Abstract
Atherosclerosis involves a specialized inflammatory process regulated by an intricate network of cytokine and chemokine signaling. Atherosclerotic lesions lead to the release of cytokines that can have multiple affects on various vascular cell functions either promoting lesion expansion or alternatively retard progression. Tumor necrosis factor-α (TNF-α) is one such cytokine that can activate both cell survival and cell death mechanisms simultaneously. Here we show that TNF-α induces apoptosis in human aortic endothelial cells (HAECs), while it promotes the proliferation of vascular smooth muscle cells (VSMCs). Both events involved the activation of the Rb–E2F1 transcriptional regulatory pathway. Stimulation of HAECs with TNF-α led to an increased expression of p73 protein and a reduction in the levels of p53. This involved apoptosis signal-regulating kinase 1 (ASK1)- mediated inactivation of Rb and its dissociation from the p73 promoter. In contrast, TNF-α stimulation of VSMCs enhanced the association of E2F1 with proliferative promoters like thymidylate synthase and cdc25A, while Rb was dissociated. ASK1 kinase has a critical role in the apoptotic process, as its depletion or dissociation from Rb reduced TNF-α-induced apoptosis. These results show that the cytokine TNF-α can elicit diametrically opposite responses in vascular endothelial cells and VSMCs, utilizing the Rb–E2F pathway.
Keywords: TNF-α, endothelial cells, apoptosis, E2F1, p73, ASK1
Tumor necrosis factor-α (TNF-α) is an inflammatory cytokine that is known to have diverse and potentially conflicting roles in cardiac function and pathology. These include beneficial effects, such as cardioprotection against ischemia, as well as potentially adverse affects, such as development of atherosclerosis, reperfusion injury, hypertrophy and heart failure. TNF-α mediates inflammatory, proliferative, cytostatic and cytotoxic effects in multiple cell types, including endothelial cells (ECs) and vascular smooth muscle cells (VSMCs).1, 2
Apoptosis induced by TNF super family requires binding of the ligand to its receptor leading to oligotrimerization of receptors.3, 4, 5 This results in aggregation of death domain containing proteins allowing recruitment of TRADD (TNF receptor 1-associated death domain protein). TRADD binds Fas associated death domain- containing protein and TNF receptor 1-associated protein 2 proteins, which in turn lead to activation of procaspase-8 and apoptosis signal-regulating kinase 1 (ASK1), respectively.6, 7, 8 TNF-α treatment leads to the activation of the ASK1-JNK/p38 death signals.8, 9, 10
Earlier studies from our laboratory had shown that the ASK1 kinase binds to Rb when cells encounter apoptotic stimuli like TNF-α or oxidative stress.11 Overexpression of Rb abrogates ASK1-mediated apoptosis by inhibiting its pro-apoptotic activity, whereas activated ASK1 could phosphorylate and inactivate Rb.11 Thus, ASK1-mediated inactivation of Rb is essential for its apoptotic activity in response to TNF-α.
Rb is believed to exert its anti-apoptotic functions by inhibiting the activity of E2F family of transcription factors, mainly E2F1.12, 13 It is established that Rb physically interacts with E2F1 and represses its transcriptional activity; inactivation of Rb by phosphorylation disrupts the binding, releasing free, transcriptionally active E2F1.12 Downstream targets of the E2F1 include genes involved in cell cycle progression (cyclin E, cdc25A and DNA polymerase α etc.), as well as pro-apoptotic genes (Apaf1, p73 and caspase 3 etc.).14, 15 Inactivation of Rb during cell cycle progression leads to the expression of proliferative E2F1 targets whereas inactivation of Rb by apoptotic signals leads to the expression of pro-apoptotic genes.
E2F-1 can function as an oncogene or a tumor suppressor;16 it can induce cell proliferation and transform cells, demonstrating its oncogenic properties, whereas E2F1 knockout mice developed tumors, suggesting tumor-suppressive properties.17 Rb knockout mice die early during embryogenesis and display extensive apoptosis, whereas mouse embryos null for both Rb and E2F-1 display reduced apoptosis. E2F1 induces apoptosis through the p53 pathway or utilizes the p53-related p73 gene, which is a transcriptional target of E2F1.18
TNF-α induces both apoptosis and G1 arrest in EC. Earlier studies have shown that the sensitivity of ECs to TNF-α is cell cycle-dependent and cytotoxicity is seen in proliferating cultures; starvation-synchronized or S- and G2/M-arrested ECs are resistant to TNF-α. It has been suggested that TNF-α inhibits E2F1 by preventing Rb phosphorylation.19 Kishore et al.20, 21 demonstrated that JNK1 and p38 MAPKs differentially affect TNF-α- mediated suppression of E2F1 in EC via two distinct and opposing mechanisms. In response to TNF-α, JNK physically associates with E2F1 and inactivates it via direct phosphorylation. In contrast, insufficient activation of p38 in TNF-α exposed EC results in reduced Rb phosphorylation and inactivation of E2F1. However, it is still unclear how TNF-α affects Rb-mediated E2F1 repression and causes apoptotic response in ECs.
Migration of VSMCs is a crucial event in the formation of vascular stenotic lesions and TNF-α modulates this process during atherosclerosis by inducing proliferative/pro-apoptotic responses in these cells.22, 23, 24 However, there are conflicting reports on the effect of TNF-α on proliferation and apoptosis of VSMCs.24 Several investigations report that TNF-α had no effect on VSMC proliferation, whereas other studies suggest that TNF-α induces proliferation of VSMCs through NF-κB.25 Similarly, the pro-apoptotic activity for TNF-α in these cells has also been unclear.26
Though E2F1 is known to mediate proliferation or cellular apoptosis, there is limited knowledge about its role in the cells involved in cardiovascular pathology. The data presented here suggest that TNF-α induces apoptosis in human aortic endothelial cells (HAECs) and proliferation in VSMCs using the Rb–E2F pathway. TNF-α-induced apoptosis involves the binding of ASK1 to Rb protein and induction of p73α gene in an E2F-dependent manner. Thus, it appears that differential effects of TNF-α on HAECs and VSMCs are mainly mediated through the Rb–E2F pathway.
Results
Apoptotic effects of TNF-α on ECs are mediated through ASK1 and p73
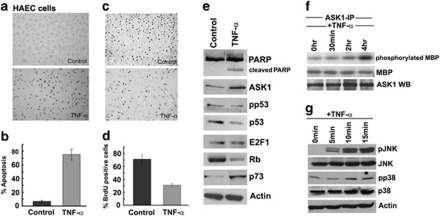
Attempts were made to evaluate the apoptotic or proliferative effect of TNF-α on primary human aortic ECs. Towards this purpose, HAECs were treated with 100 ng/ml TNF-α for 18 h; apoptosis was measured by terminal deoxynucleotidyl transferase mediated dUTP nick end labeling (TUNEL) assay. As shown in Figures 1a and b, 70±10.0% (P<0.01) cells underwent apoptosis in response to TNF-α treatment. In a similar experiment, HAECs were treated with TNF-α and cell proliferation assessed by measuring BrdU incorporation (Figures 1c and d); although asynchronous HAECs had 70±8.1% BrdU-positive cells, only 30±2.8% (P<0.01) cells showed BrdU-positive staining after TNF-α treatment, suggesting that TNF-α predominantly induces apoptosis in HAECs. Induction of apoptosis by TNF-α in HAECs was confirmed by PARP cleavage (Figure 1e). Western blots were conducted for proteins known to be involved in TNF-α-induced apoptosis, including ASK1, E2F1, Rb, p53 and p73. Stimulating HAECs with TNF-α led to an induction of p73 (1.9-fold) and ASK1 (1.95-fold), while the levels of Rb and p53 were reduced by 0.6-fold and 0.4-fold, respectively. There was no significant change in the levels of E2F1 protein. There was a slight reduction in phospho-p53 (Ser46) levels with TNF-α, indicating that p53 may not be involved in this process (Figure 1e). We next examined whether ASK1 activation was involved in TNF-α-induced apoptosis in HAEC cells. ASK1 was immunoprecipitated from untreated and TNF-α treated (30 min, 2 and 18 h) HAECs and in vitro kinase assay was conducted using myelin basic protein as the substrate. There was increased ASK1 kinase activity with TNF-α treatment at 30 min, 2 h and a marked increase at 18 h (Figure 1f).
