Abstract
The host inflammatory response to HIV invasion is a necessary component of the innate antiviral activity that vaccines and early interventions seek to exploit/enhance. However, the response is dependent on CD4+ T-helper cell 1 (Th1) recruitment and activation. It is this very recruitment of HIV-susceptible target cells that is associated with the initial viral proliferation. Hence, global enhancement of the inflammatory response by T-cells and dendritic cells will likely feed viral propagation. Mucosal entry sites contain inherent pathways, in the form of natural regulatory T-cells (nTreg), that globally dampen the inflammatory response. We created a model of this inflammatory response to virus as well as inherent nTreg-mediated regulation of Th1 recruitment and activation. With simulations using this model we sought to address the net effect of nTreg activation and its specific functions as well as identify mechanisms of the natural inflammatory response that are best targeted to inhibit viral spread without compromising initial antiviral activity. Simulation results provide multiple insights that are relevant to developing intervention strategies that seek to exploit natural immune processes: i) induction of the regulatory response through nTreg activation expedites viral proliferation due to viral production by nTreg itself and not to reduced Natural Killer (NK) cell activity; ii) at the same time, induction of the inflammation response through either DC activation or Th1 activation expedites viral proliferation; iii) within the inflammatory pathway, the NK response is an effective controller of viral proliferation while DC-mediated stimulation of T-cells is a significant driver of viral proliferation; and iv) nTreg-mediated DC deactivation plays a significant role in slowing viral proliferation by inhibiting T-cell stimulation, making this function an aide to the antiviral immune response.
Keywords: HIV, Innate inflammatory response pathway, Regulatory response pathway, Mathematical model
1 Introduction
Once HIV viremia has peaked in the lymphoid tissue there is little hope of eliminating the virus as this marks systemic spread and establishment of tissue and cellular reservoirs. Research has, therefore, been focussed on what is often referred to as the “window period”, the initial two weeks of infection, between exposure to virus and peak viremia.
The vaginal mucosa is the most common site of infection and vaccine strategies focus mainly on promoting inflammation response in this tissue. The immune response at mucosal sites, however, are characterized by the presence of anti-inflammatory factors, such as CD4+ natural regulatory T-cells (CD4+ nTreg), that allow these tissues to generally remain refractory to inflammation. The purpose of this regulation is to allow immune cells of the lungs, GI tract, nasal passages, and genital mucosa to maintain contact with innocuous foreign agents in the external environment without dwelling in a constant state of tissue-damaging hyper-inflammation. In the case of viral infection, a potential effect of anti-inflammatory nTreg is to directly and indirectly dampen key mechanisms of the protective antiviral response, such as DC activation and Natural Killer (NK) cell stimulation. The role that these anti-inflammatory factors play in initial viremia remains unclear. The effect of these interacting pathways on HIV infection is particularly complicated by the fact that the immune cells themselves are permissive hosts for this virus. Relevant questions for infection intervention strategies include Should one reduce the regulatory response in order to aid antiviral activity, i.e., NK recruitment and activation? This could unintentionally lead to greater viral proliferation through increased host recruitment and infected Th1 proliferation. Therefore, Should one enhance the regulatory response to reduce inflammation-associated recruitment and T-cell proliferation? This strategy could also aid viral proliferation by dampening antiviral NK activity as well as promoting nTreg-derived viremia.
There are a number of models that have been developed in different contexts to answer the general question of whether one should enhance or reduce the immune response in order to better control HIV. For example, a mathematical model of interaction of uninfected CD4+ T cells and the free HIV virus in the plasma is developed in [35] to investigate the effect of immunotherapy with cytokine interleukin-2 (IL-2) on an HIV-infected patient, and it was found that this type of immunotheray can be successful in delaying AIDS progression. While the authors in [16] developed mathematical models of the clinical latency stage of HIV-1 infection with the assumption that HIV-1 infection is limited either by the availability of cells that HIV can infect or by a specific anti-HIV cellular immune response. This assumption is based on the suggestions of various clinical data sets (see the references in [16] for more information) that HIV viral replication is limited by the availability of target cells. The effects of tetanus vaccination on chronically infected HIV patients were explored in [29] through a mathematical model of interaction between T cells, HIV and other antigen. The study was able to reproduce the general features of the post-vaccination rise in viral load observed in some clinical data sets (see the references in [29] for more information).
In addition, the synergistic interaction between HIV and some other pathogens were investigated by a number of researchers (e.g., [3, 34, 42, 43, 58]). In what follows, we give a brief review of some of this type of work. In [43], mathematical models of population dynamics of T helper cells, HIV, and other pathogens were formulated to address the three facets of the interactions between HIV and other pathogens: enhanced HIV replication due to immune stimulation by other pathogens; modified immune control of other pathogens due to immunological suppression by HIV; and the vicious circle formed by the positive feedback between these effects. This study indicates that there is a threshold number of activated T helper cells above which the immune system is unable to control pre-established pathogens. A mathematical model was created in [34] to describe the interaction of HIV and tuberculosis (TB) with the immune system (T cells and macrophages), and this study indicates that co-infection may indeed play a dramatic role in disease. The authors in [58] proposed a simple mathematical model for the interaction of the immune effector cells with HIV and malaria parasites in an individual host, and showed that HIV infection may increase the risk of malaria and, subsequently, malaria infection promotes the proliferation of HIV.
Recently, a mathematical model describing the dynamics of HIV, CD4+ and CD8+ T cells, and DCs interacting in a human lymph node was formulated and analyzed in [26] to investigate the dual role (enhancing HIV infection process as well as promoting an antiviral immune response) of dendritic cells in immune response to HIV infection. Here we present a study unique in that it explores the net effects of HIV-induced inflammation and subsequent nTreg-induced regulation on initial viral replication and spread to the lymph node specifically in the acute phase, i.e., upon vaginal inoculation. The resulting mathematical model is based on mechanistic knowledge of NK, Th1 and nTreg activation by dendritic cells.
1.1 Innate Inflammatory and Regulatory Pathways
Experimental data regarding the early immune response to HIV upon viral exposure in humans is difficult to obtain as it requires invasive tissue biopsies taken from the gut and genital tissues, the initial site of entry, within a very short time period post-infection. Hence, the current biological model is largely based on data gathered from rhesus macaques infected experimentally with Simian Immunodeficiency Virus (SIV) [2, 24, 46]. Based on these studies, the current biological model for early immune response to SIV and HIV infection are generally assumed to be similar and are described as follows.
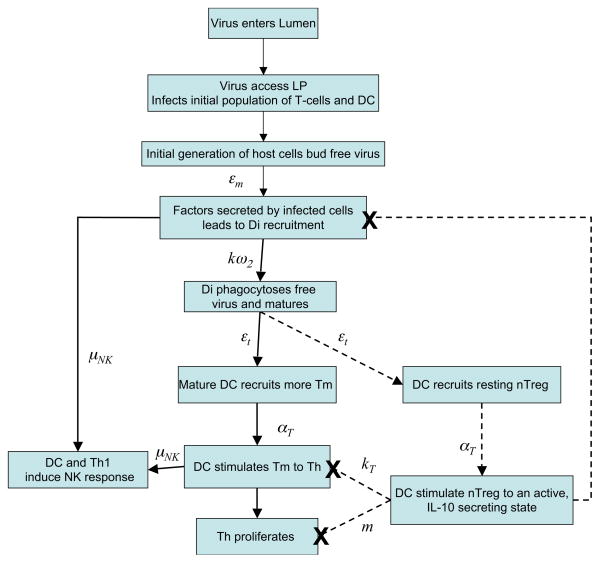
Virus enters the vaginal lumen and, within hours, gains access to susceptible immune cells: dendritic cells (DC), CD4+ T-helper cells (Th), and CD4+ natural T-regulatory cells (CD4+ nTreg) in the lamina propria (LP) [46]. This initial, small population of infected cells begins to bud virus approximately 2–4 days post-infection at which point the immune response outlined in Figure 1 is elicited. Specifically, infected cells secrete factors that trigger a pathway by which monocytes are recruited to the tissue and differentiate into immature dendritic cells (Di). Free virus, budded from infected cells, is phagocytosed by immature DC leading to their maturation. At this point mature DC secrete factors that recruit resting, or memory, T-helper cells (Tm) and present viral antigen to stimulate these T-cells to a Th1 phenotype [2] rendering them more susceptible to viral infection and promoting their proliferation, allowing enhanced viral replication in infected cells. Both mature DC and inflammatory Th1 then secrete factors such as IL-12 that activate NK cells [6]. NK cells subsequently remove infected cells from the tissue site.
Figure 1. Immune response to first generation of infected cells.
A scheme of the inflammatory (solid lines) and regulatory (dashed lines) included in the model. Arrows are labelled with parameters that determine the rate at which each event occurs.
The role of the regulatory pathway in early infection is largely unknown. Yet basic research in various in vitro and in vivo systems (reviewed in [39] and [50]) have demonstrated mechanisms that we use to construct a biological model of an HIV-dependent regulatory response. In this biological model, resting nTreg, like resting T-helper cells, are recruited and stimulated by mature, antigen-presenting DC via the T-cell receptor. Active nTreg has been shown to carry out numerous regulatory functions. In this biological model the three primary regulatory functions we assign to nTreg are: i) contact-dependent deactivation of presenting dendritic cells [47]; ii) indirect inhibition of monocyte recruitment through IL-10 secretion, which downregulates expression of inflammatory recruitment factors in surrounding cells [28]; and iii) inhibition of T-helper cell proliferation [53]. Like their inflammatory counterparts, CD4+ nTreg is also permissive to HIV infection upon activation [48].
As HIV productively infects the immune cells of these pathways with proliferating T-cells being the most productive hosts, the effect of this normally protective response is not readily predictable. For example, DC uptake of virus and active Th1 presence are critical for NK activation and subsequent removal of virus-producing cells, hindering viral replication. Yet, at the same time, the recruitment of these cells provides a higher concentration of susceptible hosts and their stimulation, inducing viral replication. Indeed, the inflammatory response has been associated with acute viral spread [2]. Given these facts, the nTreg-mediated regulatory pathway could reasonably hinder initial viral propagation by reducing the critical mechanisms of DC-mediated CD4+ T-cell recruitment and activation as well as reduce infected T-cell proliferation.
In short, from the perspective of virus success, nTreg could be equally seen as friend or foe in that it is susceptible to productive viral infection and inhibits inflammation-induced NK activity while, at the same time, inhibiting the inflammatory properties of DCs that are central to the viral lifecycle: T-cell recruitment, activation, and proliferation. Hence, the ultimate effect of nTreg presence on viral success in vivo is still unknown.
This work reports the results of simulations carried out to identify specific aspects of the inflammatory and regulatory response pathways that promote and hinder initial viral proliferation from the first generation of infected cells. This work also informs whether regulatory mechanisms could offer new avenues for HIV related interventions as the inflammatory pathway has of other pathogens.
