 1, attempts to balance on the pole but falls in <20 seconds; 2, attempts to balance on pole but falls in ≥ 20 and <40 seconds; 3, attempts to balance on pole but falls in ≥ 40 and < 60 seconds and 4, able to steadily balance itself on the wire for all 60 seconds. This test has been validated in the stroke model for young mice (Bouet et al., 2007) but has not been yet been used to evaluate deficits in aging mice.
Open Field Test
Open Field measures spontaneous locomotor activity in a novel environment and has been used in stroke models in young animals (Wahl et al., 1992). In this task, the total ambulatory activity of the mouse was assessed. The mouse was placed in the open field chamber (15″ × 15″) in a dark room in Scoville Neurobehavioral Suite at UCHC. Locomotor activity was quantified as the total number of beam breaks by a computer operated PAS Open Field system (San Diego Instruments, San Diego, CA). Each testing session was 20 minutes long and the data was collected in 60 second intervals. All the mice were acclimatized to the dark testing room for 10 minutes before the beginning of the test and the activity was recorded immediately after the mice were placed in the open field apparatus.
Corner test
This test was carried out as described by (Li et al., 2004). In short, the mouse entered a corner that was made by moving two card board pieces at an angle of 30 degrees in front of the nose. Contact with the vibrissae led to a rear and the direction in which the mouse turned was recorded. Normal mice do not exhibit a turning preference, but after ischemia, mice have a turning preference to the non-impaired side. The percentage of right turns was calculated for twenty trials in each sitting. The corner test has been used to detect both sensory and motor abnormalities in the stroke model in young animals (Li et al., 2004).
Terminal histopathology
All animals were sacrificed at 30 days after stroke with pentobarbital overdose (i.p). A separate cohort of animals (n=3/group) was sacrificed at 24 hours after 60 minutes of MCAO/Sham surgery for immunohistochemistry. Transcardial perfusion was performed with cold PBS followed by 4% paraformaldehyde; the brain was fixed for 18 h and placed in cyroprotectant (30% sucrose). The brains were cut into 30-μm free-floating sections on a freezing microtome and every eighth slice was stained by cresyl violet staining to evaluate ischemic cell damage. The images were digitalized and cerebral atrophy was analyzed using computer software (Sigma scan Pro5) as previously described (Li et al., 2004) and (Liu et al., 2009). Neuronal death was determined by measuring the tissue atrophy in both hemispheres and lateral ventricles and transforming it into mm3. Cerebral atrophy percentage was calculated by using the formula, total ipsilateral tissue/total contralateral tissue, then multiplying by 100.
Immunohistochemistry (IHC)
The brains were cut and stained as described above, and mounted onto gelatin-coated slides and allowed to air dry. The sections were then blocked in 0.1 M PB with 0.3% TX-100 (sigma) and 10% goat serum (PBTGS) for an hour and then incubated with primary antibody overnight. The primary antibody was removed with a wash in PBTGS. Secondary antibody (1:1000) and 4′, 6-diamidino-2-phenylindole, dihydrochloride (DAPI, 1:1000, Invitrogen, Carlsbad, CA) was then added to the sample for an hour. Secondary antibody was removed with 3 consecutive washes in PBTGS, 0.1 M PB, and 0.05 M PB. The signal was detected and images acquired with immunofluorescence confocal microscopy using Zeiss image acquisition software (Zeiss LSM 510). The primary antibodies used were Iba1 for microglia (1:1000, Wako, USA) and GFAP for astrocytes (1:200, Dako, Denmark). Secondary antibodies (1:1000, Invitrogen, Carlsbad, CA) were goat anti-rabbit conjugated to either Alexa-594/488. Brain slices were taken at the same distance from bregma to ensure comparison of similar structures. Four 20× fields/animal (n = 3 animals/group) were analyzed in the penumbral area of the infarct. GFAP/Iba 1 positive cells were counted using MacBiophotonics ImageJ software (NIH) with a DAPI counterstain. The total number of cells was averaged across the four fields of view for each animal. The average number of cells/field of view was used for statistical analysis as described previously (Liu et al., 2010).
Statistical analysis
The ordinal data (vertical pole, hanging wire) is expressed as median (interquartile range) and the remainder of the data is expressed as mean ± SEM. The ordinal data (vertical pole and hanging wire test) was analyzed using Friedman’s test for repeated measures. A post hoc bonferroni correction was used, when appropriate, to compare differences in groups at different time points. The open field and corner test data were analyzed with three-way (age, stroke, days) repeated measures ANOVA (between subject factors-age and stroke; within subject factor-days) and univariate ANOVA with a post hoc bonferroni was used to compare group differences at different time points. Histological outcomes and immunohistochemical quantifications were evaluated using two sample independent student t-test (two-tailed) with unequal variance. Degree of correlation between infarct size and behavioral outcome was assessed using the Pearson correlation coefficient. p<0.05 was considered statistically significant using SPSS 17.0 software.