Figure 1.
TNF-α induces apoptosis in HAECs as seen by TUNEL assay (a and b). TNF-α inhibits cell proliferation in HAECs as seen by BrdU incorporation. Error bars indicate standard deviations from two independent experiments (c and d). Stimulation with TNF-α leads to PARP cleavage and p73 upregulation confirming an apoptotic response (e). In vitro ASK1 kinase assay showing that TNF-α treatment, in particular at 18 h, leads to a strong phosphorylation of myelin basic protein (MBP) indicating ASK1 activation. Ponceau staining shows equal loading of the substrate MBP and ASK1 western blot shows equal ASK1 loading (f). Western blotting analysis shows induction of phospho-JNK and phospho-p38 at 5, 10 and 15 min of TNF-α stimulation, while total JNK and p38 levels remain the same (g)
As ASK1 activates both JNK and p38 MAP kinases by a site-specific Ser/Thr phosphorylation of their respective MKKs,27 we examined the phosphorylation of p38 and JNK in HAECs stimulated with TNF-α. Both phospho-p38 (Thr180/Tyr182) and phospho-JNK (Thr183/Tyr185) levels increased from 5 min to 15 min of TNF-α treatment, whereas total p38 and JNK levels remained unchanged (Figure 1g). This indicates that active p38 and JNK kinases work in conjunction with ASK1 to induce apoptosis in HAECs.
Proliferative effects of TNF-α on VSMCs
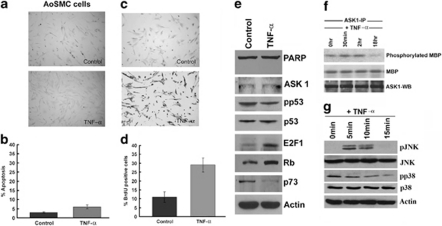
To understand how TNF-α treatment affects primary human aortic smooth muscle cells (AoSMCs), they were treated with 100 ng/ml TNF-α for 18 h. TUNEL assays showed that only 10±2.0% (P<0.05) cells were apoptotic (Figures 2a and b), comparable to that observed in untreated control cells. BrdU incorporation assays showed that TNF-α treatment led to a 3-fold increase in the number of proliferating cells (Figures 2c and d), in striking contrast to the effect of TNF-α on HAECs. Interestingly, western blots showed that TNF-α treatment led to an induction of E2F1 (2.45-fold), but a downregulation of its pro-apoptotic target, p73, by 0.5-fold (Figure 2e). Unlike HAECs, there was no reduction in the levels of p53 or Rb; however, phospho-p53 (Ser46) was reduced. Further, ASK1 levels were not altered and there was no detectable PARP cleavage (Figure 2e). ASK1 activation was also assessed in AoSMC cells upon TNF-α treatment; there was a significant reduction in ASK1 kinase activity at 18 h of TNF-α stimulation (Figure 2f). Similarly, phospho-p38 and phospho-JNK levels decreased with TNF-α treatment, demonstrating that TNF-α does not induce apoptosis in AoSMC cells (Figure 2g). It thus appears that TNF-α has opposite effects on different layers of blood vessels and these correlate with changes in the ASK1-Rb-E2F-p73 pathway.
Figure 2.
TNF-α is unable to induce apoptosis in AoSMCs as seen by TUNEL staining (a and b). TNF-α induces cell proliferation in AoSMCs as seen by BrdU incorporation. Error bars indicate standard deviations from two independent experiments (c and d). TNF-α treatment induces Rb phosphorylation in AoSMCs, however, there is no detectable PARP cleavage or change in p73 levels (e). In vitro ASK1 kinase assay showing that TNF-α treatment does not lead to phosphorylation of MBP at 18 h of stimulation. Ponceau staining shows equal loading of the substrate MBP and ASK1 western blot shows equal ASK1 loading (f). Western blotting analysis shows reduction in phospho-JNK and phospho-p38 from 5 to 15 min of TNF-α stimulation, while total JNK and p38 levels remain the same (g)
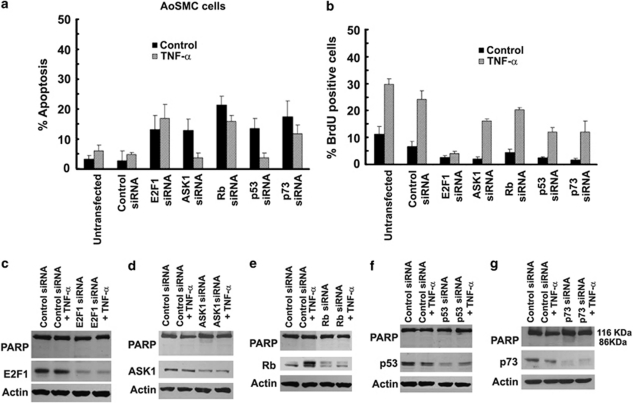
Silencing of ASK1, E2F1 and p73 protects HAECs from TNF-α-induced apoptosis
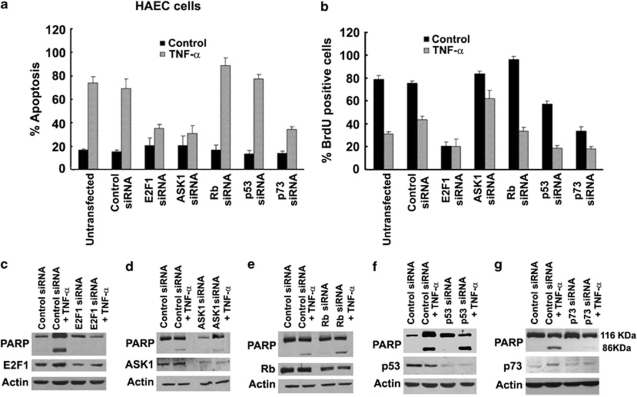
Earlier studies on Ramos cells had suggested that ASK1 modulates E2F1 activity and p73 levels to facilitate apoptosis.11 To examine the role of these proteins in TNF-α-induced apoptosis of HAECs, cells were transfected with siRNAs to E2F1, ASK1, Rb, p53, p73 or a non-targeting control siRNA. The transfected cells were treated with TNF-α (100 ng/ml) for 18 h and apoptosis was assessed by TUNEL staining. It was found that approximately 70±5.0% (P<0.01) of untransfected and control siRNA-transfected cells underwent apoptosis after TNF-α treatment (Figure 3a). Silencing of E2F1 decreased apoptosis to 38±2.1% (P<0.01); similarly, silencing of ASK1 resulted in only 35±4.0% (P<0.01) apoptotic cells. However, depletion of Rb potentiated the apoptotic response to 95±3.8% (P<0.01). Silencing p53 did not cause a significant change in apoptotic response; on the contrary, cells deprived of p73 showed a marked decrease in apoptosis (<40±1.8% P<0.01) compared with control siRNA-transfected cells upon TNF-α stimulation, suggesting that ASK1, E2F1, Rb and p73 are key factors in mediating apoptosis induced by TNF-α in HAECs (Figure 3a).
