Abstract
Susceptibility testing for common, complex adult-onset diseases is projected to become more commonplace as the rapid pace of genomic discoveries continues, and evidence regarding the potential benefits and harms of such testing is needed to inform medical practice and health policy. Apolipoprotein E (APOE) testing for risk of Alzheimer’s disease (AD) provides a paradigm in which to examine the process and impact of disclosing genetic susceptibility for a prevalent, severe and incurable neurological condition. This review summarizes findings from a series of multi-site randomized clinical trials examining psychological and behavioral responses to various methods of genetic risk assessment for AD using APOE disclosure. We discuss challenges involved in disease risk estimation and communication and the extent to which participants comprehend and perceive utility in their genetic risk information. Findings on the psychological impact of test results are presented (e.g., distress), along with data on participants’ health behavior and insurance purchasing responses (e.g., long term care). Finally, we report comparisons of the safety and efficacy of intensive genetic counseling approaches to briefer models that emphasize streamlined processes and educational materials. The implications of these findings for the emerging field of personal genomics are discussed, with directions identified for future research.
BACKGROUND
Genetic susceptibility testing for common, complex diseases
Genomic discoveries are transforming medical science, in part by providing new methods to predict the occurrence (1) or progression (2) of diseases that have a genetic basis. Because of their public health significance, there has been great interest in the identification of susceptibility genes for common, complex conditions in particular. Genetic susceptibility testing for common disease provides information about risk that is, by definition, influenced by a complex (and not always understood) interplay among genes, environment and behavior. This type of information lacks the degree of certainty of highly penetrant Mendelian variants, where the presence or absence of specific mutations is more clearly associated with the future manifestation (or not) of a given disease. Due to their limitations, there is concern that many new tests are prematurely moving into the clinical arena and the direct-to-consumer (DTC) marketplace (3, 4). Skeptics express concerns over the limited predictive value of genetic susceptibility testing, along with potential harms including psychological distress, misunderstanding of risk information, and insurance or employment bias (5, 6). On the other hand, personalized disease management and pharmaceutical regimens tailored to individuals’ genotypes represent promising applications of genomics research (7, 8). Experts forecast that genomic discoveries will increase options for prevention and treatment of common, complex health conditions such as diabetes, heart disease, and cancer (9). Some contend that personalized genomic risk information can help prompt individuals to make lifestyle changes to avoid or reduce their disease risk (10).
Although there is much debate over the likelihood and extent of benefits to be derived from genetic susceptibility testing, it appears clear that use of this modality will increase in the coming years. Such markers can often be measured with relative ease once identified, and the initiative of motivated individuals and the power of market forces may make it difficult to limit or prevent the widespread use of susceptibility genotyping in the future. Our responsibility as experts may be to understand how to use them wisely, and to articulate whether and under what circumstances they should be used. Clinical research, as opposed to ideologically based speculation, would seem to be the best guide of policy and practice in this arena. Given the vast number of genetic markers and conditions to consider, one research strategy is to select “sentinel” cases where genetic susceptibility testing for common, adult-onset conditions is available. This has been our approach, as we have used genetic susceptibility testing for Alzheimer’s disease as a paradigm in which to examine the process and impact of disclosing genetic risk information to asymptomatic adults.
Genetic testing for Alzheimer’s disease
Alzheimer’s disease (AD) is the most common type of dementia in the U.S., with a prevalence expected to increase dramatically in coming decades (11). The genetics of AD have been comprehensively summarized elsewhere. Briefly, mutations in each of 3 genes (APP, PS1 and PS2) are associated with early-onset, autosomal dominantly inherited forms of AD. Genetic testing already occurs for these rare familial forms of AD, with counseling procedures based on the Huntington’s disease testing model (12). However, these mutations account for a very small proportion of AD cases. Much more common is a variant of the susceptibility polymorphism Apolipoprotein E (APOE), a plasma protein involved in cholesterol transport. APOE has three common alleles (ε2, ε3 or ε4). The ε4 allele is a robust risk factor for sporadic and late-onset familial AD, with the degree of risk varying depending upon whether the individual carries one or two ε4 alleles (13). Discoveries of new susceptibility genes over the past decade (14, 15) have increased our knowledge of potential genetic determinants of AD, but none of these has been as frequently replicated nor has as notable a risk effect as APOE.
In the mid- to late 1990s, several expert consensus statements cautioned against the clinical use of predictive testing for AD using APOE, largely due to the fact that information about the future risk of developing AD does not change clinical care given the limited treatment and prevention options for the disease (16–19). In addition, possession of an ε4 allele is neither necessary nor sufficient to cause AD. However, each of the aforementioned statements concluded along these lines: “More research is needed on how individuals and families understand complex probabilistic genetic information and on the implications of living one’s life ‘at risk’ for developing AD” (19).