3. RESULTS
Vertical Pole test
Table 1 shows the results from the vertical pole tests. Young and aging sham mice had no deficits on this test (score=4, all days). This test was able to assess stroke-induced behavioral deficits in aging animals. Vertical pole scores were significantly lower in aging MCAO mice compared to aging sham (X2(6) =37.2, p=0.00). This test was able to differentiate between aging sham and stroke mice up to post stroke day 9 (p<0.01). A statistically significant difference in the scores was also seen in the young stroke and sham mice (X2(6) =52.164, p=0.00), however, this difference was only significant up to post surgery day 3 (p<0.01). Complete recovery on this task (score=4) was noted by post stroke day 15 in the aging and day 7 in the young. Young male stroke mice had significantly better scores than aging male stroke mice (X2(6) =86.39, p=0.00).
Table 1. The Vertical Pole test can differentiate stroke from sham animals acutely.
Vertical Pole test scores in young and aging males. The scores are represented as medians (interquartile range). There was a significant difference in the mean ranks of stroke versus sham aging animals up to day 9 post stroke. The aging animals recovered completely by day 15 (MCAO n=6; Sham n=3); There was a significant difference in the mean ranks of stroke versus sham young animals up to day 3 post stroke. The young animals recovered completely on this task by day 7 (MCAO n=14; Sham n=4).
| Day 1 | Day 3 | Day 5 | Day 7 | Day 9 | Day 11 | Day 13 | Day 15 | ||
|---|---|---|---|---|---|---|---|---|---|
| Young | Stroke | 1(0.75)* | 2.5(1)* | 2.5(2) | 4(1.75) | 4(0) | 4(0) | 4(0) | 4(0) |
| Sham | 4(0) | 4(0) | 4(0) | 4(0) | 4(0) | 4(0) | 4(0) | 4(0) | |
| Aging | Stroke | 0(0)* | 0(0.75)* | 1(0)* | 1.5(1)* | 2(0.75)* | 2.5(1.75) | 2.5(1.75) | 4(0) |
| Sham | 4(0) | 4(0) | 4(0) | 4(0) | 4(0) | 4(0) | 4(0) | 4(0) | |
Hanging wire test
Table 2 demonstrates the results of the hanging wire test. Similar to the vertical pole test, young and aging sham mice had no deficits (score=4). Aging sham and stroke mice differed significantly in their overall hanging wire scores (X2(4) =23.3, p=0.00). Individual day analysis revealed that hanging wire test can differentiate between aging sham and stroke mice up to post surgery day 3 (p<0.01). The hanging wire test could not differentiate between young stroke and sham mice at any of the time points (p>0.05), suggesting this test is relatively insensitive to behavioral deficits induced by MCAO of this duration. This test differentiated young versus aging MCAO mice (X2(4) =41.3, p=0.00) until day 5 post surgery (p=0.00) at which time aging mice became indistinguishable from sham.
Table 2. The Hanging wire test shows deficits in the aging acutely after stroke.
Median (interquartile range) scores of young and aging males on the hanging wire test. There was a significant difference in the mean ranks of aging stroke vs. sham out to day 3 after stroke. The aging animals recovered completely on this task by day 7(MCAO n=6; Sham n=3); There was no significant difference in the mean ranks of sham versus stroke young animals as they recovered very quickly on this task (MCAO n=14; Sham n=4).
| Day 1 | Day 3 | Day 5 | Day 7 | Day 9 | ||
|---|---|---|---|---|---|---|
| Young | Stroke | 3.5(1.75) | 4(0) | 4(0) | 4(0) | 4(0) |
| Sham | 4(0) | 4(0) | 4(0) | 4(0) | 4(0) | |
| Aging | Stroke | 0(0)* | 1 (0.75)* | 1.5(1) | 3.5(1.75) | 4(0) |
| Sham | 4(0) | 4(0) | 4(0) | 4(0) | 4(0) | |
Open Field Test (OFT)
There was no significant difference in the average number of beam breaks in young vs. aging before surgery (5012+/−192 young vs. 5213+/−106 in aging, p=0.6). Overall post surgery repeated measures ANOVA did show a significant effect of day [F (1.87, 84) = 7.89; p =0.002], and a surgery by day interaction [F (1.87, 84) = 8.6; p = 0.001]. Analysis at each time point revealed that the OFT significantly differentiated young MCAO and sham mice (Fig. 3B) on days 14, 21 and 28 (p<0.01) post surgery. Consistent with previous reports (Robinson and Coyle, 1980), young MCAO mice demonstrated significant hyperactivity from day 14 to day 28 after MCAO as compared to shams (p=0.00). However, the aging sham versus MCAO mice (Fig. 3A) demonstrated a significant difference in activity only post stroke day 1 (Day 1, p=0.01). In contrast to the young animals, this difference in the aging was due to the lack of stroke induced hyperactivity (Day 1 aging MCAO, 678+/−86.9; aging sham, 5414+/−926, p=0.00).
