Abstract
Considering current reliance on cancer registry data, we sought to assess the potential for bias in myelodysplastic syndrome (MDS) registration using SEER-Medicare data 2001-2005. Using a validated claims-based algorithm, we identified and compared registered and non-registered MDS patients, and found that median cumulative survival was 18 and 28 months, 74% and 64% used erythropoiesis-stimulating agents (ESAs), and average 6-month health care cost was $24,249 and $21,750, respectively. While most non-registered MDS patients showed resource utilization and survival characteristics consistent with lower-risk MDS, a subset was registered as acute myeloid leukemia (7.6%) and accounted for early mortality.
Keywords: cancer registry; MDS incidence, testing, treatment, and survival; SEER-Medicare data; ESAs
Introduction
The myelodysplastic syndromes (MDS) are a diverse group of clonal hematopoietic malignancies characterized by ineffective hematopoiesis that primarily affects older individuals. Changing definitions and classifications of hematologic malignancies, such as MDS, has complicated incidence comparisons between regions and over time.[1,2] As of 2001 in the United States, state cancer registries were legislatively mandated to begin collecting MDS incidence, stage at diagnosis, first course of treatment and survival data. By reviewing data from the Surveillance, Epidemiology, and End Results (SEER) Program and North American Association of Central Cancer Registries (NAACCR), reports of MDS incidence estimate that approximately 3.4 individuals out of 100,000 are diagnosed annually.[3,4] However, greater than 90% of the incidences were reported by hospitals and laboratories, rather than outpatient clinics where a large proportion of MDS patients are believed to be diagnosed and treated.
To ascertain the capture rate of MDS patients by population-based cancer registries, we constructed and validated a novel claims-based algorithm using SEER-Medicare data. Our algorithm discovered that approximately 1 out of 4 MDS patients 65 years old or older were not captured by cancer registries reporting to SEER.[5] Whereas, SEER registry data estimated annual MDS incidence of 20 per 100,000 individuals 65 years or older, the addition of non-registered MDS patients found by our claims-based algorithm increased the MDS incidence estimate to 75 per 100,000 individuals between 2001 and 2005. Other investigators have also reported a high rate of uncaptured MDS cases by population-based cancer registries.[6]
With such a large number of non-registered MDS patients in the United States, it is possible that case characteristics of these patients may have significant impact on our understanding of MDS diagnosis, treatment, and clinical outcomes. To determine this, we compared patient data between SEER-registered and non-registered MDS patients.
Methods
Data Sources
We conducted a retrospective review of the SEER-Medicare database, 2001 to 2005. The SEER program is a national, population-based cancer registry sponsored by the National Cancer Institute (NCI) with a catchment area equal to 26% of the US population.[7] SEER accumulates information on patient demographics, tumor characteristics, stage at diagnosis, date of diagnosis, treatment within 4 months of diagnosis, and date and cause of death.[7] Of the cancer patients 65 years or older within SEER, 93% were matched with Medicare enrollment records and claims. Medicare, administered by the Centers of Medicare and Medicaid Services (CMS), is the primary insurer for approximately 97% of the U.S. population 65 years or older.[7] All Medicare beneficiaries receive Part A coverage, which covers hospital inpatient care, skilled nursing, home healthcare, and hospice care.[7] Approximately 95% of older beneficiaries also subscribe to Part B of Medicare to obtain benefits that cover physician services, durable medical equipment, and outpatient care.[7] As an alternative to the traditional fee-for-service (FFS) Medicare, the Medicare Advantage program, Part C, is a managed care benefit that enrolls approximately 11%-14% of older Medicare beneficiaries.[8-10] Due to its reimbursement structure, administrative claims data are not available for Part C beneficiaries and will not be included in this study.