Research on genetic susceptibility testing for AD may be useful for understanding how individuals will respond to susceptibility testing for common, complex diseases in general. Genetic risk factors now being identified for other complex diseases via genome-wide associations studies are similar to APOE in that a) they are more prevalent in the general population than rare Mendelian variants, b) their positive predictive value is relatively low, and c) testing for these variants is now being marketed to the public through commercial firms. Although its lack of proven prevention options sets AD apart from modifiable conditions such as heart disease and diabetes, there is growing scientific evidence that environment, lifestyles, and social factors may influence risk of AD and related dementias (11, 20). The majority of the public believes that lifestyle, diet, and mental inactivity contributes to AD risk even while they endorse genetics as the most important risk factor for AD (21). Furthermore, individuals who seek genetic susceptibility testing for AD commonly cite disease risk reduction as a prime motivator (22). In sum, laypersons appear to view AD as more “modifiable” than current scientific evidence suggests. In the next section, we describe a series of clinical trials designed to examine the psychological and behavioral impact of providing genetic susceptibility testing for AD.
Disclosing Genetic Risk for AD: The REVEAL Study
Overview
The Risk Evaluation and Education for Alzheimer’s Disease (REVEAL) Study is a series of multi-site randomized clinical trials that examines the impact of APOE genetic susceptibility testing on asymptomatic individuals (see Table 1). An interdisciplinary team of clinicians, geneticists, genetic counselors, health psychologists, ethicists and policy scholars created protocols to evaluate the impact of APOE disclosure for evaluating AD risk. The first REVEAL Study trial compared a genetic risk assessment program that incorporated APOE genotype disclosure against a more general AD risk assessment. The second trial built on the first by expanding the participant profile to include more African Americans and to test a condensed educational and counseling protocol against a more traditional model based on genetic testing for cancer susceptibility. The third trial explored the impact of disclosing that the ε4 allele of APOE is associated with coronary artery disease in addition to AD, and also tested a telephone disclosure protocol against in-person disclosure. The fourth trial, just underway, is exploring the impact of an AD genetic risk assessment for individuals already with mild cognitive impairment (MCI).
Table 1.
Overview of REVEAL Clinical Trials
| Trial | Dates | Site Locations | Study Sample | Main Question(s) |
|---|---|---|---|---|
| REVEAL I | 2000–02 | Boston; Cleveland; New York City | 162 adult children of people with AD | What is the psychological impact of disclosure of genetic risk for AD? |
| REVEAL II | 2004–06 | Boston; Cleveland; New York City; Washington DC | 280 first-degree relatives of people with AD | Can genetic risk for AD be disclosed safely and effectively using a condensed protocol? |
| REVEAL III | 2007–09 | Boston; Cleveland; Washington; Ann Arbor MI | 257 adults with and without immediate AD family history | What is the impact of disclosure of pleiotropic disease risks associated with APOE? Can results be disclosed safely and effectively via phone? |
| REVEAL IV | 2011-present | Boston; Philadelphia; Washington; Ann Arbor MI | Persons with amnestic MCI and their study partners | What is the impact of disclosing APOE to a population at imminent risk of AD? |
In each trial, participants complete a baseline assessment and then receive pre-test education about study requirements and the risk information that will be disclosed. Following a blood draw for genotyping purposes, participants receive personalized AD risk estimates through age 85 (range: 6%-77%) depending on gender, self-identified ethnicity (REVEAL II and III, only), family history of AD and, for most participants, APOE genotype. These risks were derived from a longstanding multi-site program of genetic epidemiology research (23). Participants are followed one year to assess the impact of the risk assessment on outcomes described below.
Risk estimation
As is the case with other complex diseases, AD etiology poses numerous challenges for risk estimation. Although individual studies have suggested scores of potential risk and protective factors for AD, many have not been confirmed in prospective studies and it is unclear whether and to what extent these risk factors are additive or interactive. The risk estimates developed in REVEAL were therefore based only on well-established AD risk factors including age, gender, family history, and APOE genotype using a) sex- and family history-specific incidence curves based on a large-scale epidemiological study (24) and b) APOE genotype-specific odds ratio estimates from a meta-analysis of data over 50 studies worldwide (25). However, our estimates did not take into account other potential risk factors for the disease, including other genes, gene-gene interactions, gene-environment interactions, environmental exposures, and other demographics factors. Participants were notified of these limitations during pre-test education and when disclosed their risk of AD.
Another challenge was the issue of differing risks across racial and ethnic groups. Several epidemiological studies have suggested African Americans are at higher risk than Whites, but the underlying reasons for this disparity are not fully understood. Whether and how to disclose ethnicity-specific risk estimates raised both scientific and ethical dilemmas given the troubled history of genetic research with African Americans (26). Guided by both expert consensus and focus group data from African American community members, risk models specific to ethnicity, gender, and APOE genotype were subsequently developed for the second REVEAL trial such that African-Americans and Whites received differing risk estimates (see Table 2) (27). The experience of risk estimation in REVEAL offers lessons for how to address the ambiguity and ethical tensions inherent in risk disclosure for a complex disease whose etiology is not well-understood.
Table 2.