Figure 3.
Silencing of ASK1, E2F1 and p73 by siRNA protects HAECs from TNF-α-induced apoptosis as measured by TUNEL assay, while silencing of Rb increases the apoptosis in these cells, p53 siRNA transfection has limited effect on these cells as compared to control siRNA-transfected cells (a). Transfection with ASK1 and Rb siRNA increase proliferation of HAECs on TNF-α treatment. E2F1 siRNA decreases proliferation in both control and TNF-α treated HAECs as detected by BrdU incorporation assay (b). Error bars indicate standard deviations from two independent experiments. Western blots showing PARP cleavage in the lysates from the transfected cells showing apoptosis. Silencing of ASK1, E2F1 and p73 by siRNA protects in HAECs from TNF-α-induced apoptosis as detected by PARP cleavage, whereas silencing of Rb increases the apoptosis in these cells, p53 siRNA transfection has limited effect on these cells (c–g)
To further confirm the TUNEL data, we analyzed apoptosis by PARP cleavage in HAECs (Figures 3c-g). Depletion of E2F1 in HAECs completely abrogated PARP cleavage (Figure 3c). Similarly, depletion of ASK1 led to a reduction (0.2-fold) of uncleaved/cleaved PARP (Figure 3d). Silencing Rb and p53, on the other hand, increased the levels of uncleaved/cleaved PARP ratio by 1.17-fold (Figure 3e) and by 0.6-fold on TNF-α-induced PARP cleavage (Figure 3f). PARP cleavage was almost completely abrogated in cells transfected with p73 siRNA (Figure 3g). These results directly implicate E2F1, ASK1 and p73 in TNF-α-induced apoptosis in HAECs.
Silencing ASK1 reverses cytostatic effect of TNF-α on HAECs
As TNF-α has a cytostatic effect on HAECs as well, we examined how the above genes affected TNF-α-induced growth arrest. HAECs were transfected with specific siRNAs and treated with TNF-α (100 ng/ml) for 18 h; cell proliferation was assessed by BrdU incorporation. Although 80±2.0% (P<0.01) of untransfected and control siRNA-transfected cells showed BrdU incorporation, treatment with TNF-α decreased the number of S-phase cells to 43±1.9% (P<0.01) (Figure 3b). As expected, abrogation of E2F1 decreased the number of proliferating cells to 20±9.0% (P<0.05); interestingly, ASK1 depletion rescued the cells from the cytostatic influence of TNF-α (Figure 3b). Cells transfected with control siRNA showed only 43±1.9% (P<0.01) cells in S-phase, whereas TNF-α treatment of ASK1 depleted cells showed 65±5.0% (P<0.01) S-phase cells. Although nearly 100% cells showed S-phase entry on Rb siRNA transfection, it could not rescue the cells from cytostatic effect of TNF-α (Figure 3b). Abrogation of p53 and p73 decreased the number of cells undergoing proliferation in control as well as TNF-α-treated cells, suggesting a limited role for these proteins in proliferation. It is also possible that the ΔNp73 isoform might contribute to the proliferation, and depletion of this isoform by the pan-p73 siRNA affects proliferation. These results show that ASK1 kinase is a necessary intermediary for TNF-α-induced cell proliferation and apoptosis in HAECs.
TNF-α mediates apoptotic effects mainly through p73α in ECs
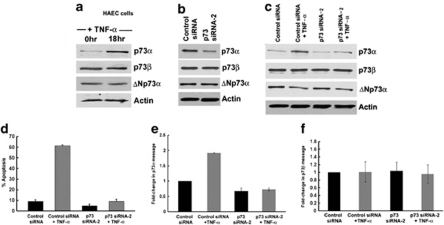
ECs showed a significant induction of p73 upon TNF-α treatment. As p73 gene expresses at least 35 different mRNA variants, of which 14 different isoforms have been described,28 we decided to determine which specific isoform mediates apoptotic effects of TNF-α. p73α and p73β are well documented for their role in the induction of apoptosis and cell cycle arrest28 and we focused on these two isoforms. Western blot analysis of HAECs treated with TNF-α for 18 h revealed a 2-fold induction of p73α but no change in p73β or ΔNp73α (Figure 4a). To further verify the involvement of p73α, cells were transfected with either control siRNA or p73siRNA-2 followed by TNF-α treatment. p73siRNA-2 significantly silenced p73α and not p73β (Figure 4b). p73α was induced 2.1-fold upon TNF-α treatment of control siRNA-transfected cells, but p73β was not induced in either control siRNA or p73siRNA-2 transfected cells (Figure 4c). We also analyzed the levels of ΔNp73α in presence of TNF-α. The ΔNp73 proteins are amino-truncated proteins containing an N-terminal domain different from p73 isoforms and act in a dominant-negative manner.28 ΔNp73α levels were marginally reduced (0.28-fold) upon TNF-α treatment of untransfected HAECs (Figure 4a). p73siRNA-2 had no effect on ΔNp73α (Figure 4b). ΔNp73α levels decreased (0.4-fold) in HAEC cells both in the presence and absence of p73α protein with TNF-α stimulation (Figure 4c).
Figure 4.
TNF-α induces apoptosis in HAECs through p73α. Western blot analysis shows induction of p73α with TNF-α treatment, no change in p73β and reduction in ΔNp73α in untransfected HAECs (a). Western blot analysis shows silencing of p73α with p73 siRNA-2 and no change in p73β or ΔNp73α protein levels (b). HAECs-transfected with control siRNA show an increase in p73α, no change in the p73β and reduction in ΔNp73α protein upon TNF-α stimulation. Abrogation of p73α with p73siRNA-2 transfection resulted in no change in p73α or p73β with TNF-α stimulation, at the same time ΔNp73α levels decreased (c). TUNEL assay shows induction of apoptotic cells upon TNF-α stimulation that is significantly reduced in the absence of p73α protein (d). Real-Time PCR showing p73α induction in the presence of TNF-α in the control siRNA-transfected HAECs. Depletion of p73α resulted in decrease in p73α in the presence and absence of TNF-α (e). There was no change in p73β mRNA upon TNF-α treatment in both control siRNA and p73siRNA-2-transfected cells (f). Error bars indicate standard deviations (d–f) from two independent experiments
TUNEL assay demonstrated that TNF-α-induced apoptosis in control siRNA-transfected cells (62±0.7% P<0.01), but significantly less in p73 siRNA-2-transfected HAECs (8.9±2.19%, P<0.05) (Figure 4d). Real-Time PCR experiments revealed that p73α, but not p73β, was induced upon TNF-α stimulation. Although there was a considerable reduction in p73α mRNA levels with p73 siRNA-2 transfection even after TNF-α treatment, p73β mRNA levels did not change (Figures 4e and f). These results ascertain that p73α is the main mediator of apoptosis in HAECs upon TNF-α stimulation.
AoSMCs are resistant to TNF-α-induced apoptosis even after silencing of ASK1, E2F1, Rb, p53 and p73
We examined whether depletion of ASK1, E2F1, Rb, p53 or p73 facilitated the induction of apoptosis by TNF-α in AoSMCs. Towards this purpose, AoSMCs were transfected with siRNA to these proteins or a control siRNA, treated with TNF-α for 18 h and apoptosis assessed by TUNEL staining. Transfection of siRNAs to E2F1, ASK1, Rb, p53 or p73 caused an increase in the number of unstimulated cells that underwent apoptosis (Figure 5a). At the same time, TNF-α stimulation did not induce significantly higher amount of apoptosis in any of these transfected cells. PARP cleavage (Figures 5c–g) confirmed that there was no induction of apoptosis in AoSMCs upon TNF-α treatment even after transfection with these siRNAs. This suggests that although these genes are necessary for maintaining the survival of AoSMCs, these cells remain resistant to TNF-α-induced apoptosis.
