Abstract
The Procedural Deficit Hypothesis (PDH) posits that Specific Language Impairment (SLI) can be largely explained by abnormalities of brain structures that subserve procedural memory. The PDH predicts impairments of procedural memory itself, and that such impairments underlie the grammatical deficits observed in the disorder. Previous studies have indeed reported procedural learning impairments in SLI, and have found that these are associated with grammatical difficulties. The present study extends this research by examining the consolidation and longer-term procedural sequence learning in children with SLI. The Alternating Serial Reaction Time (ASRT) task was given to children with SLI and typically-developing (TD) children in an initial learning session and an average of three days later to test for consolidation and longer-term learning. Although both groups showed evidence of initial sequence learning, only the TD children showed clear signs of consolidation, even though the two groups did not differ in longer-term learning. When the children were re-categorized on the basis of grammar deficits rather than broader language deficits, a clearer pattern emerged. Whereas both the grammar impaired and normal grammar groups showed evidence of initial sequence learning, only those with normal grammar showed consolidation and longer-term learning. Indeed, the grammar-impaired group appeared to lose any sequence knowledge gained during the initial testing session. These findings held even when controlling for vocabulary or a broad non-grammatical language measure, neither of which were associated with procedural memory. When grammar was examined as a continuous variable over all children, the same relationships between procedural memory and grammar, but not vocabulary or the broader language measure, were observed. Overall, the findings support and further specify the PDH. They suggest that consolidation and longer-term procedural learning are impaired in SLI, but that these impairments are specifically tied to the grammatical deficits in the disorder. The possibility that consolidation and longer-term learning are problematic in the disorder suggests a locus of potential study for therapeutic approaches. In sum, this study clarifies our understanding of the underlying deficits in SLI, and suggests avenues for further research.
Keywords: Specific Language Impairment, Procedural Memory, Consolidation, Grammar, The Procedural Deficit Hypothesis (PDH)
1. Introduction
Specific Language Impairment (SLI), which appears to affect about 7% of children (Tomblin et al., 1997), is a developmental disorder of language that cannot be accounted for by hearing problems, environmental deprivation, mental retardation, gross neurological insults, or physical abnormalities that lead to speech problems (Bishop, 1992; Leonard, 1998). However, despite the standard use of exclusionary criteria to diagnose SLI, the disorder does not appear to be limited to language. Rather, the linguistic deficits tend to co-occur with various non-linguistic problems, including impairments of motor skills and working memory (Hill, 2001; Leonard, 1998; Montgomery, Magimairaj, & Finney, 2010; Ullman & Pierpont, 2005), which have complicated the understanding of SLI (Leonard, 1998; Stromswold, 2000).
Among the various explanatory hypotheses of SLI, two broad competing perspectives can be distinguished. One perspective suggests that the disorder is caused by a deficit or delay that is specific to certain aspects of language, in particular to grammar (Clahsen, 1989; Gopnik & Crago, 1991; Rice, Wexler, & Cleave, 1995; Rice, Wexler, Marquis, & Hershberger, 2000; van der Lely, 2005). The other broad perspective posits that the underlying cause of SLI is some sort of non-linguistic processing deficit, either of a general sort (Bishop, 1994a; Kail, 1994; Leonard, McGregor, & Allen, 1992; Norbury, Bishop, & Briscoe, 2001), or one that is relatively specific, such as of working memory (Archibald & Gathercole, 2007; Montgomery, 1995) or of briefly presented stimuli or rapidly presented sequences of items (Tallal, Miller, & Fitch, 1993; Tallal & Piercy, 1973).
Hypotheses within both perspectives have been able to account for certain deficits observed in SLI. Those arguing for a purely grammatical deficit have, naturally, been successful in explaining many of the grammatical impairments observed in the disorder. However, such hypotheses cannot account for the non-linguistic problems commonly found in SLI, nor can they easily explain the full range of linguistic deficits, which typically include phonological, morphological and syntactic impairments, as well as difficulties with lexical retrieval. Conversely, hypotheses positing a general or specific processing deficit may be able to explain a number of the non-linguistic (and even some linguistic) deficits, but cannot easily account for the specific pattern of spared and impaired linguistic and non-linguistic functions observed in SLI (Ullman & Pierpont, 2005).
Crucially, all of these previously proposed hypotheses have attempted to explain SLI at a functional rather than neural level, even though it is indubitably the case that the dysfunctions in the disorder originate in the brain. It is possible that an explanatory hypothesis that takes the brain as well as behaviour into account might be better able to tie together the various apparently disparate problems of SLI by linking them to common neural substrates. The Procedural Deficit Hypothesis (PDH) takes just this approach (Ullman, 2004; Ullman & Pierpont, 2005).
According to the PDH, the pattern of impaired linguistic and non-linguistic functions associated with SLI can be largely explained by abnormalities of brain structures that constitute the procedural memory brain system (Ullman & Pierpont, 2005). This memory system is one of several brain systems involved in the implicit acquisition, storage and use of knowledge (Gabrieli, 1998; Squire & Zola, 1996; Willingham, 1998). The system involves a network of brain structures that includes the basal ganglia, the cerebellum and portions of parietal and frontal cortex, including premotor cortex and posterior parts of Broca’s area (e.g., BA 44) (Eichenbaum & Cohen, 2001; Gabrieli, 1998; Knowlton, Mangels, & Squire, 1996; Ullman, 2004; Ullman & Pierpont, 2005). The system underlies a range of perceptual, motor and cognitive skills. In particular, a large literature suggests that it subserves sequencing (Fletcher et al., 2005; Hikosaka et al., 1999; Wilkinson & Jahanshahi, 2007; Willingham, Salidis, & Gabrieli, 2002). However, it also appears to play a role in other functions (Ullman, 2004), including in the learning of probabilistic rules and categorization (Knowlton et al., 1996; Poldrack et al., 2001). Finally, accumulating evidence indicates that procedural memory also subserves the learning and use of rule-governed aspects of grammar, across syntax, morphology and phonology (Ullman, 2001, 2004; Ullman & Pierpont, 2005).
The PDH posits that abnormalities of brain structures underlying procedural memory, in particular portions of frontal/basal-ganglia circuits (especially the caudate nucleus and the region around Broca’s area) and the cerebellum, lead to impairments of the various domains and functions that depend on these structures. Most importantly, procedural memory itself is expected to be impaired, leading to deficits in implicit sequence learning, grammar, and various other tasks and functions that depend on procedural memory. Additionally, other, non-procedural, functions that depend at least in part on these brain structures should tend to be problematic, including working memory, aspects of temporal processing, and lexical retrieval (Ullman, 2004; Ullman & Pierpont, 2005).
Ullman and Pierpont (2005) accompanied their theoretical proposal with a comprehensive review of the brain and behavioural evidence regarding SLI. Overall, the data largely supported the pattern of predictions of the PDH. However, at that time no studies of learning in procedural memory in SLI had yet been published. Rather the PDH was argued on the basis of a wide range of other evidence, and it predicted impairments of procedural learning (that is, learning in procedural memory) in the disorder. We are now aware of seven published studies that have examined aspects of procedural learning in SLI (Adi-Japha, Strulovich-Schwartz, & Julius, In Press; Evans, Saffran, & Robe-Torres, 2009; Gabriel, Maillart, Guillaume, Stefaniak, & Meulemans, 2011; Kemény & Lukács, 2009; Lum, Gelgec, & Conti-Ramsden, 2010; Lum, Conti-Ramsden, Page, & Ullman, In Press; Tomblin, Mainela-Arnold, & Zhang, 2007). Four of these studies (Gabriel et al., 2011; Lum et al., 2010; Lum et al., In Press; Tomblin et al., 2007) examined procedural sequence learning using variations of the Serial Reaction Time (SRT) task (Nissen & Bullemer, 1987). Such sequence learning is also examined in the present study (see Discussion for the other procedural learning studies).
In SRT tasks, participants are typically shown four boxes or circles arranged horizontally across a computer screen. Whenever a stimulus (e.g., a smiley face) appears in one of the four positions, subjects are instructed to press one of four corresponding response keys as quickly and accurately as possible. In the implicit version of this task, participants are not told that the stimuli are presented according to a fixed sequence (usually 10 items long; e.g., 4, 2, 3, 1, 3, 2, 4, 3, 2, 1, where the numbers correspond to the four locations on the screen; Nissen & Bullemer, 1987). Sequence learning is generally operationalized as improvements in the accuracy and/or reaction times (RTs) of responses, as compared to a control condition, which is usually a randomly ordered sequence. When administered as an implicit task, learning in SRT appears to be largely, though not completely (Howard & Howard 1992; Willingham, Nissen, & Bullemer, 1989), incidental and non-conscious (Reber & Squire, 1998; Willingham et al., 2002), and depends on procedural memory brain structures, in particular the basal ganglia, cerebellum, associated motor cortical regions (e.g. pre-motor cortex), as well as portions of prefrontal and parietal cortex (Grafton, Hazeltine, & Ivry, 1995; Peigneux et al., 2000; Rauch et al., 1997) (for a review, see Doyon et al., 2009). It has also been suggested that the hippocampus (not part of the procedural memory system) may be involved in SRT learning (Schendan, Searl, Melrose, & Stern, 2003), although amnesic patients with damage the hippocampus and other parts of the medial temporal lobe or related structure typically show preserved procedural learning (Cavaco, Anderson, Allen, Castro-Caldas, & Damasio, 2004).
Tomblin et al (2007) gave a version of the SRT task to 38 adolescents diagnosed with SLI in kindergarten, and to 47 typically developing (TD) peers. Explicit knowledge of the sequence was not examined. The study reported a significant difference between the two groups in the rate and form of reaction time changes during the pattern sequence portion of the task. Whereas the TD group exhibited the expected initially rapid RT decreases followed by a gradual approach toward an asymptote (Korman, Raz, Flash, & Karni, 2003), the SLI group showed a period of slowed responses prior to rapid learning, and no evidence of an asymptote by the end of training. However, performance by the last pattern block was very similar for both groups. Overall, the findings seem to suggest that although the adolescents with SLI seemed to be able to learn the sequence, they required significantly more trials to do so. Moreover, further analyses using a re-categorization of the adolescents into those with or without either grammar or vocabulary impairments revealed that the grammar impaired but not the normal grammar adolescents showed slowed learning on the SRT task, whereas no differences in learning were found between the vocabulary impaired and normal vocabulary groups. Tomblin et al. concluded that their findings were in line with the predictions of the PDH.
