Abstract
Prostaglandin E2 constitutes a major cyclooxygenase-2-derived prostanoid produced at inflammatory sites. In vitro and in vivo data supports its role as a modulator of inflammation. Prostaglandin E2 exerts anti-inflammatory effects by binding to one of its receptors, the prostaglandin E receptor 4 (EP4), thereby modulating macrophage and T lymphocyte functions that participate crucially in innate and adaptive immunity and tissue remodeling and repair. Activation of EP4 suppresses the release of cytokines and chemokines from macrophages and T cells, inhibits the proliferation and activation of T cells, and induces T cell apoptosis. Lack of EP4 in bone marrow–derived cells accelerates local inflammation in atherosclerotic and aneurysm lesions and increases the prevalence of aneurysm formation. An EP4 agonist promotes graft survival in allograft cardiac transplantation and dampens tissue damage after myocardial ischemia. Anti-inflammatory actions of EP4 agonism may benefit other inflammatory disorders, including colitis and gastric ulcers. By contrast, EP4 acts as a pro-inflammatory mediator in encephalomyelitis, skin inflammation, and arthritis by promoting T helper (Th) 1 differentiation and Th17 expansion. Overall, EP4 activation produces powerful anti-inflammatory responses in many experimental diseases, rendering EP4 agonists attractive agents to attenuate syndromes associated with inflammation.
Introduction
The enzyme cyclooxygenase (COX) forms prostanoids, including prostaglandin D2, prostaglandin E2, prostaglandin F2α, prostacyclin (prostaglandin I2), and thromboxane A2 (Figure 1). These prostanoids possess a broad range of physiological functions and modulate inflammatory diseases. Nonsteroidal anti-inflammatory drugs (NSAIDs) have wide use to treat symptoms of inflammation. Most of these agents block the production of prostanoids through non-selective inhibition of COX, inhibiting both COX-1 and COX-2 isoforms.1,2 But the inhibitory effect of NSAIDs’ on platelet aggregation [leading to an increased bleeding risk], along with the gastrointestinal irritation that they cause limits their therapeutic usefulness.1,2
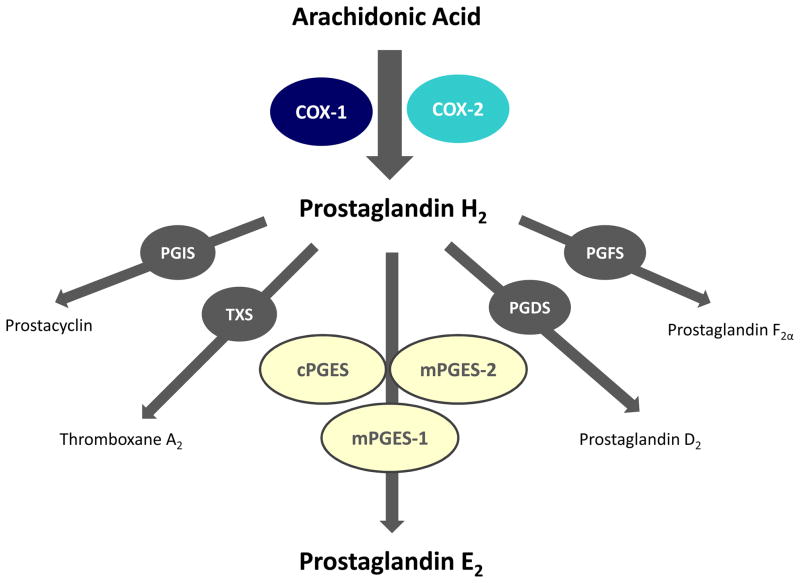
Figure 1.
Metabolism of arachidonic acid into specific prostanoids. COX-1= cyclooxygenase 1; COX-2 = cyclooxygenase 2; PGIS = prostacyclin synthase; TXS = thromboxane synthase; cPGES = cytosolic prostaglandin E synthase; mPGES-1 = microsomal prostaglandin E synthase 1; mPGES-2 = microsomal prostaglandin E synthase 2; PGDS = prostaglandin D synthase; PGFS = prostaglandin F synthase.
Studies in the early 1990s indicated that the inducible COX-2 isoform largely accounted for the formation of prostaglandins associated with inflammation, while COX-1 appears to produce mainly prostaglandins with constitutive functions.2 Accordingly, the pharmaceutical industry developed selective COX-2 inhibitors. Such agents underwent clinical deveopment: celecoxib (Celebrex®) and rofecoxib (Vioxx®) and lumiracoxib (Prexige®). These selective COX-2 inhibitors aimed to provide powerful anti-inflammatory effects, while constitutive COX-1 would continue to produce prostanoids and protect the gastric mucosa, regulate renal functions, and among other desirable functions.3 However, clinical data revealed that users of selective COX-2 inhibitors can have an increased risk of acute myocardial infarction and stroke.4,5 These unfavorable side effects led to the withdrawal of rofecoxib from the market in 2004.1 Lumiracoxib has been withdrawn from the market in several countries mostly due to hepatotoxicity concerns, but it is still sold in a few countries including Mexico, Ecuador and Dominican Republic. Celecoxib remains available for prescription, but must be delivered with a FDA-mandated “black box” warning for cardiovascular and gastrointestinal risks. The American Heart Association has declared that celecoxib should be used only as a last resort in patients who have, or are at risk for developing, cardiovascular disease.2 Indeed, findings indicated that COX-2 rather than COX-1 produces prostacyclin, the potent vasodilator and inhibitor of platelet aggregation. Hence, selective COX-2 inhibitors depress prostacyclin formation in humans, providing one possible explanation why selective COX-2 inhibitors may augment cardiovascular events. In addition, mature human platelets lack COX-2 expression, so that selective COX-2 inhibitors do not disrupt the production of thromboxane A2, which promotes platelet aggregation and vasoconstriction.1 Selective inhibition of COX-2 may favor thrombosis, predisposing to complications of atherosclerosis and other cardiovascular diseases. These findings emphasized the need to find new therapeutics that block COX-2–mediated inflammation while averting the associated cardiovascular risk.
