Abstract
Human immunodeficiency virus (HIV) remains a major public health concern despite a large education effort during the past 25 years. A persistent problem with HIV infection is the high comorbity rate of clinical depression. We previously established that increasing proinflammatory cytokines within the brain of mice induces sickness that can culminate in depressive-like behavior. Here we investigated the role of the HIV transactivator of transcription (Tat) protein in activation of brain cytokine signaling and subsequent induction of depressive-like behavior in a murine model. Adult Balb/c mice were administered a single intracerebroventricular (ICV) injection of Tat (40 ng). Social investigation of a novel juvenile was measured at 2, 4, 8 and 24h post-treatment. Mice treated with Tat did not display signs of sickness, as measured by either decreased social investigation or loss of body weight. At 24 h post-injection, mice were subjected to the forced swim test (FST). ICV administration of Tat to Balb/c mice increased immobility in the FST at 24 h post injection. A different strain of mice, C57BL/6J, responded similarly in the FST. Furthermore, adult C57BL/6J mice injected with Tat and tested in a two-bottle 1% sucrose preference test displayed reduced preference for sucrose during the 24 h post-injection period. Subsequently, brain tissues from Tat-treated and control C57BL/6J mice were collected at 4 h and 24 h post injection. CNS tissue from Tat-treated mice had increased expression of IL-1β, TNF-α, IL-6, and IDO mRNAs at 4 h post injection. These data demonstrate that a single exposure to Tat in the brain is sufficient to induce brain cytokine signaling that culminates in depressive-like behavior. The results reveal a potential role for Tat in the development of comorbid depression in HIV-infected individuals.
1. Introduction
Human immunodeficiency virus (HIV) infection remains highly prevalent in the developed world despite significant public education efforts. The Center for Disease Control estimates that approximately 1 million people are living with HIV infections or acquired immunodeficiency syndrome (AIDS) in the United States with the rate of new infections remaining stable (CDC. gov). Comorbid depression is a significant problem among the HIV infected population because these subjects have a higher incidence rate of depression than the general population (Olatunji et al., 2006). Of note, the population of individuals living with HIV infection continues to increase even with the advent of highly active antiretroviral therapy, which significantly increases the life expectancy. HIV infections with comorbid depression will likely increase as HIV treatments continue to improve.
A hallmark of HIV infection is its migration to the brain, initiating a neuroinflammatory response that contributes to the loss of neurons and AIDS dementia complex (Merrill and Chen, 1991). Immune responses in the brain during HIV infection are engaged early during infection. Infected macrophages enter the brain in low abundance during early HIV infection and activate microglial cells to initiate the production and release of cytokines (Gendelman et al., 1994). Microglia and astrocytes activated by surrounding HIV-infected cells respond with secretion of chemokines and cytokines that contribute to HIV associated dementia (Kaul et al., 2005). HIV proteins also activate macrophage-like cells in the brain, including perivascular macrophages and microglia (Rappaport et al., 1999, Kaul et al., 2001, Pu et al., 2003). The event triggers a proinflammatory cascade that ultimately leads to increased proinflammatory cytokine expression in the brain.
Strong evidence exists for an important role of peripheral and central inflammation in the development of sickness and depressive-like behaviors (reviewed in (Anisman et al., 2002, Dantzer et al., 2008)). In addition to their activity in the brain, the proinflammatory cytokines IL-1β, TNF-α, and IFN-γ activate the enzyme indoleamine 2,3-dioxygenase (IDO) (Fu et al., Henry et al., 2008, O’Connor et al., 2009b, O’Connor et al., 2009c). IDO is a tryptophan degrading enzyme that oxidizes tryptophan to n-formyl kynurenine that is then quickly converted to kynurenine. Degradation of kynurenine leads to an increase in the neuroactive metabolites quinolinic acid, 3-hydroxy kynurenine, and kynurenic acid. Evidence for increased activity of the IDO/kynurenine pathway in HIV-infected individuals is well established. The role of reduced serum tryptophan coupled with increased kynurenine in the manifestation of HIV associated neurological symptoms was presented in early research investigating peripheral macrophage activation during HIV infection (Fuchs et al., 1988). As early as 1990, Fuchs et al provided evidence for an association between neuropsychiatric symptoms and biochemical signs of IDO activation in the plasma of HIV-infected patients (Fuchs et al., 1990). The IDO-mediated reductions in tryptophan levels observed in HIV seropositive patients was highly correlated with severity of neuropsychiatric manifestations (Werner et al., 1988). Moreover, the IDO metabolite quinolinic acid increased in the cerebrospinal fluid with HIV infection (Heyes et al., 1989) and the levels of quinolinic acid in the cerebrospinal fluid correlated well with the neurological status of patients with AIDS dementia (Heyes et al., 1991). Quinolinic acid contributes to neurotoxicity and cognitive alterations via activation of N-methyl-D-aspartate (NMDA) receptors. The actions of quinolinic acid on the NMDA receptors are antagonized by kynurenic acid, which is also increased in patients with HIV infection (Heyes et al., 1992).
Induction of IDO activity and the subsequent increase in quinolinic acid in CNS cells is not dependent on infection but instead can be caused simply via the actions of viral protein products. The HIV transactivator of transcription Tat is the first HIV protein produced during viral replication and is chronically expressed during HIV infection. Tat is expressed and released by cells in the brain during HIV infection, including infected astrocytes (Bruce-Keller et al., 2003, Chauhan et al., 2003). Tat is also readily taken up by brain cells using the heparan sulfate receptor in combination with the low-density lipoprotein receptor-related protein to gain entry (Li et al., 2009). Tat protein has many effects in cells of the brain, but a prominent feature is the induction of proinflammatory cytokines in both astrocytes and microglia (Mattson et al., 2005). Because Tat is able to activate IDO in vitro (Smith et al., 2001), we hypothesized that Tat would also activate IDO and induce depression-like behaviors in vivo.