Study Population
For study inclusion, a beneficiary must have resided in a SEER region between 2001 and 2005, enrolled in FFS Medicare due to age for 13 months or more, and not participated in Medicare Advantage. The analytical sample included a 5% sample of registered (N=44,739) and non-registered (N=230,941) beneficiaries and an oversampling of beneficiaries registered in SEER with International Classification of Diseases for Oncology, Third Edition (ICD-O-3) codes 9800 to 9989 (N=23,756), which allows more in-depth examinations of hematological malignancies. Sampling weights were incorporated into the analysis to adjust for this oversampling. All study procedures were approved by the University of Florida Institutional Review Board.
Measures
Medicare administrative data contains information on beneficiary characteristics, use of supportive care services, and date of hospice entry and death. To be identified as an incident MDS case, cases were identified using the previously validated claims-based algorithm 2+BCBM.[5] For this algorithm, beneficiaries had to have (1) no MDS or unspecified anemia (ICD-9-CM 285.9) claims for 1 year (i.e., removal of prevalent cases); (2) a claim with MDS indication; (3) a second MDS claim between 1 and 12 months after the first claim or death or hospice entry within 90 days; (4) 1 or more claims for blood count (BC) within the year prior to the first claim, and (5) 1 or more claims for bone marrow (BM) biopsy within the year prior to the first claim. MDS claims were identified based on SEER guidelines in the Ninth Revision of the International Classification of Diseases codes (ICD-9-CM 284.9, 285.0, and 238.7 prior to October 2006, and 238.72-238.75 until present). Within the SEER-Medicare data, the 2+BCBM algorithm identified 5,678 MDS patients; however, 10 patients were excluded, because their bone marrow biopsy occurred prior to the introduction of SEER registration of MDS in 2001. The final analytical sample contains 2,757 registered and 2,911 non-registered MDS patients.
The analytical sample represented incident cases among older Medicare beneficiaries within SEER regions from the thirteenth month of enrollment to either their death or hospice entry. While this sample was representative of the majority of MDS patients in those regions from 2001 to 2005, it excluded the experiences of younger patients (age 65 or less) and patients diagnosed in hospice or while enrolled in Medicare Advantage. Furthermore, the algorithm required bone marrow biopsy, which is concordant with World Health Organization (WHO) and French-American-British Co-operative Group (FAB) recommendations over this period, and patients clinically diagnosed without confirmation were excluded from this analysis.
For the measurement of diagnostic and supportive care services, we examined the claims for Current Procedural Terminology (CPT) codes, which are maintained by the American Medical Association to describe the delivery of specific services (Table 1). CMS records also list dates of service, reimbursement, and beneficiary characteristics that include gender, race, ethnicity, and dates of birth, enrollment, and death. The date of most recent BM biopsy prior to MDS indication was interpreted as the date of MDS diagnosis, which allowed for a uniform 3-year period of observation after biopsy and eliminated censoring due to unequal follow-up of Medicare claims and survival.
Table 1. Services related to Myelodysplastic Syndrome.
| Services | CPT and ICD-9-CM Codes |
|---|---|
| Diagnostic Services | |
| Blood counts (BC) | CPT 85004, 85007, 85008, 85013, 85014, 85018, 85021-85025, 85027, 85044-85049, 85060, 85595, G0306, G0307 |
| Bone marrow biopsy or aspiration (BM) | CPT 38220, 38221, 85095, 85097, 85102, G0364 and ICD-9-CM 413.1 and 413.8 |
| Karyotyping | CPT 88261-88264, 88280 |
| Fluorescence in situ hybridization (FISH) | CPT 88271-88275, 88365-88368 |
| Supportive Care Services | |
| Blood transfusions | CPT 36430, C1010-C1021, P90.XX, ICD-9-CM 99.0X, and any inpatient services with blood charges |
| Erythropoiesis-stimulating agents (ESA) | CPT J0880, J0881, J0885, Q0136, Q0137, 4090F and 3160F |
| Granulocyte colony-stimulating factor (G-CSFs) | CPT J1440, J1441, J2505, and J2820 |
Abbreviations: CPT, Current Procedural Terminology; ICD-9-CM, International Classification of Diseases, Clinical Modification
Data Analysis
The demographic characteristics, diagnostic testing, supportive care treatments, cost of care, and survival time of registered patients were compared to the same variables of non-registered patients using the weighted t-test (Tables 2 and 3). To assess differential treatment, we examined the use of health services within the first 6 months, as well as illustrated month-specific means for supportive care services by group over the year subsequent to bone marrow biopsy (Figure 1). Likewise, we examined the probability of surviving 6 months after biopsy and illustrated cumulative survival over the subsequent 3 years (Figure 2). Costs were measured over the first 6 months following bone marrow biopsy and were based on Medicare reimbursement without adjustment for survival or inflation. Costs were divided by their coverage under Medicare Part A and B to aid in the economic interpretations.