Other COX-2–derived prostanoids also may protect or aggravate cardiovascular diseases. Prostaglandin E2 comprises one of the major COX-2–derived prostanoids at inflammatory sites.6,7 Accumulating in vitro and in vivo data supports the role of prostaglandin E2 as an endogenous inhibitor of inflammation.8–20 Prostaglandin E2 exerts this anti-inflammatory action by binding to one of its receptors, the prostaglandin EP4 receptor.9,12,13 Elimination of prostaglandin E2 production by selective COX-2 inhibitors would eradicate the beneficial EP4-mediated action. The loss of such endogenous anti-inflammatory signaling may additionally contribute to the adverse cardiovascular effects of selective COX-2 inhibitors. Thus, activation of EP4 receptors may serve as a novel therapeutic target to treat cardiovascular inflammatory diseases.
Prostaglandin E2 synthesis and EP receptors
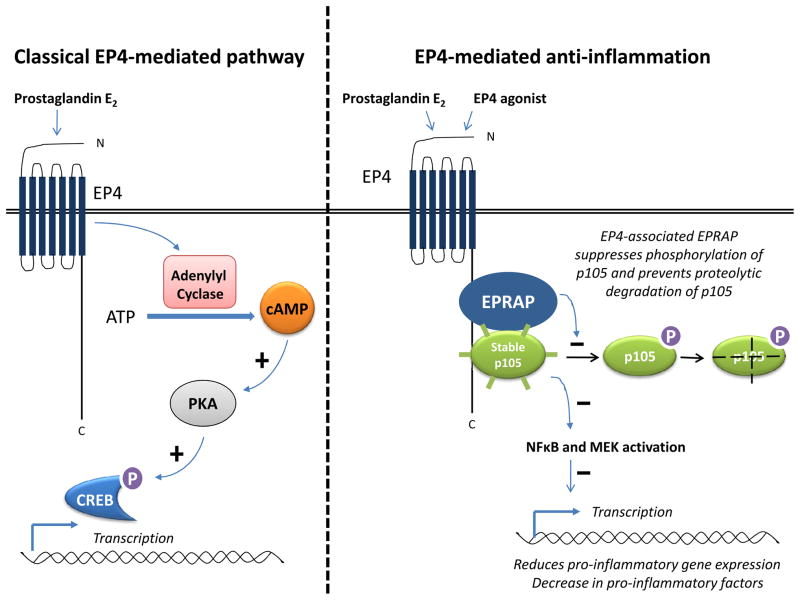
Prostaglandin E2 plays a key mediatory role in inflammation and has pleiotropic effects in a range of tissues. It is the major prostanoid secreted by various cells in response to inflammatory stimuli.2,6 Prostaglandin E2 arises from the sequential metabolism of arachidonic acid by cyclooxygenase and prostaglandin E synthases. There are three different prostaglandin E synthases: cytosolic prostaglandin E synthase (cPGES), and two microsomal prostaglandin E synthases, mPGES-1 and mPGES-2. Of these isomerases, cPGES and mPGES-2 are constitutively expressed, whereas mPGES-1 is mainly an induced enzyme.21 Prostaglandin E2 exerts its biological functions mainly via four G protein-coupled 7 transmembrane receptors — EP1, EP2, EP3, and EP4.22 The EP receptor isoforms have unique expression patterns and couple to distinct signaling pathways. EP1 receptors stimulate intracellular calcium mobilization22 and EP2 receptors initiate Gs-coupled signals stimulating adenylyl cyclase to produce cyclic adenosine monophosphate (cAMP). 22 EP3 receptor has more complex signaling. Indeed, multiple EP3 receptor isoforms are generated by alternative splicing from a single EP3 receptor gene (PTGER3), and these isoforms activate different signaling pathways, including Gi and Gs coupling, as well as calcium mobilization. 22 The action of EP4 traditionally is viewed as depending on a Gα-protein mediated transient increase in intracellular cAMP. 22 In turn, cAMP activates protein kinase A (PKA), which then phosphorylates downstream effector proteins, in particular cAMP response element binding protein (CREBP) (Figure 2).12,23 However, PKA and CREB do not mediate the anti-inflammatory action of EP4.12,13 Instead, the EP4 receptor–associated protein (EPRAP) binds to the long carboxyl terminal cytoplasmic domain of EP4, reduces stimulus-induced phosphorylation at this site, and enhances the stability of p105 [an important cytoplasmic inhibitor of the activation of nuclear factor kappa B (NFκB) and mitogen-activated protein kinase kinase (MEK)], and thus attenuates the cellular impact of NFκB and MEK (Figure 2).13,24 Ultimately, EP4 activation results in reduced NFκB-dependent inflammatory gene transcription and decreased production of pro-inflammatory factors, thereby limiting inflammatory processes. The cAMP analog 8-Br-cAMP mimicks the EP4-mediated anti-inflammatory action in human macrophages,12,25 suggesting the involvement adenylyl cyclase. Similarly, dibutyryl cAMP mimicks the EP4-mediated suppression of lipopolysacchride (LPS)-induced tumor necrosis factor-alpha (TNF-α) production in mouse peritoneal neutrophils.8 However, some proprietary EP4 agonists exhibit an anti-inflammatory activity without elevation in cAMP, at least in macrophages,13,16 suggesting that cAMP may not be necessary for the EP4-mediated anti-inflammatory action in these cells. The importance of cAMP elevation in EP4-mediated immunosuppression also remains controversial. Taken as a whole, the mechanisms underlying the anti-inflammatory actions of EP4 differ from the traditional EP4-mediated PKA, CREB-dependent cascade. The existence of a family of EP receptors coupled to distinct intracellular signals provides a molecular basis for the diverse physiological actions of prostaglandin E2.
Figure 2.
Schematic representation of the EP4-mediated classical pathway and the EP4-mediated anti-inflammatory pathway. Classical pathway: Prostaglandin E2 through EP4 transiently increases intracellular cAMP (by activation of adenylyl cyclase). cAMP activates protein kinase A (PKA), which subsequently phosphorylates the downstream cAMP response element-binding protein (CREB), which binds to certain DNA sequences and increases the transcription of downstream genes. Anti-inflammatory pathway: EPRAP binds directly to the cytoplasmic tail of EP4 and associates with p105. The EPRAP-p105 complex suppresses NFκB and MEK activation by inhibiting p105 phosphorylation and its degradation, thereby reducing pro-inflammatory gene expression.