Figure 5.
TNF-α does not induce apoptosis in AoSMCs even on transfection with various siRNAs as detected by TUNEL staining (a). TNF-α induces proliferative effect on AoSMCs, which remains unaffected in the presence of specific siRNAs for ASK1, Rb, p53 and p73, however, E2F1 siRNA inhibits the proliferation of these cells even in presence of TNF-α as measured by BrdU staining (b). Error bars indicate standard deviations from two independent experiments. Western blots showing PARP cleavage in the lysates from the transfected AoSMC cells. TNF-α does not induce apoptosis in AoSMCs on transfection with various siRNAs as detected by PARP cleavage (c–g)
Effect of siRNAs on TNF-α-induced proliferation of AoSMCs
As TNF-α was found to have a significant proliferative effect on AoSMCs, we examined whether absence of ASK1, E2F1, Rb, p53 or p73 affects its proliferative effect. AoSMCs were transfected with individual siRNAs and were treated with TNF-α (100 ng/ml) for 18 h before BrdU staining. Absence of E2F1 inhibited the proliferation of both untreated and TNF-α stimulated AoSMCs (Figure 5b). Whereas depletion of ASK1, Rb, p53 and p73 reduced the number of BrdU-positive cells in both unstimulated and TNF-α stimulated cells, there was no significant effect on TNF-α -induced proliferation. This suggests that E2F1 mediates the TNF-α -induced proliferation of AoSMCs while other components of the ASK1-Rb-p73 pathway are not necessary for the proliferative effects of TNF-α.
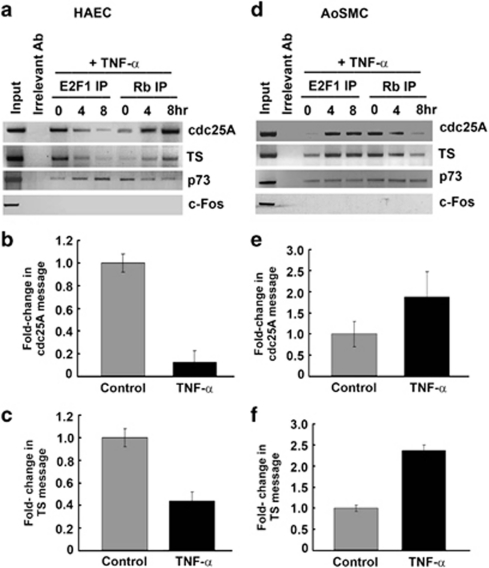
TNF-α alters the promoter occupancy of E2F1 and Rb differently in HAECs and AoSMCs
Previous studies had shown that E2F1 can induce the transcriptional activity of p73P1 promoter.29, 30 We performed chromatin immunoprecipitation (ChIP) assays to ascertain how the occupancy of E2F1 is modulated on proliferative (cdc25A and TS) and apoptotic (p73) promoters upon TNF-α treatment. E2F1 occupied the proliferative cdc25A and TS promoters in untreated HAECs, whereas there was less E2F1 on the pro-apoptotic p73 promoter (Figure 6a). TNF-α stimulation led to a progressive dissociation of E2F1 from the proliferative promoters; interestingly, there was a concomitant increase in the amount of E2F1 associating with the p73 promoter (Figure 6a). The reverse was true for Rb: TNF-α treatment led to the progressive enrichment of Rb on the proliferative promoters, with a concomitant reduction in its association with the p73 promoter. As Rb inhibits the transcriptional activity of E2F1, this indicates a repression of Cdc25A and TS upon TNF-α treatment. Real-time PCR experiments showed that indeed TNF-α treatment led to a repression of both cdc25A and TS genes (Figures 6b and c).
Figure 6.
ChIP assay showing the association of E2F1 with proliferative promoters cdc25A and TS in asynchronous HAECs, this association is lost on TNF-α treatment at the indicated time points and Rb occupies the promoter. In contrast, increasing amount of E2F1 binds to the p73 promoter while Rb is lost upon stimulation with TNF-α (a). Real-time PCR showing repression of cdc25A and TS in TNF-alpha treated HAECs (b and c). AoSMCs show opposite response to TNF-α where more E2F1 associates to the proliferative promoters cdc25A and TS while Rb is displaced. However, both E2F1 and Rb occupy the p73 promoter indicating repression of E2F1 by Rb on this promoter. PCR for c-Fos promoter, that does not bind E2F served as the control (d). Real-time PCR showing upregulation of cdc25A and TS in TNF-alpha-treated AoSMCs (e and f). TS and cdc25A were normalized to the average β-actin values for each cDNA sample. Error bars indicate standard deviations from two independent experiments
In contrast, TNF-α treatment led to the increased association of E2F1 with the cdc25A and TS promoters in AoSMCs, whereas Rb was dissociated from these promoters; there was no change in their binding to p73 promoter (Figure 6d). Real-time PCR experiments showed that TNF-α stimulation led to an induction of the E2F1-regulated cdc25A and TS genes (Figures 6e and f), facilitating cell cycle progression. Thus, TNF-α appears to elicit differential effects on HAECs and AoSMCs through the mediation of E2F1.
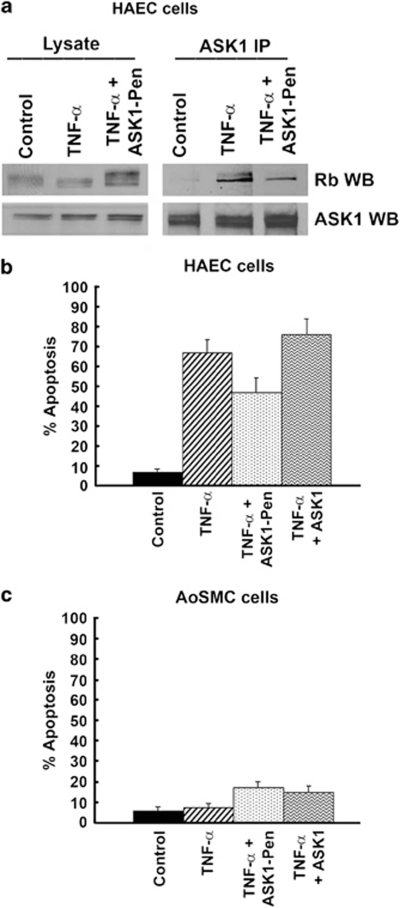
Disruption of Rb–ASK1 interaction reduces TNF-α-induced apoptosis
As ASK1 kinase contributes to TNF-α-induced apoptosis, we assessed whether disruption of the ASK1–Rb interaction affected TNF-α-induced apoptosis in HAECs. ASK1 kinase is known to bind Rb through the canonical Rb-binding motif, LXCXE.11 A 16-amino-acid peptide spanning the LXCXE motif of ASK1 was conjugated to a carrier peptide, penetratin, and delivered into HAECs using published protocols.11 The ability of this conjugate to disrupt the ASK1–Rb interaction was tested by an immunoprecipitation–western blot experiment. TNF-α treatment resulted in robust binding of ASK1 to Rb; this interaction was reduced in cells that were TNF-α stimulated after transfection of the ASK1 peptide conjugate (Figure 7a).