Figure 3. Aging mice showed transient deficits in the open field but persistent deficits were seen in the corner test.


A. Open field activity assay in aging males. The number of beam breaks is a measure of activity. Aging males showed significantly lower locomotor activity after MCAO as compared to shams on day 1 post stroke; B. Open field activity in the young males. Young males did not show any difference from the sham animals up to day 7 post stroke. The young males showed hyperactivity in the open field from day 14 to 28 post stroke and this differentiated sham from stroke animals significantly; C. Corner test in the young and aging animals. The scores are represented as the ratio of turns towards the non impaired side (R/R+L). Both young and aging shams remained at approximately 0.5, with equivalent turns to the right and left. Aging males were not testable for first seven days post stroke; therefore this test could only differentiate between aging sham and stroke from day 9 to 28(##). The corner test could differentiate between young sham and stroke out to day 29 after stroke (*). The young animals had significantly better scores than the aging males up to day 15 post MCAO (#).
Corner test
Figure 3C shows the results of the corner test in young and aging mice. Repeated measures ANOVA revealed an overall effect of day [F (4.34, 220) = 8.15; p = 0.000] and day by stroke [F (4.34, 220) = 7.54; p = 0.000]. There was a significant difference between stroke and sham animals in both young and aging at each time point (p < 0.05). A significant day by stroke interaction implies that this test can differentiate between stroke and sham animals up to four weeks after stroke. A significant effect of day is seen as the animals show a trend towards recovery over time. Aging mice did not show any active ambulation in the first week post MCAO and were not testable; therefore, the scores for the first week were not used for analysis in all the groups.
Histological Outcome
Figure 4A shows a representative coronal section of a young and aging mouse 30 days after MCAO. Surprisingly, young male mice (27.4+/−4.25) had significantly more cerebral atrophy as compared to aging mice (9.76+/−3.35) (p = 0.006), (Figure 4B). There was no significant correlation between the % tissue lost and corner test scores on post stroke day 29 in young (correlation coefficient: −0.1; p >0.05) or aging mice (correlation coefficient: −0.8; p >0.5), reflecting the relative insensitivity of histological assessments to behavioral deficits and later functional recovery.
Figure 4. Aging mice have less cerebral atrophy after stroke than young.

A. Representative cresyl violet sections of a young and aging male taken on day 30 after 60 minutes MCAO; B. Histological assessment of cerebral atrophy. Aging males had significantly lower (p<0.05) cerebral atrophy as compared to the young animals.
GFAP expression after stroke
In order to determine possible mediators of the poor behavioral recovery in aged mice despite histological evidence of smaller infarcts we examined the inflammatory milieu of young and aged mice 24 hours and 30 days after stroke. We first examined reactive astrocytes (GFAP expressing cells). Figure 5 shows representative sections from young and aging 30 days post sham surgery. The average number of GFAP positive cells per field of vision (avg/field) were significantly different in the young and aging shams (young sham 9+/− 0.7; aging sham 14.9+/− 0.9; p=0.03), Figure 5B. Aging mice had significantly higher GFAP expression compared to young mice at 24 hours (young MCAO, 16+/− 1.7; aging MCAO, 34.3+/− 3.1; p=0.00), consistent with previous reports of accelerated glial scar formation in the aging (Badan et al., 2003), Figure 5C. GFAP positive cells (avg/field) were significantly more prevalent in aging brain versus young brain on day 30 after stroke (young MCAO, 27.6+/− 3.4; aging MCAO, 57.8+/− 5.0; p=0.00), Figure 5D. Furthermore, astrocytes in the aging brain appeared reactive, with a higher number of processes (Figure 5E). An inverse correlation existed between GFAP quantification and behavior on the corner test at 30 days in aging mice (Pearson’s correlation coefficient; GFAP vs. behavior, r=−0.92 in aging, r=0.24 in young).
Figure 5. GFAP expression in coronal brain sections.