Importance of EP4 in immune cells: in vitro evidence
Prostaglandin E2 modulates several aspects of the immune response. In inflammation and related pathological conditions, its production increases following stimulation of arachidonic acid release and induction of COX-2.21 The major cells of the immune system express EP receptor isoforms — in particular, EP4.9,12 Prostaglandin E2 exerts immune suppression by binding to EP4 receptors, and thus modulating the functions of cells (including T lymphocytes and macrophages) critical to immune responses.9,12
EP4 is the predominant prostaglandin E2 receptor subtype in human macrophages.12 Prostaglandin E2, following binding to EP4, suppresses the production of inflammatory cytokines and chemokines in macrophages in vitro. It attenuates inflammatory activators of human macrophages by suppressing LPS-induced expression of cytokines, including TNF-α, interleukin-12 (IL-12), and interferon-γ (IFN-γ).26,27 In these cells, prostaglandin E2 also suppresses the production of LPS-induced chemokines, including monocyte chemoattractant protein-1 (MCP-1), IL-8, macrophage inflammatory protein-1α (MIP-1α) and -1β (MIP-1β), and interferon-inducible protein-10 (IP-10).12 These molecules all participate in leukocyte recruitment and/or the alteration of antigen-presenting ability of macrophages by inhibiting expression of MHC class II proteins. 12 Prostaglandin E2 also inhibits the TNF-α–, IFN-γ–, and IL-1β–mediated expression of these chemokines.12 The anti-inflammatory actions of prostaglandin E2 exhibit cell type selectivity, as this prostanoid suppresses chemokine expression in human macrophages but not in human vascular smooth-muscle cells exposed to LPS or pro-inflammatory cytokines.12
The suppressive action of prostaglandin E2 on cytokine and chemokine production in macrophages results almost exclusively from EP4 agonism: A selective EP4 antagonist completely reverses the suppression of MCP-1, IL-8, MIP-1α, MIP-1β and IP-10 induced by the prostanoid.12 Furthermore, prostaglandin E2 has no effect on LPS-stimulated cytokine release in EP4-deficient macrophages.24 The targeted disruption of the EP4 gene decreases prostaglandin E2–mediated inhibition of TNF-α and IL-12 in LPS-activated mouse macrophages,9,26,27 supporting the primary role of EP4 receptor subtype in the anti-inflammatory pathway in macrophages. Under some conditions, EP2 may exert a similar anti-inflammatory role as EP4.9,28 Nevertheless, this review focuses on the effects of prostaglandin E2 mediated by EP4. Indeed, the EP4-dependent suppression by prostaglandin E2 of chemokine and cytokine production in macrophages, leading to reduced infiltration of inflammatory cells, makes this subtype of prostanoid receptors a likely therapeutic target for the treatment of inflammatory diseases.
Prostaglandin E2 modulates a wide range of T-cell functions. Its ability to inhibit T-cell proliferation is well established. It suppresses the differentiation of CD4+ T helper (Th) 129–31 and the activation and proliferation of cytotoxic CD8+ T lymophocytes.32,33 The prostanoid induces apoptosis in CD4+CD8+ double-positive thymocytes34 and in resting mature T cells.35 Prostaglandin E2 affects profoundly the production of cytokines by T cells, decreases the production of IFN-γ by CD8+ T cells,36 inhibits the production of Th1 cytokines such as IFN-γ and IL-2 by Th1 cells,27 and inhibits cell-surface expression of cytokine receptors in lymphocytes.37 Thus, prostaglandin E2 plays a variety of crucial roles throughout the life of T cells, regulating proliferation, apoptosis, and cytokine production.
Studies utilizing T cells deficient in each EP subtype reveal that the immunosuppressive actions of prostaglandin E2 in T lymphocytes are mediated predominantly by EP4.9 Furthermore, a selective EP4 agonist suppresses the proliferation of CD4+ T cells in a concentration-dependent manner.9,10,16 In co-culture experiments, inhibition of T-cell proliferation occurs when the macrophages, but not when the T cells are treated with the EP4 agonist.16 Thus, EP4-mediated inhibition of T-cell proliferation depends on the activation of macrophages. IL-12 is critical to the induction of Th1 response, regulating the differentiation of Th0 to Th1 cells. Th1 cells produce pro-inflammatory cytokines such as IFN-γ and IL-2, and these cytokines in turn induce the activation and proliferation of T lymphocytes.16 Stimulation of EP4 causes reduced IL-12 release from macrophages, suggesting that the EP4 agonist suppresses T-cell proliferation through IL-12 suppression and macrophage inactivation.16 Furthermore, the inhibition of the production of IL-2 and IFN-γ from Th1 cells by EP4 may polarize cellular response towards a Th2 phenotype by enhancing IL-4 and IL-5 production.29,38 Prostaglandin E2 thus may affect the overall slant of an immune response.
The EP4-mediated anti-inflammatory action is not restricted to T cells and macrophages. It is also seen in neutrophils and dendritic cells. Thus, EP4 activation prevents N-formyl-methionyl-leucyl-phenylalanine-(fMLP)-stimulated aggregation of rat neutrophils.39 Prostaglandin E2 also suppresses TNF-α production in mouse neutrophils collected from the peritoneal cavity by casein treatment, and this suppression is dependent on EP4-activation.8 Furthermore, prostaglandin E2 inhibits IFN-α secretion and Th1 co-stimulation by human plasmacytoid dendritic cells via EP4 engagement.40 These observations support a primary role of EP4 in the anti-inflammatory response to prostaglandin E2 in immune cells.
Importance of EP4 in cardiovascular diseases: in vivo evidence
The interest in targeting EP4 to treat inflammatory diseases has emerged from the demonstration of the detrimental effects of EP4-targeted disruption or the benefits of EP4 agonists under several pathological conditions. The discussion below summarizes the potential role of EP4-activation in suppressing inflammation during myocardial ischemia/reperfusion (I/R) injury, allograft rejection after cardiac transplantation, atherosclerosis, and abdominal aortic aneurysm.