Figure 7.
Association of ASK1 with Rb is disrupted by ASK1–Pen peptide in vivo. HAECs were treated with TNF-α and ASK1–Rb interaction detected by IP-western blots using ASK1 antibody for IP and Rb antibody for the western (a). Effect of abrogating ASK1-Rb interaction on TNF-α-induced apoptosis in HAECs as evaluated by TUNEL staining (b). Effect of abrogating ASK1-Rb interaction on AoSMCs in presence of TNF-α, as evaluated by TUNEL staining (c)
The effect of abrogating ASK1–Rb interaction on TNF-α-induced apoptosis was evaluated by TUNEL staining. Approximately 70±4.7% (P<0.01) cells were apoptotic after treatment with TNF-α; however, in presence of ASK1-Penetratin peptide the apoptotic cells decreased by 22% (48±5.0% P<0.01) (Figure 7b). The unconjugated peptide did not influence the apoptotic effect of TNF-α on HAECs. This experiment suggests that the binding of ASK1 to Rb facilitates TNF-α-induced apoptosis in HAECs. ASK1 peptide conjugate had no significant effect on AoSMCs after TNF-α treatment (Figure 7c).
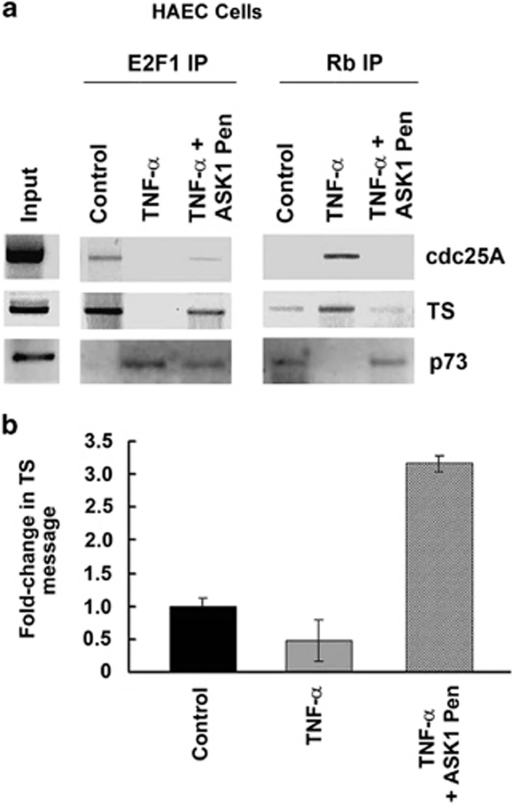
Effect of disruption of Rb–ASK1 interaction on transcription of proliferative and apoptotic genes
ChIP assays were conducted to evaluate whether disruption of ASK1–Rb interaction affected the promoter occupancy for E2F1 and Rb in HAECs. Cdc25A and TS promoters were analyzed for the binding of E2F1 and Rb after delivering ASK1-Pen peptide. E2F1 was bound to both promoters in control (unstimulated) cells; treatment with TNF-α led to the displacement of E2F1 while Rb is enriched (Figure 8a). Treatment with TNF-α in presence of ASK1-Pen peptide showed that a small amount of E2F1 still bound to both the proliferative promoters, probably because E2F1 competes with ASK1 peptide for binding to Rb. On the other hand, Rb occupies the p73 promoter while E2F1 binding is low in untreated cells; treatment with TNF-α reverses the situation, as E2F1 binds to the promoter while Rb is displaced. In presence of ASK1–Pen, treatment with TNF-α shows binding of a low amount of E2F1 while Rb is retained (Figure 8a).
Figure 8.
Differential occupancy of E2F1 and Rb on proliferative promoters cdc25A and TS, and apoptotic promoter p73 in presence of TNF-α and ASK1 peptide in HAECs (a). Real-time PCR showing effect of TNF-α and disruption of ASK1–Rb interaction on the expression of cdc25A and TS in HAECs (b)
As inhibition of ASK1–Rb interaction could reverse the TNF-α-induced silencing of proliferative promoters, we measured the mRNA levels of TS by RT-PCR. It was found that there was a marked decrease in TS expression levels upon TNF-α treatment. However, the expression levels are rescued by treating the cells with ASK1-Pen simultaneously with TNF-α (Figure 8b).
Discussion
In the case of atherosclerosis and related conditions, preservation of the endothelium with simultaneous inhibition of smooth muscle cell proliferation is beneficial to promote re-endothelialization.31 Migration, proliferation and differentiation of HAECs and AoSMCs are thus pathological responses that facilitate the development and progression of vascular lesions.32 Cytokines such as TNF-α are present at sites of vascular injury and regulate HAECs and VSMCs by transducing signals to the nucleus, where multiple genes are regulated to participate in lesion formation through proliferation, differentiation and apoptosis.33
Our studies had shown that upon TNF-α treatment, ASK1 mediates Rb inactivation in Ramos and Jurkat cells.11 We observe similar response in HAECs, where Rb is inactivated upon TNF-α treatment, facilitating upregulation of the pro-apoptotic protein p73α. The role of p53 in TNF-α-induced apoptosis has been controversial.34 We find that TNF-α had no effect on p53-mediated regulation of apoptosis in HAECs; however, ASK1 and p73 levels were found to be upregulated. We found an increase in ASK1 kinase activity with TNF-α stimulation in HAEC cells, and depletion of p73α inhibited TNF-α-induced apoptosis, implicating ASK1 and p73α to be the major contributing factors to endothelial cell apoptosis. The silencing of ASK1, E2F1 and p73 rescued HAECs from TNF-α-induced apoptosis, whereas silencing of Rb and p53 increased apoptosis suggesting an E2F1 regulated apoptotic pathway in these cells. Additionally, we found the p73α, and not p73β or ΔNp73α, to be the main mediator of apoptosis. ChIP and RT-PCR results confirm that more E2F1 is recruited on p73 promoter while it is lost from proliferative promoters upon exposure to TNF-α. The use of peptide corresponding to the LXCXE domain of ASK1 was able to decrease the recruitment of E2F1 on p73 promoter.
A very contrasting observation was made in AoSMCs; there was no effect of TNF-α on ASK1, p53 and p73 levels, and reduced ASK1 kinase activity suggests a lack of apoptotic response. In contrast we observed an increase in levels of pMEK and pERK (data not shown) and a simultaneous decrease in pp38 and pJNK suggesting a proliferative response in AoSMC cells. It has been shown that co-localization of TNF-α and ERK1/2 occurs leading to the expression of Ets-1, Egr-1 and c-Fos in neointimal lesions from rat aortae 2 weeks post balloon injury.35 The ChIP and RT-PCR experiments showed recruitment of E2F1 to proliferative promoters and increased expression of cdc25A and TS genes, respectively.
Although Rb interacts with ASK1 upon apoptotic stimuli, the Raf-1 kinase binds to Rb in response to proliferative signals, facilitating its inactivation.14 Thus, selective Rb phosphorylation by different kinases facilitates apoptotic or proliferative pathways in response to specific signaling events.36 The divergent responses of AoSMCs and HAECs to TNF-α provide unique therapeutic possibilities to target two different cell types simultaneously within the same tissue microenvironment, resulting in opposite but complimentary effects. It can be imagined that modulators of the Rb function might be good candidates for this purpose.
Materials and Methods
Cell culture and treatment
HAECs were grown in EGM medium (EBM2+ bullet kit) from Lonza (Basel, Switzerland). AoSMCs were grown in SMGM medium (SMBM+ bullet kit) from Lonza.