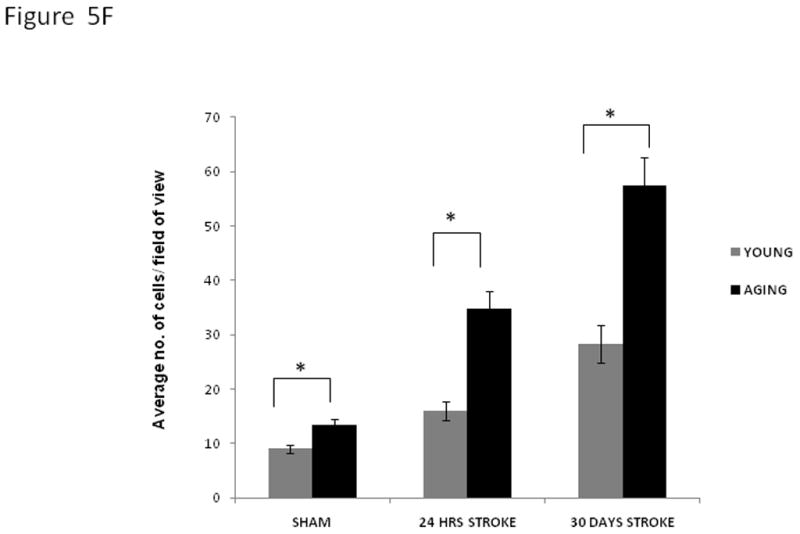
Immunofluorescent staining for GFAP (green), and DAPI (blue) in the brains of young and aging mice four weeks after MCAO; A. The pictures for immunofluorescence were taken from the penumbra, marked in the figure; B. 20× view of aging sham (top panel) and young sham (lower panel) brains showing higher GFAP expression in the aging at the baseline; C. 20× fields show the GFAP staining in the aging (top panel); and young (lower panel) 24 hours after MCAO. Aging brains show higher GFAP expression than young; D. 20× view of aging (top panel) and young (lower panel) brains 30 days after MCAO showing higher GFAP expression in the aging; E. 63× view of aging (top panel) and young (lower panel) brains 30 days after MCAO showing higher GFAP expression in the aging. The aging brain has more astrocytic processes; F. Quantification of the GFAP positive cells (average number of cells/field of view) in young and aging.
Microglial response after stroke
We next looked at the Iba1 expression in the brains of these mice. Iba1 (ionized calcium binding adaptor molecule 1) is specifically expressed in microglia (Ito et al., 1998), the resident macrophages of the brain, and has been used to evaluate the pathophysiological role of activated microglia in cerebral ischemia models (Ito et al., 2001). We first assessed Iba1 expression in sham brains of young and aging mice at 24 hours and 30 days post surgery. The average number of Iba1 positive cells per field of vision (avg/field) was significantly different in the young and aging shams (young sham 17.7+/−3; aging sham 31+/−1.9; p=0.00), Figure 6A. At 24 hours after MCAO surgery (Figure 6B), the number of Iba1 positive cells avg/field in the penumbra was significantly different in the young and aging (young MCAO, 26.1+/−0.9; aging MCAO, 51.2+/−1.9; p=0.00). Even at 30 days post MCAO (Figure 6C), the Iba1 positive cells avg/field in the penumbra were significantly higher in the aging brain (young MCAO, 26.8+/−2.7; aging MCAO, 43.6+/−3.6; p=0.00). Aging animals had higher number of Iba1 + cells which had a deramified morphology indicating enhanced activity (Figure 6D). A correlation between Iba1 numbers and behavioral deficits on the corner test at 30 days was seen in both young and aging mice (r=0.91 aging, r=0.92 young).
Figure 6. Iba1 expression in the brain sections.





Immunofluorescent staining for Iba1 (red) and DAPI (blue) in the brains of young and aging mice four weeks after MCAO; A. 20× view of aging sham (top panel) and young sham (lower panel) brains showing higher Iba1 expression in the aging at the baseline; B. 20× view of aging (top panel) and young (lower panel) brains 24 hours after MCAO showing higher Iba1 expression in the aging; C. 20× view of aging (top panel) and young (lower panel) brains 30 days after MCAO showing higher Iba1 expression in the aging; D. 63× fields show the Iba1 staining in the aging (top panel) and the young (lower panel) 30 days after MCAO; E. Quantification of the Iba1+ cells (average number of cells/field of view) in young and aging.
DISCUSSION
There are several important novel findings in this study. Firstly, we identified a simple battery of behavioral tests to evaluate chronic functional recovery in aging mice after focal ischemia. Secondly, we found that although aging male mice had worse behavior scores compared to young mice for the first two weeks after stroke, they eventually recovered to a similar level seen in young mice. Thirdly, we found that young mice had an unexpectedly higher amount of cerebral atrophy (a measure of lesion volume) than the aging mice four weeks after stroke. This was in contrast to the observed behavioral outcomes. Fourthly, GFAP and Iba1 expression is enhanced in the penumbra of the aging MCAO brain at both 24 hours and 30 days after stroke. Finally, we found higher Iba1 and GFAP expression in sham aging brain, suggesting that aging induces a pro-inflammatory milieu. This may explain the poorer behavioral outcomes in aging.
Although many behavior tests have been developed for use in young animals after stroke, this is the first study to demonstrate that a combination of behavioral tests can be used to evaluate stroke outcome in aging mice. Simple tests like the vertical pole can be used to differentiate aging sham from stroke out to nine days after stroke; others like hanging wire can be used successfully from day 1 to 3. Therefore, the vertical pole and hanging wire should only be used at acute time points, as even aging animals recover on these tasks rapidly. Both the vertical pole and hanging wire are tests of motor strength, balance and co-ordination. Since ischemic stroke causes significant motor impairments on the contralateral side, these are inexpensive tests to unmask the motor deficits in experimental murine models of stroke. In contrast to the aging mice, young animals did not show any deficits on the hanging wire after 60 minutes of ischemia, as reported previously after a 90 minute MCAO (Li et al., 2004). The vertical pole unmasked stroke deficits in the young mice only transiently (Day 1 and 3 post stroke). Other groups have reported deficits from day 2 to 8 in young mice on this test (Bouet et al., 2007). This difference could be secondary to the different strains used (Swiss mice, CERJ, France versus C57 in this study).