Myocardial Ischemia
The damage from myocardial I/R injury results from the release of oxygen free radicals and from the inflammatory response of the damaged tissues, leading to myocyte death and interstitial fibrosis, processes involved in ventricular remodeling. Inflammatory cells — including monocytes, macrophages, and neutrophils — are the main sources of inflammatory factors that fuel this event.41 EP4 activation protects the reperfused myocardium against ischemic injury in the rat.14 Pharmacological intervention with an EP4 agonist significantly reduces infarct size, and improves left ventricular contraction and dilatation as compared with vehicle-treated rats.14 The cardioprotective effect of the EP4 agonist relates largely to the attenuation of the infiltration of inflammatory cells, especially macrophages, into the ischemic myocardium.14 The EP4 agonist attenuates the release of inflammatory cytokines [including TNF-α, IL-1β, IL-6, and MCP-1] from macrophages induced by I/R. The EP4 agonist also suppresses the migration of macrophages in vitro by decreasing the level of MCP-1 production.14 Furthermore, EP4 agonists attenuate the expression and activities of MMP-2 and MMP-9 in reperfused tissue, enzymes that may decrease myocardial fibrosis.14 In accordance with these observations, mice that lack mPGES-1 exhibit adverse left ventricular dilatation and decreased left ventricular systolic and diastolic function compared with wild-type mice after acute myocardial infarction,42 suggesting that prostaglandin E2 derived from mPGES-1 causes protection and prevents I/R injury. Hence, an EP4 agonism might limit the consequences of I/R.
Allograft rejection in cardiac transplantation
In parenchymal rejection of cardiac allografts, T lymphocytes and macrophages infiltrate the myocardium and affect the myocardial cytolysis and inflammatory response that cause acute rejection.16,28 In C57BL/6 donor hearts heterotopically transplanted into BALB/c recipients [with major histocompatibility complex (MHC) mismatch], prostaglandin E2 production in the rejected grafts exceeds that in the native hearts.16,28 Administration of selective EP4 agonists to the recipient mice prevents acute cardiac rejection and prolongs graft survival compared with the vehicle-treated group.16,28 EP4 receptor activation suppresses the macrophage production of pro-inflammatory factors, [including cytokines (IFN-γ and IL-6), chemokines (IP-10, MIP-2, MIP-1α, MIP-1β, and lymphotactin)] and adhesion molecules by induction of NFκB activity, resulting in reduced macrophage infiltration and T-cell proliferation.16 These findings support a role for EP4 as an anti-inflammatory mediator in acute cardiac rejection in vivo, and highlight the effectiveness of EP4 agonists in prolonging allograft survival.
Atherosclerosis
Inflammation drives the development, progression, and clinical manifestations of atherosclerosis. The balance between persistent pro-inflammatory signals and intrinsic anti-inflammatory pathways influences all stages of this chronic inflammatory disease.43 Human atherosclerotic plaques display increased biosynthesis of prostaglandin E2.44 EP4 constitutes the predominant prostaglandin E2 receptor isoform present in human macrophages, whether studied in culture or present in atheromata.12,19 EP4 modulates inflammation and its contribution to atherosclerosis in vivo.19,45 Hypercholesterolemic low-density lipoprotein deficient mice transplanted with either EP4+/+ or EP4−/− bone marrow consumed a high-fat diet. The lack of EP4 in bone marrow–derived cells did not affect inflammation or atherosclerotic plaque size at early stages of atherosclerosis (five weeks of high-fat diet), which comprised less mature atheromata.19 But at a later stage, when mature atheromata had developed (ten weeks of high-fat diet), mice with EP4 in their bone marrow exhibited less local inflammation with decreased expression of chemotactic proteins (including MCP-1 and IP-10), reduced presence of inflammatory cells (macrophages and T cells), and an increased number of smooth-muscle cells within the plaques.19 These findings support the role of EP4 as an anti-inflammatory mediator in atherogenesis in vivo, although the interference with EP4 function did neither prevent the progression of atherosclerosis nor alter lesion size in the aorta or in the aortic root (at either early- or late-stage atherosclerosis).19 Accumulating evidence suggests that asymptomatic and symptomatic lesions differ with regard to inflammatory burden rather than plaque size. EP4 may thus suppress inflammation in established atheromata.
Abdominal aortic aneurysm
Human abdominal aortic aneurysm (AAA) tissues display increased biosynthesis of prostaglandin E2.6 The expression of EP4 abounds in mouse and human AAA tissues.20,46 These observations support a role for prostaglandin E2 and EP4 in the pathogenesis of AAA. AAA exhibit characteristics of a chronic inflammatory disorder. As remodeling of the blood vessel wall occurs, immune cells accumulate in the evolving aneurysm. Mice, of both genders, deficient in EP4 in their haemotopoietic cells have increased severity and prevalence of angiotensin II–induced aneurysms, compared to wild-type chimeric mice.20 The expression of MCP-1 in AAA lesions in EP4 bone-marrow deficient mice exceded that in those of wild-type chimeric mice.20 Accordingly, the aneurysmal lesions of the EP4-deficient bone marrow chimeric mice exhibited a markedly increased accumulation of macrophages and T cells,20 signifying an escalation of local inflammation. The lack of EP4 in bone marrow cells also augmented elastin fragmentation by enhancing the ability of MMP and cathepsins to break down elastin within the aneurysm lesions, increased the number of cells bearing markers of apoptosis, and decreased the accumulation of smooth muscle cells within AAA lesions.20 These findings demonstrate the pathophysiological importance of prostaglandin E2 signaling through EP4 receptors as an endogenous anti-inflammatory pathway involved in the prevention of aneurysm formation.
By contrast, mice with global deletion of the genes for COX-2 or mPGES-1 exhibit cardioprotective effects, with depressed prostaglandin E2 production and retarded AAA formation.47,48 Thus, EP ligands derived from COX-2 and mPGES-1 promotes AAA pathogenesis. The specific responsible EP receptor remains undefined, but activation of EP4 does not likely explain this result, since EP4 activation causes reduced incidence and severity of AAA by decreasing local inflammation.20 Because multiple EP receptors may exert opposing effects, selective inhibitors (or agonists, as in the case of EP4) for individual EP receptors may be more attractive than upstream inhibition of COX-2 or mPGES-1 for the treatment or prevention of AAA. No available therapy can prevent AAA, leaving patients with invasive treatments as the only option. Consequently, chemopreventive strategies to prevent the expansion of AAA have considerable appeal. Because of their protective effects, EP4 agonists may offer a novel approach to treat or prevent aneurysm development.