Antibodies
Polyclonal E2F1 (cat no. sc-50, for ChIP assay), monoclonal E2F1 (cat no. sc-251, for western blotting), polyclonal Rb (cat no.; sc-193, for ChIP assay), polyclonal ASK1 (cat no. sc-7931), polyclonal JNK (cat no. sc-474), monoclonal p73 (cat no. sc-17823), monoclonal p73α (cat no. sc-56194), monoclonal p53 (cat no. sc-263) antibodies were purchased from Santa Cruz biotechnology, Santa Cruz, CA, USA. Polyclonal phospho-p38 MAPK (cat no. 9211), polyclonal phospho-p53 (Ser46) (cat no. 2521), polyclonal p38 MAPK (cat no. 9212), polyclonal PARP (cat no. 9542), were purchased from Cell Signaling Technology, Danvers, MA, USA. Monoclonal beta-actin Clone AC-1 (cat no. A1978) was from Sigma-Aldrich, St. Louis, MO, USA. Monoclonal Rb (cat no. 554136) was purchased from BD Pharmingen, Franklin Lake, NJ, USA. Monoclonal p73β (cat no. ab33131) was from Abcam (Cambridge, MA, USA) and monoclonal ΔNp73α (cat no. IMG-313A) was obtained from Imgenex, San Diego, CA, USA.
TUNEL assay
HAEC and AoSMC cells were plated onto poly--lysine (Sigma, St. Louis, MO, USA) coated eight-well glass chamber slides (10 000 cells per well). The cells were treated with 100 ng/ml TNF-α for 18 h. The cells were fixed and stained according to manufacturer's instructions using Promega's DeadEnd Colorimetric TUNEL system (Promega, Madison, MI, USA). TUNEL-positive cells were visualized by microscopy and a quantitative analysis of apoptotic cells was done by counting 3 fields of 100 cells each at high power at × 200 magnification on a Leica DMILB inverted phase contrast microscope (Leica Microsystems, Wetzlar, Germany). The assay was repeated twice in triplicates and average was taken from 3 fields for each replicate. The percentage of TUNEL-positive cells was graphically represented. The assay was repeated twice in triplicates and an average of 3 fields per replicate was considered.
BrdU Incorporation assay
HAEC and AoSMC cells were plated onto poly--lysine (Sigma) coated eight-well glass chamber slides (10 000 cells per well). The cells were treated with 100 ng/ml TNF-α for 18 h. The cells were fixed and stained according to manufacturer's instructions using 5-bromo-2′-deoxy-uridine labeling and detection kit II from Roche Diagnostics Corporation (Indianapolis, IN, USA). BrdU-positive cells were visualized by microscopy and a quantitative analysis of proliferating cells was done by counting 3 fields of 100 cells each at high power at × 200 magnification on a Leica DMILB inverted phase contrast microscope (Leica Microsystems). The assay was repeated twice in triplicates and average was taken from 3 fields for each replicate. The percentage of BrdU-positive cells was graphically represented.
si RNA transfections
ASK1 (cat no. sc-29748), E2F1 (cat no. sc-29297), p53 (cat no. sc-29435), p73 (cat no.sc-36167), Rb (cat no. sc-29468) and control non-homologous (cat no. sc-37007) siRNAs were obtained from Santa Cruz Biotechnology. p73 siRNA-2 was custom synthesized from Integrated DNA Technologies (San Diego, CA, USA) (sequence: 5′-UCU GCU GAG CAG CAC CAU G-3′, used at 200 pmol concentration).37 All the other siRNAs were used at 100 pmoles concentration. The transfections were performed in HAEC and AoSMC cells using Oligofectamine (Invitrogen, Carlsbad, CA, USA). The treatment of cells with TNF-α (100 ng/ml) was started 24 h after transfections, for an additional 18 h. The cells were then subjected to TUNEL assays or BrdU proliferation assays, or western blotted for PARP, E2F1, ASK1, Rb, p53, p73α and actin. The western blots for each protein were run separately.
ASK1 in vitro kinase assay
ASK1 kinase assay was performed according to the protocol previously described.11 HAEC or AoSMC cells were untreated or treated with 100 ng/ml TNF-α for 30 min, 2 or 18 h. The cells were washed twice with cold PBS containing 1 mM sodium orthovanadate and lysed in nonionic lysis buffer. Immunoprecipitations were carried out using 200 μg protein and 1 μg of anti-rabbit ASK1 antibody. Myelin basic protein (Sigma) was used as substrate for ASK1. Immunobeads were subjected to kinase reaction in a total volume of 30 μl containing 20 mM MgCl2, 0.1 M sodium orthovanadate, 1 M dithiothreitol, 30 mM β-glycerol phosphate, 5 mM EGTA, 20 mM MOPS, 1 μM ATP, 10 μg of substrate/sample and 2.5 μCi of [γ-32P]ATP/sample. The samples were incubated at 30°C for 30 min. The reactions were terminated by the addition of sample buffer. The protein samples were separated on a 12% SDS-PAGE gel, transferred to a nitrocellulose membrane, and subjected to autoradiography. The blot was then stained with Ponceau (Bio-Rad Laboratories, Hercules, CA, USA) for myelin basic protein and was also probed with ASK1 polyclonal antibody for equal protein loading. The assay was repeated three times with three independent lysates for each cell line.
p38 and JNK western blotting
HAEC and AoSMC cells were treated with 100 ng/ml TNF-α for 0, 5, 10 and 15 min. 50 μg lysates were run on 10% SDS-PAGE, transferred onto nitrocellulose membrane and immunoblotted for phospho-p38 (Thr180/Tyr182), p38 MAPK, phospho-JNK (Thr183/Tyr185), JNK and actin.
Immunoprecipitation and immunoblotting
Cell lysates (50–200 μg) were treated with 1 μg of the appropriate primary antibody in a volume of 100 μl at 4°C for 1 h. A 3 mg aliquot of protein G-Sepharose in a 100 μl volume was added to each sample and incubated for an additional hour. The binding was performed in a buffer containing 20 mM HEPES, pH 7.9, 40 mM KCl, 1 mM MgCl2, 0.1 mM EGTA, 0.1 mM EDTA, 0.1 mM dithiothreitol, 0.1 mM NaF, 0.1 mM Na3VO4, 0.5% Igepal and 3 mg of bovine serum albumin per ml. The beads were washed six times with 600 μl of the same buffer, boiled in 20 μl of SDS sample buffer and separated on 8 or 10% polyacrylamide gels. After semi-dry transfer to supported nitrocellulose membranes, the blots were probed with the appropriate antibody. The proteins were detected by using an enhanced chemiluminescence assay system from Amersham Biosciences (Piscataway, NJ, USA).