2. Materials and Methods
2.1. Animals
Sixteen male Balb/c mice aged three months purchased from Charles Rivers Laboratories (Wilmington, MA) and fifty-eight three month old C57BL/6J male mice purchased from Jackson Laboratories (Bar Harbor, ME) were allowed to acclimate to the animal care facility for at least two weeks prior to ICV cannulation surgery. Mice were individually housed and provided with ad libitum access to Teklad 8640 chow and water. Mice were housed in a temperature (23ºC) and humidity (45%) controlled room and maintained on a 12 hour light/dark cycle (lights off at 10:00 am). After the acclimation period, mice were surgically implanted with a single guide cannula (Plastics One, Roanoke, VA) directed toward the lateral ventricle. The guide cannulas were kept clean and covered using a screw on cannula dummy for mice (Plastics One, Roanoke, VA). Coordinates for implantation were determined utilizing The Mouse Brain in Stereotaxic Coordinates (Franklin, 2001) and cannulas were placed at 1.5 mm lateral, 0.6 mm posterior, and 1.3 mm dorsal with respect to bregma. These coordinates placed the guide cannula 1 mm dorsal to the lateral ventricle. Mice were allowed to recover from surgery for two weeks before treatment. All procedures performed on the mice were in compliance with the National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee at the University of Illinois at Urbana-Champaign.
2.2. Treatments
During the two-week recovery period, mice were handled 3–4 times per week for a few minutes to habituate them to being held and manipulated. On the day of treatment, dummy cannulas were removed and mice were injected using a single internal injector cannula for mice (Plastics One, Roanoke, VA) which extended 1 mm beyond the tip of the guide cannula to reach the lateral ventricle. Injections were administered in a volume of 1 μl using a 10 μl gas-tight syringe (SGE Incorporated, Austin, TX) over a one minute time period. Injector cannulas remained in place for at least 30 seconds to allow for diffusion before being withdrawn from the ventricle. Dummy cannulas were then returned to the guide cannulas immediately following the injection and prior to behavioral testing. Mice were injected at the of the dark cycle on the treatment day. The mice received either phosphate buffered saline (PBS) or 40 ng of recombinant HIV 1 Tat protein (kindly provided by Dr. Avindra Nath as contracted at the University of Kentucky) dissolved in PBS. This dose of Tat was selected based on data generated in hippocampal slice cultures which indicated that this dose of Tat is optimal for inducing cytokine and IDO expression (Fu et al., in preparation) and on data obtained from human astrocytes which demonstrated that Tat (0–50 ng) induces robust IDO expression in a dose-dependent manner (Samikkannu et al., 2009). After treatment, 16Balb/c and 40 C57BL/6J mice were submitted to behavioral testing. At the conclusion of behavioral testing, the mice were immediately sacrificed and brain tissue was collected for PCR analysis. A separate set of 18 C57BL/6J mice were injected in the same manner but were sacrificed at four h. Brain tissue was collected and prepared for real time RT-PCR analysis.
2.3. Social Investigation Test
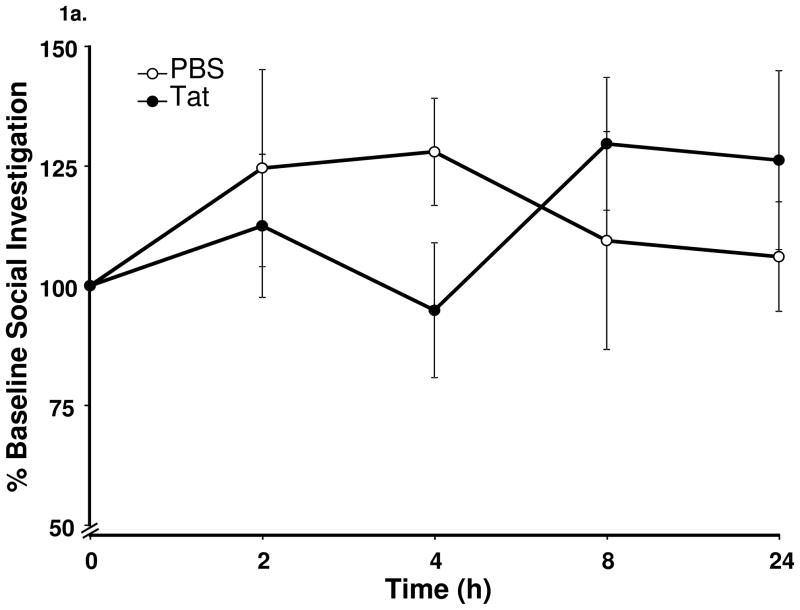
To determine whether injection of Tat or vehicle induced sickness behavior, we utilized the model of social investigation. For this behavioral test, a novel unprotected juvenile mouse of approximately three weeks of age was placed in the home cage of the treated mouse. Novelty of the juvenile was maintained by ensuring that the treated mouse was never exposed to the same juvenile more than once. After placing the juvenile in the home cage, a transparent plexiglass lid was used to cover the cage to allow for an unobstructed view of the interactions. Duration of the test was five minutes, during which time interactions between the treated mouse and the juvenile mouse were video recorded for future analysis. Treated mice were tested prior to injection for a baseline measure (time 0) and then at 2, 4, 8, and 24 h post treatment. All forms of social interaction were included in the behavioral analysis, including grooming, sniffing, aggression and sexual behavior. Interactions that were initiated and maintained by the juvenile mouse exclusively were excluded. A baseline social interaction time for each mouse was established prior to the treatments and all future social interactions were compared to the baseline time (0 h, fig.1a).
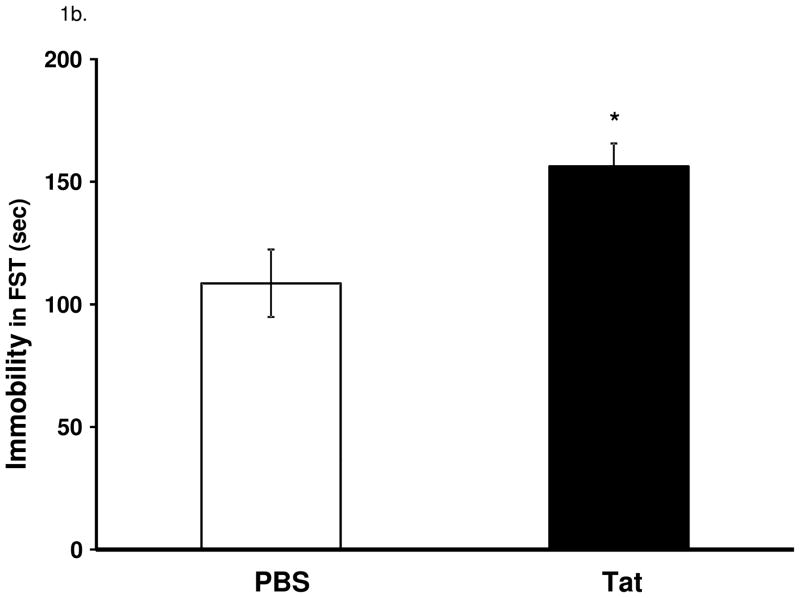
Figure 1.