Lifetime risk estimates (through age 85) for first-degree relatives of people with AD, stratified by gender, APOE genotype, and ethnic group
| Genotype | White Men | White Women | AA Men | AA Women |
|---|---|---|---|---|
| ε2/ε3 | 13% | 19% | 33% | 36% |
| ε3/ε3 | 18% | 29% | 41% | 49% |
| ε2/ε4 | 25% | 49% | 48% | 69% |
| ε3/ε4 | 29% | 52% | 56% | 73% |
| ε4/ε4 | 56% | 57% | 77% | 74% |
Note: AA = African American. ε2/ε2 estimates are not provided because there was not sufficient population prevalence of this genotype in our epidemiological studies to provide robust risk estimates
Interest in testing
The REVEAL Study has shed light on those who might express interest in genetic susceptibility testing for common complex disease. Among participants in the first REVEAL trial who had been recruited from research registries, those who were under the age of 60 and those who were college educated were more likely to progress from initial contact to randomization. Among participants who self-referred to the study after hearing about it in a memory assessment clinic, the media, or in a public presentation, the average education level was more than a college degree, and 79% were female (28). Although some of these trends may be driven by characteristics of Alzheimer’s disease rather than characteristics of testing (e.g., the need to plan for AD in middle age and gender disparities in AD caregiving), differences by gender and education have also been seen to a lesser extent in other studies of genetic susceptibility testing (29, 30).
Perceived utility of testing
As mentioned above, genetic testing in clinical practice is typically judged on whether or not it can inform medical care options. However, our findings showed that participants had numerous reasons for seeking testing that would not be classified under usual definitions of clinical utility. For example, the vast majority of participants endorsed reasons for seeking testing that included the following: arranging personal affairs; informing decisions about long-term care insurance; preparing family for the possibility of illness; and emotional relief if found to be at lower risk (31). Of note here are the perceived benefits of genetic testing for psychological well-being and advanced planning; many participants believed that information would be helpful even in the absence of proven medical care options to reduce their AD risk.
Risk recall and perceptions
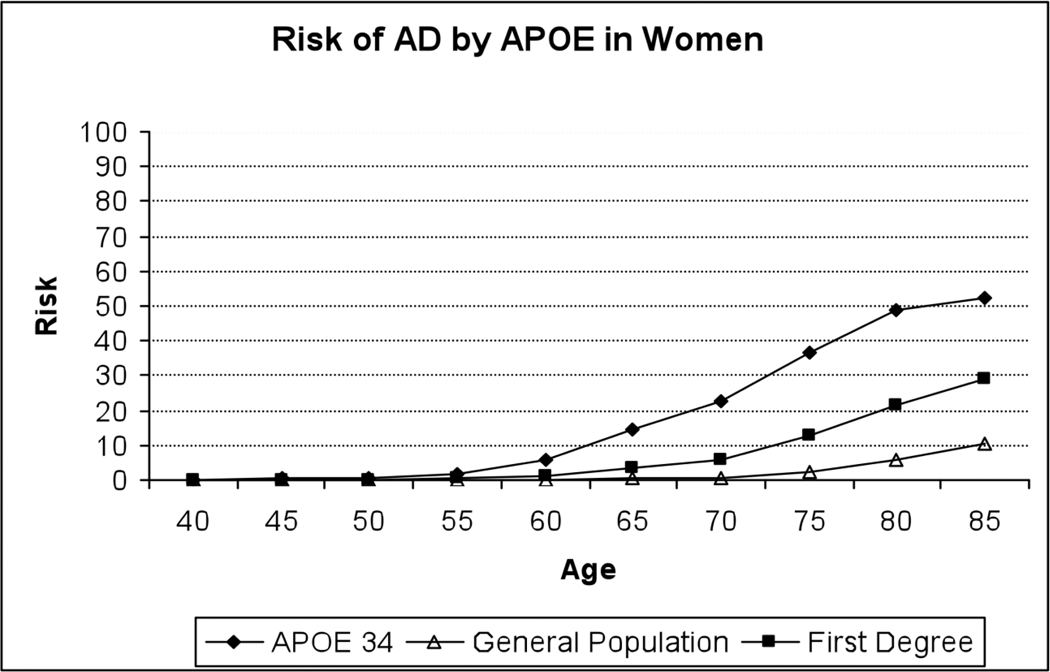
Our experience also demonstrated the challenges of conveying genetic risk information for a complex disease. Informed by the health risk communication literature, our counseling and education materials included the following: take-home written materials to reinforce information presented (32); strategies for coping with risk and resources for further information; and graphical representations of risk information to guide in-person counseling (33). For example, we used visual aids including risk curves specific to gender, ethnicity, family history and APOE genotype that conveyed risk of AD through age 85 and in comparison to reference groups including the general population (34). These curves also showed risk over time, reinforcing the importance of the interactive effect between age and APOE (see Figure 1 for an example). Yet even despite these efforts and a well-educated set of participants, many could not recall their lifetime risk information at six-week follow-up. However, the vast majority knew their APOE status, suggesting that genotype is more memorable to participants than lifetime risk and reinforcing the notion that gist-level health information is often retained instead of specific numeric estimates (35).