Realization of these goals requires a model that captures the dynamics of the true system. This eventually requires rigorous validation using time-course (longitudinal) tissue data that is yet to be found in the literature for the tissue sites of interest, the vaginal mucosa and genital lymph node. An initial step toward this goal is reported here in the form of a model that captures the dynamics of the system that is proposed in current literature and is based on the available tissue data. It is worthwhile to note that in developing a mathematical model for any complex biological system there is a balance between complexity and utility (in the context of underlying modeling philosophy, below we shall discuss further this balance between more complex models for which validating data may not currently be available and those which are simple and can be based on only already established accepted mechanisms and data sets). In this paper, we do not try to formulate a model that captures all the features of the mucosal immune response as well as all host and viral factors, but rather a model that can capture many salient features of the system and for which some parameters can be plausibly estimated based on the sparse data available. The model will however involve mechanisms that are still speculative and for which validating data is not yet available. The interested readers can refer to Appendix B of [57] for those simplifying assumptions that we made during the modeling reduction processes.
1.2 Some Remarks on Modeling Philosophy
Before proceeding with our efforts in developing a model below, we make some remarks to put our efforts in context of an overall philosophy for the use of mathematical and statistical models in scientific investigations. More detailed discussions on this topic can be found in [15, Chapter 1]. In subsequent developments we will arrive at a fairly complex model for which only a sparse amount of experimental data is available for model validation. (Indeed we are currently able to offer only a partial qualitative validation of our proposed model!) When developing such models it is natural to raise questions of how complex/simple a model one should employ in such investigations. To at least partially answer this, one should consider the underlying reasons that one might give for modeling in science and engineering. The ultimate goal of modeling is not the model itself. Rather modeling is simply a means for providing a conceptual framework in which real systems may be investigated. The modeling process itself is, when properly done, most often an iterative process and often involves efforts over time (and possibly by different research groups). Numerous rationales may be given (to aid in simplification, preciseness, formulation of hypotheses, design of critical experiments) but perhaps the most fundamental rationale is that modeling leads to an organization of inquiry. Properly done, it tends to polarize one’s thinking and aid in posing basic questions concerning what one does and does not know for certain about the real system. Whatever the reasons that have been advanced to justify modeling attempts, it is sufficient perhaps to note that the primary goal must be enlightenment, that is, to gain a better understanding of the real system, and the success or lack thereof of any modeling attempt should be judged with this in mind. Thus, it is not the model itself that is the goal but rather increased understanding of the physical or biological system under investigation. In any modeling attempt, one can seek a simple model that can be validated with existing data and justified in terms of accepted mechanisms. This would result in the simplest model that can explain current theory and data. An alternative approach is to seek a more complex model that stretches our current understanding and poses new questions to be pursued with new conceptual and new experimental investigations. As the reader will see in our subsequent developments below, this latter approach is our choice here. But this will lead to large complex models with a large number of parameters, many of which have not been discussed in the literature. The analysis and attempted validation of such models leads to difficult and ill-posed inverse or parameter estimation problems. We have encountered such difficulties previously, even in earlier HIV infection models (see [4, 9]) and have successfully used an iterative process to alleviate certain aspects of the model analaysis/validation and ill-posedness aspects of the parameter estimation procedures. As explained below, we do that again here.
In related and potentially important research, new methods (illustrated on one of our earlier HIV models in [8]) are currently being developed to formulate and solve such parameter estimation problems. These selection methods involve use of sensitivity functions and information matrices effectively with data sets and complex dynamic models to rank parameters in a model in roughly the order of their importance in the ability of the model to describe (fit) a given data set. As development of these methods matures, they will undoubtedly be of significant value in modeling investigations such as that presented in this paper.
1.3 Model Components
The model describes HIV progression among CD4+ cell populations of conventional T-cells, CD4+ natural T-regulatory cells (CD4+ nTreg), and dendritic cells (DC) as they transition between location and phenotype compartments in response to virus. As such, the only active CD4+ T-helper cells represented are of an inflammatory Th1 phenotype based on cytokine profiles indicating a type 1 response elicited in SIV models [2]. Model variables (compartments) are listed in Table 1 below, and all variables are in units of cells per mL.
Table 1.
Populations represented in the model.
| Di | Immature dendritic cells | |
| DI | Non-infected active dendritic cells in the GLN | |
| DI* | Infected active dendritic cells in the GLN | |
| DE | Non-infected active dendritic cells in the LP | |
| DE* | Infected active dendritic cells in the LP | |
| Tm | Non-infected CD4+ Memory T-cells | |
|
|
Latently infected CD4+ Memory T-cells | |
| TI | Non-infected active CD4+ T helper 1 cells in the GLN | |
| TI* | Infected active CD4+ T helper 1 cells in the GLN | |
| TE | Non-infected active CD4+ T helper 1 cells in the LP | |
| TE* | Infected active CD4+ T helper 1 cells in the LP | |
| Rm | Non-infected memory CD4+ natural T regulatory cells | |
|
|
Latently infected memory CD4+ natural T regulatory cells | |
| RI | Non-infected active CD4+ natural T regulatory cells in the GLN | |
| RI* | Infected active CD4+ natural T regulatory cells in the GLN | |
| RE | Non-infected active CD4+ natural T regulatory cells in the LP | |
| RE* | Infected active CD4+ natural T regulatory cells in the LP |
Cell populations are compartmentalized by four phenotypes; i) resting, generally memory or immature immune cells, denoted with the subscripts ‘m’ or ‘i’; ii) active, no subscript; iii) infected, denoted with the superscript ‘*’; iv) not infected. These are then further compartmentalized into two tissue sites: i) The vaginal lamina propria (LP), more generally termed the effector site of the immune response and denoted by the superscript ‘E’; ii) the genital lymph node (GLN), the inductive site of the immune response and denoted by the superscript ‘I’.
2 Model Equations
2.1 Dendritic Cells
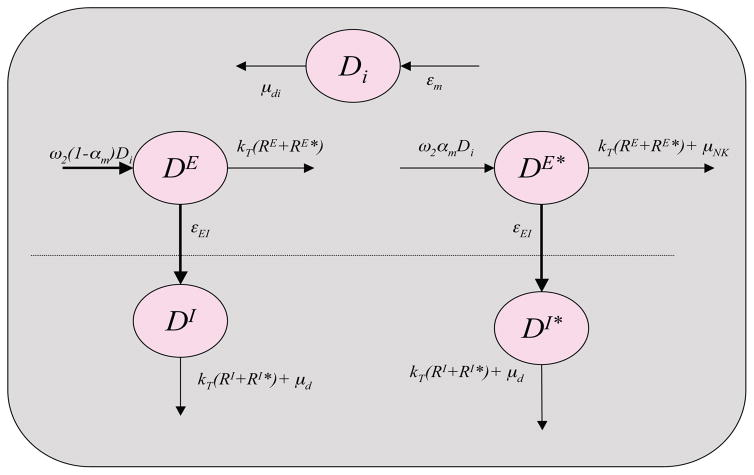
The scheme for the dynamics of dendritic cells is illustrated in Figure 2. The corresponding compartment model for describing its dynamics is given by
Figure 2.
Scheme for dynamics of dendritic cell populations represented.
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
where Di is the concentration of immature dendritic cells, DI and DI* are, respectively, the concentrations of non-infected and infected activated dendritic cells in the inductive site (GLN), and DE and DE* are the concentrations of non-infected and infected activated dendritic cells in the effector site (LP). Immature dendritic cells are present in the lamina propria (Di, Equation (1)) at a constant value of d0 representing that each Di that transfers to one of the activated dendritic cell compartments is replaced from an unlimited monocyte pool in the blood [54]. The constant parameter μdi denotes the death rate of the immature dendritic cells. The functional parameter εm represents the rate of recruitment of monocytes by inflammatory factors secreted by active immune cells [28] and is a function of productively infected cells, TE* and DE*, given by
| (6) |
where cm is a positive constant. This function is based on the rationale that virus presence induces various inflammatory responses of cells not explicitly represented that promote secretion of recruitment factors and that active CD4+ nTreg secretes IL-10, which can inhibit the expression of these recruitment factors [28]. It is assumed that the IL-10 secretion by productively infected CD4+ nTreg (RE*) cancels out its ability to induce monocyte recruit and is not included in the equation.
Dendritic cells contact and phagocytose free virus at the rate of ω2 = kvE. This is the rate of contact with free virus in the effector site where k is the rate of contact between any two cells or cells and virions in both tissue sites and vE is the amount of free virus in the effector site. Specifically, vE is a function of productively infected cells given by
| (7) |
where NE is the burst size from T-cells and ND is the burst size from dendritic cells. Upon phagocytosis all virus is successfully degraded in the presentation process, with a probability of 1 − αm, and the cell becomes an active, presenting dendritic cell (DE, Equation (4)) that is capable of stimulating resting T-cells, promoting NK cytolytic activity, and recruiting resting T-cells from the blood to the tissue. After a period in the LP, corresponding to the migration rate εEI, the presenting dendritic cells migrate to the GLN joining the DI population (Equation (2)). Here they can stimulate resting T-cells until they die at a rate of μd.
In the case that virions are able to escape degradation and establish infection in a dendritic cell, with a probability of αm, the cell enters the DE* population (Equation (5)). As with non-infected dendritic cells, those that are infected will migrate to the GLN in the same manner joining the DI* population (Equation (3)).
Active nTreg, that are either infected or not infected, deactivate dendritic cells upon contact rendering them incapable of cytokine secretion or stimulation of resting T-cells [47]. We represent this by removing them from the system upon contact with cells of the active CD4+ nTreg population (RE, RE*) with the terms kT (RE + RE*) in Equations (4) and (5) and kT (RI* + RI) in Equations (2) and (3), respectively. Here kT is the contact rate between dendritic cells and nTreg, which was estimated as greater than that between all other cells, k, in agreement with experimental evidence that nTreg have a higher affinity for dendritic cells than do T-helper cells [47].
Similar to all infected cells in the effector site, those of the DE* population may be eliminated by cytolytic NK cells at a rate of μN K given by
| (8) |
which is a functional parameter dependent on the concentration of active Th1 and DC based on that fact that IL-12, IL-15, IL-18, IL-21, and interferons (IFN) induce NK cell survival and proliferation, and can promote cytotoxic function. These cytokines are primarily secreted by presenting dendritic cells [6], but IL-12 is also secreted by Th1 T-cells [28]. The constant parameter cN K determines the maximum value μN K may reach as the concentrations of activated DC and Th1 increase and λ determines the rate at which μN K rises to this maximum value in proportion to the rise of DC and Th1 concentrations.
Studies have shown that when an immature dendritic cell is infected, its immune functions of antigen presentation and cytokine production may be impaired [22]. Specifically, HIV-infected human DCs in vitro have been shown to secrete IL-10 instead of IL-12 and IL-18 and have reduced CD80 expression. However, whether this effect of viral infection occurs in vivo and in the acute phase is unknown. To accommodate this possibility the model includes the coefficient δ as seen in Equation (8). This parameter determines the extent to which viral infection impairs the ability of DC to induce NK activation as well as recruit and stimulate T-cells (discussed below). In this context, a value of δ < 1 represents virus-induced inhibition of IL-12 and IL-18 expression reducing the ability of infected DC to recruit and activate NK cells.
NK cells in lymphoid tissue have not been shown to have cytolytic activity in vivo [6]. Therefore, those of the DI* population are not considered susceptible to NK-mediated elimination.