Central administration of Tat induces depressive-like behavior in the absence of overt sickness behavior. (a) Tat (40 ng) protein did not cause a change (p = 0.30) in time spent investigating a novel juvenile measured at 2, 4, 8 and 24 h post Tat (b) Tat-treated mice displayed increased (p < 0.05) time mice spent immobile in the FST at 24 h. Data represent mean ± S.E.M. (n = 8 mice per group).
2.4. Forced Swim Test
To determine whether ICV injection of Tat induces depressive-like behavior in mice, we utilized a modified version of the Porsolt Forced Swim Test (Porsolt et al., 2001). Mice were placed in a white or black plastic container (depending on mouse hair color) which was partially filled (20 cm diameter x 24 cm tall) with 24 ± 0.5ºC water. Test duration was five min and the mice were video recorded for future analysis. The forced swim test was administered twenty-four h after the treatment following the conclusion of all other behavioral measures.
2.5. Sucrose Preference Test
To determine whether preference for sweetened solutions is impacted by exposure to Tat, we employed a two-bottle sucrose preference test. Approximately one week prior to treatment, C57BL/6J mice were trained to drink either water or 1% sucrose from two test bottles during a twenty-four h period. Bottles were weighed prior to being placed on the cage lids of the home cage and mice were allowed ad libitum access to the bottles. After twenty-four h the bottles were weighed to determine the amount of sucrose and water that had been consumed. Preference was calculated as a percentage of sucrose consumed compared to the total fluid consumption. Once all mice displayed a preference for the 1% sucrose solution, treatments were administered. The final sucrose and water bottle weights were measured immediately before the mice were submitted to the FST. On the day of treatment, 1% sucrose solution and water were provided immediately following treatment and consumption was measured over the twenty-four h period following injection.
2.6. Tissue Collection
After mice had been subjected to the forced swim task, they were euthanized in a CO2 chamber. Mice were quickly dissected and the heart was exposed. A knick was made in the right atrium, and this was followed by rapid perfusion of ~30 ml ice-cold PBS via the left ventricle. After the perfusion was complete brains of mice were rapidly removed and placed in a vial stored on dry ice. The brain tissue was stored frozen at −80ºC until processing.
2.7. Tissue Processing & Real Time RT-PCR Analysis
One hemisphere of the brain tissue was removed from storage and 2 ml of cold Trizol reagent (Invitrogen, Carlsbad, CA) were added. The tissue sample in Trizol was then homogenized in the Trizol reagent using an ultrasonic tissue disruptor (Sonics and Materials Inc., Newborn, CT). The RNA was isolated according to the protocol provided with the Trizol reagent. The RNA was quantified and measured for purity (OD 260/280) using a Nanodrop instrument (Nanodrop Products, Wilmington, DE) and submitted to reverse transcription using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA). These cDNA samples were analyzed using real-time quantitative RT-PCR on an Applied Biosystems Prism7900. The TaqMan gene expression assay system was used for the detection of TNF- (catalog no. Mm00443258_m1), IL-1β (catalog no. Mm00434228_m1), IL-6 (catalog no. Mm00446190_m1), IDO (catalog no. Mm00492586_m1), CD11b (Mm00434455_m1), Iba1 (Mm00479862_g1), MHCII (Mm00439226_m1), GFAP (Mm00546086_m1) and GAPDH (catalogno. Mm999999_g1). All primers were purchased from Applied Biosystems. Duplicate samples were analyzed using 125 ng of cDNA template for each reaction according to the manufacturer’s instructions. Relative quantitative measurement of target gene levels was performed using the ΔΔCt method, where Ct is the threshold concentration. GAPDH was used as the endogenous housekeeping control gene.
2.8. Statistical Analysis
Data are represented as the means ± SEM. All measures were analyzed using a one-way analysis of variance (ANOVA). Repeated measures ANOVA was used for social investigation, and when appropriate post-hoc analysis using Fishers protected least significant difference test was employed.
3. Results
3.1. Tat induces depressive-like behavior in BALB/c mice in the absence of overt sickness behavior
To determine whether Tat protein administered ICV induces sickness behavior, we assessed the amount of time spent socially investigating a novel juvenile. Additionally, we measured body weight loss during the 24 h following treatment as a further measure of sickness in response to Tat. Tat-treated mice demonstrated no reduction in the time spent investigating a novel juvenile (repeated measures ANOVA, social investigation x treatment, Figure 1a. F4,52 = 1.9, p > 0.05). The twenty-four hour changes in body weight did not differ according to treatment (Tat; 0.05 ± 0.1 g vs PBS; 0.175 ± 0.1 g; F1,14 = 0.8, p > 0.05).
At the conclusion of the social investigation test, mice were submitted to the FST and immobility was measured as time spent floating. Mice were video recorded and behavior was scored manually by an observer blinded to the treatments. Tat-treated mice displayed increased duration of immobility at 24 h after treatment compared to control mice (F1,14 = 8.4, p < 0.05, Figure 1b.).
3.2. Tat reduces sucrose preference in C57BL/6J mice and induces depressive-like behavior
To determine whether Tat-induced depressive-like behavior extended to mice on a different genetic background, experiments were carried out on C57BL6/J mice. These mice were submitted to the FST as well as to a sucrose preference test during the first 24 h post treatment.