Figure 1.
Sample risk curve presented to REVEAL participants
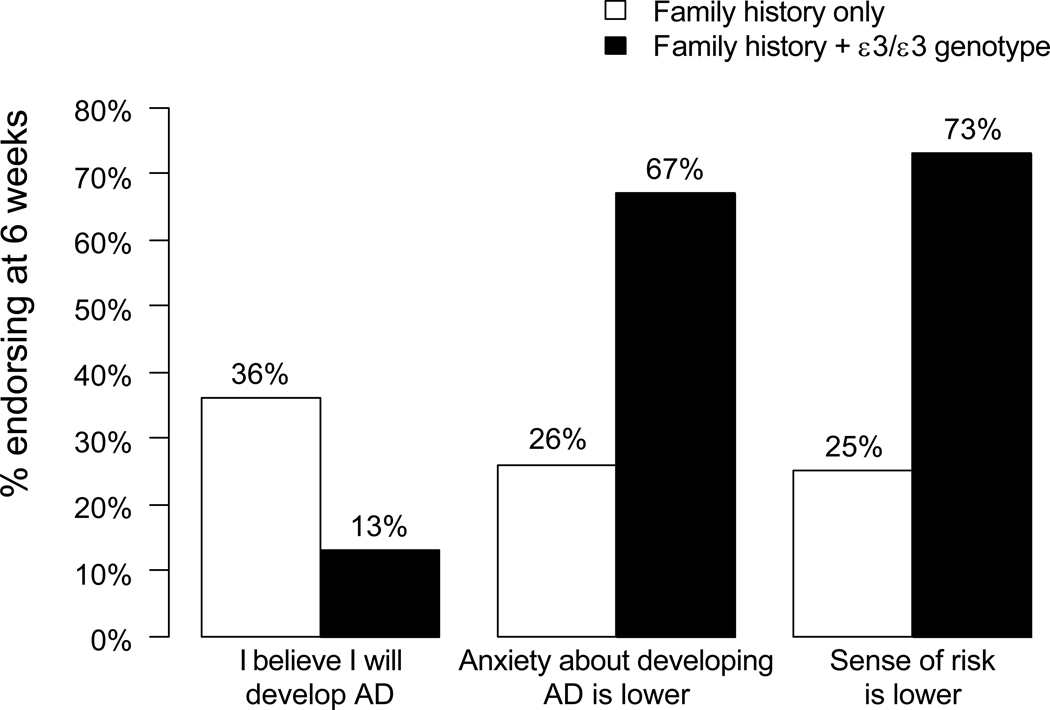
Results also suggested that genotype information has an outsized influence on risk perceptions, even when offered within a multi-factorial risk assessment. For example, we compared women with ε4-negative test results to a control group of women who received an identical lifetime risk estimate but who were not disclosed their APOE status. Although both groups received the same “take-home” message about lifetime risk of AD (i.e., 29% probability), the ε4-negative women perceived their risk as lower, reported testing as having a more positive impact, endorsed less strongly the belief that they might develop AD, and reported a greater reduction in anxiety about AD (see Figure 2) (36). Ongoing analyses in REVEAL are continuing to explore the extent to which “negative” genetic test results contribute to a potentially false sense of reassurance about disease risk.
Figure 2.
Proportion of participants receiving identical 29% numerical risk estimates that endorsed perceived risk survey items, stratified by risk disclosure group
Data also suggest that calculated risk estimates are often not taken at face value. Approximately half of participants in the second REVEAL trial who could accurately recall their numerical risk estimates asserted that their lifetime risk for AD was either lower or higher than what was calculated (37). Some participants appeared to be “anchored” to their initial, pre-test AD risk perceptions. Others may have adjusted for personal risk or protective factors not accounted for in our risk estimates.
Psychological impact of APOE disclosure
A prominent concern about genetic testing for AD is the potential of psychological harm when disclosing risk information for a severe, incurable disorder (18). In our first trial, clinical symptoms of anxiety, depression and test-related distress were assessed up to one year following disclosure using validated self-reported measures, including the Beck Anxiety Inventory (38), Center for Epidemiologic Studies-Depression Scale (39), and the Impact of Events Scale (40), respectively. Results showed no difference in changes in the time-averaged measures between the intervention (risk assessment with APOE genotype disclosure) and control (risk assessment with APOE disclosure) arms. Secondary comparisons between those receiving ε4-positive results and controls also found no significant differences. Results suggest that APOE genotype disclosure under carefully controlled circumstances to adult children of persons with AD did not pose significant psychological risks, even for those who learned they were ε4-positive (41). These results are largely consistent with studies of the impact of genetic testing for other adult-onset disorders such as Huntington’s disease and hereditary cancer syndromes; this research suggests that a) baseline psychological functioning is a better predictor of post-test response than the actual test result itself, and b) test-specific distress, while sometimes significant, is usually transient if patients are provided proper post-test counseling (42, 43). It should be pointed out, however, that these results may not generalize to current modes of APOE disclosure that are now occurring via DTC genetic testing companies. The latter model not only lacks in-person counseling but also discloses APOE information simultaneously with risks for numerous other conditions. This format would seemingly raise the chances that ε4-positive results could come “out of the blue” and thereby prove more psychologically distressing than as observed in our studies.