The open field test has been well validated in a permanent cerebral ischemia model in rats (Robinson and Coyle, 1980). Similar to previous reports (Robinson and Coyle, 1980), young mice exhibited post-stroke hyperactivity in the open field from day 14 to 28 after stroke, and this differentiated sham from stroke animals. However, no significant hyperactivity was noted in the aging animals. In fact, aging mice had significant locomotor suppression as seen in OFT on day one after stroke and in the corner test for seven days after stroke. This finding is interesting as it has been well documented that ischemic stroke increases motor activity in young animals, the cause of which is not well understood. Some studies suggest this hyperactivity to be due to the habituation deficits (Plamondon and Khan, 2005, Milot and Plamondon, 2009), while others report it to be due to non associative memory loss (Colbourne and Corbett, 1995). The exploratory activity of an animal in an open field is a reflection of its emotional and motivational state (Katz et al., 1981). Therefore, one possible explanation for the initial hypoactivity seen in the aging mice, is the loss of incentive motivation/“loss of interest” (Roth and Katz, 1979). It is possible that this reflects post-stroke anhedonia (Roth and Katz, 1979, Katz et al., 1981, Wang et al., 2009) in the aging, which will be investigated in future studies. As the importance of age as a factor in the physiological response to stroke is recognized, more sophisticated tests of learning and memory will need to be assessed and developed for use in aging animals. Coupling behavioral outcomes with measurement of neuronal function (i.e., electrophysiology) or structure (in vivo microscopy) are increasingly used in young animals, and transitioning these methods into aging models should be a priority.
Unlike the hanging wire and vertical pole tests, which exclusively measure motor function, the corner test detects integrated motor and sensory input that is more likely damaged in infarction of the MCA territory and underlying sensorimotor cortex (Li et al., 2004). The rearing response is motor but the response is also dependent on vibrissae stimulation (sensory) caused by the tactile sensations elicited by the two ends of the board. The corner test was the most reliable test for showing chronic behavioral deficits in the aging mice from day 7 (as aging animals did not show activity in the corner for first week after stroke) to thirty days after stroke. In the young, this test could be used to differentiate between stroke and sham animals from day 1 to day 30 after stroke. Persistence of deficits has been reported up to post stroke day 90 (Zhang et al., 2000) in young mice. Since the mice do not rapidly recover on this test; this is the best test to assess chronic functional outcomes after stroke in both young and aging mice.
So what is a reasonable battery of behavioral tests for use in aging mice after stroke? Our findings suggest that a combination of vertical pole (post stroke day1-9) and corner test (post stroke day 9 to 30) can be used to assess post stroke functional outcomes in aging mice. These can be supplemented with hanging wire and open field to assess deficits at very acute end points. All these tests are simple and inexpensive and show deficits even with repeated testing. It should be noted at this point in the lifespan (late middle age and equivalent to humans in the 5th decade) the balance and co-ordination of aging animals was not impaired at the baseline. It is likely that baseline deficits in locomotion and balance exist in very old mice (~ 24 months). The median lifespan of a C57BL6 mouse is 851 days (~28 months) (Harrison et al,) and a 50% spontaneous mortality is expected in this age group. A middle aged mouse is 10–14 months old (equivalent to 38–47 years old human) and an aged mouse is 18–24 months old (equivalent to 56–69 years old human) (Flurkey, Harrison et al, 2007). We specifically chose to examine aging mice (15–16 months old) rather than aged mice for several reasons. Aged animals (18–24 months) are near the end of their lifespan, often have a high rate of tumors, and are considerably more fragile than 16 month old mice (Miller and Nadon, 2000). Moreover, aged mice often have other comorbidities and illnesses making it difficult to interpret the results as an effect of age or sickness (Miller and Nadon, 2000). It is likely that these confounders and baseline deficits would make the assessment of stroke recovery more difficult to interpret. We anticipate that behavioral recovery would be slower or non-existent in very old mice, which will be investigated in future studies.