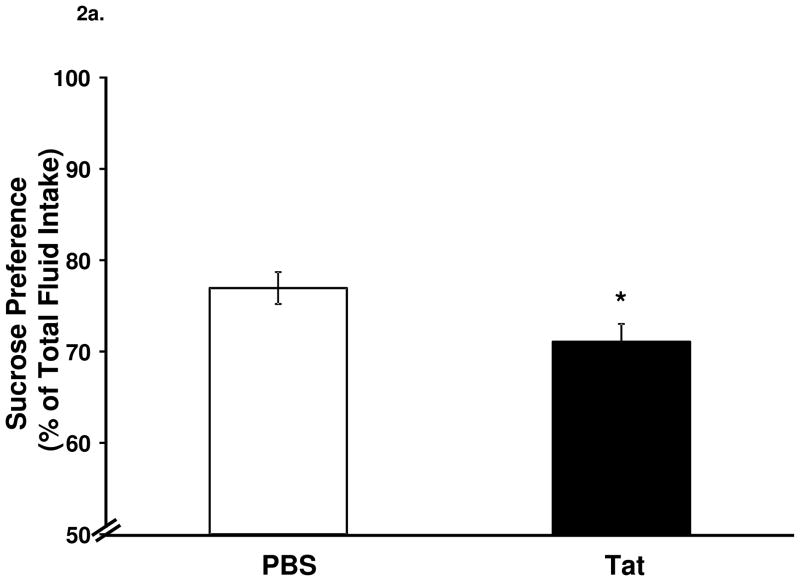
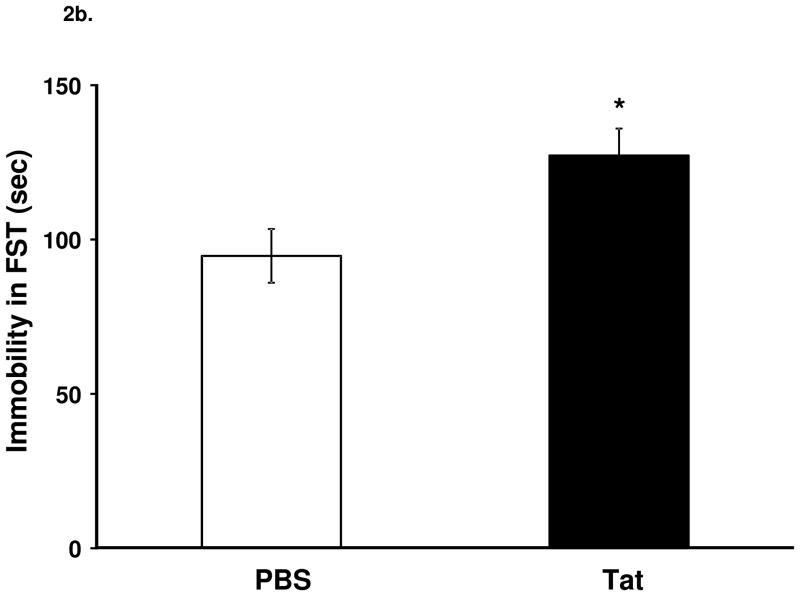
Tat significantly decreased sucrose preference (Fig. 2a, F1,26 = 5.0, p < 0.05) and increased duration of immobility in the FST (Fig. 2b, F1,26 = 7.0, p < 0.05,).
Figure 2.
Central injection of Tat reduces sucrose preference and induces depressive-like behavior. (a) Tat injection reduced (p < 0.05) sucrose preference compared to control injected mice. (b) Tat-injected mice had increased (p < 0.05) immobility in the FST 24 h later. Data represent mean ± S.E.M. (n = 14 mice per group).
3.3. Tat increases proinflammatory cytokine and IDO mRNA in the brain of C57BL/6J mice
After confirming that mice develop depressive-like behavior in response to Tat protein, we then sought to determine whether Tat caused a change in proinflammatory cytokine expression in the brain tissue of these mice at 4 h and 24 h after treatment. We employed the real-time RT-PCR to measure expression of IL-1β, TNF-α, IL-6 and IDO mRNA in the brains of C57BL6/J mice.
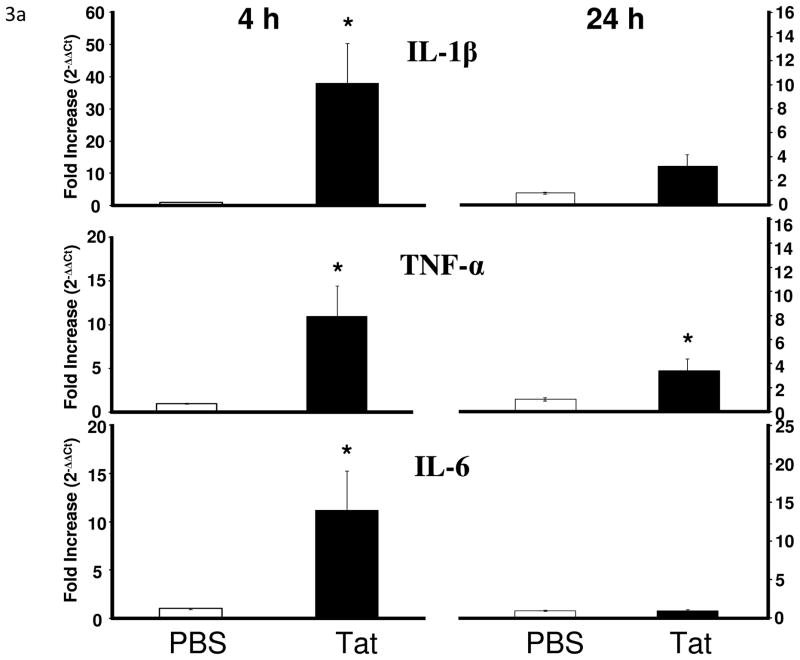
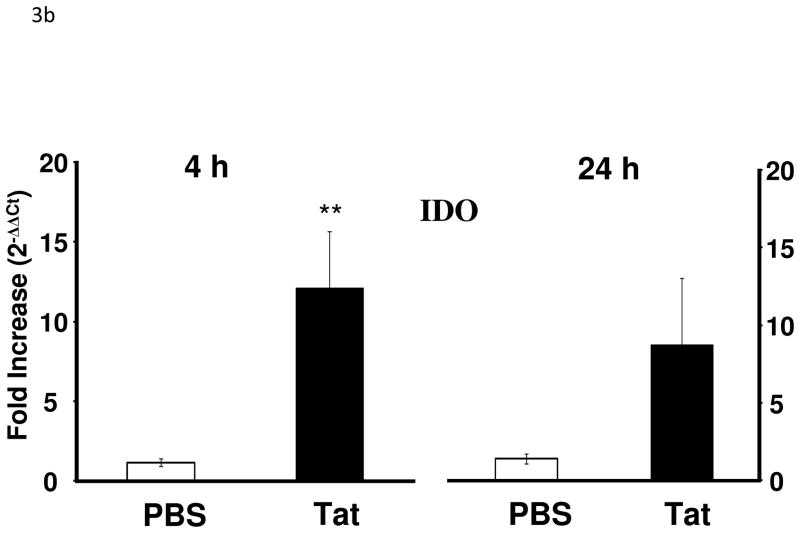
Tat treatment significantly increased IL-1β (F1,17 = 7.8, p < 0.05), TNF-α (F1,17 = 5.5, p < 0.05), and IL-6 mRNA (F1,17 = 7.3, p < 0.05) expression in the brains at 4 h but not 24 h [IL-1β (F1,15 = 4.2, p < 0.10); IL-6 (F1,15 = 0.01, p > 0.05)] post injection with the exception of TNF-α (F1,15 = 4.5, p < 0.05) (Fig. 3a). There was also a significant increase in brain IDO mRNA that was apparent at 4 h (F1,16 = 9.4, p < 0.01) but had subsided at 24 h (F1,15 = 2.5, p > 0.10) after treatment with Tat (Fig. 3b). Although Tat increased cytokine expression, we confirmed that a single acute dose of Tat (40 ng) protein administered ICV did not induce any neuronal damage as measured by fluorojade B labeling (data not shown).