2.2 Conventional CD4+ T-cells
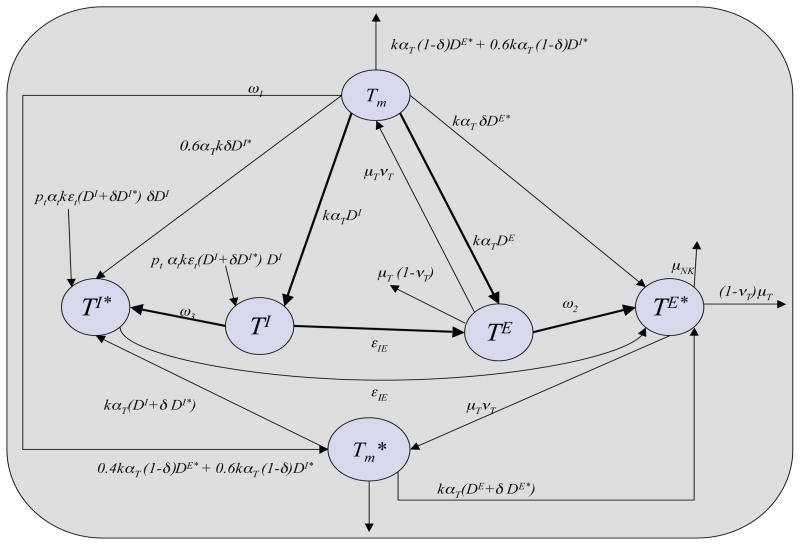
The scheme for the dynamics of conventional CD4+ T cells is depicted in Figure 3. The corresponding compartment model for describing its dynamics is given by
Figure 3.
Scheme for dynamics of T-helper cell populations represented.
| (9) |
| (10) |
| (11) |
| (12) |
| (13) |
| (14) |
The model includes a resting T-cell population that represents both effector memory T-cells that occupy the LP and central memory T-cells that occupy the GLN. This population is compartmentalized into non-infected cells (Tm, (Equation (9)) and latently infected memory T-cells ( , Equation (10)). All resting T-cells are non-infected at the time of viral introduction.
Memory T-cells may be infected directly by virus at a rate of ω1 = kαv(vE + vI), entering the population. The coefficient αv represents the reduced likelihood of resting T-cell infection due to lower expression of the the CD4 surface receptor compared to active T-cells, vE is the amount of free virus in the LP (Equation (7)), and vI represents the amount of free virus in the GLN given by
| (15) |
Infected cells of the population may also be created from de-activated infected T-helper cells (discussed below), represented by the term νTμT TE* in Equation (10). The model assumes infected, presenting DC does not pass virus to resting T-cells upon transient association in the absence of T-cell receptor (TcR) stimulation.
Upon contact with antigen-presenting dendritic cells, a resting T-cell, infected or non-infected, is stimulated to an active Th1 in either the inductive site or the effector site. TI (Equation (11)) and TI* (Equation (12)) are the respective concentrations of non-infected and infected Th1 cells in the inductive site. TE (Equation (13)) and TE* (Equation (14)) are the concentrations of non-infected and infected Th1 in the effector site. As an example, this stimulation is represented in Equation (11) by the term 0.6ptkαT TmDI, where pt is the number of daughter cells produced from one central memory T-cell upon stimulation and subsequent proliferation, αT is the probability of stimulation representing the antigen specificity of the T-cell receptor, and 0.6 is the fraction of T-cells that are central memory T-cells and, therefore, in contact with dendritic cells of the inductive site, DI. This fraction is based on a study in mice that showed 60% of CD8+ memory T-cells created in response to lymphocytic choriomeningitis virus were CD62L+, a marker for central memory versus effector memory T-cells [41]. Such in vivo data was not found for CD4+ T-cells specifically. Similarly, the term 0.4pT kαT TmDE in Equation (13) represents T-cell stimulation in the effector site, where the number of daughter cells produced from one stimulated effector memory T-cell, pT, differs from that of central memory T-cells such that pT < pt [21]. Both pT and pt are functions of nTreg concentrations representing the fact that activated nTreg inhibit conventional T-cell proliferation [50], and they are given by
| (16) |
Here m is a positive constant used to determine the effect of nTreg on Th1 proliferation, pt0 and pT 0 denote the number of daughter cells produced by one proliferating T-cell (without inhibition) in the GLN and LP, respectively.
The term εt(DI + δDI*)DI in Equation (11) represents additional naive T-cells that may be recruited by factors secreted by presenting dendritic cells, where the parameter εt is the number of T-cells recruited by one active dendritic cell. The parameter δ represents the extent to which HIV infection impairs secretion of recruitment factors by dendritic cells as well as their ability to stimulate T-cells to inflammatory phenotype. These cells may then be stimulated with a probability of αt, generally less than the probability αT of memory T-cell stimulation [28]. The rates at which resting T-cells infiltrate the tissue from the blood is assumed to be independent of the current tissue concentration. De-activation of active T-cells occurs at a rate of μT. Upon de-activation a fraction, νT, of T-cells re-enter the memory T-cell population and the remainder are removed through apoptosis.
T-cells in either tissue site may enter the infected compartments, TE* or TI*, through the following pathways: i) Direct infection by virus. This occurs at a rate of ω2 = kve and ω3 = kvI, the rate of contact with free virus in the effector and inductive sites, respectively. As described, vE and vI are functional parameters representing the amount of free virus in the different tissue sites. ii) Stimulation of naive T-cells and cells of the Tm population by virus-harboring individuals of the DI* and DE* populations represented by the first and second terms of Equation (12) and the second term in Equation (14). iii) Re-stimulation of latently infected memory T-cells of the (Equation (10)) population represented by the fourth term in Equation (12) and Equation (14). As mentioned above, the coefficient δ is included in the stimulation terms as the study conducted in [22] specifically demonstrated that HIV infected dendritic cells isolated from chronically infected patients have a reduced ability to stimulate T-cells to an inflammatory phenotype in vitro. The model does not include passage of virus directly from a presenting, infected DC to an active Th1 or nTreg. Exclusion of this mode of infection assumes that a presenting DC would not associate with a T-cell that is already stimulated and in association with another DC.
2.3 CD4+ Natural T-regulatory Cells
The scheme for the dynamics of CD4+ nTreg cells is depicted in Figure 4. The corresponding compartment model for describing its dynamics is given by
Figure 4.
Scheme for dynamics of CD4+ nTreg cell populations represented.
| (17) |
| (18) |
| (19) |
| (20) |
| (21) |
| (22) |
The life cycle of non-infected and infected CD4+ nTreg is similar to that of T-helper cells, described above, where Rm represents the non-infected memory CD4+ nTreg population (Equation (17)), RI represents non-infected active CD4+ nTreg in the inductive site (Equation (19)), and RE represents non-infected active CD4+ nTreg in the effector site (Equation (21)). Memory CD4+ nTreg (Rm) are assumed equally distributed into effector memory cells and central memory cells in the LP and GLN respectively. This is reflected in the terms 0.5kαT RmDI in Equation (19) and 0.5kαT RmDE in Equation (21). The model assumes the same μT and νT for CD4+ nTreg as for T-helper cells. However, there is evidence that CD4+ nTreg may not undergo activation-induced cell death (apoptosis) as readily in cell culture [51]. Stimulation of each implied naive CD4+ nTreg results in pr daughter cells that may differ from that of conventional T-cells, pt, and memory CD4+ nTreg do not proliferate upon re-stimulation by DC.
The model seeks to explore the net effect when CD4+ nTreg stimulation is coupled with the inflammatory response to HIV presence. As such, the same number of CD4+ nTreg is recruited by virus-presenting DC as T-helper cells (εt) and resting CD4+ nTreg are stimulated with the same probability (αT). Though the mucosal site is believed to include a constant level of active CD4+ nTreg stimulated by self antigen, this baseline population is not included in the model. Including this constitutively active CD4+ nTreg population, independent of HIV presence, is assumed to have little impact on model predictions according to lack of sensitivity of model variables to initial values for RE(0) and RI (0).
Infected DC that are less able to stimulate T-helper cells to an inflammatory phenotype, discussed above, may still be able to efficiently stimulate resting CD4+ nTreg cells. This is due to the fact that impairment of DC activity includes lower levels of CD80 expression and secretion IL-10, all of which have been implicated in inducing Treg phenotypes in resting T-cells [50]. A value of 1 assigned to the parameter δr allows infected DC to retain the full capacity to stimulate CD4+ nTreg cells even when the ability to induce a Th1 phenotype is reduced, i.e., δ < 1.
3 Model Calibration and Analysis
The model contains 17 variables and 25 constant parameters. Equations (1)–(5), (9)–(14) and (17)–(22) were first written as a vector system
where , θ̄ is the vector for model parameters, and x̄0 is the set of initial conditions. We followed standard practice (e.g., see [4, 9]) and solutions were determined for a log-transformed system as this resolves the problem of states becoming unrealistically negative due to computer round-off errors. As values of the model parameters are in dramatically different scales, from 10−8 to 108, all of the parameter values are also transformed to the log scale.
Therefore, let xi = log10(x̄i) and x0i = log10(x̄0i), where i = 1, 2, 3, …, 17 and θi = log10(θ̄i) for i = 1, 2, 3, …, 25. Then we have
| (23) |
where f = (f1, f2, …, f17)T is given by
3.1 Simulation Setup
Simulations were carried out by solving the log transformed versions of Equations (1)–(5), (9)–(14) and (17)–(22) in MATLAB with ODE solver 23 (ode23tb) over a time course of 60 units, corresponding to two weeks where one time unit is 6 hours. Parameter values used are listed in the third column of Table 6 of Appendix. The values of these parameters were either derived from published experimental studies (a process that is described in more detail in the Appendix) or arbitrarily assigned if there is no studies related to them. For those parameters whose values can be found or derived from the literature, the reference numbers were given after their values, and the species from which in vivo these measurements were taken or cells were isolated for measurements in vitro were indicated after the reference numbers with “M” for “mouse”, “H” for “human”, and “NS” for “not specified”. Ideally, one would have parameter values based exclusively on in vivo, human data. However, as previously mentioned such data is sparse in the current literature. Therefore, though our primary interest is to model the dynamics of early HIV infection in humans, we found it necessary to use the values obtained from other mammalian species, and assume that humans and other mammalian species have the similar parameter values.
Simulations begin at the time of viral budding from the small first generation of infected host cells, approximately on day 3 post-infection, the time at which SIV DNA is detected in cells of infected rhesus macaque tissue [46, 24]. Initial cell concentrations are given in Table 5 and are chosen so that only resting immune cells are present at the time of infection assuming no significant immune activation at the time of infection in an otherwise healthy individual. The initial infected cell population is seeded in the LP and is evenly divided among conventional T-helper cells, dendritic cells, and CD4+ nTreg cells as the demographics of this first generation of hosts is not known. The initial infected cell concentrations are based on experimental measurements taken from rhesus macaques infected with SIV reported in [46] in which the authors quantified vRNA levels, a marker of free virus and productively infected cells, in vaginal mucosa and lymph nodes during the first days of infection. The infected cell population was weighted in the LP compartment based on the observations in the same study that during the first 3 days of infection this is the site where most productively infected cells were detected with only a very small number in the vaginal lymphoid tissues.
Table 5.