Behavioral impact of disclosure
Genetic risk information alone is typically insufficient to engender complex behavior changes such as smoking cessation and modification of dietary and exercise habits (42, 44). However, some studies (45, 46) suggest that genetic risk information may enhance patient preferences for biological interventions (e.g., medications) over health behavior changes (e.g., lifestyle changes) (47). Indeed, in the first two REVEAL trials, the most common health behavior change reported by participants was the addition of vitamins or nutritional supplements (e.g., vitamin E) (48). This finding may suggest a need to scrutinize emerging commercial services that are using genetic test results to market nutriceuticals of unproven benefit.
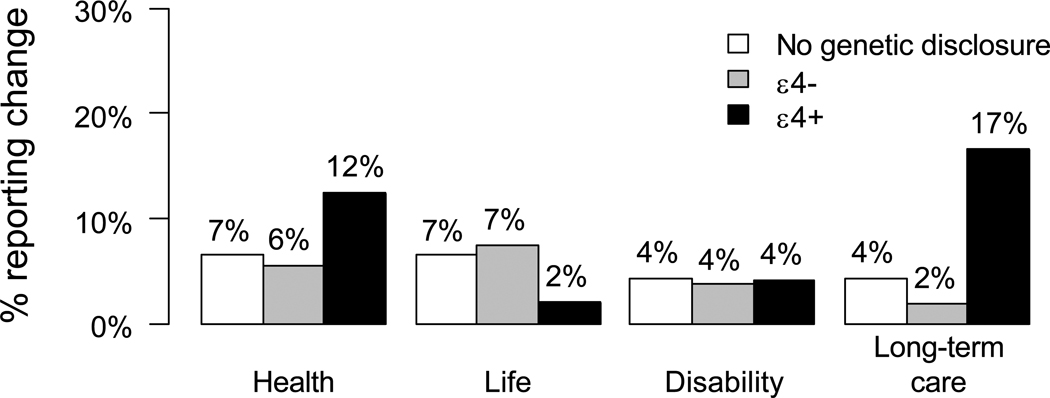
Another domain of interest was purchasing of long-term care (LTC) insurance. LTC insurance is relevant because AD often results in nursing home placement and lengthy inpatient stays. In our first trial, ε4-positive participants were approximately four times more likely than controls to report LTC insurance changes during the one-year follow-up (see Figure 3) (49), a finding that was replicated in our second trial (50). If APOE testing is utilized by a significant number of consumers to inform their LTC insurance purchasing, then insurers may be within their rights to address this adverse selection by increasing premiums or denying coverage based on APOE results. Policymakers will then have to decide whether to expand the Genetic Information Nondiscrimination Act to address not only health insurance, but also other domains including LTC.
Figure 3.
Proportion of participants in the first REVEAL Study trial reporting insurance change during 12 months following genetic risk disclosure, stratified by randomization status/APOE genotype and insurance domain
Study limitations
REVEAL findings should be interpreted with several study limitations in mind. Treatment and prevention options for AD are very limited compared to those for many other common, complex conditions. Indeed, expectations and attitudes about AD susceptibility testing and responses to results disclosure may change dramatically as proven strategies to reduce AD risk emerge. In addition, results may not generalize to broader segments of the population, given that participants were predominantly of higher socioeconomic status and generally highly motivated to pursue risk information. We also screened out participants who demonstrated moderate to severe psychiatric and/or cognitive difficulties at baseline. Behavioral changes were primarily assessed through self-report and only up to one year following disclosure; future studies should seek more objective measures of these domains, with longer-term follow-up. Finally, research presented here was conducted primarily in the U.S. There is limited cross-national work in this area, although one survey comparing the beliefs of Italians and Americans about genetic susceptibility testing for AD suggested stronger beliefs among Americans about the likelihood of developing AD, greater AD knowledge, stronger beliefs that susceptibility testing can help with planning for the future, and greater fears about the possibility of insurance and employment discrimination (51). Such findings suggest that cultural differences in lifestyles, social systems and health policies may impact attitudes towards susceptibility testing.
FUTURE DIRECTIONS
Alternative service delivery models
The time-intensive, traditional genetic counseling model will need to be adapted to accommodate the needs of a growing number of patients seeking genetic testing for adult-onset conditions (52). Leaders in the field have called for a model of care emphasizing briefer protocols and use of supplementary educational media (53, 54). In this spirit, the second REVEAL trial examined the impact of a condensed clinical risk communication protocol for first-degree relatives of people with AD, as compared to the extended protocol developed in our first trial. The condensed protocol was delivered in fewer sessions and required less face-to-face time (mean = 33 minutes vs. 76 minutes) with the study clinician. In addition, participants did not differ by protocol in terms of depression and anxiety symptoms or by rates of risk recall and comprehension at any point in the yearlong follow-up period (55). We are also continuing to address emerging trends in provision of genetic test results with ongoing analyses of the third REVEAL trial, where telephone disclosure of APOE results were compared to an in-person model. The trial also examined the impact of disclosure of pleiotropic disease risks (i.e., AD and coronary artery disease) associated with APOE.