Figure 3.
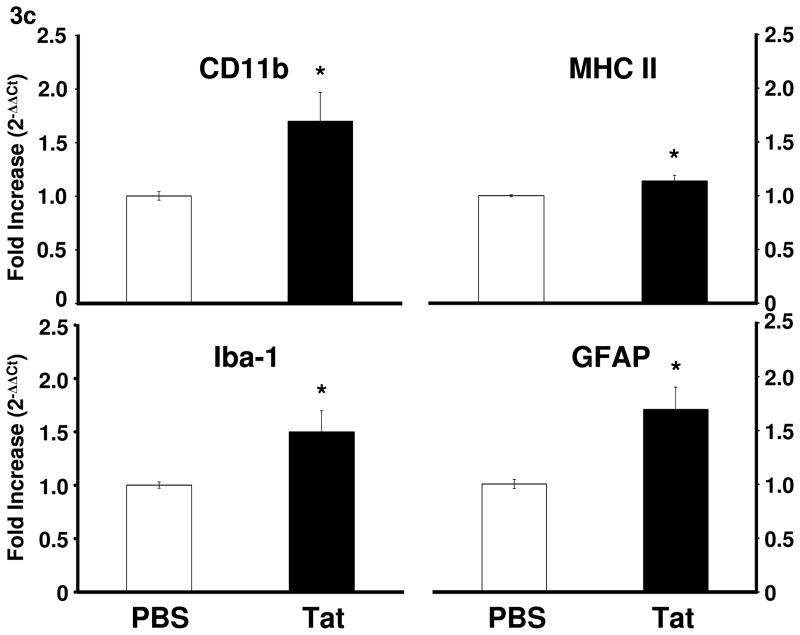
Tat protein administration into the CNS increases proinflammatory cytokine and IDO mRNA in the CNS. (a) Tat injection increased brain expression of IL-1β (p < 0.05), TNF-α (p < 0.05) and IL-6 (p < 0.05) at 4 h post injection. Mean Ct values for PBS group were: IL-1β, 32.5 ± 0.1; TNF-α, 32.5 ± 0.1; IL-6, 32.3 ± 0.1. Tat increased TNF-α (p = 0.05) expression at 24 h after injection. (b) IDO mRNA expression increased at 4 h after Tat treatment (p < 0.01; **) Data represent mean ± S.E.M. (n = 9 mice per group). Mean IDO Ct value for PBS group was 38.9 ± 0.5 (c) Tat increased activation markers of microglia and astrocytes 24 h post-injection (p < 0.05). Data represent mean ± S.E.M. (n = 6 mice per group). Mean Ct values for PBS group were CD11b, 21.3 ± 0.3; MHCII, 22.2 ± 0.4; Iba-1, 22.3 ± 0.4; GFAP, 17.7 ± 0.4.
3.3. Tat increases expression of astrocyte and microglia activation markers in the brain of C57BL/6J mice
To assess the possibility the involvement of glia in response to Tat, we utilized real-time RT-PCR to measure expression of microglial activation markers CD11b, Iba-1, and MHC II and the astrocyte activation marker GFAP in brain tissue. Tat-treated mice displayed increased expression of CD11b (F1,10 = 6.5, p < 0.05), Iba-1 (F1,10 = 6.2, p < 0.05), MHC II (F1,10 = 6.7, p < 0.05), and GFAP (F1,10 = 11.0, p < 0.01) at 24 h after treatment (Fig. 3c), indicating that glial cells are activated by Tat.
4. Discussion
These experiments were designed to determine whether Tat given ICV can induce sickness and depressive-like behavior in mice and the possible reasons for this change. This study was based on the hypothesis that Tat, which is expressed in the brains of HIV-infected individuals (Li et al., 2009) even during HAART therapy, is able to activate brain cytokine signaling and lead to increased expression of IDO. Our findings indicate that a single ICV injection of Tat increases brain IL-1β, TNF-α, IL-6 and IDO expression and results in depressive-like behavior but not sickness behavior.
Prior to the present study on Tat-induced depression-like behavior, the ability of Tat to induce behavioral alterations had not yet been reported, which is in contrast to other HIV protein components such as gp120. Administration of the HIV protein gp120 has been shown to induce sickness and depressive–like behaviors. For example, repeated ICV injection of the HIV protein gp120 caused a reduction in locomotor activity and a loss of body weight in rats. Further, central administration of HIV gp120 reduced preference for saccharin, which was interpreted as evidence of anhedonia (Barak et al., 2002a). These behavioral responses to central administration of gp120 were associated with increased brain expression of IL-1β and TNF-α (Barak et al., 2002b).