Lum et al. (2010) used another variation of the SRT task to investigate procedural sequence learning in 15 children (7-8 years old) with SLI and 15 TD control children. Sequence specific learning was assessed by subtracting RTs on correct trials in the final pattern block from those in the subsequent control (random) block. Children with SLI showed significantly less sequence learning as compared to TD children. The findings were taken to suggest a procedural learning impairment in SLI. In a more recent study, Lum and colleagues (In Press) examined working memory, declarative memory and procedural memory in 51 children with SLI (mean age 10) and 51 TD control children. Children with SLI were again found to be impaired at procedural sequence learning, even when composite measures of working memory were held constant. Importantly, in both of these studies, none of the children displayed any explicit knowledge of the sequence.
In the fourth study, a probabilistic version of the SRT task was given to 15 children with SLI (ages 7-13) and 15 TD control children (Gabriel et al., 2011). Explicit sequence knowledge was not examined. A group by block interaction that was reported for the pattern blocks may have been consistent with procedural learning problems among the SLI children (see figure on page 340), though no follow up analyses to this interaction were reported. In a linear polynomial comparison over the same blocks, no group differences were found. The authors also reported that the children with SLI were borderline significantly less accurate than the TD children in the final pattern block, although the difference between this block and the subsequent control block did not differ between the groups. The authors conclude that the findings suggest that children with SLI do not display “global” procedural memory deficits.
The present study was designed to fill several important gaps in the literature. First, whereas all previous studies of procedural sequence learning focused on initial sequence learning, that is, within a single test session, we extended the investigation to also examine consolidation (i.e., improvement due to presumed consolidation) (e.g. Doyon et al., 2009; Robertson, Pascual-Leone, & Miall, 2004) and retention of sequence knowledge. Crucially, previous evidence suggests that slower or even intact initial procedural learning may be accompanied by impairments of consolidation and longer-term retention of this knowledge. For example, Vicari et al. (2005) found that whereas children with dyslexia and TD children both showed intact initial procedural learning (in a mirror drawing task), one day later the children with dyslexia showed deficits as compared to the TD group. In another study, Adi-Japha et al (In Press) found that in a grapho-motor procedural learning task, children with SLI showed a later onset of rapid learning in the practice phase, and a lack of the consolidation gains in performance 24 hours post training displayed by the TD group. Second, whereas most studies of procedural learning in SLI have focused on the group differences between SLI and TD children, here we additionally investigated, following Tomblin et al. (2007), the PDH’s specific prediction that grammar deficits, but not vocabulary or broader language deficits, should be associated with procedural learning problems. Third, whereas previous studies have generally excluded language impaired children whose performance IQ (PIQ) fell below the traditional diagnostic cutoff for SLI of -1 SD, we broadened the investigation to include not only children who fell within this range but also those whose PIQ extended to -1.5 SD. This allowed us to test whether or not PIQ plays a role in the procedural learning effects of interest, in order to examine whether the traditional cutoff of -1 SD is useful in studies of procedural learning, and perhaps more generally in the study of SLI (for discussion, see Bishop, 1994b; Bishop & Snowling, 2004; Pearce, James, & McCormack, 2010). Note that for simplicity, here we refer to all such children as SLI.
Finally, instead of a traditional SRT task, we employed the Alternating Serial Reaction Time task (ASRT; Howard, Howard, Japikse et al., 2004; Howard & Howard, 1997; Song, James, Howard, & Howard, 2007). ASRT differs from the original SRT task in that, instead of having pattern and random blocks, random items are interspersed with the pattern throughout the task, e.g., 1-r-2-r-4-r-3 (where the numbers correspond to locations on the screen and r stands for random locations among the four positions). Unlike the SRT task, which sometimes leads to explicit knowledge even when administered as an implicit task (Howard, 1992; Willingham et al., 1989), ASRT learning elicits no explicit knowledge at all, and thus is more likely to be learned in procedural rather than declarative memory (Howard, Howard, Japikse et al., 2004; Howard, Howard, Japikse, & Eden, 2006; Howard & Howard, 1997). Additionally, the alternating random/pattern structure of this task allows one to examine procedural sequence learning continuously, over the course of the task, rather than just at fixed points such as the end of the task in SRT tasks in which one compares pattern and random blocks.
2. Methods
2.1 Participants
Thirty-one children with Specific Language Impairment (SLI) and 31 TD children participated in the study (see Table 1 for participants characteristics of the final set of children included in statistical analysis). The children were recruited from schools in Iowa by the Child Language Research Center at the University of Iowa. Parents or legal guardians provided written consent. Children were initially categorized as having potentially impaired or normal language development on the basis of their scores on classroom-administered listening and reading tasks. They were then given a set of diagnostic language tests (see Table 2). Following the EpiSLI standard (Tomblin et al., 1997; Tomblin, Records, & Zhang, 1996), children who fell at or below -1.14 SD on a Language Composite score (based on the diagnostic language tests and subtests in Table 2) were considered language impaired (SLI), whereas those above this cutoff were considered to have normal language (TD). This composite was computed by dividing the sum of the (sub)test z-scores by the standard deviation of the combined variances and covariances (Crocker & Algina, 1986). Diagnostic testing occurred one to two years before the participants were given the procedural learning task described in the present paper.
Table 1.
Participant characteristics
| Variable | SLI (n = 21)
|
TD (n = 27)
|
Comparison
|
|||
|---|---|---|---|---|---|---|
| M | SD | M | SD | t | p | |
| Age | 10.05 | 1.08 | 9.92 | 1.06 | 0.41 | .686 |
| Handedness | 0.74 | 0.53 | 0.67 | 0.66 | 0.43 | .671 |
| LComp | -1.45 | 0.29 | 0.18 | 0.71 | 9.87 | <.001 |
| PIQ | 90.07 | 11.23 | 105.81 | 17.32 | 3.61 | <.001 |
Note: Abbreviations: LComp = Language Composite Score; PIQ = Performance IQ. Age is expressed in years. Handedness scores are based on the Edinburgh Handedness Inventory (Oldfield, 1971). LComp and PIQ have a mean of 100 and standard deviation of 15.
Table 2.
Tests used for categorization of children into SLI and TD groups.
| Ages 7:0-8:11 | Ages ≥9:0 |
|---|---|
| PPVT-R | PPVT-R |
| CELF-3: | CELF-3: |
|
|
| Nonword Repetition Task | Nonword Repetition Task |
Note: Abbreviations and references: PPVT-R = Peabody Picture Vocabulary Test – Revised (Dunn & Dunn, 1997); CELF-3 = Clinical Evaluation of Language Fundamentals − 3 (Semel, Secord, & Wiig, 1995); Nonword Repetition Task (Dollaghan & Campbell, 1998).
All children were monolingual American English speakers. Apart from their language impairment, none had any known sensory or developmental disorders, including autism, mental retardation, and cerebral palsy, and none had had any head trauma requiring medical care. Additionally, the ADHD Rating Scale-IV, home-version (DuPaul, Power, Anastopoulos, & Reid, 1998) was used to exclude 11 children (7 SLI, 4 TD) with ADHD, yielding 24 SLI and 27 TD children without ADHD. All children had normal hearing (Association, 1997).
2.2 Stimuli and Procedure
Procedural memory, specifically sequence learning, was tested with a version of the Alternating Serial Reaction Time task (ASRT, see above; Howard, Howard, Japikse et al., 2004; Howard & Howard, 1997; Song et al., 2007). As with classic SRT tasks, a sequence of visual stimuli appeared in one of four horizontally arranged locations (indicated by open circles) on the computer screen. The stimulus was a picture of a dog. Participants were asked to press one of four horizontally arranged buttons whenever the stimulus appeared in the corresponding location on the screen. Specifically, they were asked to “catch the dog” as quickly and accurately as possible by pressing the button corresponding to the circle in which the dog appeared. They were instructed to use the middle and index fingers of both hands, with a PST Serial Response Box. Stimuli were presented (on a CRT screen) and response times were acquired with E-Prime version 1.2.
Target locations were determined by a repeating eight-element structure in which fixed and random locations alternated. All participants received the same 8-item sequence pattern, 1r2r4r3r. That is, the dog appeared in position 1 (the left-most circle), then randomly in any of the four circles, then in position 2, and so on. Each stimulus presentation and response constituted one trial. Trials were organized into blocks of 85 trials: 5 warm-up random trials (not included in analyses) followed by 10 repetitions of the 8-item sequence (Howard, Howard, Japikse et al., 2004; Song et al., 2007). Participants were encouraged not to stop during a block, but to take a break for 20 seconds or more after each block. Feedback was given at the end of each block, to guide subjects to an accuracy level of about 92%. All instructions and feedback were displayed visually on the screen as well as read aloud to the participant. The task was self-paced, such that the correct button had to be pressed before a new stimulus would appear on the screen. However, the experimenter controlled the beginning of each new block by clicking the mouse.
Analyses were based on “epochs”, each of which is consisted of 5 consecutive blocks. On the first day of testing (Session 1) subjects completed four epochs (20 blocks), for the examination of initial sequence learning. Session 2, consisting of a single epoch (5 blocks), followed an average of about three days after Session 1, to test for consolidation and retention as well as longer-term sequence learning.
Even though previous studies using the ASRT task have consistently shown that participants (including adults) do not develop explicit knowledge of the pattern (e.g., Howard, Howard, Japikse et al., 2004; Howard et al., 2006; Howard & Howard, 1997), we interviewed each participant after the completion of both test sessions to probe for any signs of explicit awareness of the sequence. As expected, no participant was able to describe any aspect of the pattern.