Immunosuppressive effect of EP4 in non-cardiovascular related inflammatory diseases
The usefulness of EP4 activation to limit inflammatory diseases extends beyond those discussed above, and may apply to other conditions. For example: 1) peripheral EP4 activation protects the brain from systemic inflammation.18 In vivo, conditional deletion of EP4 in macrophages and microglia increases lipid peroxidation and pro-inflammatory gene expression in the brain and in isolated adult microglia following peripheral LPS administration.18 Peripheral pharmacological treatment with a selective EP4 agonist leads to a decrease in pro-inflammatory gene expression in the plasma and in the hippocampus of LPS-treated mice,18 demonstrating that EP4 signaling attenuates inflammation in the central nervous system by suppressing the transmission of systemic immune responses to the brain. 2) EP4 activation maintains intestinal homeostasis, retaining mucosal integrity by suppressing innate immunity. Thus, mice deficient in EP4 developed severe colitis when administered 3% dextran sulphate sodium (DSS) while the same treatment only induced marginal colitis in wild-type mice.10 The administration of an EP4 selective antagonist mimicked this phenotype in wild-type mice.10 Likewise, administration of an EP4 selective agonist to wild-type mice ameliorated severe colitis normally induced with 7% DSS, while administration of the EP4 antagonist suppressed the recovery from colitis and induced a significant proliferation of CD4+ T cells.10 3) EP4 agonists accelerated chronic gastric ulcer healing, suppressed inflammatory cell infiltration in the granulation tissue, and reduced gastric bleeding and inflammation. EP4 agonists augmented ulcer healing by promoting proliferation and survival of mucous epithelial cells.15
Pro-inflammatory responses mediated by EP4
In spite of the numerous reports of the immunosuppressive effect of EP4 activation, some findings indicate that EP4 activation may stimulate immune responses under certain conditions. Indeed, prostaglandin E2 increases cytotoxic responses against allogeneic cells.49 Prostaglandin E2, by activating EP4 receptors, also augments Th1 proliferation and IL23-induced Th17 proliferation.50 The occurrences of EP4-mediated pro-inflammatory responses are also observed in vivo. In mice subjected to experimental autoimmune encephalomyelitis or contact hypersensitivity, the administration of a selective EP4 antagonist decreases the accumulation of both Th1 and Th17 cells in regional lymph nodes and the production of IFN-γ and IL-12 by lymph node cells, and suppresses disease progression.50 As a result, prostaglandin E2 and EP4 activation promote immune inflammation by causing Th1 differentiation and Th17 expansion. Likewise, in two separate experimental arthritis models — collagen-induced arthritis and glucose-6-phosphate isomerase-induced arthritis — the activation of EP4 with prostaglandin E2 promoted Th1 differentiation and Th17 expansion, exacerbating arthritis and promoting inflammatory pain.51 The oral administration of an EP4 antagonist suppressed Th1 and Th17 cytokine production and reduced arthritis and inflammatory pain in these rodents.51 These findings support the pro-inflammatory role of EP4 activation in vivo during contact hypersensitivity, in encephalomyelitis and in arthritis.
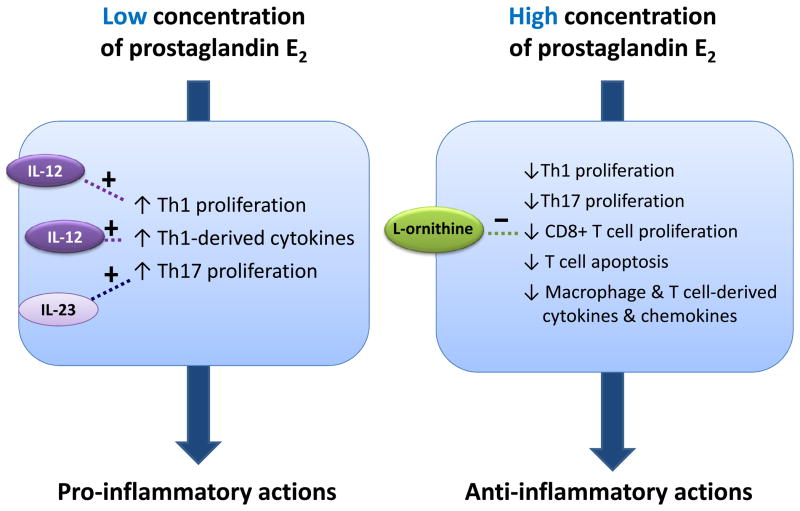
Why prostaglandin E2 exerts pro-inflammatory effects through EP4 in some circumstances, and anti-inflammatory actions in others is unclear. This apparent paradox could relate to the prevailing concentration of prostaglandin E2 or EP4 agonist. For example, at nanomolar concentrations, prostaglandin E2 enhances Th1 differentiation, promoting IFN-γ through activation of EP4.29 Such Th1 differentiation-promoting activities do not pertain to higher prostaglandin E2 concentrations.29 These findings propose a bimodal effect of prostaglandin E2 on immune responses, promoting inflammation at lower but attenuating it at higher concentrations (Figure 3). In this case, the immune-modulation fate of prostaglandin E2 would depend largely on its local concentration in vivo. Given this, it is worth noting that the concentration of prostaglandin E2 often increases as disease progresses or worsens, as is the case in cardiac transplantation allografts,16 atherosclerotic lesions19, and aortic aneurysms.20 When comparing the results of diverging studies with the administration of exogenous prostaglandin E2, it remains difficult to compare the absolute amount of prostaglandin E2 present from one experiment to another because the endogenous formation of the prostanoid varies and alter substantially to the actual prostaglandin E2 level present at the cellular level within the different systems.
Figure 3.
Schematic representation of the proposed bi-modal effect of prostaglandin E2. In the presence of certain modulators, such as IL-12 or IL-23, a low concentration of prostaglandin E2 results in pro-inflammatory signals. Conversely, a high concentration of prostaglandin E2 leads to anti-inflammatory responses. Modulators such as L-ornithine decrease proliferation of CD8+ T cells, thereby preventing anti-inflammatory action.