Conclusions
Experts forecast that genetic susceptibility testing for common adult-onset diseases will become much more commonplace in the future, and AD and APOE testing provide a fruitful paradigm in which to examine its potential benefits and harms. Results indicate that many individuals are interested in genetic testing for non-medical reasons and that provision of test results does not generally result in adverse psychological effects if delivered by trained professionals using appropriate educational approaches. Furthermore, our results suggest that modifications to streamline the genetic counseling process in this context can be made without increasing the likelihood of participant distress or misunderstanding. However, our findings also demonstrate numerous challenges associated with the estimation and communication of genetic risk for AD, and they suggest a potential future need to develop policies to address genetic discrimination in LTC insurance. Lessons from the REVEAL Study may be useful to bear in mind as the field of personal genomics continues to expand. However, despite our attempts to keep pace with evolving practices, the rapid pace of scientific discoveries and commercial efforts to market genetic tests is overwhelming our ability to generate a clinical research evidence base to guide practice and policy. Already being provided are all-in-one personal genomic services that include carrier screening, pharmacogenetic tests, and susceptibility testing for scores of health conditions. Understanding and teasing apart reactions to genetic information provided in such a format will undoubtedly prove vexing and may strain the limits of our traditional clinical trials designs. Yet such research will be critical if we are to responsibly integrate genomic discoveries into both healthcare and society at large.
Acknowledgements
The authors acknowledge members of the REVEAL Study Group, including teams at Boston University (Clara Chen, Adrienne Cupples, Lindsay Farrer), Case Western Reserve University (Peter Whitehouse and Melissa Barber Butson), Weill Medical College of Cornell University (Norman Relkin), Duke University (Robert Cook-Deegan, Charmaine Royal), Howard University (Thomas Obisesan), and the University of Michigan (Erin Linnenbringer, Wendy Uhlmann). The authors would also like to thank Lindsay Zausmer for her assistance with manuscript preparation.
Sources of support: This work was supported by grants from the National Human Genome Research Institute (R01 HG02213 and R01 HG005092) and the national Alzheimer’s Association (IIRG-07–58189).
Footnotes
Conflict of interest statement: The authors report no conflicts related to the work presented herein.
References
- 1.Burke W, Psaty BM. Personalized medicine in the era of genomics. JAMA. 2007;298:1682–1684. doi: 10.1001/jama.298.14.1682. [DOI] [PubMed] [Google Scholar]
- 2.Urquidi V, Goodison S. Genomic signatures of breast cancer metastasis. Cytogenet Genome Res. 2007;118:116–129. doi: 10.1159/000108292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hudson K, Javitt G, Burke W, et al. ASHG statement on direct-to-consumer genetic testing in the United States. Am J Hum Genet. 2007;81:635–637. doi: 10.1097/01.AOG.0000292086.98514.8b. [DOI] [PubMed] [Google Scholar]
- 4.Offit K. Genomic profiles for disease risk: Predictive or premature? JAMA. 2008;299:1353–1355. doi: 10.1001/jama.299.11.1353. [DOI] [PubMed] [Google Scholar]
- 5.Burke W. Clinical validity and clinical utility of genetic tests. Curr Protoc Hum Genet. 2009;60:9.15.11–19.15.17. doi: 10.1002/0471142905.hg0915s60. [DOI] [PubMed] [Google Scholar]
- 6.Hubbard R, Lewontin RC. Pitfalls of genetic testing. N Engl J Med. 1996;334:1192–1193. doi: 10.1056/NEJM199605023341812. [DOI] [PubMed] [Google Scholar]
- 7.Evans JP. Health care in the age of genetic medicine. JAMA. 2007;298:2670–2672. doi: 10.1001/jama.298.22.2670. [DOI] [PubMed] [Google Scholar]
- 8.Secretary’s Advisory Committee on Genetics, Health and Society. A roadmap for the integration of genetics and genomics into health and society. Washington, DC: Department of Health & Human Services; 2004. [Google Scholar]
- 9.Collins FS, Green ED, Guttmacher AE, et al. A vision for the future of genomics research. Nature. 2003;422:835–847. doi: 10.1038/nature01626. [DOI] [PubMed] [Google Scholar]
- 10.Go VLW, Wong DA, Wang Y, et al. Diet and cancer prevention: evidence-based medicine to genomic medicine. J Nutr. 2004;134:3513S–3516S. doi: 10.1093/jn/134.12.3513S. [DOI] [PubMed] [Google Scholar]