Initial conditions.
| Di | Immature dendritic cells | 108 cells/mL [26] | |
| DI | Non-infected active dendritic cells in the GLN | 10−10 cells/mL | |
| DI* | Infected active dendritic cells in the GLN | 1 cells/mL | |
| DE | Non-infected active dendritic cells in the LP | 10−10 cells/mL | |
| DE* | Infected active dendritic cells in the LP | 10 cells/mL | |
| Tm | Non-infected CD4+ memory T-cells | 108 cells/mL [23] | |
|
|
Latently infected CD4+ Memory T-cells | 10−10 cells/mL | |
| T I | Non-infected active CD4+ Th1 cells in the GLN | 10−10 cells/mL | |
| T I* | Infected active CD4+ Th1 cells in the GLN | 1 cells/mL | |
| T E | Non-infected active CD4+ Th1 cells in the LP | 10−10 cells/mL | |
| T E* | Infected active CD4+ Th1 cells in the LP | 10 cells/mL | |
| Rm | Non-infected memory CD4+ nTreg cells | 107 cells/mL [7] | |
|
|
Latently infected memory CD4+ nTreg cells | 10−10 cells/mL | |
| RI | Non-infected active CD4+ nTreg cells in the GLN | 10−10 cells/mL | |
| RI* | Infected active CD4+ nTreg cells in the GLN | 1 cells/mL | |
| RE | Non-infected active CD4+ nTreg cells in the LP | 10−10 cells/mL | |
| RE* | Infected active CD4+ nTreg cells in the LP | 10 cells/mL |
3.2 Sensitivity Analysis
In practice, one may often be in the situation of estimating a large number of unknown parameters with a limited set of data (which is true for our case). To mitigate some of this difficulty, sensitivity analysis has been widely used in inverse problem investigations (e.g., [8, 10, 11, 12, 13, 15] and the references therein) to identify the model parameters and/or initial conditions to which the model outputs (observations) are most sensitive and for which one can readily construct confidence intervals when they are estimated (i.e., which are the most reliably estimated values). Moreover, recently developed methods [8] for parameter subset selection have proven quite useful in systematically identifying the parameters or subsets of parameters that one can reasonably expect to estimate with a given data set. Some parts of this recently developed methodology [12, 13] also include new methods to assist in design of experiments in the context of investigations such as those initiated here.
To compute the sensitivities of model outputs to the model parameters and initial conditions, one need to know the sensitivity of each model state to each parameter and initial condition. Sensitivities of each variable xi to each parameter θj can be determined by (see, for example, [10] and the references therein)
| (24) |
Corresponding sensitivities of each variable, xi, to each initial condition, x0j (the jth component of x0) can be determined by
| (25) |
where , i = 1, 2, …, 17 and j = 1, 2, …, 17. These sensitivities were calculated by solving Equations (23), (24), and (25) simultaneously in MATLAB with ODE solver 15s using initial concentrations and parameter values listed in Table 5 and the third column of Table 6, respectively. Results of the sensitivity analysis informed which parameters were estimated as well as the confidence intervals for these estimated parameters are described in the section below.
Table 6.
Parameters used in the model. The third column is for the values of the parameters used in the simulations, and the fourth column is for the ranges of those parameters whose ranges can be found in the literature. For those parameters whose values can not be found in the literature an arbitrary value was assigned. While for those parameters whose values or ranges can be found in the literature, the reference numbers were given after the values or ranges, and the species from which in vivo these measurements were taken or cells were isolated for measurements in vitro were indicated after the reference numbers with “M” for “mouse”, “H” for “human”, and “NS” for “not specified”.
|
Birth/death
| |||
| μT | rate of active T-cell deactivation number of daughter cells from one proliferating | 0.05/6 hr [41, M] | |
| pt0 | T-cell in the GLN, uninhibited number of daughter cells from one proliferating | 512 [21, H] | |
| pT 0 | T-cell in the LP, uninhibited | 128 [21, H] | |
| μdi | death rate of immature dendritic cells | 0.0006/6hr [31, M] | |
| μd | death rate of dendritic cells | 0.12/6 hr [37, NS] | |
| d0 | baseline number of immature dendritic cells | 108 cells/m L [26, NS] | |
| pr | number of daughter cells from one proliferating naive CD4+ nTreg | 512 | |
| NE | number of free virus produced by one T-cell | 200 | 100 – 300 [25, 28, 19, H] |
| ND | number of free virus produced by one DC | 5 | |
| cN K | the maximum value of μN K | 75/6hr | |
| λ | the rate at which μN K rises to its maximum coefficient to determine effect of CD4+ nTreg | 10−8 mL/cells | |
| m | on Th1 proliferation | 10−10 mL/cells | |
|
Migration
| |||
| ε IE | rate of cell migration from GLN to LP | 0.125/6hr [28, NS] | |
| ε EI | rate of cell migration from LP to GLN | 0.25/6hr | |
| ε t | Number of naive T-cells recruited by one active DC | 100 | 100 – 300 [52, M] |
| cm | constant in em function | 1 cells/(mL · 6hr) | |
|
Contact/interactions
| |||
| α t | probability of naive T-cell stimulation by DC | 10−4 | 10−4 – 10−7 [28, NS] |
| α T | probability of memory T-cell stimulation | 10−4 | 10−5 – 10−3 [28, NS] |
| νT | fraction of T-cells that become memory T-cells | 0.02 | 0.02 – 0.1 [30, M] |
| δ | fraction of D* able to stimulate resting T-cells | 1 | |
| δr | fraction of D* able to stimulate resting nTreg | 1 | |
| α v | probability of memory T-cell infection by virus on contact | 0.1 | |
| α m | probability of viral infection of Di upon uptake of virion | 0.01 | 0.01 – 0.5 [40, H] |
| k | rate of contact between individuals | 10−7 mL/(cells · 6hr) | |
| kT | rate of contact between CD4+ nTreg and DC | 10−6 mL/(cells · 6hr) | (10−9 – 10−3 ) [47, M] |
3.3 Parameter Estimation
Proper calibration and validation of this model for the use of predicting HIV dynamics requires longitudinal data of infected and non-infected immune cells in vaginal and gut tissue during acute infection. As mentioned earlier such data was not found in the literature. Rather, we use aggregate vRNA levels obtained by euthanizing monkeys infected with SIV on different days post-infection [46]. The generally accepted biological model for acute viremia assumes a continual time course on which these averaged measurements lie and is described in Haase [24]. Following this biological model, we fit a general curve through three data points for vRNA concentration in the LP and GLN at days 3, 7, 15 and days 3, 10, 15 post-infection, respectively. These data points are estimated averages of multiple, divergent samples taken from different monkeys and are reported in Miller et al. [46]. The vRNA concentrations reported in [46] were in units of (μg vRNA)/(1μg total tissue RNA). To map these concentrations to model variables, we make the approximation that (1μg vRNA)/(1μg total tissue RNA) = (1 infected cell or virion)/(1μg tissue), and use the conversion that 1μg of tissue = 10−6mL assuming that 1g of tissue = 1mL [23].
The mathematical model was calibrated using two synthetic data sets composed of points on these curves, which conform to the trajectory proposed by Haase[24] and Miller et al. [46] for total amount of free virus and productively infected cells in the vaginal LP and GLN during the acute phase of infection. Synthetic data set 1 (depicted by ‘+’ in Figure 5) contains values of vRNA in the vaginal LP at various time points over a 2 week period, and synthetic data set 2 (depicted by diamonds in Figure 5) contains values of vRNA in the GLN over the same time period. As shown in Figure 5, the trajectory specifies an exponential growth from an initial value of approximately 104mL−1 on day 3 post-infection to a peak value of 109mL−1 on day 8 post-infection in the LP and a similar rise to 1010mL−1 in the GLN.
Figure 5. vRNA levels.
Solid and dashed lines indicate vRNA levels predicted by the mathematical model in the LP and GLN, respectively. The marker ‘+’ indicates synthetic data set 1, and diamonds represent synthetic data set 2. Circled points indicate those estimated by Haase et al. [24] directly from experimental data where vRNA values were averaged over widely varied samples reported in Miller et al [46].
Note that the synthetic data include the total amount of free virus and productively infected cells (i.e., total amount of vRNA) in the LP and the total amount of free virus and productively infected cells in the GLN. For system (1)–(5), (9)–(14) and (17)–(22), the total amount of free virus and productively infected cells in the LP is given by
| (26) |
and the total amount of free virus and productively infected cells in the GLN is
| (27) |
Specifically, the sum of the first two terms in (26) an (27) are used to account for the total amount of free virus in the LP and GLN, respectively, and the sum of the last three terms are the total amount of productively infected cells in the LP and GLN, respectively. Note that all the equations in the system (1)–(5), (9)–(14) and (17)–(22) are coupled together. Hence, the values of z̄1 and z̄2 depend on all the model parameters and initial conditions, either strongly or weakly.
Let zi = log10(z̄i), where i = 1, 2. Then the norm of sensitivities of each zi to each parameter, θj, and each initial condition x0j was calculated with
and
respectively, where i = 1, 2. Based on the values of ẑ1 and ẑ2, the 11 parameters to which z1 and z2 are most sensitive are k, NE, αT, cN K, pT 0, αm, δ, εIE, εEI, λ and kT (in the descending order), and these parameters will be estimated during the first iteration of parameter estimation process (see details below).
Let , i = 1, 2, …, n1 and , i = 1, 2, …, n2 denote the measurement time points for synthetic data sets 1 and 2, respectively, be the observed total amount of vRNA in LP at time point , i = 1, 2, …, n1, and be the observed total amount of vRNA in GLN at time point , i = 1, 2, …, n2. We define , i = 1, 2, …, n1, and , i = 1, 2, …, n2. The statistical model is assumed to have the form
| (28) |
where q ∈ ℝ denotes vector of the estimated parameters (κ is a positive integer, and denotes the number of estimated parameters), and the observation errors , i = 1, 2, …, n1 and , i = 1, 2, …, n2 are independent and identically distributed with zero mean and variance (log transforms are commonly used in the literature to provide nearly uniform variance, for example, they have been used in [4, 9] for other HIV models). With this assumption, q can be estimated by using an ordinary least squares (OLS) technique
| (29) |
where
 is some compact set in ℝk. Then the bias adjusted estimate for
(e.g., see [10]) is given by
is some compact set in ℝk. Then the bias adjusted estimate for
(e.g., see [10]) is given by
As expected, we have a large parameter set with little experimental data. Hence, to have initial estimates, the parameter estimation was implemented in an iterative process similar to that used in [4, 9] (this approach was also required for those modeling efforts even though substantially more longitudinal data was available to support those modeling efforts). We first estimate those 11 parameters deemed most influential (by sensitivity analysis) on z1 and z2 with all the other parameters remained with the values assigned in the third column of Table 6. We then fix 3 of these 11 parameters at their OLS estimates and re-estimate the remaining 8 parameters, and the OLS estimates for these 3 fixed parameters (δ, εIE and εEI) are illustrated in the first three rows of Table 2. Lastly, we fix 3 of these 8 parameters at their new OLS estimates and re-estimate the remaining 5 parameters, and the OLS estimates for these 3 newly fixed parameters (pT 0, αm and λ) and these 5 re-estimated ones (k, NE, αT, cN K and kT) are given respectively in the middle three rows of Table 2 and the last five rows of this table. The choice of the parameters to be fixed during the last two iterations is based on the combination of sensitivity analysis, the confidence we had in the estimated value as well as the knowledge we had on these parameters. The values for these 11 parameters illustrated in Table 2 will be used in the subsequent simulations where all the other parameters remain with the values assigned in the third column of Table 6.