2.3 Statistical Analysis
Three children with SLI were excluded from the analysis. One child was excluded due to missing data, and two children were excluded because they were outliers: one whose RTs were greater than 2 SDs above the mean of all subjects (in fact, 4.6 SDs above the mean), and the other whose change in RTs during the initial training period (epochs 1-4) showed the opposite pattern to that of all other participants, that is, it increased rather than decreased. Thus, the final set of participants on which analyses were performed was composed of 21 children in the SLI group and 27 children in the TD group. The two groups did not differ in age and handedness (Table 1), or in test session interval (i.e., the number of days between the two test sessions; SLI M = 3.05, SD = 2.36, TD M = 3.52, SD = 4.80, t(46)= 0.41, p = .682) or sex (SLI: 12 boys, 9 girls; TD: 14 boys, 13 girls; χ2 = 0.133, p = .715). Performance IQ (PIQ), which was assessed with the Picture Completion and Block Design subtests of the Wechsler Intelligence Scale for Children, Third edition (Wechsler, 1991), was not matched between the groups (Table 1), and therefore was included as a covariate in all analyses (see below).
Following previous ASRT studies (e.g., Howard & Howard, 1997; Howard, Howard, Dennis, Yankovich, & Vaidya, 2004; Song et al., 2007) sequence learning and memory were examined with “triplet” based analyses (for a detailed description, see Howard, Howard, Dennis et al., 2004). All trials were categorized into either high or low frequency triplets. High frequency (HF) triplets were those in which the trial was the third in a sequence in which the first and third items were consistent with pattern (fixed) items in the sequence (1r2r4r3r). High frequency triplets thus consisted of all triplets ending on a pattern trial as well as those random trials that by chance formed a structure-consistent triplet (e.g., the four possible 1r2 triplets, namely 112, 122, 132, 142). Low frequency (LF) triplets, by contrast, were those in which the trial was the third in a sequence in which this was not the case, and thus the triplet was “structure-inconsistent” (e.g., the sixteen r2r triplets). Repetitions (e.g., 111) and trills (e.g., 121) were excluded from analysis (Howard, Howard, Dennis et al., 2004).
Following previous studies (Howard, Howard, Dennis et al., 2004; Song et al., 2007), the median RTs for HF triplets and LF triplets were calculated separately for each block and for each participant. Next, the mean of these block medians was computed for each participant for each epoch (5 blocks), that is, for epochs 1 through 5 (epochs 1-4 in Session 1, and epoch 5 in Session 2), again separately for HF and LF triplets. Sequence learning was defined as an increasing difference between LF and HF RTs over epochs, reflecting increasing knowledge of HF triplets as compared to LF triplets. RTs for all correct responses, not just for first responses that were correct, were included in these computations. Since each trial proceeds to the next one only when a correct response is made (whether or not it is the first response), RTs for all trials were included in this “final RT” approach. As compared to only including RTs of first responses that were correct, this approach has the advantage of including more data, and thus it more accurately reflects the distribution of responses. Note that with this approach there is no need for separate accuracy analyses: since all trials are included in the RT analyses, those trials with incorrect first responses will simply tend to have longer RTs (due to the fact that their RTs will be from a subsequent response). Finally, since using the simple RT difference between LF and HF triplets may result in participants (and groups) with overall longer response times falsely displaying more learning, the difference between LF and HF RTs was normalized (Dye, Green, & Bavelier, 2009; Faust, Balota, Speiler, & Ferraro, 1999; Madden, Pierce, & Allen, 1996). This was done by dividing, for each participant, the RT difference between LF and HF triplets for each epoch by the average RT (that is, over both LF and HF triplets) for this same epoch: (LF mean RT for epoch X − HF mean RT for epoch X) / ((LF mean RT for epoch X + HF mean RT for epoch X) / 2). Thus, sequence learning was operationalized as an increase over epochs in the normalized RT difference between LF and HF triplets.
Initial sequence learning (i.e., over all four epochs in Session 1) was examined with a mixed-design 2 (between-subjects; Group: SLI vs. TD) × 4 (within-subjects; Epoch: 1 through 4) ANCOVA, with PIQ included as a covariate that also interacted with Epoch (to control for and examine the effect of PIQ on procedural learning). The consolidation (and retention) of sequence learning between Session 1 and 2 was examined with a mixed-design 2 (Group: SLI vs. TD) × 2 (Epoch: 4 vs. 5; that is, the last epoch in Session 1 vs. the single epoch in Session 2) ANCOVA, with the same covariate structure as above. Finally, we examined longer-term sequence learning with another 2 (Group) × 2 (Epoch: 1 vs. 5; that is, the first epoch in Session 1 vs. the single epoch in Session 2) ANCOVA, again with the same covariate structure.
Planned comparisons followed up significant (p < .05) interactions, or main effects with more than one level. Specifically, a significant Group × Epoch interaction was followed up with between-subject (SLI vs. TD) one-way ANCOVAs for all Epochs in the model, as well as within-subject one-way ANCOVAs between the Epochs for each of the two groups (in all cases, with PIQ included as a covariate). Any significant main effect of Epoch in the examination of initial sequence learning (the only circumstance in which a main effect had more than two levels) was followed up with one-way ANCOVA comparisons of epoch 1 to all later epochs (i.e., 2, 3, and 4), across both groups, to examine by which epoch sequence learning had occurred.
3. Results
3.1 SLI versus TD Groups
To examine initial sequence learning (that is, within Session 1), we performed an ANCOVA (Figure 1a) with the factors Group (SLI vs. TD) and Epoch (1-4); see Methods for details. This revealed a main effect of initial sequence learning, over both groups (main effect of Epoch: F(3, 135) = 4.04, p=.009, ), with a medium effect size (i.e., ((2 = .059((2 ;Cohen, 1988). The two groups did not differ in their degree of initial sequence learning (Group × Epoch interaction: F(3, 135) = 0.18, p = .911, ), nor did they differ in the normalized RT differences between LF and HF triplets across all four epochs (main effect of Group: F(1, 45) =0.38, p = .542, ). Planned comparisons from the main effect of Epoch revealed significant differences, over both groups, between epochs 1 and 3 (F(1, 45) = 7.69, p = .008, ) and between epochs 1 and 4 (F(1, 45) = 10.80, p = .002, ), with large effect sizes ( ). The results suggest that both groups showed initial sequence learning by epoch 3.
Figure 1.
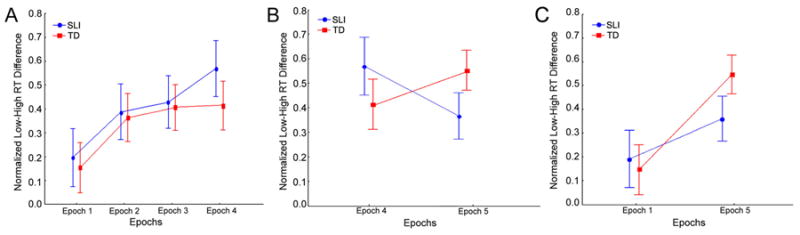
Sequence learning and consolidation for the Specific Language Impairment (SLI) and Typically Developing (TD) groups of children. For both groups, the figure displays the means and standard errors, for each epoch, of the normalized reaction time (RT) difference between Low Frequency and High Frequency triplets, which indicates sequence specific knowledge. These are shown, with Performance IQ (PIQ) covaried out, for (A) initial sequence learning (epochs 1-4); (B) consolidation and retention (epoch 4 vs. epoch 5); and (C) longer-term learning (epoch 1 vs. epoch 5).
To examine consolidation of sequence learning between the final epoch of Session 1 and the single epoch of Session 2, we performed an ANCOVA with Group (SLI vs. TD) and Epoch (4 vs. 5). See Figure 1b. This yielded no main effects of either Group (F(1, 45) = 0.02, p = .886, ) or Epoch (F(1, 45) = 0.18, p = .677, ). However, it revealed a marginally significant Group × Epoch interaction (Epochs 4 vs 5: F(1, 45) = 3.13, p = .083, ), with a medium effect size. Although the lack of significance precludes follow-up comparisons, Figure 1b suggests that this effect was due to an increase in sequence knowledge between epochs 4 and 5 – that is consolidation – for the TD group, but a decrease for the SLI group, suggesting the possibility of a loss of knowledge.
Finally, to examine overall sequence learning, that is, between epoch 1 (in Session 1) and epoch 5 (in Session 2), we performed an ANCOVA with Group (SLI vs. TD) and Epoch (1 vs. 5). See Figure 1c. This approach revealed that the two groups showed longer-term sequence learning, with a large effect size (main effect of Epoch: F(1, 45) = 9.02, p= .004, ). There was no main effect of Group (F(1, 45) = 0.42, p = .522, ) and no Group × Epoch interaction (F(1, 45) = 1.25, p = .270, ), which yielded a small effect size (i.e., ).
3.2 Grammar Impaired versus Normal Grammar Groups
To test whether grammar impairment specifically, rather than language impairment more broadly defined, is associated with deficits at sequence learning and/or consolidation, all children were re-categorized into Grammar Impaired (GI) and Normal Grammar (NG) groups. Following Tomblin et al (2007), children with z-scores at or below -1.14 on a composite of grammar tests were defined as GI (n = 19), whereas those with z-scores above-1.14 were defined as NG (n = 29). The grammar composite was based on the CELF-3 Word Structure, Recalling Sentences, and Sentence Structure subtests for children ages 7 to 8, and on the CELF-3 Formulated Sentences and Recalling Sentences subtests for children 9 to 14 years old at the time of diagnostic testing. The composite score was computed analogously to the Language Composite score described above. This re-categorization resulted in four children changing their language status: one child who was originally categorized as TD was categorized as GI and 3 children who were originally categorized as SLI were categorized as NG. The GI and NG groups did not differ significantly in age (GI M = 10.14, SD = 0.98, NG M = 9.88, SD = 1.11, t(46) = -0.82, p =.414), handedness (GI M = 0.64, SD = 0.65, NG M = 0.74, SD 0.57, t(46) = 0.54 p= .590), sex (GI: 8 girls,11 boys; NG: 14 girls, 15 boys, χ2 = 0.176, p = .675), or in the number of days between the two test sessions (GI M = 2.79, SD = 2.20, NG M = 3.66, SD = 4.68, t(46) = 0.75, p = .456). The difference in PIQ between groups remained significant (GI M = 87.74, SD = 8.33, NG M = 106.26, SD = 16.97, t(46) = 4.4, p < .0001).