Table 2. Characteristics of Patients with Myelodysplastic Syndrome by SEER Registration*.
| SEER Registration | |||
|---|---|---|---|
| Registered | Not Registered | ||
| Characteristics | (N=2,757) | (N=2,911) | P |
| Age (years) in January 2001 | |||
| [median (interquartile range)] | 79 (74-83) | 78 (73-83) | 0.005 |
| Gender, % | |||
| Male | 56.9% | 48.3% | <0.001 |
| Female | 43.1% | 51.7% | |
| Race and Ethnicity,% | |||
| White | 89.6% | 88.5% | 0.411 |
| Black | 5.2% | 5.4% | 0.799 |
| Other | 1.1% | 1.2% | 0.731 |
| Asian | 2.8% | 3.0% | 0.841 |
| Hispanic | 1.1% | 1.7% | 0.247 |
| North American Native | <0.4% | <0.4% | 0.855 |
| Unknown | <0.4% | <0.4% | 0.571 |
| Registered with acute myeloid leukemia | <0.4% | 7.6% | <0.001 |
NOTE: P values represent group comparisons on weighted t-tests. Percentages less than 0.4% are suppressed to protect patient anonymity.
MDS Patients identified using validated claims-based algorithms.
Table 3. Differences between Registered and Not Registered MDS Patients within 6 Months of Bone Marrow Biopsy*.
| SEER Registration | |||
|---|---|---|---|
| Events within 6 months of Bone Marrow Biopsy | Registered | Not Registered | |
| (N=2,757) | (N=2,911) | P | |
| Survival, % | 73.7% | 74.8% | 0.531 |
| Cytogenetic Testing, % | |||
| Karyotyping | 51.8% | 47.4% | 0.033 |
| FISH | 5.4% | 8.4% | 0.005 |
| Supportive Care,% | |||
| Blood Transfusion | 59.6% | 43.4% | <0.001 |
| ESA | 74.2% | 63.6% | <0.001 |
| G-CSFs | 11.3% | 6.4% | <0.001 |
| Cost to Medicare, $ | |||
| Total | $ 24,249 | $ 21,750 | 0.007 |
| Part A | $ 13,427 | $ 12,859 | 0.466 |
| Part B | $ 10,822 | $ 8,890 | <0.001 |
Note: P values represent group comparisons on weighted t-tests.
Abbreviations: MDS, myelodysplastic syndrome. FISH, fluorescence in situ hybridization. ESA, erythropoiesis-stimulating agents. G-CSFs, granulocyte colony-stimulating factors.
MDS Patients identified using validated claims-based algorithms.
Figure 1. Supportive Care by Month since Bone Marrow Biopsy and SEER Registration.