Table 2.
The OLS estimates for those estimated parameters.
| Parameter | Symbol | OLS Estimate |
|---|---|---|
| Coefficient determining effect of infection on DC functions | δ | 0.74 |
| Rate of cell migration from GLN to the LP | εIE | 0.155/6hr |
| Rate of cell migration from LP to the GLN | εEI | 0.11/6hr |
|
| ||
| Number of daughter cells produced by one T-cell in the LP, uninhibited | pT 0 | 299 |
| Probability of infection of DC | αm | 0.04 |
| The rate at which μN K rises to its maximum | λ | 1.31 · 10−8 mL/cells |
|
| ||
| General contact rate | k | 1.08 · 10−8 mL/(cells · 6hr) |
| Number of free virus produced by one T-cell | NE | 300 |
| Probability of memory T-cell stimulated by DC | αT | 10−3 |
| The maximum value of μN K | cN K | 51.2/6hr |
| Contact rate between active DC and CD4+ nTreg | kT | 2.38 · 10−5 mL/(cells · 6hr) |
Even though the data we have are not from experiments, we provide a way (which can be employed once the experimental observations are available) to quantify the uncertainty in our parameter estimation. There are two methods that have been widely used in the literature to quantify uncertainty in parameter estimates, one is bootstrapping and the other is asymptotic theory. These two methods have been investigated and computationally compared in [14] for problems with different form and level of noise. It was found that asymptotic theory is always faster computationally than bootstrapping, and there is no clear advantage in using the bootstrapping and asymptotic theory when the constant variance data using OLS is assumed. Based on these findings, we will use asymptotic theory in this paper to quantify the uncertainty in parameter estimation.
Let
. Then the sensitivity matrix χ (q) is (n1 + n2) × κ matrix with its (i, j)th element being defined by
, i = 1, 2, …, n1 + n2 and j = 1, 2, …, κ, where
 i is the ith element of
i is the ith element of
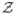 , and qj is the jth element of q. Then by the asymptotic theory (e.g., see [10, 49] and the references therein), we know that the OLS estimator Q̂ is asymptotically normally distributed
, and qj is the jth element of q. Then by the asymptotic theory (e.g., see [10, 49] and the references therein), we know that the OLS estimator Q̂ is asymptotically normally distributed
| (30) |
Here q0 denotes the true value of the estimated parameter vector q, and Σ0 is given by
Since the true value q0 and the variance are unknown, we follow the standard statistical practice by using the OLS estimates q̂ and ŝ2 for q0 and , respectively. Hence, the asymptotic properties of the least squares estimator Q̂ can be approximated by
| (31) |
where
Then the standard error Std(Q̂ j) for the jth element of Q̂ is calculated by , where Σjj is the (j, j)th entry of covariance matrix Σ̂. Hence, the endpoints of the confidence intervals for Q̂j (the jth element of Q̂), j = 1, 2, …, κ, are given by
where t1−α/2 is a distribution value that is determined from a statistical table for Student’s t-distribution based on the level of significance α.
For the results we report in the following, we choose α = 0.05, which corresponds to t1−α/2 = 1.96 when the number of degrees of freedom is greater than or equal to 30 (e.g., see [11] and the references therein), which is true for our case (n1 = 31, n2 = 24 and κ = 5). Table 3 illustrates the endpoints of the 95% confidence intervals (i.e., α = 0.05) for those 5 estimated parameters illustrated in last five rows of Table 2 (i.e., those 5 parameters that are estimated during the last iteration of parameter estimation process). From this table, we see that the confidence intervals for all these estimated parameters are reasonably small in comparison to their corresponding estimated values.
Table 3.
The endpoints of the 95% confidence intervals for q.
| Parameters | q̂ j ± 1.96 Std(Q̂j) | Parameters | q̂j ± 1.96 Std(Q̂j) |
|---|---|---|---|
| log10(NE) | 2.477 ± 0.078 | log10(cN K) | 1.709 ± 0.025 |
| log10(αT) | −3.000 ± 0.069 | log10(k) | −7.966 ± 0.027 |
| log10(kT) | −4.623 ± 0.455 |
Figure 5 illustrates the fits to the data. From this figure, we see that we have pretty good fits to the data. Hence, by this figure and Table 3 we may infer that the goodness-to-fit is reasonably well.
3.4 Partial Qualitative Validation
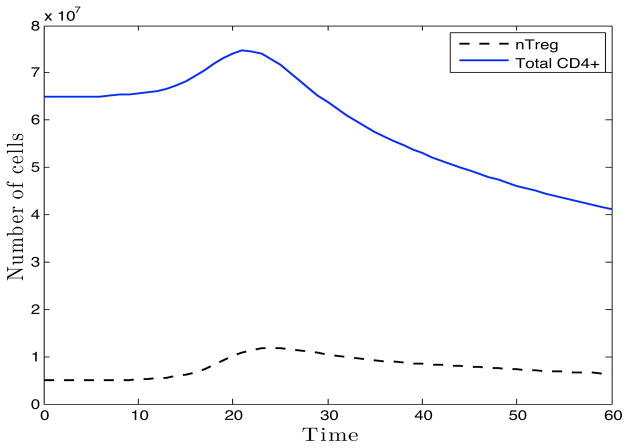
As we have already mentioned, sufficient experimental data is not available to fully validate our proposed model. Nonetheless, we make a first attempt to partially support and qualitatively validate this initial modeling effort with synthetic data produced from limited experimental data in the literature. The model was fit to log vRNA levels. The partial qualitative validation is carried out by comparing the underlying cell dynamics. Specifically, those for T-cells in the lymphoid tissue (Figure 6) were compared to those observed in SIV models of acute infection. In agreement with Li et al., [38] and Estes et al., [20], the model predicts an initial influx of CD4+ T-cells, both helper and regulatory, to the site of infection. This influx is followed by a decrease in the total CD4+ T-cell population of the lymph node as observed by Karlsson et al., [32]. This study reported an average 20% reduction in total CD4+ T-cells in non-specific lymphoid tissue by day 14 and 50% on day 28 post-infection. Our model predicts a reduction of 22% by day 14 post-infection that continues to a 46% reduction in lymphoid T-cells by day 21 post-infection, the last time point of the simulation. In addition, non-human primate infection referenced in Hogue et al. [26] measured 1.7 · 106mL−1 infected cells in the lymph node on day 6 post-infection. Our model predicts 8.64 · 105mL−1 productively infected CD4+ T-cells in the GLN on this day.
Figure 6.
Simulated timecourse of total CD4+ T-cells (solid line) and CD4+ nTreg (dashed line) in lymphoid tissue following infection of first host generation.
To further test the validity of our model we observed the effect of CD4+ nTreg depletion, represented in the model as reduced resting CD4+ nTreg at the time of infection (Rm(0)), on the course of viral proliferation. Though it has been observed that Treg cells from hosts infected with HIV and FIV (feline immunodeficiency virus) suppress antiviral responses during the chronic stage of infection [1, 33, 45], a recent study in FIV shows that Treg cell depletion of approximately 78% prior to infection does not significantly impact viral load or CD4+ T cell levels in tissues [44]. When simulations were carried out with reduced Rm(0) values from 107mL −1 to 105mL −1 and then to 10 −10mL−1, there was no change in the time of viral peak in either tissue site and < 8% increase in peak vRNA values in the GLN and ≤ 13.5% increase in peak vRNA value in the LP, in accordance with these FIV results.
4 Simulations and Results
For each experimental condition described in the following sections, four measurements were made to determine the rate and extent of viral proliferation: i) The maximum vRNA levels z̄1max in the LP, that is, , where z̄1 is given by Equation (26); ii) the maximum vRNA levels z̄2max in the GLN, given by with z̄2 defined by Equation (27); iii) the time tpeakLP at which the vRNA level peaks in the LP, that is, ; and iv) the time tpeakGLN at which estimated vRNA peaks in the GLN, that is, . The latter two measurements can be interpreted as the estimated “window period” between the start of simulation and the time at which peak viremia is reached in each tissue.
As we noted earlier that all the equations in the system (1)–(5), (9)–(14) and (17)–(22) are coupled together. Hence, the values of the peak vRNA levels in LP and GLN as well as the corresponding time at which their levels peak depend on all the model parameters and initial conditions, either strongly or weakly. In this section, we will compare these four values for the null model with those representing different immunological backgrounds and interventions of interest. These include the effect of early inflammatory versus regulatory response on initial viremia, the role of CD4+ nTreg on initial viral spread, as well as the impact of inflammatory mechanisms.
4.1 Effect of Early Inflammatory vs. Regulatory Response on Initial Viremia
To assess the net effect of a enhanced inflammatory or regulatory immune response, in terms of expediency and strength, to HIV production we observed the correlation between viral spread and initial concentration of active immune cells in the LP; DE(0), T E(0), and RE(0). A set of initial concentration values for each variable were sampled from 10−10 to 1010, where 10−10 is the value in the null model.
Results
In Table 4 we report estimated peak vRNA levels and their corresponding time points for the null model and in the cases of elevated initial concentration of each active cell population to 106mL−1, approximately the expected concentration under normal inflammatory conditions [18]. It can be seen that none of these increases of the active immune cell populations has a significant impact on peak vRNA levels in either tissue with ≤ 0.1% difference even though the initial concentration is raised by over 6 orders of magnitude. However, all have a significant impact on tpeakLP and tpeakGLN, representing the “window period”. Specifically we show in Figure 7 that the initial level of all active immune cell populations is negatively correlated with tpeakLP and tpeakGLN indicating that as their concentrations increase, virus is able to spread more rapidly. This effect is particularly pronounced for the T-cell populations, both inflammatory and regulatory, and coincides with earlier markers of the inflammatory response. Specifically, values for the functional parameter μN K and εm, reach maximum values sooner and begin to decrease sooner (see Table 4 in [57] for more details).
Table 4.
Effect of immunological background on initial viremia.
Figure 7. Effect of enhanced immune response on the rate of viral spread.
Here the predicted values of tpeakLP (A) and tpeakGLN (B) are plotted against initial values of active immune cell concentrations in the LP.
Conclusions
These results have multiple implications.
An increased initial concentration of T E can represent a recent immune response from which residual Th1 cells are still present or a very rapid inflammatory response that allows entrance of Th1 prior to viral replication in the initial host population; a possible consequence of vaccine exposure.
In 2005 the notorious STEP vaccine trial was carried out, in which high-risk participants were administered HIV protein within an adenovirus vector. This trial failed returning results indicating that vaccinated individuals were more likely to be infected with HIV [55]. The prevailing hypothesis for this surprising result was that the immune response elicited, by either the adenovirus vector or the HIV antigen itself, created an inflammatory environment predisposing individuals to infection upon exposure of viable HIV. The effect of enhanced T E(0) in our simulations supports this hypothesis. A vaccine-elicited inflammatory response would facilitate viral spread predisposing the host to infection if exposure occurs while it is still active. This is a probable scenario for individuals in high-risk positions such as those who received the vaccine during the trial.
The results also indicate that inducing the CD4+ nTreg response at the time of infection will likely expedite infection. The decrease in tpeakGLN and tpeakLP seen with elevated RE(0) indicates that, indeed, active CD4+ nTreg has a net effect of aiding viral proliferation and dissemination, not necessarily by increasing viral production, but by expediting it. Furthermore, that this increase is not due to nTreg-mediated dampening of NK activation, but rather its ability to replicate virus.