First, we investigated initial sequence learning, that is, within Session 1, with an ANCOVA with the factors Group (GI vs. NG) and Epoch (1 through 4). See Figure 2a. This analysis produced a pattern parallel to that observed for the SLI and TD groups in initial sequence learning: a main effect of Epoch, with a medium effect size (F(3, 135)= 3.54, p =.017, ), no Group × Epoch interaction (F(3, 135) = 0.77, p = .517, , small effect size) and no main effect of Group (F(1, 45) = 0.48, p = .492, , small effect size). Planned comparisons for the main effect of Epoch revealed significant differences between epochs 1 and 3 (F(1, 45) = 5.65, p = .023, ) and between epochs 1 and 4, (F(1, 45) = 9.74, p= .003, ), with medium to large effect sizes. These analyses suggest that the GI and NG groups did not differ in their initial sequence learning, and that both learned by Epoch 3.
Figure 2.
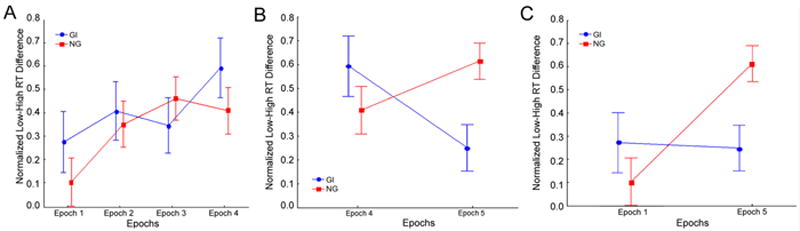
Sequence learning and consolidation for the Grammar Impaired (GI) and Normal Grammar (NG) groups of children. For both groups, the figure displays the means and standard errors, for each epoch, of the normalized reaction time (RT) difference between Low Frequency and High Frequency triplets, which indicates sequence specific knowledge. These are shown, with Performance IQ (PIQ) covaried out, for (A) initial sequence learning (epochs 1-4); (B) consolidation and retention (epoch 4 vs. epoch 5); and (C) longer-term learning (epoch 1 vs. epoch 5).
To examine the consolidation of sequence learning, we performed an ANCOVA with Group (GI vs. NG) and Epoch (4 vs. 5). See Figure 2b. This revealed no main effects of either Group (F(1, 45) = 0.56, p = .459, , small effect size) or Epoch (F(1, 45) = 0.81, p = .371, , small effect size). However, there was a significant Group × Epoch interaction, with a large effect size (F(1, 45) = 7.74, p = .008, ). Consistent with Figure 2b, planned comparisons revealed significantly worse performance by the GI than NG group at epoch 5, with a large effect size (F(1, 45) = 7.41, p = .009, ) but not at epoch 4 (F(1, 45) = 1.10, p = .300, ), which in fact showed the opposite (though non-significant) pattern, with a small effect size. Additionally, the NG group showed some evidence of consolidation, that is, an improvement of performance between Epochs 4 and 5, with a medium effect size, though this effect did not reach statistical significance (F(1, 27) = 2.81, p = .105, ), whereas the GI group showed some evidence of a loss of sequence knowledge during this period, with a borderline significant decrement between Epochs 4 and 5, with a large effect size (F(1, 17) = 3.99, p = .062, ).
The difference between the GI and NG groups in overall sequence learning was also striking (Figure 2c). The Group (GI vs. NG) × Epoch (1 vs. 5) ANCOVA produced a significant main effect of Epoch, with a medium to large effect size (F(1, 45) = 6.56, p = .014, ), though this was qualified by a significant Group × Epoch interaction, also with a medium to large effect size (F(1, 45) = 6.56, p= .014, ) (sic; the values for this interaction are in fact identical to those of the main effect). There was no main effect of Group (F(1, 45) = 0.67, p = .41, ; small effect size). Planned follow-up comparisons for the interaction were consistent with Figure 2c. As we have seen above, the NG group performed significantly better than the GI group in epoch 5. In contrast, the two groups did not differ in epoch 1 (F(1, 45) = 0.89, p = .350, , small effect size). Additionally, whereas the NG group showed robust overall sequence learning, with a large effect size (F(1, 45) = 17.48, p = .0003, ), the GI group did not differ at all between epochs 1 and 5 (F(1, 45) = 0.07, p = .793, ).
It is possible that the observed differences between the GI and NG groups with respect to procedural learning and consolidation were not due to grammar impairments, but to co-occurring impairments of vocabulary. To control for this possibility, we performed the same set of analyses reported above (ANCOVAs for Epochs 1-4, 4 vs. 5, and 1 vs. 5, together with all planned comparisons) but with a vocabulary measure (z-scores from the standardized scores of the PPVT-R; see Table 2) as a covariate (as with PIQ, also interacting with Epoch) in addition to PIQ. These analyses produced precisely the same pattern of significance as the ANCOVAs and planned comparisons with only PIQ as a covariate. Moreover, vocabulary did not interact with Epoch in any of the ANCOVAs (ps > .685). These results suggest that vocabulary deficits do not account for the observed differences between the GI and NG groups on procedural learning and consolidation.
It might also be argued that the observed effects could be explained not just by co-occurring vocabulary deficits, but by broader language impairments other than grammar. We therefore performed the same set of analyses again, but while covarying out such a broader measure instead of just vocabulary. This measure was computed from the linear regression of grammar z-scores predicting the full Language Composite z-scores, yielding a residualized composite language measure from which the variance predicted by the grammar z-scores was removed. This residual represents the full Language Composite (which was used to categorize SLI vs.TD), after removing those aspects of the composite attributable to the grammar measure. With this non-grammar language composite included as a covariate (also interacting with Epoch), in addition to PIQ, the exact same pattern of significance was obtained for all ANCOVAs and planned comparisons as for those analyses with PIQ alone, or with both PIQ and vocabulary as covariates. Additionally, the non-grammar language composite did not interact with Epoch in any of the ANCOVAs (ps > .630). These results suggest that the observed GI/NG group differences on procedural learning and consolidation were not explained by non-grammatical aspects of language impairment.
3.3 Grammar as a Continuous Variable
The analyses presented in the previous section suggest that grammar impairments, and not vocabulary or broader language deficits, predict the observed procedural learning and consolidation problems. Nevertheless, this conclusion might be strengthened by analyses in which vocabulary and the non-grammar composite are treated in an analogous manner to grammar, that is, as dichotomous variables in additional analyses (see Tomblin et al., 2007). However, such an approach was not possible. First, a cutoff of -1.14 for vocabulary z-scores yielded only 8 children in the vocabulary impaired group, too few for a reliable analysis. Additionally, an analogous cutoff could not be computed for the non-grammar composite measure, which consists of residuals of z-scores.
Therefore, we took a different approach for directly comparing the grammar and non-grammar measures in the same way, that is, we treated both as continuous variables – just as vocabulary and the non-grammar language composite, but not grammar, were treated in the analyses above. Importantly, these analyses can tell us whether grammar or non-grammar measures influence procedural learning and consolidation independent of somewhat arbitrary group cutoffs. Additionally, introducing grammar as a continuous variable avoids problems with dichotomous variables, such as spurious effects (false positives) (MacCallum, Zhang, Preacher, & Rucker, 2002).
First, we performed ANCOVAs with Epoch as a categorical variable and Grammar and Vocabulary as continuous variables, as well as PIQ as a continuous covariate. All continuous variables were allowed to interact with Epoch. These ANCOVAs and their associated planned comparisons produced the same pattern of results as the models above in which Grammar group (GI vs. NG) was included as a categorical variable. For initial sequence learning (Epochs 1-4), there was a borderline significant main effect of Epoch, with a small-to-medium effect size (F(3, 132) = 2.58, p= .056, ), and no Grammar × Epoch (F(3, 132) = 0.17, p = .919, ) or Vocabulary × Epoch (F(3, 132) = 0.28, p = .837, ) interactions. Planned comparisons for the main effect of Epoch (which we performed despite the finding that p > .05 because this effect was predicted, based on the analogous findings reported above) yielded differences between Epochs 1 and 3 (F(1, 44) = 5.54, p = .023, , medium effect size) and Epochs 1 and 4 (F(1, 44) = 6.06, p = 0.018, , medium effect size). For the consolidation of sequence learning (Epochs 4 vs. 5), the ANCOVA produced a Grammar × Epoch interaction (F(1, 44) = 6.11, p = .017, , medium effect size), but no Vocabulary × Epoch interaction (F(1, 44) = 0.06, p = .802, ) and no main effect of Epoch (F(1, 44) = 2.24, p = .141, , small effect size). Planned comparisons for the Grammar × Epoch interaction revealed that Grammar had a significant effect in Epoch 5 (F(1, 44) = 4.75, Beta = .453; p = .035, , medium effect size; Beta was computed in an analogous multiple regression; see Figure 3c), such that higher grammar z-scores were associated with larger normalized RT differences between LF and HF triplets, that is, with greater sequence knowledge. In contrast, there was no such effect of Grammar in Epoch 4 (F(1,44) = 1.24, Beta = -.239, p = .272, , small effect size; Figure 3b). Finally, for overall sequence learning (Epochs 1 vs. 5), the ANCOVA yielded a main effect of Epoch (F(1, 44) = 16.80, p = .0002, , large effect size), which was qualified by a Grammar × Epoch interaction (F(1, 44) = 5.74, p = .021, , medium effect size); there was no Vocabulary × Epoch interaction (F(1, 44) = 0.32, p = .572, ). Planned comparisons for the Grammar × Epoch interaction showed a significant effect of Grammar in Epoch 5 (see just above), but not in Epoch 1 (F(1, 44) = 1.30, Beta = -.247, p = .261, , small effect size; Figure 3a).