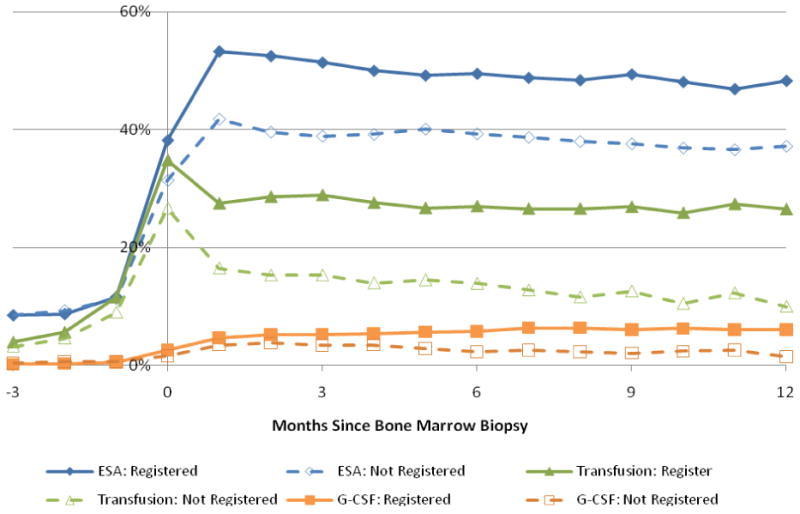
Abbreviations: MDS, myelodysplastic syndrome. ESA, erythropoiesis-stimulating agents. G-CSF, Granulocyte colony-stimulating factor
Figure 2. Survival of MDS Patients by SEER Registration.
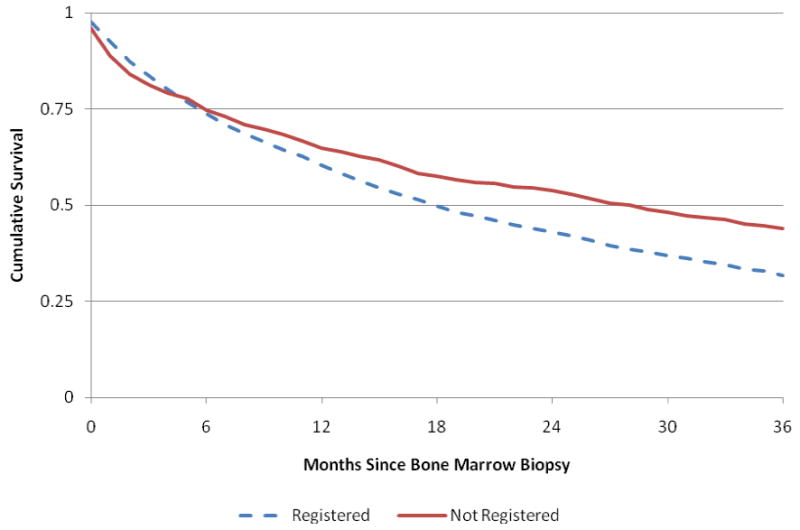
Abbreviations: MDS, myelodysplastic syndrome.
Results
Table 2 shows that registered MDS patients were older and more likely to be male than non-registered MDS patients. Although the registered sample had a greater proportion of White patients, no statistically significant differences between registration and race and ethnicity categories were found.
A key difference between the registered and non-registered MDS patients was the proportion of patients registered with AML. Whereas only 6 of the 2,757 registered MDS patients (0.2%) were also registered with AML between 2001 and 2005, a much greater proportion (7.6%) of the non-registered MDS patients was registered as AML.
Table 3 shows the events within the first 6 months following bone marrow biopsy. For both registered and non-registered MDS patients, 48.2% and 52.6% were not evaluated for cytogenetic abnormalities in the bone marrow, respectively; therefore, no karyotype information was available on these patients for prognostic scoring (International Prognostic Scoring System)[11] or fully informed decision-making for therapy (e.g., if del(5q) then consideration for lenalidomide).[12,13] Although the difference in 6-month survival was not significant, non-registered patients received less health services than registered patients within the first 6 months following diagnosis. Figure 1 further illustrates the decreased service use that occurred during the calendar month of bone marrow biopsy and trends stabilized by the second month. There were a small but statistically greater proportion of non-registered MDS patients that had FISH testing of bone marrow as compared to the registered MDS patient cohort (8.4% vs. 5.4%).