The indication is that a more rapid CD4+ nTreg response would likely not be protective against viral proliferation. This is relevant as CD4+ nTreg has been shown to promote and inhibit a successful antiviral response in different species. Specifically, SIV pathogenesis is greatly reduced in African green monkeys compared to that in rhesus macaques. It has been suggested that this may be due to the fact that the CD4+ nTreg response in African green monkeys is quicker, leading to reduced susceptible T-cell recruitment and stimulation by DC in the infection site [23, 36]. In our simulations, increasing the initial concentration of RE(0) represents an immediate CD4+ nTreg response elicited prior to viral production by the first generation of infected cells. These simulation results suggest this is not a plausible explanation for reduced viremia in green monkeys as an earlier CD4+ nTreg response has little effect on dampening the extent of viral proliferation. Rather, the sooner active CD4+ nTreg is present, even prior to the inflammatory response, the quicker virus will propagate.
4.2 The Role of CD4+ nTreg Functions in Initial Viral Spread
It was seen that active CD4+ nTreg has the effect of increasing the rate of viral dissemination. We then sought to identify the net effect of specific CD4+ nTreg functions: contact-dependent deactivation of presenting dendritic cells, governed by the parameter kT, and inhibition of Th1 proliferation, governed by the parameter m. This was done by plotting peak vRNA levels, z̄1max and z̄2max (Figure 8(A) and (B)) as well as their corresponding time points tpeakLP and tpeakGLN (Figure 8(C) and (D)) by increasing values for these parameters. Specifically, values for m, the coefficient for functional parameters pt and pT (defined in Equation (16)), that determines the extent to which CD4+ nTreg inhibits T-helper cell proliferation, were sampled from the range [10−10, 10−1]. Values for kT, the rate of contact and de-activation of DC by CD4+ nTreg were taken from the range [2.3821 · 10−10, 2.3821 · 10−1].
Figure 8. Effect of CD4+ nTreg regulatory functions on viral spread.
Here the four measurements of viral spread are plotted by increasing values of the parameter m (circles) and kT (+ sign); (A) peak level of vRNA in the LP, z̄1max, (B) peak level of vRNA reached in the GLN, z̄2max, (C) time of peak vRNA level reached in the LP, tpeakLP, (D) time of peak vRNA level reached in the GLN, tpeakGLN. Circled points represent parameter values in the null model.
Results
Plots in Figure 8 clearly show a negative correlation between both functions and peak vRNA levels indicating that enhancement of either has a net effect of reducing viral proliferation, with kT having a tighter correlation. In relation to the “window period”, the negative effect of kT is also clearly demonstrated. In Figure 8(C) and (D) it can be seen that as kT increases from its default value, the values of tpeakLP and tpeakGLN increases indicating that CD4+ nTreg-mediated DC de-activation has a net effect of prolonging the window period. Though these values are also higher than that in the null model when kT is severely reduced, this corresponds with very high viral load that likely continues to rise uninhibited. The relationship between the window period and m appears more complex. Like kT, as m increases from the default value the window period is increased. However, there is a range for m, 10−3–10−2, in which the beneficial effect is much less significant, even non-existent in the LP. This highlights the complex, non-linear relationship between m and the response of the over all system. For example, RE is, itself, an indirect function of m as an increased m leads to decreased TE and TE*, which affects the functional parameters μ N K and em that directly influence the levels of RE* and RE.
We next sought to determine whether such enhancement of CD4+ nTreg functions could change its relationship to increased viral proliferation, i.e., whether enhancement of these specific functions could render the net effect of CD4+ nTreg activation to be protective. Specifically, we sought a value for these parameters at which one could clearly observe a negative correlation between peak vRNA levels, z̄1max and z̄2max, and the initial active CD4+ nTreg concentration, RE(0), as well as a positive correlation with the window periods, tpeakLP and tpeakGLN. To this end, we observed the four measurements for viral proliferation against increasing values for initial active nTeg concentration, sampled from the range [10−10, 1010] with different values for m and kT.
Results
For all values assigned to m, from the range [10−10, 10−1], the estimated peak vRNA levels remained positively correlated with initial active CD4+ nTreg concentration, if affected at all, and the estimated window periods remained negatively correlated (as observed from Figure 9 in [57]). In the case of enhanced ability to deactivate DC, the effect is largely just as insignificant. For most values of kT the relationship between active CD4+ nTreg and vRNA levels remains positive and that with the window period remains negative. However, it can be see in Figure 9 that for higher values of kT, there is a specific range of initial CD4+ nTreg concentrations (about 102 –104mL−1) that inhibit the propagation of the first generation of free virus produced by the initial host population. In other words, viremia does rise above the concentration of approximately 104mL−1. This effect on viremia in the GLN, specifically, is observed over a wider range of CD4+ nTreg concentrations than those in the LP.
Figure 9. Relationship between initial active CD4+ nTreg concentration and viral proliferation given enhanced DC-deactivation capacity.
Here the following factors are plotted by increasing values of RE(0) given different values of kT as indicated in the legend; (A) peak level of vRNA in the LP, z̄1max, (B) peak level of vRNA reached in the GLN, z̄2max, (C) time of peak vRNA level reached in the LP, tpeakLP, (D) time of peak vRNA level reached in the GLN, tpeakGLN. The thicker dotted line represents the value of kT used in the null model. Solid lines indicate kT values for which initial viremia is successfully controlled and does not rise significantly above concentration of the first generation of budded virus.
Conclusions
CD4+ nTreg-mediated inhibition of DC is a useful aspect of the regulatory response that one wants to maintain offering the most promising avenue for intervention. Furthermore, with an enhanced capacity to de-activate DC, there is a range of CD4+ nTreg activation that could be protective at the time of infection.
However, manipulation of CD4+ nTreg-mediated inhibition of Th1 proliferation could have unpredictable, unintended consequences. Hence, this function is not a strong candidate for exploitation in reducing viral spread as simulations indicate that enhancing the capacity to inhibit Th1 proliferation, does not change the net effect of CD4+ nTreg activation as it continues to expedite viral proliferation due to its ability to replicate virus.
With these findings we recommend conducting carefully designed experiments to determine potential beneficial effect of promoting CD4+ nTreg-mediated DC inhibition in an in vivo setting of acute infection. In a similar vein, it would be interesting to experimentally observe the net effect of enhancing CD4+ nTreg-mediated inhibition of Th1 proliferation given the anti-intuitive dynamics reported here.
4.3 Impact of Inflammatory Mechanisms
The model includes an inflammatory response to viral production that is comprised of four main mechanisms: i) stimulation of both resting conventional T-cells and CD4+ nTreg at the rate of αT, ii) recruitment of both resting conventional T-cells and CD4+ nTreg to the inductive site at the rate εt, iii) the recruitment of monocytes, immature dendritic cell precursors, at the rate εm (Equation (6)), mediated by the parameter cm, and iv) removal of infected cells by NK cells at the rate μN K (Equation (8)), mediated by the parameters cN K, the maximum value of the removal rate, and λ, the rate of increased removal as DC and Th1 concentrations rise. In order to clarify those mechanisms that most influenced the net positive impact of the inflammatory response on HIV spread we sought to correlate the peak vRNA levels with each the parameters governing these four mechanisms. To do this we plotted estimated peak vRNA levels in the LP (z̄1max) and in the GLN (z̄2max) by values for each parameter. Values for εt were sampled from the range [10−10, 1000], those for cm from the range [10−10, 1000], those for αT from the range [10−10, 1], those cN K from the range [5.12 · 10−10, 5.12 · 103], and those for λ from the range [10−10, 10−1].
Results
Plots in Figure 10 show that monocyte recruitment has little effect on viremic levels, with T-cell recruitment having a slightly anti-intuitive, negative effect on viral proliferation. NK activity and Th1 stimulation are the processes that play the most critical role in determining the rate of viral proliferation. Specifically, enhanced NK activity is intuitively beneficial. DC-mediated stimulation of regulatory and inflammatory T-cells, however, has a significant impact in aiding viral proliferation.
Figure 10. Relationship between inflammatory mechanisms and acute viral spread.
Here the following factors are plotted by increasing values of cm, εt, αT, cN K, and λ. (A) peak level of vRNA in the LP, z̄1max, (B) peak level of vRNA reached in the GLN, z̄2max.
Conclusion
The positive correlation between the inflammatory response (shown in section 4.1) and expedited viremia is largely attributed to DC-mediated stimulation of T-cells, both Th1 and CD4+ nTreg. However, NK cells can be a very effective fighter against viral spread, which is significant as their activity may be elicited prior to the CD8 response through DC activation and potentially independent of CD4+ Th1 activation.
These results suggest that one would want to be specific in choosing the inflammatory function to be targeted for enhancement as an intervention strategy. T-cell recruitment by DCs may be slightly protective, but if this is coupled with Th1 stimulation, the impact can be quite negative for the host. In terms of determining inflammatory mechanisms to inhibit in order to quell viral spread, targeting DC-mediated recruitment of T-cells is not recommended as it appears to be a necessary component of the antiviral response. A better strategy would be targeted disruption of DC contact with and/or stimulation of T-cells (helper and regulatory). Indeed, simulations with reduced αT to 10−10 leads to much lower viremia with no noticeable T-cell loss (results not shown).
5 Discussion
Here we present a model of the HIV-specific inflammatory pathway leading to Th1 activation and the nTreg-mediated regulatory pathway that suppresses it. Simulations based on this model and its component mechanisms suggest that a global activation of either the adaptive regulatory or inflammatory response would expedite viral dissemination, overcoming the innate antiviral response, as both lead to activation of HIV-permissive Th1 and nTreg. This result holds even over a wide range of conditions that would enhance or weaken the influence of nTreg on DC-deactivation and Th1 proliferation. Analysis of the most effective regulatory functions and harmful inflammatory mechanisms specify DC-mediated stimulation of CD4+ T-cells as the key mechanism driving viral proliferation. The indication is that once DC carries HIV to the lymph node to present to T-cells, the infection has little chance of being stopped as the virus is now in an environment with a plentitude of highly permissive hosts, has access to the blood stream, and is largely protected from NK mediated removal. Hence, any vaccine-primed responses that require this presentation upon viral challenge will occur too late and be ineffective, if not harmful. This includes a typical CD8 response which relies on the activation and proliferation of CD4+ T-cells for cytotoxic T-lymphocyte (CTL) activation. Indeed such obstacles are what have made finding an effective and protective vaccine so difficult.
This work suggests that such strategies will continually fail to control systemic spread and viremia. Rather, there should be a focus on responses that may lead to viral removal prior to interaction between a presenting infected DC and resting T-cell takes place. This may include neutralizing antibody that could, theoretically, eliminate virus prior to contact with DC by preventing transcytosis from vaginal lumen to LP or antibody-dependent cell cytotoxicity that would enhance the ability of NK cells to eliminate the initial host cell population at site of infection prior to spread.
Ideal intervention strategies would be those that enhance the innate response without a need for T-cell expansion. As an example, this could involve NK activation through selective recruitment of plasmacytoid DCs (pDCs) from the blood to the infection site as this DC subset does not play a significant role in T-cell expansion, but is effective in inducing NK response through secretion of IFN-α [5]. Though pDC has been associated with immune dysregulation, this association is seen with established viral infection in the chronic disease phase. Other strategies to enhance the effective NK response is to target viral proteins that inhibit innate immune response such as nef, which leads to downregulation of NKG2D ligands on the surface of infected cells [5].