Figure 3.
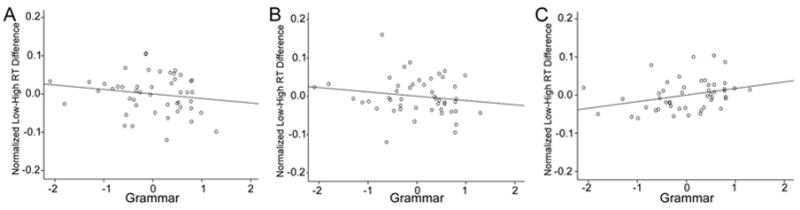
Partial effects of Grammar z-scores on the normalized reaction time (RT) difference between Low Frequency and High Frequency triplets (shown here standardized), which indicates sequence specific knowledge. These are shown, with Performance IQ (PIQ) and Vocabulary covaried out, for (A) epoch 1, (B) epoch 4, and (C) epoch 5.
Second, in analogous analyses, we examined the effects of grammar and the non-grammar language composite, that is, in ANCOVAs with the categorical variable Epoch, and the continuous variable Grammar, Non-Grammar Composite, and PIQ. These produced the same pattern of results obtained in the analyses described just above. For initial sequence learning (Epochs 1-4), the ANCOVA produced a marginally significant main effect of Epoch (F(3, 132) = 2.32, p = .078, , small-to-medium effect size), and no Grammar × Epoch (F(3, 132) = 0.28, p = .837, ) or Non-Grammar Composite × Epoch (F(3, 132) = 0.19, p = .904, ) interactions. Planned comparisons for the main effect of Epoch revealed significant differences between Epochs 1 and 3 (F(1, 44) = 4.75, p = .035, , medium effect size) and Epochs 1 and 4 (F(1, 44) = 5.30, p = .026, , medium effect size). For the consolidation of sequence learning (Epochs 4 vs. 5), the ANCOVA elicited a significant Grammar × Epoch interaction (F(1, 44) = 5.86, p = .020, , medium effect size) but no Non-Grammar Composite } Epoch interaction (F(1, 44) = 0.07, p = .786, ) and no main effect of Epoch, (F(1, 44) = 2.29, p = .138, , small effect size). Planned comparisons for the Grammar × Epoch interaction revealed a marginally significant effect of Grammar in Epoch 5 (F(1, 44) = 3.61, Beta = .411, p = .064, ), with higher grammar levels associated with greater sequence knowledge, but no effect of Grammar in Epoch 4 (F(1, 44) = 1.62, Beta = -.284, p = .210, , small effect size). (The scatterplots for these associations are very similar to those displayed in Figure 3, and thus are not presented here.) For overall sequence learning (Epochs 1 vs. 5), the ANCOVA elicited a main effect of Epoch (F(1, 44) = 15.41, p = .0003, , large effect size), which was qualified by a Grammar × Epoch interaction, (F(1, 44) = 4.40, p = .042, , medium effect size); there was no Non-Grammar Composite × Epoch interaction (F(1, 44) = 0.06, p = .814, ). Planned comparisons for the Grammar × Epoch interaction revealed a marginally significant effect of grammar in Epoch 5 (see just above), but no effect of grammar in Epoch 1 (F(1, 44) = 1.01, Beta = -.227, p = .320, , small effect size).
4. Discussion
In this study, the Alternating Serial Reaction Time task was used to test probabilistic sequence learning and consolidation in a group of children with SLI, as compared to a group of typically developing (TD) control children. Initial sequence learning, that is, within the first test session, was observed in both of these groups, with no differences between them. However, analyses of the consolidation of sequence learning, defined as an improvement in sequence knowledge between the last epoch of Session 1 and the single epoch in Session 2, which took place an average of about three days later, yielded a marginally significant difference between the groups, apparently due to a tendency for the TD but not SLI group to show consolidation. Nevertheless, the two groups showed statistically equivalent longer-term sequence learning, defined as the improvement of sequence knowledge between the first epoch of Session 1 and the single epoch of Session 2.
To test the prediction of the PDH that procedural memory deficits should be associated with grammar impairments specifically, rather than broader language impairments, we re-categorized all of the children on the basis of grammatical ability, that is, as Grammar Impaired (GI) or Normal Grammar (NG). Both of these groups showed initial sequence learning, which was statistically equivalent in the two groups. However, the groups differed in their consolidation of sequence learning and in their longer-term learning, with only the NG group showing signs of either. Indeed, whereas the NG group showed robust longer-term learning, by Session 2 the GI group appeared to have lost whatever sequence knowledge they had gained in Session 1, and showed not the slightest indication of longer-term learning. These findings held even when a measure of vocabulary or a broader non-grammar language measure were controlled for. When grammar was examined as a continuous variable over all children, the same relationship between grammar and procedural memory was observed, even when the vocabulary or the broader non-grammar language measures were controlled for. In these analyses grammar correlated positively with sequence knowledge in Session 2, but not in Session 1, indicating that better grammar was associated with better longer-term procedural sequence learning, but not with better initial sequence learning.
These findings were not accounted for by age, education, handedness or test session interval, none of which differed between either the SLI and TD or the GI and NG groups. As we have seen, the observed effects were also not explained by deficits in either vocabulary or broader non-grammatical aspects of language. Finally, the pattern of results was not explained by performance IQ, which was included as a covariate in all analyses. PIQ did not predict sequence learning or consolidation in any analysis except in the examination of longer-term learning in the comparison between the GI and NG groups, which yielded a significant interaction between PIQ and Epochs 1 vs. 5 (F(1, 45) = 4.72, p = .035, ); planned comparisons showed that PIQ predicted sequence knowledge in Session 2 (Epoch 5: F(1, 45) = 5.0, Beta = -.365, p = .031, ) but not in the first epoch of Session 1 (Epoch 1: F(1, 45) = 0.74, Beta = .151, p = .396, ). Crucially however, these analyses revealed that higher PIQ was associated with lower levels of sequence knowledge in Session 2, whereas better grammar predicted higher levels, as we have seen above. Thus, not only did grammar predict sequence learning with the effects of PIQ covaried out, but the direction of the effect of PIQ in Session 2 was opposite to that of grammar, and this was found only in one analysis. These findings suggest that performance IQ did not explain the observed group differences (SLI vs. TD or GI v. NG) or the effect of grammar on consolidation and longer-term learning.
The present study extends the literature on procedural sequence learning in children with SLI. First, whereas all four previous studies focused on initial sequence learning (Gabriel et al., 2011; Lum et al., 2010; Lum et al., In Press; Tomblin et al., 2007), here we extended the investigation to include consolidation and longer-term learning. The results suggest that both consolidation and longer-term sequence learning may be dysfunctional in SLI. Moreover, as predicted by the PDH, such problems appear to be specifically associated with grammar impairments, rather than with vocabulary or broader non-grammatical language deficits. The findings suggest that children with grammar impairments not only fail to consolidate sequence learning in this task, but actually lose the sequence knowledge that was initially learned. This leads to a lack of any longer-term sequence learning, that is, a failure to retain any sequence knowledge after an average of about three days following initial learning. These findings are broadly consistent with the one other study of the consolidation of procedural learning in SLI (Adi-Japha et al., In Press). That study, which examined grapho-motor procedural learning (see Introduction), observed consolidation gains in TD children 24 hours post-training, but not in children with SLI.
Second, whereas most previous studies of procedural sequence learning in SLI have reported impairments in initial learning, i.e., within the first test session, the analyses presented above did not reveal any such deficits. Rather, no interactions were observed between Epoch on the one hand, and SLI/TD, GI/NG or grammar as a continuous variable, on the other, with learning observed in all cases. In contrast, of the four previous studies of procedural sequence learning, initial learning impairments were found for children with SLI in three studies (Lum et al., 2010; Lum et al., In Press; Tomblin et al., 2007), and may have been present in the fourth (see Introduction; Gabriel et al., 2011). Additionally, initial learning impairments were reported in the study of procedural grapho-motor learning (Adi-Japha et al., In Press), in a study examining probabilistic category learning (Kemény & Lukács, 2009), and in a study of the statistical learning of verbal stimuli (Evans et al., 2009) that appears to rely on procedural memory (McNealy, Mazziotta, & Dapretto, 2010). Thus, the preponderance of previous research, if not all such work, has observed initial procedural learning deficits in SLI.
It is unclear why the previously observed pattern of impaired initial procedural learning in SLI was not found in the present study. It is important to emphasize, however, that the lack of group differences (and the lack of an effect of grammar) on initial sequence learning corresponds to a null effect, which could be due to multiple factors. Such factors might affect group differences in initial sequence learning, but not (or less) differences in consolidation and longer-term learning, perhaps because these effects are simply more robust. It might be suggested that one such factor is insufficient power, due to the small number of subjects. But though a lack of power could reasonably explain marginally significant effects such as in the SLI/TD differences in consolidation, none of the analyses examining initial sequence learning showed any hint of group differences (or any effect of grammar when introduced as a continuous variable), suggesting that lower power on its own did not account for the null effects. Moreover, several of the previous studies that did report group differences had even fewer participants (Adi-Japha et al., In Press; Gabriel et al., 2011; Kemény & Lukács, 2009; Lum et al., 2010). It is also possible that the children with SLI in the present study were simply less impaired at procedural memory than those in previous studies. For example, here we examined a population-based sample of children with language impairment, who were identified for this study in schools. These children could plausibly have been less impaired than children identified on the basis of a previous clinical diagnosis, who constituted the target group in at least some previous studies (Adi-Japha et al., In Press; Gabriel et al., 2011; Kemény & Lukács, 2009). Additionally, because the children here were identified as language impaired one to two years before the present study, some of them might not have still qualified as having such impairments. Moreover, the finding that lower PIQ may be associated with better procedural memory (at least for consolidation) suggests that the inclusion of children with lower PIQ might actually reduce the strength of any procedural memory deficits, though presumably covarying out PIQ eliminated most such effects. Finally, it is possible that initial sequence learning is for some reason easier for children with SLI in the probabilistic ASRT task than in traditional deterministic SRT tasks. Indeed, Gabriel et al. (2011) also did not report clear initial sequence learning deficits with a probabilistic sequence learning task, whereas such deficits were evident in the three other previous sequence learning studies, all of which used deterministic sequences (Lum et al., 2010; Lum et al., In Press; Tomblin et al., 2007). Nevertheless, the notion that probabilistic sequences are easier to learn than deterministic sequences seems somewhat counterintuitive, and to our knowledge is not supported by any independent findings. Further studies may shed light on this issue.