In the present experiment, Tat-treated mice did not display the transient sickness episode that has been observed in other models of inflammation-induced depressive-like behavior. This finding is not unique to our model of Tat-induced depressive-like behavior. Young adult mice treated centrally with a single administration of gp120 had normal social interaction behavior (Abraham et al., 2008). This was in contrast to aged mice that responded to gp120 by decreased social investigation, probably because of the increased sensitivity of their primed microglial cells to gp120. The consideration of other indices of sickness other than loss of body weight and decreased social investigation is unlikely to account for the apparent lack of effect of Tat on sickness. These two indices have been repeatedly shown to be very sensitive to inflammatory stimuli administered either peripherally or centrally (see (Dantzer, 2001) for a review). In contrast to its inability to induce sickness behavior, Tat clearly increased duration of immobility in the FST and decreased sucrose preference, two well-accepted measures of depressive-like behavior (Porsolt et al., 1977, Willner et al., 1987).
In this model of acute Tat injection given icv, we found that development of depressive-like behavior occurred in the absence of any major neurodegenerative change. Tat protein has been implicated as a causative agent in the neurodegenerative process associated with prolonged HIV disease (Irish et al., 2009, Li et al., 2009). Indeed, chronic exposure to Tat protein leads to profound neuronal loss as demonstrated in Tat over-expressing mice (Kim et al., 2003, Zhou et al., 2004). In order to discard this possibility, we used fluorojade B labeling 24 h after Tat injection to confirm that neurons are not undergoing degenerative events (Schmued and Hopkins, 2000).
A single acute exposure to Tat protein induced a robust increase in the expression of pro-inflammatory cytokines, as has been demonstrated by others using in vitro systems. Addition of Tat to primary human fetal astrocytes, human peripheral blood mononuclear cells, macrophages, and astrocytic and macrophage cell lines as well as rat microglial primary cultures increases the production of TNFα (Mayne et al., 1998, Nicolini et al., 2001). IL-1β was also found to be produced by Tat-stimulated rat microglial cultures (Nicolini et al., 2001). We chose to study the expression of cytokines and IDO mRNA at the whole brain level rather than in discrete brain areas. The reasons are that we do not know yet where these molecules must be expressed in the brain in order to induce depressive-like behavior. Secondly, brain distribution of the cellular type that is likely to be responsible for the expression of IDO, microglia, is diffuse. Many investigators study the regulation of these molecules in brain areas that are supposed to be critical for development of depression, e.g., the hippocampus, frontal cortex and basal ganglia (see for instance (Norman et al., 2010)). However, there remains a clear lack of evidence that blocking cytokine expression in these brain areas and only in these brain areas is necessary and sufficient for abrogating inflammation-induced depression. In addition, there is already evidence in the case of other brain actions of cytokines that they are expressed and act at distant sites form the brain area in which the response originates. Inflammation-induced activation of the hypothalamic-pituitary-adrenal axis is a typical example. Cytokines do not directly act on CRH-containing neurons in the paraventricular nucleus but rather act at the level of the brain stem the ascending catecholaminergic neurons originating from the ventrolateral medulla (Ericsson et al., 1994).
In the present study, Tat was able not only to increase the expression of brain proinflammatory cytokines but also to induce brain IDO expression. This effect that is consistent with what has been observed in vitro using organotypic cultures of murine hippocampal slices (Fu et al., in preparation). We measured IDO at the mRNA level because its increase is a valid marker of increased IDO enzymatic activity (Curreli et al., 2001, Andre et al., 2008). Although not tested in the context of Tat administration, we have already shown that induction of the tryptophan degrading enzyme IDO in response to inflammation is pivotal in the development of depressive-like behavior in mice (O’Connor et al., 2009a, O’Connor et al., 2009b, O’Connor et al., 2009c).
The lack of a sickness response to Tat occurred despite the fact that Tat induced proinflammatory cytokines in the brain. In view of this lack of effect it is somewhat surprising that Tat was potent enough to induce the expression of IDO and cause development of depression-like behavior. Previous experiments with lipopolysaccharide or Bacillus Calmette-Guerin have repeatedly demonstrated that increased expression of cytokines induces sickness behavior and ultimately leads to depressive-like behavior in response to activation of IDO (O’Connor et al., 2009a, O’Connor et al., 2009b, O’Connor et al., 2009c). It could be argued that the present dissociation between the cytokine response and sickness behavior was due to an insufficient magnitude and/or duration of the cytokine response to Tat. However, it is difficult to envision an infra-threshold cytokine response to Tat that could still lead to IDO activation and depressive-like behavior since these last two responses are supposed to be the result of a too intense or prolonged inflammatory response (Dantzer et al., 2008). An alternative interpretation is that the induction of IDO by Tat and the subsequent development of depressive-like behavior are independent of the cytokine response to Tat.
IDO is normally activated by proinflammatory cytokines including IFN-γ and TNF-α. However, IFN-γ independent pathways of activation of IDO have been described in response to LPS (Fujigaki et al., 2001, Wang et al., 2010) as well as to HIV infection (Maneglier et al., 2009). The IDO gene has several interferon-stimulated response elements (ISRE) and interferon-γ-activated sequences (GAS) elements in its promoter region that are normally required for activation of IDO by IFN-γ. The IDO promoter region also contains consensus sequences for transcriptional factors including AP-1, NF-κβ and NF-IL-6 (Fujigaki et al., 2006). There is already evidence that HIV-1 Tat can activate NF-κβ in brain cells both in vivo (Flora et al., 2005) and in vitro (e.g., (Nicolini et al., 2001).
The cell types in which IDO is activated in response to Tat and which could be responsible for the development of Tat-induced depression-like behavior were not investigated in the present study. Activation of IDO and subsequent alterations in tryptophan metabolism leading to production of the excitotoxin quinolinic acid in macrophages and microglia have already been proposed to play a pivotal role in neuro AIDS (Heyes et al., 1998, Smith et al., 2001). We confirm here that cells of macrophage lineage that are known to reside in the brain, including monocytes, perivascular macrophages and parenchymal microglia, display increased expression of activation markers CD11b, Iba1 and MHC class II expression in response to Tat and are likely to be the cells responsible for producing increased IDO and cytokine expression. Astrocytes could also be implicated since they show increased GFAP expression in response to Tat and they have been shown to respond to Tat by increased IDO (Samikkannu et al., 2009).