Along with lower resource utilization, total cost to Medicare was 11% ($2,499) lower among the non-registered patients than the registered patients, and this difference is largely attributable to Part B costs (physician services, outpatient care, and durable medical equipment), which was 22% lower ($1,832).
Figure 2 provides a more complete description of the association between survival and SEER registration. Overall, the median survival of older MDS patients diagnosed between 2001 and 2005 was 26 months after bone marrow biopsy. Among the registered patients, cumulative median survival was lower than among non-registered MDS patients (18 months vs. 28 months, P < 0.001). However, registered MDS patients were more likely to survive the first 3 months after bone marrow biopsy compared to non-registered patients (0.837 vs. 0.812, P=0.095), but the difference was not statistically significant. Among the non-registered MDS patients who were registered for AML (7.6% of the cohort) were less likely to survive the first 3-months (0.566 vs. 0.832, P<0.001) than patients registered as MDS in SEER. In summary, non-registered MDS patients had better median survival than registered MDS patients, but were less likely to survive the first 3 months, particularly MDS patients registered with AML (i.e., late-stage diagnoses).
Discussion
Considering the important use of cancer registry data in understanding MDS incidence and outcomes,[3,4,14] we questioned whether the uncaptured MDS cases exhibited significantly different characteristics than the registered cases. Using a validated claims-based algorithm, we found that MDS patients not registered in SEER received fewer services, incurred less costs and had better survival than SEER-registered MDS patients. Thus, the high number of uncaptured MDS cases would have had a significant impact on cancer registry data.
Evidence of less resource utilization, decreased cost of care and better survival among non-registered patients suggests that the majority of non-registered patients had a lower grade MDS that was less life threatening and required fewer services. A recent study of the NAACCR showed that only 4% of the MDS incident cases were reported to registries by physicians' offices, which is a surprisingly low proportion given that MDS is more commonly diagnosed and managed outside the hospital setting.[4] The systematic bias in population-based registration toward inpatient settings may explain why registered patients appeared to require more medical intervention than non-registered patients.
Additionally, we found a subset (7.6%) of AML cases in the non-registered MDS cohort. Based on the 2001 SEER guidelines, only one malignancy in the myeloid lineage was registered. Therefore, it was possible for patients to present with AML after a history of unregistered MDS. This co-registration is significant, as evidenced by the reduced survival seen in the first 3 months of the non-registered MDS patients due to the AML subset. In 2010, SEER changed its guidelines and permitted the co-registration of acute and chronic myeloid malignancies.
Several considerations must be made when interpreting claims data. First, the 2+BCBM algorithm required that patients undergo bone marrow biopsy. However, bone marrow pathology is not required for SEER registration. Therefore, comparisons between MDS patients who do or may not undergo bone marrow biopsy could produce different outcomes based on the patients' willingness to undergo this procedure and/or the physicians' adherence to WHO and FAB recommendations. Second, service use, cost and survival estimates were only generalizable to older Medicare beneficiaries after their thirteenth month of enrollment and do not characterize MDS patients enrolled in Medicare Advantage or who are age 65 or younger. Third, the claims-based analysis potentially missed tests and supportive care services that were not covered by Medicare or under alternative codes. For example, inpatient claims under the prospective payment system of Medicare Part A were classified under 1 of approximately 500 diagnosis-related groups (DRGs) that were expected to have similar hospital resource use. Because hospital payments were based on DRGs (e.g., pneumonia) and not on services, the use of services in the hospital setting may have been censored. Injectable drugs, such as ESAs and G-CSFs, are typically reimbursed outside of DRGs, and blood transfusions require specific blood charges that must be indicated as a separate entry for inpatient claims; therefore, the potential for this type of censoring is minimal. The use of drugs for the treatment of MDS, such as azacitidine and decitabine, was not largely available until 2004. A key advantage of administrative data is 100% follow-up for 3 years following diagnosis through the Social Security Administration data and the transparency of the policy implications in terms of the expected costs to the Medicare program.