Concentrations of other modulators also likely impact the specific fate of EP4-mediated immune modulation (Figure 3). EP4 mediates Th1 differentiation-promoting effect in vitro, but this effect depends on the presence of IL-12 in the culture medium.50 Without this cytokine, prostaglandin E2 has little effect on Th1 differentiation.50 This facilitation of T-cell differentiation by prostaglandin E2 is specific to the Th1 subset.50 Prostaglandin E2 does not enhance, but instead suppresses Th17 differentiation from CD4+CD62L+ naïve T cells under any T-cell receptor stimulation condition.50 But the presence of sufficient IL-23 in the culture medium, prostaglandin E2 initiates Th17 cell expansion by EP4 receptor activation.50 In contrast to the EP4-mediated anti-inflammatory actions, EP4-mediated Th1 differentiation does not require cAMP or protein kinase A, but instead depends on phosphoinositide 3-kinase (PI3K).50,51 Not all immune-promoting effects resulting from EP4 signaling depend on PI3K. Indeed, EP4-dependent, IL-23-induced Th17 expansion does not require activation of this kinase and depends instead on the production of cAMP.50 L-ornithine can also control the direction of the immune regulatory response to prostaglandin E2. L-ornithine is an immune-regulatory substance that accumulates in supernatants of activated macrophages as a consequence of the high arginase [which converts L-arginine into urea and L-ornithine] activity of these cells.49 High concentrations of L-ornithine counteract the inhibitory effect of prostaglandin E2 on CD8+ T cell activation, and vice versa prostaglandin E2 counteracts the inhibitory effect of high doses of L-ornithine. Prostaglandin E2 and L-ornithine therefore are interdependent and antagonistic.49 For these reasons, the presence of other modulators influences the ultimate fate of EP4-mediated actions.
Conclusion
Although prostaglandin E2 exerts well recognized immunosuppressive actions, the adverse effects related to its broad biological activities resulting from the activation of its four EP receptors render it an impractical therapeutic agent. Compounds that interact specifically with the immune-suppressive EP4 receptor may allow a wider, more practical exploitation in the therapy of inflammatory diseases. Experimental studies illustrate the potential merit of EP4 in hampering inflammation in various disorders. Such investigations have demonstrated anti-inflammatory effects of EP4 in atherosclerosis and AAA using chimeric mice that lack EP4, but the efficacy of EP4 agonists in these animal models remains speculative. An EP4 agonist has promoted graft survival in allograft cardiac transplantation and dampened tissue damage after myocardial ischemia, but the efficacy and safety of EP4 activation in humans remains largely unknown. Accumulating in vivo data demonstrate that anti-inflammatory therapy by activation of EP4 receptor may benefit not only cardiovascular diseases, but also other inflammatory disorders — in particular colitis, endotoxic shock, and gastric ulcers. Conversely, EP4 acts as a pro-inflammatory mediator in encephalomyelitis, skin inflammation, and arthritis by promoting Th1 differentiation and Th17 expansion. Overall, the suppression of immune responses following activation of EP4 receptors presents an attractive therapeutic target for treating a broad range of inflammatory diseases. EP4 agonists may become anti-inflammatory drugs that mitigate COX-2-mediated inflammation, without augmenting cardiovascular risks.
Acknowledgments
Sources of Funding: This review was supported in parts by grants HL-34636 and HL-80472 (Dr. Libby) from NIH, and by the Seed Funding Programme for Basic Research (Dr. Tang) from The University of Hong Kong.
References
- 1.Funk CD, FitzGerald GA. COX-2 inhibitors and cardiovascular risk. J Cardiovasc Pharmacol. 2007;50:470–479. doi: 10.1097/FJC.0b013e318157f72d. [DOI] [PubMed] [Google Scholar]
- 2.Amer M, Bead VR, Bathon J, Blumenthal RS, Edwards DN. Use of nonsteroidal anti-inflammatory drugs in patients with cardiovascular disease – a cautionary tale. Cardiology in Rev. 2010;18:204–212. doi: 10.1097/CRD.0b013e3181ce1521. [DOI] [PubMed] [Google Scholar]
- 3.Fries JF. Selective cyclooxygenase inhibition: promise for future NSAID therapy? Scand J Rheumatol Suppl. 1996;102:23–28. [PubMed] [Google Scholar]
- 4.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286:954–959. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 5.Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA. Use of nonsteroidal anti-inflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115:1634–1642. doi: 10.1161/CIRCULATIONAHA.106.181424. [DOI] [PubMed] [Google Scholar]
- 6.Holmes DR, Wester W, Thompson RW, Reilly JM. Prostaglandin E2 synthesis and cyclooxygenase expression in abdominal aortic aneurysms. J Vasc Surg. 1999;25:810–815. doi: 10.1016/s0741-5214(97)70210-6. [DOI] [PubMed] [Google Scholar]
- 7.Giuliano F, Warner TD. Origins of prostaglandin E2: Involvements of cyclooxygenase (COX)-1 and COX-2 in human and rat systems. J Pharmacol Exp Therap. 2002;302:1001–1006. doi: 10.1124/jpet.102.041244. [DOI] [PubMed] [Google Scholar]
- 8.Yamane H, Sugimoto Y, Tanaka S, Ichikawa A. Prostaglandin E2 receptors, EP2 and EP4, differentially modulate TNF-α and IL-6 production induced by lipopolysaccharide in mouse peritoneal neutrophils. Biochem Biophy Res Commun. 2000;278:224–228. doi: 10.1006/bbrc.2000.3779. [DOI] [PubMed] [Google Scholar]