In conclusion, these experiments establish that Tat induces a profile of activation of brain cytokines and IDO expression that leads to behavioral disturbances characteristic of human depressive symptoms. These findings confirm the possibility that a biological basis exists that contributes to the increased incidence of depression during HIV infection.
Research Highlight.
HIV Tat-induced neuroinflammation is associated with depressive-like behavior.
Acknowledgments
This work was supported by Grant R01 MH079829 to RD and R01 AG029573 to KWK.
Footnotes
Conflict of interest
Robert Dantzer has received honorarium from Astra-Zeneca, Bristol-Myers-Squibb and Lundbeck Laboratories and is working as a consultant for Lundbeck Laboratories. Keith W. Kelley has received honorarium from Astra-Zeneca.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, et al. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiol Aging. 2008;29:614–21. doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre C, O’Connor JC, Kelley KW, Lestage J, Dantzer R, Castanon N. Spatio-temporal differences in the profile of murine brain expression of proinflammatory cytokines and indoleamine 2,3-dioxygenase in response to peripheral lipopolysaccharide administration. Journal of neuroimmunology. 2008;200:90–9. doi: 10.1016/j.jneuroim.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H, Kokkinidis L, Merali Z. Further evidence for the depressive effects of cytokines: anhedonia and neurochemical changes. Brain, behavior, and immunity. 2002;16:544–56. doi: 10.1016/s0889-1591(02)00011-9. [DOI] [PubMed] [Google Scholar]
- Barak O, Goshen I, Ben-Hur T, Weidenfeld J, Taylor AN, Yirmiya R. Involvement of brain cytokines in the neurobehavioral disturbances induced by HIV-1 glycoprotein 120. Brain research. 2002a;933:98–108. doi: 10.1016/s0006-8993(02)02280-1. [DOI] [PubMed] [Google Scholar]
- Barak O, Weidenfeld J, Goshen I, Ben-Hur T, Taylor AN, Yirmiya R. Intracerebral HIV-1 glycoprotein 120 produces sickness behavior and pituitary-adrenal activation in rats: role of prostaglandins. Brain, behavior, and immunity. 2002b;16:720–35. doi: 10.1016/s0889-1591(02)00025-9. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Chauhan A, Dimayuga FO, Gee J, Keller JN, Nath A. Synaptic transport of human immunodeficiency virus-Tat protein causes neurotoxicity and gliosis in rat brain. J Neurosci. 2003;23:8417–22. doi: 10.1523/JNEUROSCI.23-23-08417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A, Turchan J, Pocernich C, Bruce-Keller A, Roth S, Butterfield DA, et al. Intracellular human immunodeficiency virus Tat expression in astrocytes promotes astrocyte survival but induces potent neurotoxicity at distant sites via axonal transport. The Journal of biological chemistry. 2003;278:13512–9. doi: 10.1074/jbc.M209381200. [DOI] [PubMed] [Google Scholar]
- Curreli S, Romerio F, Mirandola P, Barion P, Bemis K, Zella D. Human primary CD4 + T cells activated in the presence of IFN-alpha 2b express functional indoleamine 2,3-dioxygenase. J Interferon Cytokine Res. 2001;21:431–7. doi: 10.1089/107999001750277916. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain, behavior, and immunity. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora G, Pu H, Lee YW, Ravikumar R, Nath A, Hennig B, et al. Proinflammatory synergism of ethanol and HIV-1 Tat protein in brain tissue. Exp Neurol. 2005;191:2–12. doi: 10.1016/j.expneurol.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Franklin GPaKBJ. Stereotaxic Coordinates. 2. San Diego: Academic Press; 2001. The Mouse Brain. [Google Scholar]
- Fu X, Zunich SM, O’Connor JC, Kavelaars A, Dantzer R, Kelley KW. Central administration of lipopolysaccharide induces depressive-like behavior in vivo and activates brain indoleamine 2,3 dioxygenase in murine organotypic hippocampal slice cultures. Journal of neuroinflammation. 7:43. doi: 10.1186/1742-2094-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs D, Werner ER, Dierich MP, Wachter H. Cellular immune activation in the brain and human immunodeficiency virus infection. Annals of neurology. 1988;24:289. doi: 10.1002/ana.410240227. [DOI] [PubMed] [Google Scholar]
- Fuchs D, Moller AA, Reibnegger G, Stockle E, Werner ER, Wachter H. Decreased serum tryptophan in patients with HIV-1 infection correlates with increased serum neopterin and with neurologic/psychiatric symptoms. Journal of acquired immune deficiency syndromes. 1990;3:873–6. [PubMed] [Google Scholar]
- Fujigaki H, Saito K, Fujigaki S, Takemura M, Sudo K, Ishiguro H, et al. The signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines. J Biochem. 2006;139:655–62. doi: 10.1093/jb/mvj072. [DOI] [PubMed] [Google Scholar]
- Fujigaki S, Saito K, Sekikawa K, Tone S, Takikawa O, Fujii H, et al. Lipopolysaccharide induction of indoleamine 2,3-dioxygenase is mediated dominantly by an IFN-gamma-independent mechanism. Eur J Immunol. 2001;31:2313–8. doi: 10.1002/1521-4141(200108)31:8<2313::aid-immu2313>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Gendelman HE, Lipton SA, Tardieu M, Bukrinsky MI, Nottet HS. The neuropathogenesis of HIV-1 infection. Journal of leukocyte biology. 1994;56:389–98. doi: 10.1002/jlb.56.3.389. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, et al. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. Journal of neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes MP, Rubinow D, Lane C, Markey SP. Cerebrospinal fluid quinolinic acid concentrations are increased in acquired immune deficiency syndrome. Annals of neurology. 1989;26:275–7. doi: 10.1002/ana.410260215. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Brew BJ, Martin A, Price RW, Salazar AM, Sidtis JJ, et al. Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: relationship to clinical and neurological status. Annals of neurology. 1991;29:202–9. doi: 10.1002/ana.410290215. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Brew BJ, Saito K, Quearry BJ, Price RW, Lee K, et al. Inter-relationships between quinolinic acid, neuroactive kynurenines, neopterin and beta 2-microglobulin in cerebrospinal fluid and serum of HIV-1-infected patients. Journal of neuroimmunology. 1992;40:71–80. doi: 10.1016/0165-5728(92)90214-6. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Saito K, Lackner A, Wiley CA, Achim CL, Markey SP. Sources of the neurotoxin quinolinic acid in the brain of HIV-1-infected patients and retrovirus-infected macaques. Faseb J. 1998;12:881–96. doi: 10.1096/fasebj.12.10.881. [DOI] [PubMed] [Google Scholar]