ChIP assay
ChIP assay was performed using two confluent plates of HAECs or AoSMCs (about 1 × 106 cells per plate) for each immunoprecipitation reaction, as described previously.11 Briefly, cells were cross-linked with 1% formaldehyde for 20 min at room temperature; the cells were harvested and lysates were prepared. The Rb polyclonal and E2F1 polyclonal antibodies were used for immunoprecipitations. HA antibody IP was used as negative control. Immuneprecipitates were analyzed for the presence Rb and E2F1 on cdc25A, TS and p73 promoter. Rabbit anti-mouse secondary antibody was used as the control for all reactions. The PCR were then performed using 5 μl of the DNA from the immunoprecipitation reactions or 1 μl of DNA from the input reaction as template. PCR cycling conditions were as follows: 94°C for 2 min; then 35 cycles of 94°C for 30 s, 56°C (Cdc25a, fos, TS)/53°C (for p73) for 30 s and 68°C for 30 s, followed by 68°C for 2 min. The sequences of the PCR primers used in the PCR reactions were as follows:
p73 promoter (forward primer) 5′-GCCGGGAGGAGACCTTGG-3′
p73 promoter (reverse primer) 5′-GTTTCGCTGCGTCCCCTTCG-3′
Cdc25A promoter (forward primer) 5′-TCTGCTGGGAGTTTTCATTGACCTC-3′
Cdc25A promoter (reverse primer) 5′-TTGGCGCCAAACGGAATCCACCAATC-3′
TS promoter (forward primer) 5′-TGGCGCACGCTCTCTAGAGC-3′
TS promoter (reverse primer) 5′-GACGGAGGCAGGCCAAGTG-3′
c-Fos promoter (forward primer) 5′-TGTTGGCTGCAGCCCGCGAGCAGTTC-3′
c-Fos promoter (reverse primer) 5′-GGCGCGTGTCCTAATCTCGTGAGCAT-3′.
RNA isolation
HAECs and AoSMCs were treated with TNF-α. Unstimulated asynchronous cells were used as control. Total RNA was isolated by RNeasy mini-prep kit (cat no. 74104) from Qiagen (Valencia, CA, USA) following the manufacturer's protocol.
Real-time PCR
RT-PCR was performed using one confluent plate of HAECs for each treatment. One μg RNA was DNase-treated using RQ1 DNase (Promega), followed by first-strand cDNA synthesis using the iScript cDNA synthesis kit (Bio-Rad). 1/20th of the final cDNA reaction volume was used in each PCR reaction. Primers sequences are as follows:
TS (forward primer) 5′-CTGCCAGCTGTACCAGAGAT-3′
TS (reverse primer) 5′-ATGTGCATCTCCCAAAGTGT-3′
Cdc25A (forward primer) 5′-AACCTGACCGTCACTATGGA-3′
Cdc25A (reverse primer) 5′-GAATCTGTTGACTCGGAGGA-3′
β-actin (forward primer) 5′-ATCCTCACCCTGAAGTACCC-3′
β-actin (reverse primer) 5′-TAGAAGGTGTGGTGCCAGAT-3′.
All primers were run at an annealing temperature of 58°C. Real-time PCR reactions were performed in a volume of 25 μl, which included 200 nM of each forward and reverse primer and iQ SYBR Green Supermix (Bio-Rad). Reactions were run in duplicate using an iCycler and iQ software (Bio-Rad). Average threshold cycles (Ct) for TS and Cdc25A were normalized to the average β-actin Ct values for each cDNA sample and relative levels of p73, TS and Cdc25A were calculated by the ΔΔCt method:38 2−TS−β-actin for TS expression or 2−Cdc25a−β-actin for Cdc25a expression. Fold-change was determined and plotted as histograms.
Similarly, Real-Time PCR was performed for p73α and β.39 RNA was made from HAECs transfected with 200 pmol each of either control siRNA or p73α siRNA followed by treatment with TNF-α for 18hr. The primers used were as follows:
p73α (forward primer) 5′-GCACCACGTTTGAGCACCTCT-3′
p73α (reverse primer) 5′-GCAGATTGAACTGGGCCATGA-3′
p73β (forward primer) 5′-CCGACCCCAGCCTCGTCAG-3′
p73β (reverse primer) 5′-CTGAGCCGCCGATGGA-3′.
The primers were run at an annealing temperature of 60°C. The rest of the protocol remained as described above.
Delivery of ASK1 peptide to cells
We have synthesized a peptide corresponding to the LXCXE domain of ASK1 (AKAFILKCFEPDPDKRAC), which is essential for binding to Rb with a cysteine residue added at the C terminus for coupling to the carrier molecule, penetratin.40 10 mM ASK1 peptide was conjugated to 1 mM penetratin (QBiogene, Carlsbad, CA, USA) in the presence of 10 mM tris- (2-carboxyethyl) phosphine as described previously.40 The final conjugate was diluted to 150 μM and aliquoted and stored in −80°C until further use. Approximately 10 000 HAECs will be plated on eight-well poly--lysine-coated chamber slides; the cells were treated with TNF-α (100 ng/ml) for 18 h in the presence or absence of 5 μM ASK1 peptide conjugated to penetratin. For the negative control, cells were exposed to the same concentration of unconjugated peptide. The effect of abrogation of interaction of Rb and ASK1 on apoptosis of HAECs was evaluated by TUNEL assay and assessing PARP cleavage in the lysates.
Statistical and densitometric analysis
For all data shown, Student's t-test was conducted to evaluate for differences among control and TNF-α treatments and P≤0.05 was considered significant. Data shown are mean±S.D. Densitometric analysis was done on the western blots as indicated using AlphaEaseFC software and the fold-change was determined.
Acknowledgments
This study was supported by Grant no. CA63136 from the NCI to SPC. SR is a recipient of a post-doctoral award from the AHA (Grant no. 0425904B).
Glossary
- AoSMC
aortic smooth muscle cells
- ASK1
apoptosis signal-regulating kinase 1
- EC
endothelial cells
- HAEC
human aortic endothelial cells
- TNF-α
tumor necrosis factor-α
- VSMC
vascular smooth muscle cells
The authors declare no conflict of interest.