- 9.Nataraj C, Thomas DW, Tilley SL, Nguyen M, Mannon R, Koller BH, Coffman TM. Receptors for prostaglandin E2 that regulate cellular immune response in the mouse. J Clin Invest. 2001;108:1229–1235. doi: 10.1172/JCI13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kabashima K, Saji T, Murata T, Nagamachi M, Matsuoka T, Segi E, Tsuboi K, Sugimoto Y, Kobayashi T, Miyachi Y, Ichikawa A, Narumiya S. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J Clin Invest. 2002;109:883–893. doi: 10.1172/JCI14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakamoto A, Matsumur J, Mii S, Gotoh Y, Ogawa R. A prostaglandin E2 receptor subtype EP4 agonist attenuates cardiovascular depression in endotoxin shock by inhibiting inflammatory cytokines and nitric oxide production. Shock. 2004;22:76–81. doi: 10.1097/01.shk.0000129338.99410.5d. [DOI] [PubMed] [Google Scholar]
- 12.Takayama K, Garcia-Cardena G, Sukhova GK, Comander J, Gimbrone MA, Libby P. Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J Biol Chem. 2002;277:44147–44154. doi: 10.1074/jbc.M204810200. [DOI] [PubMed] [Google Scholar]
- 13.Takayama K, Sukhova GK, Chin MT, Libby P. A novel prostaglandin E receptor 4-associated protein participates in anti-inflammatory signaling. Circ Res. 2006;98:499–504. doi: 10.1161/01.RES.0000204451.88147.96. [DOI] [PubMed] [Google Scholar]
- 14.Hishikari K, Suzuki J, Ogawa M, Isobe K, Takahashi T, Onishi M, Takayama K, Isobe M. Pharmacological activation of the prostaglandin E2 receptor EP4 improves cardiac function after myocardial ischaemia/reperfusion injury. Cardiovasc Res. 2009;81:123–132. doi: 10.1093/cvr/cvn254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang GL, Im WB, Donde Y, Wheeler LA. EP4 agonist alleviates indomethacin-induced gastric lesions and promotes chronic gastric ulcer healing. World J Gastroenterol. 2009;15:5149–5156. doi: 10.3748/wjg.15.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogawa M, Suzuki J, Kosuge H, Takayama K, Nagai R, Isobe M. The mechanism of anti-inflammatory effects of prostaglandin E2 receptor 4 activation in murine cardiac transplantation. Transplantation. 2009;87:1645–1653. doi: 10.1097/TP.0b013e3181a5c84c. [DOI] [PubMed] [Google Scholar]
- 17.Scher JU, Pillinger MH. The anti-inflammatory effects of prostaglandins. J Investig Med. 2009;57:703–308. doi: 10.2310/JIM.0b013e31819aaa76. [DOI] [PubMed] [Google Scholar]
- 18.Shi J, Johansson J, Woodling NS, Wang Q, Montine TJ, Andreasson K. The prostaglandin E2 E-prostanoid 4 receptor exerts anti-inflammatory effects in brain innate immunity. J Immunol. 2010;184:7207–7218. doi: 10.4049/jimmunol.0903487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang EH, Shimizu K, Christen T, Rocha VZ, Shvartz E, Tesmenitsky Y, Sukhova G, Shi GP, Libby P. Lack of EP4 receptors on bone marrow-derived cells enhances inflammation in atherosclerotic lesions. Cardiovasc Res. 2010 doi: 10.1093/cvr/cvq262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang EHC, Shvartz E, Shimizu K, Rocha VZ, Zheng C, Fukuda D, Shi GP, Sukhova G, Libby P. Deletion of EP4 on bone marrow-derived cells enhances inflammation and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2010 doi: 10.1161/ATVBAHA.110.216580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samuelsson B, Morgenstern R, Jakobsson PJ. Membrane prostaglandin E synthase-1: A novel therapeutic target. Pharmacol Rev. 2007;59:207–224. doi: 10.1124/pr.59.3.1. [DOI] [PubMed] [Google Scholar]
- 22.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 23.Hwang D. Fatty acids and immune responses – a new perspective in searching for clues to mechanism. Annu Rev Nutr. 2000;20:431–456. doi: 10.1146/annurev.nutr.20.1.431. [DOI] [PubMed] [Google Scholar]
- 24.Minami M, Shimizu K, Okamoto Y, Folco E, Ilasaca ML, Feinberg MW, Aikawa M, Libby P. Prostaglandin E2 receptor type 4-associated protein interacts directly with NK-κB1 and attenuates macrophage activation. J Biol Chem. 2007;15:9692–9703. doi: 10.1074/jbc.M709663200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong WW, Burke PA, Drotar BME, Chavali SR, Forse RA. Effects of prostaglandin E2, cholera toxin and 8-bromo-cyclic AMP on lipopolysaccharide-induced gene expression of cytokines in human macrophages. Immmunology. 1995;84:446–452. [PMC free article] [PubMed] [Google Scholar]
- 26.van der Pouw Kraan TC, Boeije LC, Smeenk RJ, Wijdenes J, Aarden LA. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995;181:775–779. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hilkens C, Snijders A, Vermeulen H, van der Meide P, Wierenga E, Kapsenberg M. Accessory cell-derived interleukin-12 and prostaglandin E2 determine the level of interferon-gamma produced by activated human CD4+ T cells. Ann N Y Acd Sci. 1996;795:349–350. doi: 10.1111/j.1749-6632.1996.tb52689.x. [DOI] [PubMed] [Google Scholar]
- 28.Nomi T, Sho M, Akahori T, Kanehiro H, Nakajima Y. Protective effect of prostaglandin E2 receptor EP2 and EP4 in alloimmune response in vivo. Transplant Proc. 2006;38:3209–3210. doi: 10.1016/j.transproceed.2006.10.118. [DOI] [PubMed] [Google Scholar]
- 29.Betz M, Fox BS. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunology. 1991;146:108–113. [PubMed] [Google Scholar]
- 30.Gold KN, Weyand CM, Goronzy JJ. Modulation of helper T cell function by prostaglandins. Arthritis Rheum. 1994;37:925–933. doi: 10.1002/art.1780370623. [DOI] [PubMed] [Google Scholar]
- 31.Hilkens CM, Vermeulen H, van Neerven RJ, Snijdewint FG, Wierenga EA, Kapsenberg ML. Differential modulation of T helper 1 (Th1) and T helper type 2 (Th2) cytokine secretion by prostaglandin E2 critically depends on interleukin-2. Eur J Immunol. 1995;25:59–63. doi: 10.1002/eji.1830250112. [DOI] [PubMed] [Google Scholar]