Another important finding of this study is the clear evidence of differences in recovery rate in the young and aging mice. The vertical pole, hanging wire and corner test demonstrated that the aging animals had worse behavior scores than the young for the first two weeks after stroke. This is consistent with clinical reports and has been seen by others in rat models (Badan et al., 2003). Although, the aging mice had worse behavioral deficits (in the early recovery phase), stroke related histological damage to the brain (computed by percentage cerebral atrophy) was significantly lower in aging animals versus the young. There are inconsistencies in literature regarding the size of the infarct after stroke in the aging with reports of increased, decreased, or equivalent stroke damage (Wang et al., 2003, DiNapoli et al., 2008, Liu et al., 2009). We are the first to demonstrate these findings at a chronic end point (30 days after stroke). Our results are consistent with findings by (Shapira et al., 2002) and (Liu et al., 2009) at acute end points. There are various possible explanations for the differences seen between histological and behavioral outcomes. Firstly, there was an enhanced astrocytic response (as evident by enhanced GFAP expression) in the aging brain at both 24 hours (accelerated astrocytic response) and 30 days after stroke. These reactive astrocytes release various inflammatory cytokines and chemokines in the injured brain (Ridet et al., 1997, Little and O’Callagha, 2001, Anderson et al., 2003). Moreover, it has been well described that the proliferation of reactive astrocytes after injury leads to glial scar formation (Fawcett and Asher, 1999, McGraw et al., 2001, Silver and Miller, 2004). By isolating the injured area, viable tissue could be spared from toxic substances released from cells that are irreversibly damaged. However, the injured area is also isolated from cells and molecules which are vital for synaptogenesis (leading to acute stroke recovery), neurogenesis (a more chronic mechanism contributing to recovery) and subsequent repair (Stroemer et al., 1995, Anderson et al., 2003). Significantly enhanced GFAP expression was evident in the aging brain at 24 hours after MCAO, suggesting early and more rapid formation of the glial scar. This could impede the development of synaptic connections (Rudge and Silver, 1990) and lead to poor initial recovery, as was seen in the aging (Badan et al., 2003). We hypothesize that rapid formation of a glial scar in the aging brain not only contributes to the paradoxically smaller infarcts, by reducing infarct expansion; but also leads to poor behavioral recovery by acutely reducing exposure to substances that could salvage damaged tissue. GFAP expression and recovery rates were negatively correlated in the aging brain, suggesting that enhanced astrocytosis could contribute to slower recovery. In our model, aging mice were indistinguishable from young at 30 days, suggesting although recovery is slower, it is complete, at least as measured by the testing in this study. This suggests that more chronic mechanisms of recovery, such as neurogenesis, may be less impaired.
Secondly, there was an enhanced microglial response seen in the aging brain. Microglia are the sensors in the brain that can acutely reduce ischemic damage by production of neurotrophic factors like TNF (Lambertsen et al., 2009) or by phagocytosing the necrotic debris. However, activated microglia also produce cytotoxic and inflammatory mediators, which contribute to cell death. Enhanced and prolonged microglial responsiveness in the aged brain (Ridet et al., 1997) hampers neuronal recovery. We have shown that more amoeboid/phagocytic microglia are evident in the aging brain both at one day and four weeks after stroke and more activated microglia are present at baseline in the uninjured aging brain, suggesting that aged animals are primed to have a detrimental neuroimmune response to injury. Enhanced baseline microglia numbers has been reported previously in rats (Ogura et al., 1994), monkeys (Peters et al., 1991, Sheffield and Berman, 1998) and humans (Dickson et al., 1992). It has been well documented that oxidative stress and inflammatory cytokine exposure across the lifespan (Godbout and Johnson, 2009) leads to a change in microglial phenotype and “priming” (Sparkman and Johnson, 2008). These “primed” microglia (with shortened processes and cell surface expression of markers of activation), get induced rapidly and release higher amounts of cytokines as compared to activated microglia (Boche et al., 2003, Perry et al., 2003, Frank et al., 2007, Sparkman and Johnson, 2008). We speculate that the rapid and exaggerated inflammatory cytokine production by these microglia (primed with age), may explain the worse behavior in the aging mice (Sparkman and Johnson, 2008). Further evidence suggests that inflammation related biomarkers are enhanced in older stroke patients and these predict worse outcomes after stroke (Smith et al., 2004, De Martinis et al., 2006). We have found similar patterns in aging male mice after MCAO with an enhanced pro-inflammatory IL-6 response (Liu et al., 2009). This enhanced inflammatory pattern could contribute to stroke induced behavioral deficits.
In conclusion, aging mice can be successfully tested for long term functional recovery repeatedly after stroke using a battery of behavior tests which include vertical pole, hanging wire, open field and corner test. Aging males show smaller infarcts but worse initial recovery after stroke; this may be attributed to the pro-inflammatory milieu of the aging brain and formation of glial scar. The disassociation of behavior and histological outcome emphasizes the need for pre-clinical stroke studies to include assessment of functional outcomes, especially when aging animals are used.
Research Highlight.
Aging enhances the neuroimmune response to experimental stroke and impairs functional recovery.
Acknowledgments
We would like to thank Dr. Lisa Conti for help with the statistical analysis. This work is funded by RO1 NS050505, NS055215 and NS0 66406 (to LDM).