Alternatively, there might in fact be group differences in initial sequence learning in the present study that were simply not captured by the analyses presented above. One intriguing possibility is that the children with SLI did not show saturation of learning. A typical learning curve shows saturation, as reflected in an asymptotic shape, with rapid learning followed by a reduction of learning and a flattening of the curve (Adi-Japha, Karni, Parnes, Loewenschuss, & Vakil, 2008; Hauptmann, Reinhart, Brandt, & Karni, 2005). In the present study, the shape of the learning curves seemed to differ between groups, in particular between the normal grammar (NG) and grammar impaired (GI) groups. As can be seen in Figure 2a, the NG group showed a typical pattern of rapid early learning followed by a flattening of the curve by the end of Session 1, whereas there was no indication of such an asymptote in the GI group. Indeed, post-hoc statistical analyses suggested saturation of learning in the NG group, as reflected by similar levels of sequence knowledge in the last two epochs of Session 1 (Epoch 3: M = 0.0434, SD = 0.0397; Epoch 4: M = 0.0403, SD = 0.0559; t(28) = 0.30, p = .765). In contrast, the GI group showed more than a fifty percent increase in the measure of sequence knowledge between these epochs, though this difference did not reach statistical significance (Epoch 3 M = 0.0390, SD = 0.0554, Epoch 4 M = 0.0604, SD = 0.0371, t (18) = 1.72, p = .102).
Such a possible lack of saturation of learning could also help explain the observed deficits in consolidation and longer-term sequence learning. Previous studies of procedural memory suggest that if the asymptotic phase is not attained (e.g., if training is interrupted before this point), later consolidation gains may not occur (Hauptmann & Karni, 2002; Hauptmann et al., 2005; Karni, 1996), and previously learned sequence knowledge may even be lost (Adi-Japha, Fox, & Karni, 2011), as was found in the present study. Indeed, such a loss has been reported in a study of individuals with ADHD (Adi-Japha et al., 2011), which is highly comorbid with SLI, and whose procedural deficits may have a common neural basis (Tagarelli, Pullman, & Ullman, 2011; Ullman, 2004; Ullman & Pierpont, 2005). In Adi-Japha et al. (2011), both TD individuals and those with ADHD showed initial procedural learning (in the Finger Opposition Task), but only the ADHD individuals showed signs of non-asymptotic initial learning, as well as a loss of procedural knowledge 24 hours later. Thus, in the present study, the observed impairments of consolidation and longer-term learning in SLI could be due to abnormal procedural learning mechanisms, as suggested by the apparently non-asymptotic initial learning curve. Indeed, analyses or visual examination of the learning curves in at least some previous studies of procedural learning in SLI also hint at the possibility that the asymptotic (power law) curve may be abnormal in initial procedural learning (Adi-Japha et al., In Press; Lum et al., In Press; Tomblin et al., 2007). Further studies examining this issue seem warranted.
Third, the present study extends previous research on procedural memory in children with language impairment by demonstrating that deficits of procedural memory are not due to lower PIQ. Quite the opposite, if there is any effect at all of PIQ, it appears to go in the opposite direction, with lower PIQ associated with better procedural consolidation and longer-term learning – though even this pattern must be treated with caution, since it was observed in only one analysis. Thus, the evidence suggests that including language impaired children somewhat below the traditional PIQ cutoff of -1 SD does not lead to spurious effects that would falsely suggest procedural memory impairments in SLI. Rather, their inclusion is likely to either not affect observed procedural memory deficits, or, possibly, it may weaken such effects, resulting in false negatives rather than false positives. Indeed, the lack of an effect of PIQ on procedural memory is consistent with previous studies on other populations, including children with other developmental disorders (Vicari, Verucci, & Carlesimo, 2007; Vinter & Detable, 2003) and unimpaired adults (Gebauer & Mackintosh, 2007; Reber, Walkenfeld, & Hernstadt, 1991). More generally, the absence of any clear impact of PIQ on procedural memory in language impaired children in this study is consistent with previous evidence suggesting that individuals with language impairment with PIQ scores above -1 SD and those with scores below this traditional cutoff do not represent qualitatively different disorders (Bishop, 1994b; Bishop & Snowling, 2004; Pearce et al., 2010).
Overall, the findings support and further refine the PDH. They confirm the prediction that aspects of procedural memory are impaired in SLI. They specifically suggest that the impairments may occur not only in initial learning (e.g., Adi-Japha et al., In Press; Evans et al., 2009; Kemény & Lukács, 2009; Lum et al., 2010; Lum et al., In Press; Tomblin et al., 2007), but also and perhaps even primarily in consolidation and longer-term learning. Moreover, the results are consistent with the prediction of the PDH that procedural impairments in SLI are linked specifically to grammar, rather than to vocabulary or broader language deficits.
The findings also shed light on other explanatory theories of SLI. Impairments on non-linguistic tasks, such as the one used in this study, challenge the strong view that SLI is specific to language, in particular to grammar (Rice, 2000; van der Lely, 2005). The finding that procedural memory impairments are associated with grammar deficits is particularly problematic for such views, as well as for related hypotheses that explain SLI in terms of purely grammatical impairments (Clahsen, 1989; Gopnik & Crago, 1991). The results of the present study also do not seem to be easily explained by other explanatory accounts of SLI. The pattern of procedural memory deficits, as well as the associations between procedural memory and grammar impairments, do not seem to be expected by hypotheses positing that the language impairments in SLI are explained by phonological deficits (Joanisse, 2004) or problems with working memory (Archibald & Gathercole, 2007; Montgomery, 1995). They also do not seem to be accounted for by processing difficulties, either related to general capacity (Bishop, 1994a; Kail, 1994; Leonard et al., 1992; Norbury et al., 2001) or to briefly presented stimuli (Tallal et al., 1993; Tallal & Piercy, 1973), particularly since the ASRT task was self-paced, and thus allowed participants to proceed at a comfortable speed.
The present study has a number of limitations that may be addressed by future studies. First, although the participants displayed no indication of explicit knowledge of the sequences (after either test session), and the ASRT task has been associated with procedural memory brain structures (Bo, Peltier, Noll, & Seidler, 2011; Nemeth et al., Under Preparation), we cannot rule out the possibility that at least some of the observed learning may have taken place in declarative memory – in particular, since declarative memory brain structures have been implicated in implicit learning (Chun & Phelps, 1999; Henke, 2010), and show effects of consolidation (Marshall & Born, 2007; Van Strien, Cappaert, & Witter, 2009). Indeed, the PDH predicts that children with SLI should rely on declarative memory to compensate for grammatical and other deficits caused by procedural memory impairments (Ullman & Pierpont, 2005). In the present study, it is at least plausible that aspects of both grammar and sequence learning may have relied at least in part on declarative memory brain structures, leading to the observed associations between them. Future studies should elucidate this issue. Second, although the present study examined associations between grammar and procedural memory, it did not investigate any causal effects of the hypothesized reliance of grammar on procedural memory. Third, although we demonstrated that grammatical deficits predict impairments in procedural consolidation and longer-term learning with the ASRT task, it remains to be seen if similar findings are obtained with other tasks probing procedural memory. Fourth, here we examined consolidation and longer-term learning an average of three days from initial learning. It would seem warranted to also investigate much longer-term effects, perhaps also with additional training, to see whether individuals with SLI may eventually come to learn in procedural memory, or whether observed language improvements during development (Aram, L., & Nation, 1984; Leonard, 1998) are due to other mechanisms, such as declarative memory (Ullman & Pierpont, 2005). Finally, it seems worthwhile for future studies to examine the possibility, as suggested from this and other studies, that at least one potential procedural memory abnormality in SLI may be related to problems with saturation of learning.
In conclusion, the present study, together with previous research, seems to suggest the following. Children with SLI generally show impairments of procedural memory. These seem to affect not only initial learning, but also consolidation and longer-term learning, perhaps resulting in a loss of any initial learning that may have taken place. At least some of these procedural memory deficits in SLI, both in initial learning (Tomblin et al., 2007), and in consolidation and longer-term learning (in the present study), appear to be associated with the grammar impairments, but not with vocabulary or broader language problems. Overall, these findings support and further specify the PDH, but are not expected by and may be problematic for other explanatory accounts of SLI. The possibility that consolidation and longer-term learning are problematic in the disorder suggests a locus of potential study for therapeutic approaches. In sum, this study clarifies our understanding of the underlying deficits in SLI, and suggests avenues for further research.
Highlights.
Children with Specific Language Impairment (SLI) show procedural memory impairments, as examined with a probabilistic procedural sequence learning task.
The results suggest that consolidation and longer-term procedural learning are impaired in SLI.
These impairments are specifically tied to grammatical deficits, and not to vocabulary or broader language problems.
The results support and further specify the Procedural Deficit Hypothesis (PDH) of SLI.
Acknowledgments
This work was supported by Uppsala University, the Sven Jerring Foundation, the Sunnerdahl Foundation, the Mable H. Flory Charitable Trust, and NIH R01 HD049347. The authors wish to thank Darlene Howard, Jim Howard, Dezso Nemeth, Connie Ferguson, Marlea O’Brien, Marcia ST Clair, Håkan Aldskogius, Lena Karlsson, Matthew Gelfand, and Kaitlyn Litcofsky for various contributions. The authors are deeply grateful to all the participating children and their parents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jonas Persson, Email: jonas.persson@psychology.su.se.