From the perspective of the Medicare program, these results suggest that MDS incidence is less costly than originally estimated based on SEER registered samples and that these costs are occurred at a younger age, for a longer duration, and with more of an equal balance of men and women. From the perspective of the SEER program, the interpretation of patterns in MDS incidence and service use must account for biases incurred from the 2001 guidelines. However, the 2010 guidelines should improve the representativeness of this sample. Also, greater resources are needed for cancer registries to pursue innovations in outpatient registration such as continuous and targeted surveillance of Medicare records to reduce the under-registration of lower grade MDS patients.
Acknowledgments
The authors thank the staff in Dr. Craig's lab at H. Lee Moffitt Cancer Center & Research Institute for their contributions to the research and creation of this paper: Riddhi Patel (research assistance) and Carol Templeton (copy editing).
Funding Source: Funding support for this research was provided by the NIH Infrastructure grant, “Developing Information Infrastructure Focused on Cancer Comparative Effectiveness Research” (RC2-CA148332; PI: Fenstermacher); and Dr. Craig's NCI Career Development Award (K25 -CA122176).
Footnotes
Authors' Contributions: B.M.C. designed the concept, analyzed data, and wrote the manuscript. C.R.C. designed the concept and wrote and approved the manuscript. D.E.R. and A.F.L. edited and approved the manuscript.
Conflict of Interest: All the authors declare no competing financial interests and have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sant M, Allemani C, Tereanu C, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116:3724–3734. doi: 10.1182/blood-2010-05-282632. [DOI] [PubMed] [Google Scholar]
- 2.Steensma DP. Myelodysplastic Syndromes: Pathobiology and Clinical Management. Second. Informa Healthcare; 2008. [Google Scholar]
- 3.Ma X, Does M, Raza A, et al. Myelodysplastic syndromes: incidence and survival in the United States. Cancer. 2007;109:1536–42. doi: 10.1002/cncr.22570. [DOI] [PubMed] [Google Scholar]
- 4.Rollison DE, Howlader N, Smith MT, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001-2004, using data from the NAACCR and SEER programs. Blood. 2008;112:45–52. doi: 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- 5.Cogle CR, Craig BM, Rollison DE, et al. Incidence of the Myelodysplastic Syndromes using a Novel Claims-Based Algorithm: High Number of Uncaptured Cases by Cancer Registries. Blood. 2011 doi: 10.1182/blood-2011-02-337964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg SL, Chen E, Corral M, et al. Incidence and clinical complications of myelodysplastic syndromes among United States medicare beneficiaries. J Clin Oncol. 2010;28:2847–52. doi: 10.1200/JCO.2009.25.2395. [DOI] [PubMed] [Google Scholar]
- 7.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002:IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 8.Foundation THJKF. Medicare+Choice Fact Sheet. April 2003. 2003. [Google Scholar]
- 9.Foundation THJKF. Medicare Advantage FactSheet. 2004. [Google Scholar]
- 10.Foundation THJKF. Medicare Advantage Factsheet. 2005. [Google Scholar]
- 11.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–88. [PubMed] [Google Scholar]
- 12.List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. New England Journal of Medicine. 2006;355:1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 13.List A, Kurtin S, Roe DJ, et al. Efficacy of lenalidomide in myelodysplastic syndromes. New England Journal of Medicine. 2005;352:549–557. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- 14.Neukirchen J, Schoonen WM, Strupp C, et al. Incidence and prevalence of myelodysplastic syndromes: Data from the Dusseldorf MDS-registry. Leuk Res. 2011 doi: 10.1016/j.leukres.2011.06.001. [DOI] [PubMed] [Google Scholar]