- 32.Leung KH, Mihich E. Prostaglandin modulation of development of cell-mediated immunity in culture. Nature. 1980;288:597–600. doi: 10.1038/288597a0. [DOI] [PubMed] [Google Scholar]
- 33.Hendricks A, Leibold W, Kaever V, Schuberth HJ. Prostaglandin E2 is variably induced by bacterial superantigens in bovine mononuclear cells and has a regulatory role for the T cell proliferative response. Immunobiology. 2000;201:493–505. doi: 10.1016/S0171-2985(00)80069-8. [DOI] [PubMed] [Google Scholar]
- 34.Mastino A, Piacentini M, Grelli S, Favalli C, Autuori F, Tentori L, Oliverio S, Garaci E. Induction of apoptosis in thymocytes by prostaglandin E2 in vivo. Dev Immunol. 1992;2:263–271. doi: 10.1155/1992/80863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pica F, Franzese O, D’Onofrio C, Bonmassar E, Favalli C, Garaci E. Prostaglandin E2 induces apoptosis in resting immature and mature human lymphocytes: a c-Myc-dependent and Bcl-2 independent associated pathway. J Pharmacol Exp Ther. 1996;277:1793–1800. [PubMed] [Google Scholar]
- 36.Ganapathy V, Gurlo T, Jarstadmarken HO, von Grafenstein H. Regulation of TCR-induced IFN-gamma release from islet-reactive non-obese diabetic CD8(+) T cells by prostaglandin E(2) receptor signaling. Int Immunol. 2000;12:851–860. doi: 10.1093/intimm/12.6.851. [DOI] [PubMed] [Google Scholar]
- 37.Goetzl EJ, An S, Zeng L. Specific suppression by prostaglandin E2 of activation-induced apoptosis of human CD4+CD8+ T lymphoblasts. J Immunol. 1995;154:1041–1047. [PubMed] [Google Scholar]
- 38.Katamura K, Shintaku N, Yamauchi Y, Fukui T, Ohshima Y, Mayumi M, Furusho K. Prostaglandin E2 at priming of naïve CD4+ T cells inhibits acquisition of ability to produce IFN-gamma and IL-2, but not IL-4 and IL-5. J Immunol. 1995;155:4604–4612. [PubMed] [Google Scholar]
- 39.Wise H. Activation of the prostaglandin EP4-receptor subtype is highly coupled to inhibition of N-formyl-methionyl-leucyl-phenylalanine-stimulated rat neutrophil aggregration. Prostaglandins Leukot Essent Fatty Acids. 1998;58:77–84. doi: 10.1016/s0952-3278(98)90133-8. [DOI] [PubMed] [Google Scholar]
- 40.Fabricius D, Neubauer M, Mandel B, Schutz C, Viardot A, Vollmer A, Jahrsdorfer B, Debatin KM. Prostaglandin E2 inhibits IFN-alpha secretion and Th1 costimulation by human plasmacytoid dendritic cells via E-prostanoid 2 and E-prostanoid 4 receptor engagement. J Immunol. 2010;184:677–684. doi: 10.4049/jimmunol.0902028. [DOI] [PubMed] [Google Scholar]
- 41.Frangogiannis NG. Targeting the inflammatory response in healing myocardial infarcts. Curr Med Chem. 2005;13:1877–1893. doi: 10.2174/092986706777585086. [DOI] [PubMed] [Google Scholar]
- 42.Degousee N, Fazel S, Angoulvant D, Stefanski E, Pawelzik SC, Korotkova M, Arab S, Liu P, Lindsay TF, Zhuo S, Butany J, Li RK, Audoly L, Schmidt R, Angioni C, Geisslinger G, Jakobsson PJ, Rubin BB. Microsomal prostaglandin E2 synthase-1 deletions leads to adverse left ventricular remodeling after myocardial infarction. Circulation. 2008;117:1701–1710. doi: 10.1161/CIRCULATIONAHA.107.749739. [DOI] [PubMed] [Google Scholar]
- 43.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010;74:213–220. doi: 10.1253/circj.cj-09-0706. [DOI] [PubMed] [Google Scholar]
- 44.Rolland PH, Jouve R, Pellegrin E, Mercier C, Serradimigni A. Alteration in prostaglandin and prostaglandin E2 production. Correlation with changes in human aortic atherosclerotic disease. Arteriosclerosis. 1984;4:70–78. doi: 10.1161/01.atv.4.1.70. [DOI] [PubMed] [Google Scholar]
- 45.Babaev VR, Chew JD, Ding L, Davis S, Breyer MD, Breyer RM, Oates JA, Fazio S, Linton MF. Macrophage EP4 deficiency increases apoptosis and suppresses early atherosclerosis. Cell Metab. 2008;8:492–501. doi: 10.1016/j.cmet.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bayston T, Ramessur S, Reise J, Jones KG, Powell JT. Prostaglandin E2 receptors in abdominal aortic aneurysm and human aortic smooth muscle cells. J Vasc Surg. 2003;38:354–359. doi: 10.1016/s0741-5214(03)00339-2. [DOI] [PubMed] [Google Scholar]
- 47.Gitlin JM, Trivedi DB, Langenbach R, Loftin CD. Genetic deficiency of cyclooxygenase-2 attenuates abdominal aortic aneurysm formation in mice. Cardiovasc Res. 2007;73:227–236. doi: 10.1016/j.cardiores.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 48.Wang M, Lee E, Song W, Ricciotti E, Rader DJ, Lawson JA, Pure E, FitzGerald GA. Microsomal prostaglandin E synthase-1 deletion suppresses oxidative stress and angiotensin II-induced abdominal aortic aneurysm formation. Circulation. 2008;117:1302–1309. doi: 10.1161/CIRCULATIONAHA.107.731398. [DOI] [PubMed] [Google Scholar]
- 49.Hacker-Shahin B, Droge W. Antagonistic regulator of cytotoxic T lymphocyte activation by prostaglandin E2 and L-ornithine. Immunopharmacology. 1986;11:57–60. doi: 10.1016/0162-3109(86)90065-2. [DOI] [PubMed] [Google Scholar]
- 50.Yao C, Sakata D, Esaki Y, Li Y, Matsuoka T, Kuroiwa K, Sugimoto Y, Narumiya S. Prostaglandin E2-EP4 signaling promotes immune inflammation through TH1 cell differentiation and TH17 cell expansion. Nat Med. 2009;15:633–640. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- 51.Chen Q, Muramoto K, Masaaki N, Ding Y, Yang H, Mackey M, Li W, Inoue Y, Ackermann K, Shirota H, Matsumoto I, Spyvee M, Schiller S, Sumida T, Gusovsky F, Lamphier M. A novel antagonist of the prostaglandin E2 EP4 receptor inhibits Th1 differentiation and Th17 expansion and is orally active in arthritis models. Br J Pharmcol. 2010;160:292–310. doi: 10.1111/j.1476-5381.2010.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]