Antoine Tremblay, Email: trea26@gmail.com.
Esther Adi-Japha, Email: japhae@mail.biu.ac.il.
João Veríssimo, Email: jlverissimo@fp.ul.pt.
Cristina D. Dye, Email: c.dye@salford.ac.uk.
Per Alm, Email: per.alm@neuro.uu.se.
Margareta Jennische, Email: margareta.jennische@neuro.uu.se.
J. Bruce Tomblin, Email: j-tomblin@uiowa.edu.
References
- Adi-Japha E, Fox O, Karni A. Atypical acquisition and atypical expression of memory consolidation gains in a motor skill in young female adults with ADHD. Research in Developmental Disabilities. 2011;32:1011–1020. doi: 10.1016/j.ridd.2011.01.048. [DOI] [PubMed] [Google Scholar]
- Adi-Japha E, Karni A, Parnes A, Loewenschuss I, Vakil E. A shift in task routines during the learning of a motor skill: Group-averaged data may mask critical phases in the individuals’ acquisition of skilled performance. Journal of Experimental Psychology: Learning Memory and Cognition. 2008;34:1544–1551. doi: 10.1037/a0013217. [DOI] [PubMed] [Google Scholar]
- Adi-Japha E, Strulovich-Schwartz O, Julius M. Delayed motor skill acquisition in children with language impairment. Research in Developmental Disabilities. doi: 10.1016/j.ridd.2011.05.005. In Press. [DOI] [PubMed] [Google Scholar]
- Aram DM, E BL, Nation JE. Preschoolers with Language Disorders: 10 Years Later. Journal of Speech and Hearing Research. 1984;27:232–244. doi: 10.1044/jshr.2702.244. [DOI] [PubMed] [Google Scholar]
- Archibald LMD, Gathercole SE. The complexities of complex memory span: Storage and processing deficits in specific language impairment. Journal of Memory and Language. 2007;57:177–194. [Google Scholar]
- Association, A.S.-L.-H. Guidelines for audiologic screening. 1997 Available from www.asha.org/policy.
- Bishop DVM. The underlying nature of specific language impairment. Journal of Child Psychology and Psychiatry. 1992;33:3–66. doi: 10.1111/j.1469-7610.1992.tb00858.x. [DOI] [PubMed] [Google Scholar]
- Bishop DVM. Grammatical errors in specific language impairment: Competence or performance limitations? Applied Psycholinguistics. 1994a;15:507–550. [Google Scholar]
- Bishop DVM. Is Specific Language Impairment a valid diagnostic category? Genetic and psycholinguistic evidence. Philosophical Transactions of the Royal Society London B: Biological Sciences. 1994b;346:105–111. doi: 10.1098/rstb.1994.0134. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, Snowling MJ. Developmental dyslexia and specific language impairment: Same or different? Psychological Bulletin. 2004;130:858–886. doi: 10.1037/0033-2909.130.6.858. [DOI] [PubMed] [Google Scholar]
- Bo J, Peltier SJ, Noll DC, Seidler RD. Symbolic representations in motor sequence learning. NeuroImage. 2011;54:417–426. doi: 10.1016/j.neuroimage.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaco S, Anderson SW, Allen JS, Castro-Caldas A, Damasio H. The scope of preserved procedural memory in amnesia. Brain. 2004;127:1853–1867. doi: 10.1093/brain/awh208. [DOI] [PubMed] [Google Scholar]
- Chun MM, Phelps EA. Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nature Neuroscience. 1999;2:844–847. doi: 10.1038/12222. [DOI] [PubMed] [Google Scholar]
- Clahsen H. The grammatical characterization of developmental dysphasia. Linguistics. 1989;27:897–920. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Crocker L, Algina J. Introduction to classical and modern test theroy. Fort Worth, TX: Holt, Rinehart and Winston; 1986. [Google Scholar]
- Dollaghan CA, Campbell TF. Nonword repetition and child language impariment. Journal of Speech Langauge and Hearing Research. 1998;41:1136–1146. doi: 10.1044/jslhr.4105.1136. [DOI] [PubMed] [Google Scholar]
- Doyon J, Bellec P, Amsel R, Penhune VB, Monchi O, Carrier J, et al. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behavioural Brain Research. 2009;199:61–75. doi: 10.1016/j.bbr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Examiner’s Maunal for the PPVT-III. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD rating scale - IV: Checklists, norms, and clinical interpretation. New York, NY: Guilford Press; 1998. [Google Scholar]
- Dye MWG, Green CS, Bavelier D. The development of attention skills in action video game players. Neuropsychologia. 2009;47:1780–1789. doi: 10.1016/j.neuropsychologia.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: Memory systems of the brain. New York: Oxford University Press; 2001. [Google Scholar]
- Evans JL, Saffran JR, Robe-Torres K. Statistical learning in children with specific language impairment. Journal of Speech, Language, and Hearing Research. 2009;52:321–355. doi: 10.1044/1092-4388(2009/07-0189). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust ME, Balota DA, Speiler DH, Ferraro FR. Individual differences in information-processing rate and amount: Implications for group differences in response latency. Psychological Bulletin. 1999;125:777–799. doi: 10.1037/0033-2909.125.6.777. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Zafiris O, Frith CD, Honey RAE, Corlett PR, Zilles K, et al. On the benefits of not trying: brain activity and connectivity reflecting the interactions of explicit and implicit sequence learning. Cerebral Cortex. 2005;15:1002–1015. doi: 10.1093/cercor/bhh201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel A, Maillart C, Guillaume M, Stefaniak N, Meulemans T. Exploration of serial structure procedural learning in children with language impairment. Journal of the International Neuropsychological Society. 2011;17:336–343. doi: 10.1017/S1355617710001724. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD. Cognitive neuroscience of human memory. Annual Review of Psychology. 1998;49:87–115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- Gebauer GF, Mackintosh NJ. Psychometric intelligence dissociates implicit and explicit learning. Journal of Experimental Psychology: Learning Memory and Cognition. 2007;33:34–54. doi: 10.1037/0278-7393.33.1.34. [DOI] [PubMed] [Google Scholar]
- Gopnik M, Crago M. Familial aggregation of a developmental language disorder. Cognition. 1991;39:1–50. doi: 10.1016/0010-0277(91)90058-c. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, Ivry R. Functional mapping of sequence learning in normal humans. Journal of Cognitive Neuroscience. 1995;7:497–510. doi: 10.1162/jocn.1995.7.4.497. [DOI] [PubMed] [Google Scholar]
- Hauptmann B, Karni A. From primed to learn: the saturation of repetition priming and the induction of long-term memory. Cognitive Brain Research. 2002;13:313–322. doi: 10.1016/s0926-6410(01)00124-0. [DOI] [PubMed] [Google Scholar]
- Hauptmann B, Reinhart E, Brandt SA, Karni A. The predictive value of the leveling off of within-session performance for procedural memory consolidation. Cognitive Brain Research. 2005;24:181–189. doi: 10.1016/j.cogbrainres.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Henke K. A model for memory systems based on processing modes rather than consciousness. Nature Reviews Neuroscience. 2010;11:523–532. doi: 10.1038/nrn2850. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K, et al. Parallel neural networks for learning sequential procedures. TINS. 1999;22:464–471. doi: 10.1016/s0166-2236(99)01439-3. [DOI] [PubMed] [Google Scholar]
- Hill EL. Non-specific nature of specific language impairment: A review of the literature with regard to concomitant motor impairments. International Journal of Language and Communication Disorders. 2001;36:149–171. doi: 10.1080/13682820010019874. [DOI] [PubMed] [Google Scholar]
- Howard DV, Howard JH, Jr, Japikse KC, DiYani C, Thompson A, Somberg R. Implicit sequence learning: Effects of level of structure, adult age, and extended practice. Psychology and Aging. 2004 doi: 10.1037/0882-7974.19.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard DV, Howard James H. Adult age differences in the rate of learning serial patterns: Evidence from direct and indirect tests. Psychology and Aging. 1992;7:232–241. doi: 10.1037//0882-7974.7.2.232. [DOI] [PubMed] [Google Scholar]
- Howard JH, Howard DV, Japikse KC, Eden GF. Dyslexics are impaired on implicit high-order sequence learning, but not on implicit spatial context learning. Neuropsychologia. 2006;44:1131–1144. doi: 10.1016/j.neuropsychologia.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Howard JH, Jr, Howard DV. Age differences in implicit learning of higher-order dependencies in serial patterns. Psychology and Aging. 1997;12(4):634–656. doi: 10.1037//0882-7974.12.4.634. [DOI] [PubMed] [Google Scholar]
- Howard JH, Jr, Howard DV, Dennis NA, Yankovich H, Vaidya CJ. Implicit spatial contextual learning in healthy aging. Neuropsychology. 2004;18:124–134. doi: 10.1037/0894-4105.18.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joanisse MF. Specific language impairments in children: Phonology, semantics and the english past tense. Current Directions in Psychological Science. 2004;13:156–160. [Google Scholar]
- Kail R. A method for studying the generalized slowing hypothesis in children with specific language impairment. Journal of Speech and Hearing Research. 1994;37:418–421. doi: 10.1044/jshr.3702.418. [DOI] [PubMed] [Google Scholar]
- Karni A. The acquisition of perceptual and motor skills: a memory system in the adult human cortex. Cognitive Brain Research. 1996;5(1-2):39–48. doi: 10.1016/s0926-6410(96)00039-0. [DOI] [PubMed] [Google Scholar]
- Kemény F, Lukács Á. Impaired procedural learning in language impairment: Results from probabilistic categorization. Journal of Clinical and Experimental Neuropsychology. 2009;32:249–258. doi: 10.1080/13803390902971131. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Korman M, Raz N, Flash T, Karni A. Multiple shifts in the representation of a motor sequence during the acquisition of skilled performance. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12492–12497. doi: 10.1073/pnas.2035019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard L, McGregor K, Allen G. Grammatical morphology and speech perception in children with specific language impairment. Journal of Speech and Hearing Research. 1992;35:1076–1085. doi: 10.1044/jshr.3505.1076. [DOI] [PubMed] [Google Scholar]