- 11.Alzheimer’s Association. 2011 Alzheimer's disease facts and figures. Alzheimers Dement. 2011;7:208–244. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Steinbart EJ, Smith CO, Poorkaj P, et al. Impact of DNA testing for early-onset familial Alzheimer disease and frontotemporal dementia. Arch Neurol. 2001;58:1828–1831. doi: 10.1001/archneur.58.11.1828. [DOI] [PubMed] [Google Scholar]
- 13.Mihaescu R, Detmar SB, Cornel MC, et al. Translational research in genomics of Alzheimer's disease: a review of current practice and future perspectives. J Alzheimers Dis. 2010;20:967–980. doi: 10.3233/JAD-2010-1410. [DOI] [PubMed] [Google Scholar]
- 14.Bertram L, Lange C, Mullin K, et al. Genome-wide association analysis reveals putative Alzheimer's disease susceptibility loci in addition to APOE. Am J Hum Genet. 2008;83:623–632. doi: 10.1016/j.ajhg.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollingworth P, Harold D, Sims R, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet. 2011;43:429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrer LA, Brin MF, Elsas L, et al. Statement on use of apolipoprotein E testing for Alzheimer disease. JAMA. 1995;274:1627–1629. [PubMed] [Google Scholar]
- 17.Relkin NR, Gandy S. Consensus statements on the use of ApoE genotyping in Alzheimer's Disease. Neurology Alert. 1996;14:58–59. [Google Scholar]
- 18.Post SG, Whitehouse PJ, Binstock RH, et al. The clinical introduction of genetic testing for Alzheimer disease. An ethical perspective. JAMA. 1997;277:832–836. doi: 10.1001/jama.277.10.832. [DOI] [PubMed] [Google Scholar]
- 19.McConnell LM, Koenig BA, Greely HT, et al. Genetic testing and Alzheimer disease: has the time come? Nat Med. 1998;4:757–759. doi: 10.1038/nm0798-757. [DOI] [PubMed] [Google Scholar]
- 20.Morris JC. Dementia update 2005. Alzheimer Dis Assoc Disord. 2005;19:100–117. doi: 10.1097/01.wad.0000167923.56275.d8. [DOI] [PubMed] [Google Scholar]
- 21.Roberts JS, Connell CM. Illness representations among first-degree relatives of people with Alzheimer disease. Alzheimer Dis Assoc Disord. 2000;14:129–136. doi: 10.1097/00002093-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Christensen KD, Roberts JS, Uhlmann WR, et al. Changes to perceptions of the pros and cons of genetic susceptibility testing after APOE genotyping for Alzheimer disease risk. Genet Med. 2011;13:409–414. doi: 10.1097/GIM.0b013e3182076bf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lautenschlager NT, Cupples LA, Rao VS, et al. Risk of dementia among relatives of Alzheimer's disease patients in the MIRAGE study: What is in store for the oldest old? Neurology. 1996;46:641–650. doi: 10.1212/wnl.46.3.641. [DOI] [PubMed] [Google Scholar]
- 24.Farrer LA, Cupples LA, Blackburn S, et al. Interrater agreement for diagnosis of Alzheimer's disease: the MIRAGE study. Neurology. 1994;44:652–656. doi: 10.1212/wnl.44.4.652. [DOI] [PubMed] [Google Scholar]
- 25.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 26.Washington HA. Medical apartheid: the dark history of medical experimentation on Black Americans from colonial times to the present. New York: Doubleday; 2006. [Google Scholar]
- 27.Christensen KD, Roberts JS, Royal CDM, et al. Incorporating ethnicity into genetic risk assessment for Alzheimer disease: the REVEAL Study experience. Genet Med. 2008;10:207–214. doi: 10.1097/GIM.0b013e318164e4cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts JS, Barber M, Brown TM, et al. Who seeks genetic susceptibility testing for Alzheimer's disease? Findings from a multisite, randomized clinical trial. Genet Med. 2004;6:197–203. doi: 10.1097/01.gim.0000132688.55591.77. [DOI] [PubMed] [Google Scholar]
- 29.Esplen MJ, Madlensky L, Aronson M, et al. Colorectal cancer survivors undergoing genetic testing for hereditary non-polyposis colorectal cancer: motivational factors and psychosocial functioning. Clin Genet. 2007;72:394–401. doi: 10.1111/j.1399-0004.2007.00893.x. [DOI] [PubMed] [Google Scholar]
- 30.Alford SH, McBride CM, Reid RJ, et al. Participation in genetic testing research varies by social group. Public Health Genomics. 2011 doi: 10.1159/000294277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts JS, LaRusse SA, Katzen H, et al. Reasons for seeking genetic susceptibility testing among first-degree relatives of people with Alzheimer disease. Alzheimer Dis Assoc Disord. 2003;17:86–93. doi: 10.1097/00002093-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Woloshin S, Schwartz LM. How can we help people make sense of medical data? Eff Clin Pract. 1999;2:176–183. [PubMed] [Google Scholar]
- 33.Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations. Med Decis Making. 2007;27:696–713. doi: 10.1177/0272989X07307271. [DOI] [PubMed] [Google Scholar]