In terms of prevention treatments that seek to exploit the innate immune response, our conclusions recommend those that could disrupt DC: T-cell interactions upon HIV entry, allowing NK activity to occur through DC stimulation alone. Such strategies may involve treatment with tolerogenic commensal bacterial strains in the vaginal lumen, inhibition of antigen presentation by DC, and targeted inhibition of DC-TcR interactions.
Knowledge of the mechanisms involved in this model is evolving. Specifically, those governing DC-mediated delivery of virus, HIV-specific NK activation, and HIV-specific activation and suppressive functions of nTreg continue to be a source of controversy in the experimental HIV immunology field.
As stated in Section 2.2, it may be possible for infected DC to deliver viable virus to resting T-cells upon transient association in the absence of TcR recognition and subsequent stimulation. By not including this mechanism of contact-dependent infection of resting T-cells our model may underestimate the number of latently infected memory T-cells that may be established by a single infected DC in the early stage of infection. The possible error in size of this reservoir population presumably has little impact on the dynamics of early infection and the initial viremic spike, but will be relevant in future models that seek to encompass the chronic disease stage following the acute phase.
This model assumes that HIV-specific nTreg suppressor functions are dependent upon TcR stimulation indicated by in vitro observations [50] and focus on the theoretical consequences of HIV-solicited regulatory response coupled to the inflammatory response. The true mucosal system likely includes HIV-nonspecific nTreg activation pathways that may be relevant. As mentioned, the mucosal site is believed to include a constant level of active CD4+ nTreg stimulated by self antigen and commensal bacteria. This constitutively active CD4+ nTreg population could be represented as a constant in-flow into the RE population. In addition, there may be a polyclonal nTreg population present that can be activated by TGF-β secreted from neighboring nTreg [50]. This could be represented by an influx into the RE population proportional to the current RE population, enhancing the effect of nTreg cell concentration on viral proliferation and NK suppression. Hence, we presume that exclusion of these mechanisms leads to simulation results that underestimate the contribution of nTreg presence to viral proliferation. The significance of such HIV-independent, polyclonal populations and circumstances governing their activation in vivo is largely unknown and may be incorporated into later versions of the model as more information is gathered.
Studies have indicated nTreg may have a direct cytolytic effect on NK cells. This mode of suppression was not included as it was demonstrated in a minority of nTreg in specific in vivo conditions of a tumor microenvironment and has not been reproducible in other situations [50, 17]. Including this mechanism would likely enhance the positive effect of nTreg activation on viral dissemination and, therefore, is not presumed to change the net effect of the regulatory response reported here. Specifically, it ultimately promotes viral proliferation.
The assumptions made here regarding mechanisms represented and excluded in the model are a subset of those currently in the literature making this model a foundation for studying various hypothesized details of each mechanism in future versions.
Given the lack of data in the current literature, this modeling effort constitutes a preliminary exploration into the role of the regulatory pathway and how it may be exploited to prevent HIV proliferation. The current model is capable of addressing conceptual questions relevant to devising intervention to quell HIV spread such as those addressed here: Should one promote the regulatory response?, Should one inhibit the regulatory response? and What specific mechanisms of inflammation would be best to suppress in a targeted manner?
However, the model is not adequate for making quantitative predictions as this requires calibration to specific experimental data. Future work involves using this preliminary model to suggest specific experiments with which such data can be collected. A model calibrated to adequate data could then be used to make quantitative predictions of viral load and timing of systemic spread within a satisfactory level of confidence.
Highlights.
Mathematical model of inflammatory and regulatory responses to initial HIV invasion.
We address net effect of nTreg activation and its specific functions on HIV spread.
We identify mechanisms of the natural inflammatory response to inhibit viral spread.
A global activation of inflammatory response expedites viral dissemination.
A global activation of adaptive regulatory response expedites viral dissemination.
Acknowledgments
This research was supported in part by Grant Number R01A I071915-07 from the National Institute of Allergy and Infectious Diseases and in part by grants NSF HSD Grant SES-0729441, NIH MIDAS project 2U01GM070694-7, NSF PetaApps Grant OCI-0904844, DTRA CNIMS Grant HDTRA1-07-C-0113, NIH MIEP HHSN272201000056C, NIH Grant 1R56AI089313-01A1, NIH/NIAID 5R01AI47707 and grant UNC CFAR P30 AI50410. In addition, the authors are grateful to the two anonymous referees for their helpful comments and constructive suggestions which led to an improved version of the paper.
Appendix: Initial Conditions, Parameters and Rate Functions
Initial variable concentrations are given in Table 5. In general, subscripts denote phenotype. The alphanumeric superscript denotes location and the superscript ‘*’ indicates infectious counterpart.
Parameters required by the model are listed in Table 6 and fall roughly into three categories: i) birth/death processes, ii) migration, and iii) contact/interaction processes. Units for rates are per 6 hours as this is approximately the length of time a T-cell takes to proliferate in association with the stimulating DC [52]. When possible, constant values were assigned according to published experimental data. For example, pt0, the uninhibited rate of T-cell proliferation in the GLN, is based on an in vitro study of CD8+ T-cells and their capacity to proliferate in response to TCR stimulation [21]. This study reported that a stimulated naive CD8+ T-cell or central memory T-cell undergoes a maximum of 9 doublings. Hence 1 T-cell will end-up giving birth to 29 = 512 T-cells. The parameter pT 0, the uninhibited rate of T-cell proliferation in the LP comes from observations in the same study that, upon stimulation, effector memory T-cells proliferate to a lower extent than do central memory T-cells resulting in a concentration ratio of roughly 1:4. Proliferation of naive nTreg may be reduced compared to T-helper cells as the express lower levels of IL2R [56]. For this reason, pr was included as a separate parameter assigned the range 128–512. By default, we assumed that naive nTreg proliferate to the same extent as naive T-helper cells.
The range for εt was derived based on the observation of Stoll et al., [52] that there are 3.35 times more naive T-cells in the lymph nodes with presenting dendritic cells compared to lymph nodes where they are absent. As the concentration of naive T-cells in lymphoid tissue in non-inflammatory conditions is on the order of 108 [23] this would require an increase of approximately 2 · 108 naive T-cells under inflammatory conditions for a total of approximately 3 · 108. Given that the expected number of activated dendritic cells under inflammatory conditions is approximately 106 in the LN [18], one dendritic cell is estimated to recruit approximately 2 · 102 T-cells, giving εt a range of 102 to 3 · 102.
The parameter d0 represents the number of immature DC in the naive system. The value assigned was borrowed from a previously published model [26], though an experimental measurement was not cited.
For transition rates such as death, de-activation, and migration rates: μT, μdi, μd, εIE, and εEI, measurements from the literature were in the form of an average lifespan or dwell time for an individual cell type in a particular location. In order to avoid introducing the complication of delay-differential equations, we used the common strategy of modeling cell death and movement as affecting a fixed proportion of cells in each time interval, resulting in an exponential distribution of lifetimes with the experimentally observed mean, x, or a half-life of x ln 2.
For other parameters such direct data was not found and a range was estimated based on less direct biological measurements and knowledge. In the case of αm, it is not known with what frequency viable virions are able to escape the intra-cellular phagosomal degradation and enter the cytoplasm to effectively infect the dendritic cell. Therefore, we assigned a range based on the ratio of viral protein p24 in the cytoplasm to that in the phagosome after uptake by macrophages [40]. However, p24 is a marker of the virion capsule, not necessarily an intact, viable virion. In the case of αv, the probability that a resting memory T-cell in infected upon contact with virus, a value was assigned based on the knowledge that the viral receptor protein CD4 is upregulated only in active T-cells and present at lower levels on the surface of resting cells [28]. However, a quantitative comparison of CD4 density between resting and active T-cells was not found in the literature.
The parameter NE represents the amount of viable virus budded from T-cells. Viral burst size in T-cells is approximately 1100 virions/cell per day in vitro [28]. However, only 10% of virus produced is estimated to be viable in vivo [19]. Hence, the amount of viable virus produced by a single T-cell is estimated to be on the order to 102 per day. ND represents the amount of virus budded from each dendritic cell. This is known to be less than that of T-cells and the value is assigned based on data that the burst size in macrophages, cells of the same lineage as dendritic cells, is roughly 0.1 that of the burst size in T-cells [27].
In the case where the quantitative value of a parameter does not have a direct correlation with experimental data, as in the cases of cN K, λ, m, k, kT, and cm, a value was arbitrarily chosen.
The parameter dependent rate functions are summarized in Table 7.
Table 7.
Parameter dependent rate functions.