- Leonard LB. Children with specific language impairment. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Lum JA, Gelgec C, Conti-Ramsden G. Procedural and declarative memory in children with and without specific language impairment. International Journal of Language & Communication Disorders. 2010;45:96–107. doi: 10.3109/13682820902752285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum JAG, Conti-Ramsden G, Page D, Ullman MT. Working, declarative and procedural memory in Specific Language Impairment. Cortex. doi: 10.1016/j.cortex.2011.06.001. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychological Methods. 2002;7:19–40. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Pierce TW, Allen PA. Adult age differences in the use of distractor homogeneity during visual search. Psychology and Aging. 1996;11:454–474. doi: 10.1037//0882-7974.11.3.454. [DOI] [PubMed] [Google Scholar]
- Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends in Cognitive Sciences. 2007;11:442–450. doi: 10.1016/j.tics.2007.09.001. [DOI] [PubMed] [Google Scholar]
- McNealy K, Mazziotta JC, Dapretto M. The neural basis of speech parsing in children and adults. Developmental Science. 2010;13:385–406. doi: 10.1111/j.1467-7687.2009.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery JW. Sentence comprehension in children with specific language impairment: The role of phonological working memory. Journal of Speech and Hearing Research. 1995;38:187–199. doi: 10.1044/jshr.3801.187. [DOI] [PubMed] [Google Scholar]
- Montgomery JW, Magimairaj BM, Finney MC. Working memory and Specific Language Impairment: An update on the relation and perspectives on assessment and treatment. American Journal of Speech-Language Pathology. 2010;19:78–94. doi: 10.1044/1058-0360(2009/09-0028). [DOI] [PubMed] [Google Scholar]
- Nemeth D, Howard DV, Howard JH, Jr, Dye C, Song SS, Gardian G, et al. Impaired implicit sequence learning in pre-symptomatic Huntington’s disease. Parkinson’s Disease and Related Disorders. Under Preparation. [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: Evidence from performance measures. Cognitive Psychology. 1987;19:1–32. [Google Scholar]
- Norbury CF, Bishop DVM, Briscoe J. Production of English finite verb morphology: A Comparison of SLI and mild-moderate hearing impairment. Journal of Speech Language and Hearing Research. 2001;44:165–178. doi: 10.1044/1092-4388(2001/015). [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pearce WM, James D, McCormack P. A comparison of oral narratives in children with specific language and non-specific language impairment. Clinical Linguistics & Phonetics. 2010;24:622–645. doi: 10.3109/02699201003736403. [DOI] [PubMed] [Google Scholar]
- Peigneux P, Maquet P, Meulemans T, Destrebecqz A, Laureys S, Degueldre C, et al. Striatum forever, despite sequence learning variability: A random effect analysis of PET data. Human Brain Mapping. 2000;10:179–194. doi: 10.1002/1097-0193(200008)10:4<179::AID-HBM30>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Clark J, Pare-Blagoev EJ, Shohamy D, Moyano JC, Myers C, et al. Interactive memory systems in the human brain. Nature. 2001;414:546–550. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Savage CR, Curran T, Kendrick A, Brown HD, et al. Striatal recruitment during an implicit sequence learning task as measured by functional magnetic resonance imaging. Human Brain Mapping. 1997;5:124–132. [PubMed] [Google Scholar]
- Reber AS, Walkenfeld FF, Hernstadt R. Implicit and explicit learning: Individual differences and IQ. Journal of Experimental Psychology: Learning Memory and Cognition. 1991;17:888–896. doi: 10.1037//0278-7393.17.5.888. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Squire LR. Encapsulation of implicit and explicit memory in sequence learning. Journal of Cognitive Neuroscience. 1998;10:248–263. doi: 10.1162/089892998562681. [DOI] [PubMed] [Google Scholar]
- Rice ML. Grammatical symptoms of specific language impairment. In: Bishop DVM, Leonard LB, editors. Speech and language impairments in children: Causes, characteristics, intervention and outcome. East Sussex, UK: Psychology Press Ltd; 2000. pp. 17–34. [Google Scholar]
- Rice ML, Wexler K, Cleave PL. Specific language impairment as a period of extended optional infinitive. Journal of Speech and Hearing Research. 1995;38:850–863. doi: 10.1044/jshr.3804.850. [DOI] [PubMed] [Google Scholar]
- Rice ML, Wexler K, Marquis J, Hershberger S. Acquisition of irregular past tense by children with specific language impairment. Journal of Speech, Language, and Hearing Research. 2000;43:1126–1145. doi: 10.1044/jslhr.4305.1126. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Pascual-Leone A, Miall RC. Current concepts in procedural consolidation. Nature Reviews Neuroscience. 2004;5:576–582. doi: 10.1038/nrn1426. [DOI] [PubMed] [Google Scholar]
- Schendan H, Searl M, Melrose R, Stern C. An fMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. 2003;37:1013–1025. doi: 10.1016/s0896-6273(03)00123-5. [DOI] [PubMed] [Google Scholar]
- Semel E, Secord WA, Wiig EH. Clinical evaluation of language fundamentals. Vol. 3. San Antonio, TX: Psychological Corporation; 1995. [Google Scholar]
- Song S, Howard James H, Jr, Howard DV. Implicit probabilistic sequence learning is independent of explicit awareness. Learning & Memory. 2007;14:167–176. doi: 10.1101/lm.437407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Structure and function of declarative and nondeclarative memory systems. Proceedings of the National Academy of Science USA. 1996;93:13515–13522. doi: 10.1073/pnas.93.24.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromswold K. The cognitive neuroscience of language acquisition. In: Gazzaniga MS, editor. The new cognitive neurosciences. Cambridge, MA: MIT Press; 2000. pp. 909–932. [Google Scholar]
- Tagarelli KM, Pullman MY, Ullman MT. ADHD and language lmpairment: A new perspective on comorbidity. Journal of Cognitive Neuroscience, Supplement. 2011:227. [Google Scholar]
- Tallal P, Miller S, Fitch RH. Neurobiological Basis of speech: A case for the preeminence of temporal processing. Annals of the New York Academy of Sciences. 1993;682:27. doi: 10.1111/j.1749-6632.1993.tb22957.x. [DOI] [PubMed] [Google Scholar]
- Tallal P, Piercy M. Developmental aphasia: Impaired rate of non-verbal processing as a function of sensory modality. Neuropsychologia. 1973;11:389–398. doi: 10.1016/0028-3932(73)90025-0. [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Mainela-Arnold E, Zhang X. Procedural Learning in Adolescents With and Without Specific Language Impairment. Language Learning and Development. 2007;3:269–293. [Google Scholar]
- Tomblin JB, Records NL, Buckwalter P, Zhang X, Smith E, O’Brien M. Prevalence of specific language impairment in kindergarten children. Journal of Speech, Language and Hearing Research. 1997;40:1245–1261. doi: 10.1044/jslhr.4006.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin JB, Records NL, Zhang X. A system for the diagnosis of specific language impairment in kindergarten children. Journal of Speech and Hearing Research. 1996;39:1284–1294. doi: 10.1044/jshr.3906.1284. [DOI] [PubMed] [Google Scholar]
- Ullman MT. A neurocognitive perspective on language: The declarative/procedural model. Nature Reviews Neuroscience. 2001;2:717–726. doi: 10.1038/35094573. [DOI] [PubMed] [Google Scholar]
- Ullman MT. Contributions of memory circuits to language: The declarative/procedural model. Cognition. 2004;92:231–270. doi: 10.1016/j.cognition.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Ullman MT, Pierpont EI. Specific language impairment is not specific to language: the procedural deficit hypothesis. Cortex. 2005;41:399–433. doi: 10.1016/s0010-9452(08)70276-4. [DOI] [PubMed] [Google Scholar]
- van der Lely HKJ. Domain-specific cognitive systems: insight from Grammatical-SLI. Trends in Cognitive Sciences. 2005;9:53–59. doi: 10.1016/j.tics.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Van Strien NM, Cappaert NLM, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal–hippocampal network. Nature Reviews Neuroscience. 2009;10:272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- Vicari S, Finzi A, Menghini D, Marotta L, Baldi S, Petrosini L. Do children with developmental dyslexia have an implicit learning deficit? J Neurol Neurosurg Psychiatry. 2005:1–8. doi: 10.1136/jnnp.2004.061093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari S, Verucci L, Carlesimo GA. Implicit memory is independent from IQ and age but not from etiology: Evidence from Down and Williams syndromes. Journal of Intellectual Disability Research. 2007;51:932–941. doi: 10.1111/j.1365-2788.2007.01003.x. [DOI] [PubMed] [Google Scholar]
- Vinter A, Detable C. Implicit learning is children and adolescents with mental retardation. American Journal on Mental Retardation. 2003;108:94–107. doi: 10.1352/0895-8017(2003)108<0094:ILICAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Wechsler DL. Wechsler Intelligence Scale for Children, Third Edition. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- Wilkinson L, Jahanshahi M. The striatum and probabilistic implicit sequence learning. Brain Research. 2007;1137:117–130. doi: 10.1016/j.brainres.2006.12.051. [DOI] [PubMed] [Google Scholar]
- Willingham D, Salidis J, Gabrieli JD. Direct comparison of neural systems mediating conscious and unconscious skill learning. Journal of Neurophysiology. 2002;88:1451–1460. doi: 10.1152/jn.2002.88.3.1451. [DOI] [PubMed] [Google Scholar]
- Willingham DB. A neuropsychological theory of motor skill learning. Psychological Review. 1998;105:558–584. doi: 10.1037/0033-295x.105.3.558. [DOI] [PubMed] [Google Scholar]
- Willingham DB, Nissen MJ, Bullemer P. On the development of procedural knowledge. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1989;15:1047–1060. doi: 10.1037//0278-7393.15.6.1047. [DOI] [PubMed] [Google Scholar]