- 34.Roberts JS, Cupples LA, Relkin NR, et al. Genetic risk assessment for adult children of people with Alzheimer's disease: the Risk Evaluation and Education for Alzheimer's Disease (REVEAL) Study. J Geriatr Psychiatry Neurol. 2005;18:250–255. doi: 10.1177/0891988705281883. [DOI] [PubMed] [Google Scholar]
- 35.Eckert SL, Katzen H, Roberts JS, et al. Recall of disclosed apolipoprotein E genotype and lifetime risk estimate for Alzheimer's disease: the REVEAL Study. Genet Med. 2006;8:746–751. doi: 10.1097/01.gim.0000250197.44245.a3. [DOI] [PubMed] [Google Scholar]
- 36.LaRusse S, Roberts JS, Marteau TM, et al. Genetic susceptibility testing versus family history-based risk assessment: Impact on perceived risk of Alzheimer disease. Genet Med. 2005;7:48–53. doi: 10.1097/01.gim.0000151157.13716.6c. [DOI] [PubMed] [Google Scholar]
- 37.Linnenbringer E, Roberts JS, Hiraki S, et al. "I know what you told me, but this is what I think:" perceived risk of Alzheimer disease among individuals who accurately recall their genetics-based risk estimate. Genet Med. 2010;12:219–227. doi: 10.1097/GIM.0b013e3181cef9e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beck AT, Epstein N, Brown G, et al. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 39.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 40.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Green RC, Roberts JS, Cupples LA, et al. A randomized trial of APOE genotype disclosure for risk of Alzheimer’s disease: the REVEAL Study. N Engl J Med. 2009;361:245–254. doi: 10.1056/NEJMoa0809578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heshka JT, Palleschi C, Howley H, et al. A systematic review of perceived risks, psychological and behavioral impacts of genetic testing. Genet Med. 2008;10:19–32. doi: 10.1097/GIM.0b013e31815f524f. [DOI] [PubMed] [Google Scholar]
- 43.Meiser B. Psychological impact of genetic testing for cancer susceptibility: An update of the literature. Psychooncology. 2005;14:1060–1074. doi: 10.1002/pon.933. [DOI] [PubMed] [Google Scholar]
- 44.Marteau TM, Lerman C. Genetic risk and behavioural change. BMJ. 2001;322:1056–1059. doi: 10.1136/bmj.322.7293.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marteau T, Senior V, Humphries SE, et al. Psychological impact of genetic testing for familial hypercholesterolemia within a previously aware population: A randomized controlled trial. Am J Med Genet A. 2004;128A:285–293. doi: 10.1002/ajmg.a.30102. [DOI] [PubMed] [Google Scholar]
- 46.Phelan JC, Yang LH, Cruz-Rojas R. Effects of attributing serious mental illnesses to genetic causes on orientations to treatment. Psychiatr Serv. 2006;57:382–387. doi: 10.1176/appi.ps.57.3.382. [DOI] [PubMed] [Google Scholar]
- 47.Senior V, Marteau TM. Causal attributions for raised cholesterol and perceptions of effective risk-reduction: Self-regulation strategies for an increased risk of coronary heart disease. Psychol Health. 2007;22:699–717. [Google Scholar]
- 48.Chao S, Roberts JS, Marteau TM, et al. Health behavior changes after genetic risk assessment for Alzheimer disease: the REVEAL Study. Alzheimer Dis Assoc Disord. 2008;22:94–97. doi: 10.1097/WAD.0b013e31815a9dcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zick CD, Mathews CJ, Roberts JS, et al. Genetic testing for Alzheimer's disease and its impact on insurance purchasing behavior. Health Aff. 2005;24:483–490. doi: 10.1377/hlthaff.24.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor DH, Jr, Cook-Deegan RM, Hiraki S, et al. Genetic testing for Alzheimer's and long-term care insurance. Health Aff. 2010;29:102–108. doi: 10.1377/hlthaff.2009.0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Binetti G, Benussi L, Roberts S, et al. Areas of intervention for genetic counselling of dementia: cross-cultural comparison between Italians and Americans. Patient Educ Couns. 2006;64:285–293. doi: 10.1016/j.pec.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 52.Resta RG. Defining and redefining the scope and goals of genetic counseling. Am J Med Genet. 2006;142:269–275. doi: 10.1002/ajmg.c.30093. [DOI] [PubMed] [Google Scholar]
- 53.Guttmacher AE. Human Genetics on the Web. Annu Rev Genomics Hum Genet. 2001;2:213–233. doi: 10.1146/annurev.genom.2.1.213. [DOI] [PubMed] [Google Scholar]
- 54.Guttmacher AE, Jenkins J, Uhlmann WR. Genomic medicine: who will practice it? A call to open arms. Am J Med Genet. 2001;106:216–222. doi: 10.1002/ajmg.10008. [DOI] [PubMed] [Google Scholar]
- 55.Green RC, Roberts JS, Chen C, et al. Comparing the impact of a condensed vs extended protocol for disclosure of APOE to relatives of patients with AD: The REVEAL Study. Alzheimers Dement. 2007;3:S184. [Google Scholar]