|
Birth/death
|
| Death rate of infected cells by NK: μN K = cN K(1− e−λ (DE+δDE*+TE+TE*)) |
| Daughter cells produced from one proliferating T-cell in the GLN, inhibited: |
| Daughter cells produced from one proliferating T-cell in the LP, inhibited: |
|
Migration
|
| Recruitment rate of Di by inflammatory factors: |
|
Contact/interactions
|
| Rate of infection of memory T-cells: ω1 = kαv (vE + vI) |
| Rate of contact with virus in the LP: ω2 = kvE |
| Rate of contact with virus in the GLN: ω3 = kvI |
| The concentration of free virus in the LP: vE = NE (TE*+ RE*) + ND DE* |
| The concentration of free virus in the GLN: vI = NE (TI* + RI*) + ND DI* |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aandahl EM, Michaelsson J, Moretto WJ, Hecht FM, Nixon DF. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J Virol. 2004;78(5):2454–9. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abel K, Rocke DM, Chohan B, Fritts L, Miller CJ. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J Virol. 2005;79(19):12164–72. doi: 10.1128/JVI.79.19.12164-12172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abu-Raddad LJ, Patnaik P, Kublin JG. Dual infection with HIV and Malaria fuels the spread of both diseases in sub-Saharan Africa. Science. 2006;314:1603–1606. doi: 10.1126/science.1132338. [DOI] [PubMed] [Google Scholar]
- 4.Adams BM, Banks HT, Davidian M, Rosenberg ES. Model fitting and prediction with HIV treatment interruption data. Bulletin of Mathematical Biology. 2005;69:563–584. doi: 10.1007/s11538-006-9140-6. [DOI] [PubMed] [Google Scholar]
- 5.Altfeld M, Fadda L, Frleta D, Bhardwaj N. Dcs and nk cells: critical effectors in the immune response to hiv-1. Nature Reviews Immunology. 2011;11(3):176–186. doi: 10.1038/nri2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andoniou CE, Andrews DM, Degli-Esposti MA. Natural killer cells in viral infection: more than just killers. Immunol Rev. 2006;214:239–50. doi: 10.1111/j.1600-065X.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 7.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. Journal of Experimental Medicine. 1996;184(2):387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banks HT, Cintron-Arias A, Kappel F. Parameter selection methods in inverse problem formulation. Proceedings Biomedical Modeling and Cardiovascular-Respiratory Control: Theory and Practice Summer School and Workshop: A Marie Curie Training Event, Springer Lecture Notes, 2011, to appear. Center for Research in Scientific Computation Report, CRSC-TR10-03; November, 2010; Raleigh: NCSU; [Google Scholar]
- 9.Banks HT, Davidian M, Hu Shuhua, Kepler GM, Rosenberg ES. Modelling HIV immune response and validation with clinical data. J Biological Dynamics. 2008;2:357–385. doi: 10.1080/17513750701813184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banks HT, Davidian M, Samuels JR, Jr, Sutton KL. An inverse problem statistical methodology summary. In: Chowell Gerardo, Hyman Mac, Hengartner Nick, Bettencourt Luis MA, Castillo-Chavez Carlos., editors. Statistical Estimation Approaches in Epidemiology; Center for Research in Scientific Computation Report, CRSC-TR08-01; January, 2008; Berlin Heidelberg New York: Raleigh: Springer; NCSU; 2009. pp. 249–302. [Google Scholar]
- 11.Banks HT, Davis JL. Quantifying uncertainty in the estimation of probability distributions. Math Biosci Eng; Center for Research in Scientific Computation Report, CRSC-TR07-21; December, 2007; Raleigh: NCSU; 2008. pp. 647–667. [DOI] [PubMed] [Google Scholar]
- 12.Banks HT, Dediu Sava, Ernstberger Stacey L, Kappel Franz. Generalized sensitivities and optimal experimental design. J Inverse and Ill-posed Problems. 2010;18:25–83. Center for Research in Scientific Computation Report, CRSC-TR08-12, (Revised) November, 2009, NCSU, Raleigh. [Google Scholar]
- 13.Banks HT, Holm KJ, Kappel F. Comparison of optimal design methods in inverse problems. Inverse Problems; Center for Research in Scientific Computation Report, CRSC-TR10-11; July, 2010; Raleigh: NCSU; 2011. p. 075002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banks HT, Holm KJ, Robbins D. Standard error computations for uncertainty quantification in inverse problems: asymptotic theory vs. bootstrapping. Mathematical and Computer Modelling. 2009;52:1610–1625. doi: 10.1016/j.mcm.2010.06.026. Center for Research in Scientific Computation Report, CRSC-TR09-13, Revised, May, 2010, NCSU, Raleigh. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banks HT, Tran HT. Mathematical and Experimental Modeling of Physical and Biological Processes. CRC Press; Boca Raton, FL: 2009. [Google Scholar]
- 16.De Boer RJ, Perelson AS. Target cell limited and immune control models of HIV infection: a comparison. J Theor Biol. 1998;190:201–214. doi: 10.1006/jtbi.1997.0548. [DOI] [PubMed] [Google Scholar]
- 17.Cao XF, Cai SF, Fehniger TA, Song JL, Collins LI, Piwnica-Worms DR, Ley TJ. Granzyme b and perforin are important for regulatory t cell-mediated suppression of tumor clearance. Immunity. 2007;27(4):635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Catron DM, Itano AA, Pape KA, Mueller DL, Jenkins MK. Visualizing the first 50 hr of the primary immune response to a soluble antigen. Immunity. 2004;21(3):341–347. doi: 10.1016/j.immuni.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Dimitrov DS, Willey RL, Sato H, Chang LJ, Blumenthal R, Martin MA. Quantitation of human immunodeficiency virus type 1 infection kinetics. J Virol. 1993;67(4):2182–90. doi: 10.1128/jvi.67.4.2182-2190.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estes JD, Li Q, Reynolds MR, Wietgrefe S, Duan L, Schacker T, Picker LJ, Watkins DI, Lifson JD, Reilly C, Carlis J, Haase AT. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J Infect Dis. 2006;193(5):703–12. doi: 10.1086/500368. [DOI] [PubMed] [Google Scholar]
- 21.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101(11):4260–6. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 22.Granelli-Piperno A, Golebiowska A, Trumpfheller C, Siegal FP, Steinman RM. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL-10 production and T cell regulation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(20):7669–7674. doi: 10.1073/pnas.0402431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haase AT. Population biology of HIV-1 infection: Viral and CD4(+) T cell demographics and dynamics in lymphatic tissues. Annual Review of Immunology. 1999;17:625–656. doi: 10.1146/annurev.immunol.17.1.625. [DOI] [PubMed] [Google Scholar]
- 24.Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005;5(10):783–92. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- 25.Haase AT, Henry K, Zupancic M, Sedgewick G, Faust RA, Melroe H, Cavert W, Gebhard K, Staskus K, Zhang ZQ, Dailey PJ, Jr, Balfour HH, Erice A, Perelson AS. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science. 1996;274(5289):985–9. doi: 10.1126/science.274.5289.985. [DOI] [PubMed] [Google Scholar]
- 26.Hogue IB, Bajaria SH, Fallert BA, Qin S, Reinhart TA, Kirschner DE. The dual role of dendritic cells in the immune response to human immunodeficiency virus type 1 infection. J Gen Virol. 2008;89(Pt 9):2228–39. doi: 10.1099/vir.0.83600-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Igarashi T, Brown CR, Endo Y, Buckler-White A, Plishka R, Bischofberger N, Hirsch V, Martin MA. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): Implications for HIV-1 infections of humans. Proc Natl Acad Sci U S A. 2001;98(2):658–63. doi: 10.1073/pnas.021551798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janeway CA, Travers P, Walport M, Shlomchik M. Immunobiology: The immune system in health and disease. 5. Garland Publishing; 2001. [Google Scholar]
- 29.Jones LE, Perelson AS. Modeling the effects of vaccination on chronically infected HIV-positive patients. Journal of Acquired Immune Deficiency. 2002;6:369–377. doi: 10.1097/00126334-200212010-00001. [DOI] [PubMed] [Google Scholar]
- 30.Kaech SM, Ahmed R. Immunology: CD8 T cells remember with a little help. Science. 2003;300(5617):263–5. doi: 10.1126/science.1084511. [DOI] [PubMed] [Google Scholar]
- 31.Kamath AT, Henri S, Battye F, Tough DF, Shortman K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 2002;100(5):1734–1741. [PubMed] [Google Scholar]
- 32.Karlsson I, Malleret B, Brochard P, Delache B, Calvo J, Le Grand R, Vaslin B. Dynamics of T-cell responses and memory T cells during primary simian immunodeficiency virus infection in cynomolgus macaques. J Virol. 2007;81(24):13456–68. doi: 10.1128/JVI.01619-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinter AL, Horak R, Sion M, Riggin L, McNally J, Lin Y, Jackson R, O’Shea A, Roby G, Kovacs C, Connors M, Migueles SA, Fauci AS. CD25+ regulatory T cells isolated from HIV-infected individuals suppress the cytolytic and nonlytic antiviral activity of HIV-specific CD8+ T cells in vitro. AIDS Res Hum Retroviruses. 2007;23(3):438–50. doi: 10.1089/aid.2006.0162. [DOI] [PubMed] [Google Scholar]
- 34.Kirschner D. Dynamics of co-infection with M. tuberculosis and HIV-1. Theoretical Population Biology. 1999;55:94–109. doi: 10.1006/tpbi.1998.1382. [DOI] [PubMed] [Google Scholar]
- 35.Kirschner DE, Webb GF. Immunotherapy on HIV-1 infection. Journal of Biological Systems. 1998;6:71–83. [Google Scholar]
- 36.Kornfeld C, Ploquin MJ, Pandrea I, Faye A, Onanga R, Apetrei C, Poaty-Mavoungou V, Rouquet P, Estaquier J, Mortara L, Desoutter JF, Butor C, Le Grand R, Roques P, Simon F, Barre-Sinoussi F, Diop OM, Muller-Trutwin MC. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J Clin Invest. 2005;115(4):1082–91. doi: 10.1172/JCI23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106(3):263–6. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 38.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, Nephew KR, Pambuccian S, Lifson JD, Carlis JV, Haase AT. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009 doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li S, Gowans EJ, Chougnet C, Plebanski M, Dittmer U. Natural regulatory t cells and persistent viral infection. J Virol. 2008;82(1):21–30. doi: 10.1128/JVI.01768-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marechal V, Prevost MC, Petit C, Perret E, Heard JM, Schwartz O. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J Virol. 2001;75(22):11166–77. doi: 10.1128/JVI.75.22.11166-11177.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6(8):793–9. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLean AR, Kirkwood TBL. A model of human immunodeficiency virus infection in T helper cell clones. J Theor Biol. 1990;147:177–203. doi: 10.1016/s0022-5193(05)80051-7. [DOI] [PubMed] [Google Scholar]
- 43.McLean AR, Nowak MA. Models of interactions between HIV and other pathogens. J Theor Biol. 1992;155:69–102. doi: 10.1016/s0022-5193(05)80549-1. [DOI] [PubMed] [Google Scholar]
- 44.Mikkelsen SR, Long JM, Zhang L, Galemore ER, VandeWoude S, Dean G. Regulatory T cell depletion prior to acute feline immunodeficiency virus infection does not alter disease pathogenesis. PLoS ONE. doi: 10.1371/journal.pone.0017183. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mikkelsen SR, Reckling SK, Egan EA, Dean GA. In vivo depletion of CD4(+)CD25(hi) regulatory T cells is associated with improved antiviral responses in cats chronically infected with feline immunodeficiency virus. Virology. 2010;403(2):163–72. doi: 10.1016/j.virol.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller CJ, Li Q, Abel K, Kim EY, Ma ZM, Wietgrefe S, La Franco-Scheuch L, Compton L, Duan L, Shore MD, Zupancic M, Busch M, Carlis J, Wolinsky S, Haase AT. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79(14):9217–27. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3(+) natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(29):10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oswald-Richter K, Grill SM, Shariat N, Leelawong M, Sundrud MS, Haas DW, Unutmaz D. Hiv infection of naturally occurring and genetically reprogrammed human regulatory t-cells. PLoS Biol. 2004;2(7):E198. doi: 10.1371/journal.pbio.0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seber GAF, Wild CJ. Nonlinear Regression. Wiley; 1989. [Google Scholar]
- 50.Shevach EM. Mechanisms of foxp3+ t regulatory cell-mediated suppression. Immunity. 2009;30(5):636–45. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 51.Singh N, Yamamoto M, Takami M, Seki Y, Takezaki M, Mellor AL, Iwashima M. CD4(+)CD25(+) regulatory T cells resist a novel form of CD28- and Fas-dependent p53-induced T cell apoptosis. J Immunol. 2010;184(1):94–104. doi: 10.4049/jimmunol.0900753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296(5574):1873–6. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- 53.Thornton AM, Shevach EM. Suppressor effector function of cd4(+)cd25(+) immunoregulatory t cells is antigen nonspecific. Journal of Immunology. 2000;164(1):183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 54.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nature Reviews Immunology. 2003;3(11):867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 55.Walker BD, Burton DR. Toward an AIDS vaccine. Science. 2008;320(5877):760–4. doi: 10.1126/science.1152622. [DOI] [PubMed] [Google Scholar]
- 56.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198(2):249–58. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wendelsdorf K, Dean G, Hu S, Nordone S, Banks HT. Technical Report CRSC-TR10-18. Center for Research in Scientific Computation, North Carolina State University; Dec, 2010. Host immune responses that promote initial HIV spread. [Google Scholar]
- 58.Xiao D, Bossert WH. An intra-host mathematical model on interaction between HIV and Malaria. Bulletin of Mathematical Biology. 2010;72:1892–1911. doi: 10.1007/s11538-010-9515-6. [DOI] [PubMed] [Google Scholar]