- Irish BP, Khan ZK, Jain P, Nonnemacher MR, Pirrone V, Rahman S, et al. Molecular Mechanisms of Neurodegenerative Diseases Induced by Human Retroviruses: A Review. American journal of infectious diseases. 2009;5:231–58. doi: 10.3844/ajidsp.2009.231.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–94. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell death and differentiation. 2005;12 (Suppl 1):878–92. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. The American journal of pathology. 2003;162:1693–707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Li G, Steiner J, Nath A. Role of Tat protein in HIV neuropathogenesis. Neurotoxicity research. 2009;16:205–20. doi: 10.1007/s12640-009-9047-8. [DOI] [PubMed] [Google Scholar]
- Maneglier B, Malleret B, Guillemin GJ, Spreux-Varoquaux O, Devillier P, Rogez-Kreuz C, et al. Modulation of indoleamine-2,3-dioxygenase expression and activity by HIV-1 in human macrophages. Fundam Clin Pharmacol. 2009;23:573–81. doi: 10.1111/j.1472-8206.2009.00703.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell death and differentiation. 2005;12 (Suppl 1):893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- Mayne M, Bratanich AC, Chen P, Rana F, Nath A, Power C. HIV-1 tat molecular diversity and induction of TNF-alpha: implications for HIV-induced neurological disease. Neuroimmunomodulation. 1998;5:184–92. doi: 10.1159/000026336. [DOI] [PubMed] [Google Scholar]
- Merrill JE, Chen IS. HIV-1, macrophages, glial cells, and cytokines in AIDS nervous system disease. Faseb J. 1991;5:2391–7. doi: 10.1096/fasebj.5.10.2065887. [DOI] [PubMed] [Google Scholar]
- Nicolini A, Ajmone-Cat MA, Bernardo A, Levi G, Minghetti L. Human immunodeficiency virus type-1 Tat protein induces nuclear factor (NF)-kappaB activation and oxidative stress in microglial cultures by independent mechanisms. Journal of neurochemistry. 2001;79:713–6. doi: 10.1046/j.1471-4159.2001.00568.x. [DOI] [PubMed] [Google Scholar]
- Norman GJ, Karelina K, Zhang N, Walton JC, Morris JS, Devries AC. Stress and IL-1beta contribute to the development of depressive-like behavior following peripheral nerve injury. Molecular psychiatry. 2010;15:404–14. doi: 10.1038/mp.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Andre C, Wang Y, Lawson MA, Szegedi SS, Lestage J, et al. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci. 2009a;29:4200–9. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Briley EM, Szegedi SS, Lestage J, et al. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J Immunol. 2009b;182:3202–12. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Molecular psychiatry. 2009c;14:511–22. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olatunji BO, Mimiaga MJ, O’Cleirigh C, Safren SA. Review of treatment studies of depression in HIV. Top HIV Med. 2006;14:112–24. [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Archives internationales de pharmacodynamie et de therapie. 1977;229:327–36. [PubMed] [Google Scholar]
- Porsolt RD, Brossard G, Hautbois C, Roux S. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. In: Crawley Jacqueline N, et al., editors. Current protocols in neuroscience / editorial board. Unit 8. Chapter 8. 2001. p. 10A. [DOI] [PubMed] [Google Scholar]
- Pu H, Tian J, Flora G, Lee YW, Nath A, Hennig B, et al. HIV-1 Tat protein upregulates inflammatory mediators and induces monocyte invasion into the brain. Molecular and cellular neurosciences. 2003;24:224–37. doi: 10.1016/s1044-7431(03)00171-4. [DOI] [PubMed] [Google Scholar]
- Rappaport J, Joseph J, Croul S, Alexander G, Del Valle L, Amini S, et al. Molecular pathway involved in HIV-1-induced CNS pathology: role of viral regulatory protein, Tat. Journal of leukocyte biology. 1999;65:458–65. doi: 10.1002/jlb.65.4.458. [DOI] [PubMed] [Google Scholar]
- Samikkannu T, Saiyed ZM, Rao KV, Babu DK, Rodriguez JW, Papuashvili MN, et al. Differential regulation of indoleamine-2,3-dioxygenase (IDO) by HIV type 1 clade B and C Tat protein. AIDS research and human retroviruses. 2009;25:329–35. doi: 10.1089/aid.2008.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain research. 2000;874:123–30. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Smith DG, Guillemin GJ, Pemberton L, Kerr S, Nath A, Smythe GA, et al. Quinolinic acid is produced by macrophages stimulated by platelet activating factor, Nef and Tat. Journal of neurovirology. 2001;7:56–60. doi: 10.1080/135502801300069692. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lawson MA, Dantzer R, Kelley KW. LPS-induced indoleamine 2,3-dioxygenase is regulated in an interferon-gamma-independent manner by a JNK signaling pathway in primary murine microglia. Brain, behavior, and immunity. 2010;24:201–9. doi: 10.1016/j.bbi.2009.06.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner ER, Fuchs D, Hausen A, Jaeger H, Reibnegger G, Werner-Felmayer G, et al. Tryptophan degradation in patients infected by human immunodeficiency virus. Biological chemistry Hoppe-Seyler. 1988;369:337–40. doi: 10.1515/bchm3.1988.369.1.337. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psycho pharmacology. 1987;93:358–64. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Zhou BY, Liu Y, Kim B, Xiao Y, He JJ. Astrocyte activation and dysfunction and neuron death by HIV-1 Tat expression in astrocytes. Molecular and cellular neurosciences. 2004;27:296–305. doi: 10.1016/j.mcn.2004.07.003. [DOI] [PubMed] [Google Scholar]