Footnotes
Edited by V De Laurenzi
References
- Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- Wang X, Yue TL, Barone FC, White RF, Gagnon RC, Feuerstein GZ. Concomitant cortical expression of TNF-alpha and IL-1 beta mRNAs follows early response gene expression in transient focal ischemia. Mol Chem Neuropathol. 1994;23:103–114. doi: 10.1007/BF02815404. [DOI] [PubMed] [Google Scholar]
- Chen J, Fujii K, Zhang L, Roberts T, Fu H. Raf-1 promotes cell survival by antagonizing apoptosis signal-regulating kinase 1 through a MEK-ERK independent mechanism. Proc Natl Acad Sci USA. 2001;98:7783–7788. doi: 10.1073/pnas.141224398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy JC, Granville DJ, Hunt DW, McManus BM. Endothelial cell apoptosis: biochemical characteristics and potential implications for atherosclerosis. J Mol Cell Cardiol. 2001;33:1673–1690. doi: 10.1006/jmcc.2001.1419. [DOI] [PubMed] [Google Scholar]
- Madge LA, Pober JS. TNF signaling in vascular endothelial cells. Exp Mol Pathol. 2001;70:317–325. doi: 10.1006/exmp.2001.2368. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- Ichijo H. Molecular mechanisms for cell life and cell death. Kokubyo Gakkai Zasshi. 1998;65:155–163. doi: 10.5357/koubyou.65.155. [DOI] [PubMed] [Google Scholar]
- Matsuzawa A, Ichijo H. Molecular mechanisms of the decision between life and death: regulation of apoptosis by apoptosis signal-regulating kinase 1. J Biochem (Tokyo) 2001;130:1–8. doi: 10.1093/oxfordjournals.jbchem.a002947. [DOI] [PubMed] [Google Scholar]
- Takeda K, Matsuzawa A, Nishitoh H, Ichijo H. Roles of MAPKKK ASK1 in stress-induced cell death. Cell Struct Funct. 2003;28:23–29. doi: 10.1247/csf.28.23. [DOI] [PubMed] [Google Scholar]
- Dasgupta P, Betts V, Rastogi S, Joshi B, Morris M, Brennan B, et al. Direct binding of apoptosis signal-regulating kinase 1 to retinoblastoma protein: novel links between apoptotic signaling and cell cycle machinery. J Biol Chem. 2004;279:38762–38769. doi: 10.1074/jbc.M312273200. [DOI] [PubMed] [Google Scholar]
- Harbour JW, Dean DC. Rb function in cell-cycle regulation and apoptosis. Nat Cell Biol. 2000;2:E65–E67. doi: 10.1038/35008695. [DOI] [PubMed] [Google Scholar]
- Liu DX, Nath N, Chellappan SP, Greene LA. Regulation of neuron survival and death by p130 and associated chromatin modifiers. Genes Dev. 2005;19:719–732. doi: 10.1101/gad.1296405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta P, Sun J, Wang S, Fusaro G, Betts V, Padmanabhan J, et al. Disruption of the Rb--Raf-1 interaction inhibits tumor growth and angiogenesis. Mol Cell Biol. 2004;24:9527–9541. doi: 10.1128/MCB.24.21.9527-9541.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour JW, Dean DC. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 2000;14:2393–2409. doi: 10.1101/gad.813200. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Vousden KH. E2F-1 induced apoptosis. Apoptosis. 2001;6:173–182. doi: 10.1023/a:1011332625740. [DOI] [PubMed] [Google Scholar]
- Tonini T, Hillson C, Claudio PP. Interview with the retinoblastoma family members: do they help each other. J Cell Physiol. 2002;192:138–150. doi: 10.1002/jcp.10117. [DOI] [PubMed] [Google Scholar]
- Kinkade R, Rizwani W, Chellappan S.E2F transcription factors in cell proliferation, apoptosis, senescence and cancerIn: Yoshida K (ed). Control of Cellular Physiology by Transcription Factors E2F Research Signpost Press: Trivandrum, India, 2007 [Google Scholar]
- Lopez-Marure R, Bernal AE, Zentella A. Interference with c-myc expression and RB phosphorylation during TNF-mediated growth arrest in human endothelial cells. Biochem Biophys Res Commun. 1997;236:819–824. doi: 10.1006/bbrc.1997.7056. [DOI] [PubMed] [Google Scholar]
- Goukassian DA, Kishore R, Krasinski K, Dolan C, Luedemann C, Yoon YS, et al. Engineering the response to vascular injury: divergent effects of deregulated E2F1 expression on vascular smooth muscle cells and endothelial cells result in endothelial recovery and inhibition of neointimal growth. Circ Res. 2003;93:162–169. doi: 10.1161/01.RES.0000082980.94211.3A. [DOI] [PubMed] [Google Scholar]
- Kishore R, Luedemann C, Bord E, Goukassian D, Losordo DW. Tumor necrosis factor-mediated E2F1 suppression in endothelial cells: differential requirement of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase signal transduction pathways. Circ Res. 2003;93:932–940. doi: 10.1161/01.RES.0000102400.22370.20. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Nishikawa O, Yukawa S, Yoshimoto M, Nishide I. Effects of hemodialysis membrane on serum lipid profile of maintenance hemodialysis patients] Nippon Jinzo Gakkai Shi. 1999;41:1–7. [PubMed] [Google Scholar]
- Wang Z, Rao PJ, Castresana MR, Newman WH. TNF-alpha induces proliferation or apoptosis in human saphenous vein smooth muscle cells depending on phenotype. Am J Physiol Heart Circ Physiol. 2005;288:H293–H301. doi: 10.1152/ajpheart.00165.2004. [DOI] [PubMed] [Google Scholar]
- Selzman CH, Shames BD, Reznikov LL, Miller SA, Meng X, Barton HA, et al. Liposomal delivery of purified inhibitory-kappaBalpha inhibits tumor necrosis factor-alpha-induced human vascular smooth muscle proliferation. Circ Res. 1999;84:867–875. doi: 10.1161/01.res.84.8.867. [DOI] [PubMed] [Google Scholar]
- Geng YJ, Azuma T, Tang JX, Hartwig JH, Muszynski M, Wu Q, et al. Caspase-3-induced gelsolin fragmentation contributes to actin cytoskeletal collapse, nucleolysis, and apoptosis of vascular smooth muscle cells exposed to proinflammatory cytokines. Eur J Cell Biol. 1998;77:294–302. doi: 10.1016/S0171-9335(98)80088-5. [DOI] [PubMed] [Google Scholar]
- Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- Murray-Zmijewski F, Lane DP, Bourdon JC. p53/p63/p73 isoforms: an orchestra of isoforms to harmonise cell differentiation and response to stress. Cell Death Differ. 2006;13:962–972. doi: 10.1038/sj.cdd.4401914. [DOI] [PubMed] [Google Scholar]
- Lissy NA, Davis PK, Irwin M, Kaelin WG, Dowdy SF. A common E2F-1 and p73 pathway mediates cell death induced by TCR activation. Nature. 2000;407:642–645. doi: 10.1038/35036608. [DOI] [PubMed] [Google Scholar]
- Pediconi N, Ianari A, Costanzo A, Belloni L, Gallo R, Cimino L, et al. Differential regulation of E2F1 apoptotic target genes in response to DNA damage. Nat Cell Biol. 2003;5:552–558. doi: 10.1038/ncb998. [DOI] [PubMed] [Google Scholar]
- Vallieres K, Petitclerc E, Laroche G. On the ability of imatinib mesylate to inhibit smooth muscle cell proliferation without delaying endothelialization: an in vitro study. Vascul Pharmacol. 2009;51:50–56. doi: 10.1016/j.vph.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Schwartz SM, Virmani R, Rosenfeld ME. The good smooth muscle cells in atherosclerosis. Curr Atheroscler Rep. 2000;2:422–429. doi: 10.1007/s11883-000-0081-5. [DOI] [PubMed] [Google Scholar]
- Introna M, Mantovani A. Early activation signals in endothelial cells. Stimulation by cytokines. Arterioscler Thromb Vasc Biol. 1997;17:423–428. doi: 10.1161/01.atv.17.3.423. [DOI] [PubMed] [Google Scholar]
- Chau BN, Chen TT, Wan YY, DeGregori J, Wang JY. Tumor necrosis factor alpha-induced apoptosis requires p73 and c-ABL activation downstream of RB degradation. Mol Cell Biol. 2004;24:4438–4447. doi: 10.1128/MCB.24.10.4438-4447.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetze S, Kintscher U, Kaneshiro K, Meehan WP, Collins A, Fleck E, et al. TNFalpha induces expression of transcription factors c-fos, Egr-1, and Ets-1 in vascular lesions through extracellular signal-regulated kinases 1/2. Atherosclerosis. 2001;159:93–101. doi: 10.1016/s0021-9150(01)00497-x. [DOI] [PubMed] [Google Scholar]
- Wang S, Nath N, Minden A, Chellappan S. Regulation of Rb and E2F by signal transduction cascades: divergent effects of JNK1 and p38 kinases. Embo J. 1999;18:1559–1570. doi: 10.1093/emboj/18.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LM, Nugent JK, Zhao X, Irwin MS. HDM2 antagonist Nutlin-3 disrupts p73-HDM2 binding and enhances p73 function. Oncogene. 2008;27:997–1003. doi: 10.1038/sj.onc.1210707. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Horvilleur E, Bauer M, Goldschneider D, Mergui X, de la Motte A, Benard J, et al. p73alpha isoforms drive opposite transcriptional and post-transcriptional regulation of MYCN expression in neuroblastoma cells. Nucleic Acids Res. 2008;36:4222–4232. doi: 10.1093/nar/gkn394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem. 1994;269:10444–10450. [PubMed] [Google Scholar]