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe T, Kunz A, Shimamura M, Zhou P, Anrather J, Iadecola C. The neuroprotective effect of prostaglandin E2 EP1 receptor inhibition has a wide therapeutic window, is sustained in time and is not sexually dimorphic. J Cereb Blood Flow Metab. 2009;29:66–72. doi: 10.1038/jcbfm.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MF, Blomstrand F, Blomstrand C, Eriksson PS, Nilsson M. Astrocytes and stroke: networking for survival? Neurochem Res. 2003;28:293–305. doi: 10.1023/a:1022385402197. [DOI] [PubMed] [Google Scholar]
- Badan I, Buchhold B, Hamm A, Gratz M, Walker LC, Platt D, Kessler C, Popa-Wagner A. Accelerated glial reactivity to stroke in aged rats correlates with reduced functional recovery. J Cereb Blood Flow Metab. 2003;23:845–854. doi: 10.1097/01.WCB.0000071883.63724.A7. [DOI] [PubMed] [Google Scholar]
- Boche D, Cunningham C, Gauldie J, Perry VH. Transforming growth factor-beta 1-mediated neuroprotection against excitotoxic injury in vivo. J Cereb Blood Flow Metab. 2003;23:1174–1182. doi: 10.1097/01.WCB.0000090080.64176.44. [DOI] [PubMed] [Google Scholar]
- Bouet V, Freret T, Toutain J, Divoux D, Boulouard M, Schumann-Bard P. Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse. Exp Neurol. 2007;203:555–567. doi: 10.1016/j.expneurol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Colbourne F, Corbett D. Delayed postischemic hypothermia: a six month survival study using behavioral and histological assessments of neuroprotection. J Neurosci. 1995;15:7250–7260. doi: 10.1523/JNEUROSCI.15-11-07250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol. 2006;80:219–227. doi: 10.1016/j.yexmp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Crystal HA, Mattiace LA, Masur DM, Blau AD, Davies P, Yen SH, Aronson MK. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging. 1992;13:179–189. doi: 10.1016/0197-4580(92)90027-u. [DOI] [PubMed] [Google Scholar]
- DiNapoli VA, Huber JD, Houser K, Li X, Rosen CL. Early disruptions of the blood-brain barrier may contribute to exacerbated neuronal damage and prolonged functional recovery following stroke in aged rats. Neurobiol Aging. 2008;29:753–764. doi: 10.1016/j.neurobiolaging.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M, Engelhardt B, Koistinaho J, Lindvall O, Meairs S, Mohr JP, Planas A, Rothwell N, Schwaninger M, Schwab ME, Vivien D, Wieloch T, Dirnagl U. Improving outcome after stroke: overcoming the translational roadblock. Cerebrovasc Dis. 2008;25:268–278. doi: 10.1159/000118039. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- Flurkey K, Currer JM, Harrison DE. The Mouse in Aging Research. In: Fox JG, et al., editors. The Mouse in Biomedical Research. 2. American College Laboratory Animal Medicine (Elsevier); Burlington, MA: 2007. pp. 637–672. [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Johnson RW. Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Immunol Allergy Clin North Am. 2009;29:321–337. doi: 10.1016/j.iac.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Harrison http://research.jax.org/faculty/harrison/ger1vi_LifeStudy1.html.
- Hattori K, Lee H, Hurn PD, Crain BJ, Traystman RJ, DeVries AC. Cognitive deficits after focal cerebral ischemia in mice. Stroke. 2000;31:1939–1944. doi: 10.1161/01.str.31.8.1939. [DOI] [PubMed] [Google Scholar]
- Howard G, Toole JF, Frye-Pierson J, Hinshelwood LC. Factors influencing the survival of 451 transient ischemic attack patients. Stroke. 1987;18:552–557. doi: 10.1161/01.str.18.3.552. [DOI] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998;57:1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- Ito D, Tanaka K, Suzuki S, Dembo T, Fukuuchi Y. Enhanced expression of Iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke. 2001;32:1208–1215. doi: 10.1161/01.str.32.5.1208. [DOI] [PubMed] [Google Scholar]
- Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci Biobehav Rev. 1981;5:247–251. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- Lambertsen KL, Clausen BH, Babcock AA, Gregersen R, Fenger C, Nielsen HH, Haugaard LS, Wirenfeldt M, Nielsen M, Dagnaes-Hansen F, Bluethmann H, Faergeman NJ, Meldgaard M, Deierborg T, Finsen B. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci. 2009;29:1319–1330. doi: 10.1523/JNEUROSCI.5505-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187:94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Little AR, O’Callagha JP. Astrogliosis in the adult and developing CNS: is there a role for proinflammatory cytokines? Neurotoxicology. 2001;22:607–618. doi: 10.1016/s0161-813x(01)00032-8. [DOI] [PubMed] [Google Scholar]
- Liu F, Akella P, Benashski SE, Xu Y, McCullough LD. Expression of Na-K-Cl cotransporter and edema formation are age dependent after ischemic stroke. Exp Neurol. 2010;224:356–361. doi: 10.1016/j.expneurol.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, McCullough LD. Middle cerebral artery occlusion model in rodents: methods and potential pitfalls. J Biomed Biotechnol. 2011:464701. doi: 10.1155/2011/464701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab. 2009;29:792–802. doi: 10.1038/jcbfm.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay J, Mensch G. The atlas of heart disease and stroke. 2004. [Google Scholar]
- McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280:20493–20502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- McGraw J, Hiebert GW, Steeves JD. Modulating astrogliosis after neurotrauma. J Neurosci Res. 2001;63:109–115. doi: 10.1002/1097-4547(20010115)63:2<109::AID-JNR1002>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Miller RA, Nadon NL. Principles of animal use for gerontological research. J Gerontol A Biol Sci Med Sci. 2000;55:B117–123. doi: 10.1093/gerona/55.3.B117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milot MR, Plamondon H. Time-dependent effects of global cerebral ischemia on anxiety, locomotion, and habituation in rats. Behav Brain Res. 2009;200:173–180. doi: 10.1016/j.bbr.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. The influence of age on stroke outcome. The Copenhagen Stroke Study. Stroke. 1994;25:808–813. doi: 10.1161/01.str.25.4.808. [DOI] [PubMed] [Google Scholar]
- Ogura K, Ogawa M, Yoshida M. Effects of ageing on microglia in the normal rat brain: immunohistochemical observations. Neuroreport. 1994;5:1224–1226. doi: 10.1097/00001756-199406020-00016. [DOI] [PubMed] [Google Scholar]
- Paylor R, Nguyen M, Crawley JN, Patrick J, Beaudet A, Orr-Urtreger A. Alpha7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: a behavioral characterization of Acra7-deficient mice. Learn Mem. 1998;5:302–316. [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4:103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- Peters A, Josephson K, Vincent SL. Effects of aging on the neuroglial cells and pericytes within area 17 of the rhesus monkey cerebral cortex. Anat Rec. 1991;229:384–398. doi: 10.1002/ar.1092290311. [DOI] [PubMed] [Google Scholar]
- Plamondon H, Khan S. Characterization of anxiety and habituation profile following global ischemia in rats. Physiol Behav. 2005;84:543–552. doi: 10.1016/j.physbeh.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- Robinson RG, Coyle JT. The differential effect of right versus left hemispheric cerebral infarction on catecholamines and behavior in the rat. Brain Res. 1980;188:63–78. doi: 10.1016/0006-8993(80)90557-0. [DOI] [PubMed] [Google Scholar]
- Roth KA, Katz RJ. Stress, behavioral arousal, and open field activity--a reexamination of emotionality in the rat. Neurosci Biobehav Rev. 1979;3:247–263. doi: 10.1016/0149-7634(79)90012-5. [DOI] [PubMed] [Google Scholar]
- Rudge JS, Silver J. Inhibition of neurite outgrowth on astroglial scars in vitro. J Neurosci. 1990;10:3594–3603. doi: 10.1523/JNEUROSCI.10-11-03594.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira S, Sapir M, Wengier A, Grauer E, Kadar T. Aging has a complex effect on a rat model of ischemic stroke. Brain Res. 2002;925:148–158. doi: 10.1016/s0006-8993(01)03270-x. [DOI] [PubMed] [Google Scholar]
- Sheffield LG, Berman NE. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol Aging. 1998;19:47–55. doi: 10.1016/s0197-4580(97)00168-1. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Emsley HC, Gavin CM, Georgiou RF, Vail A, Barberan EM, del Zoppo GJ, Hallenbeck JM, Rothwell NJ, Hopkins SJ, Tyrrell PJ. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004;4:2. doi: 10.1186/1471-2377-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman NL, Johnson RW. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 2008;15:323–330. doi: 10.1159/000156474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroemer RP, Kent TA, Hulsebosch CE. Neocortical neural sprouting, synaptogenesis, and behavioral recovery after neocortical infarction in rats. Stroke. 1995;26:2135–2144. doi: 10.1161/01.str.26.11.2135. [DOI] [PubMed] [Google Scholar]
- Wahl F, Allix M, Plotkine M, Boulu RG. Neurological and behavioral outcomes of focal cerebral ischemia in rats. Stroke. 1992;23:267–272. doi: 10.1161/01.str.23.2.267. [DOI] [PubMed] [Google Scholar]
- Wang RY, Wang PS, Yang YR. Effect of age in rats following middle cerebral artery occlusion. Gerontology. 2003;49:27–32. doi: 10.1159/000066505. [DOI] [PubMed] [Google Scholar]
- Wang SH, Zhang ZJ, Guo YJ, Zhou H, Teng GJ, Chen BA. Anhedonia and activity deficits in rats: impact of post-stroke depression. J Psychopharmacol. 2009;23:295–304. doi: 10.1177/0269881108089814. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chen J, Li Y, Zhang ZG, Chopp M. Quantitative measurement of motor and somatosensory impairments after mild (30 min) and severe (2 h) transient middle cerebral artery occlusion in rats. J Neurol Sci. 2000;174:141–146. doi: 10.1016/s0022-510x(00)00268-9. [DOI] [PubMed] [Google Scholar]


