Abstract
The B subunit (RTB) of ricin toxin is a galactose-/N-acetyl galactosamine-specific lectin that promotes attachment and entry of ricin into host cells. RTB is also the archetype of the so-called R-type lectin family, whose members include haemagglutinins of botulinum neurotoxin (BoNT) progenitor toxins, as well as the binding subunits of cytolethal distending toxins. Although RTB is an appealing subunit vaccine candidate, as well as a potential target for immunotherapeutics, the degree to which RTB immunization elicits protective antibodies against ricin toxin remains unresolved. To address this issue, groups of mice were immunized with RTB and then challenged with 5xLD50s of ricin administered intraperitoneally. Despite high RTB-specific serum antibody titers, groups of RTB immunized mice were only partially immune to ricin challenge. Analysis of a collection of RTB-specific B cell hybridomas suggested that only a small fraction of antibodies against RTB have demonstrable neutralizing activity. Two RTB-specific neutralizing monoclonal IgG1 antibodies, 24B11 and SylH3, when passively administered to mice, were sufficient to protect the animals against a 5xLD50 dose of ricin. Both 24B11 and SylH3 blocked ricin attachment to terminal galactose residues and prevented toxin binding to the surfaces of bone marrow-derived macrophages (BMM), suggesting that they function by steric hindrance and recognize epitopes located on RTB’s carbohydrate recognition sub-domains (1α or 2γ). These data raise the possibility of using specific RTB sub-domains, rather than RTB itself, as antigens to more efficiently elicit neutralizing antibodies and protective immunity against ricin.
1. Introduction
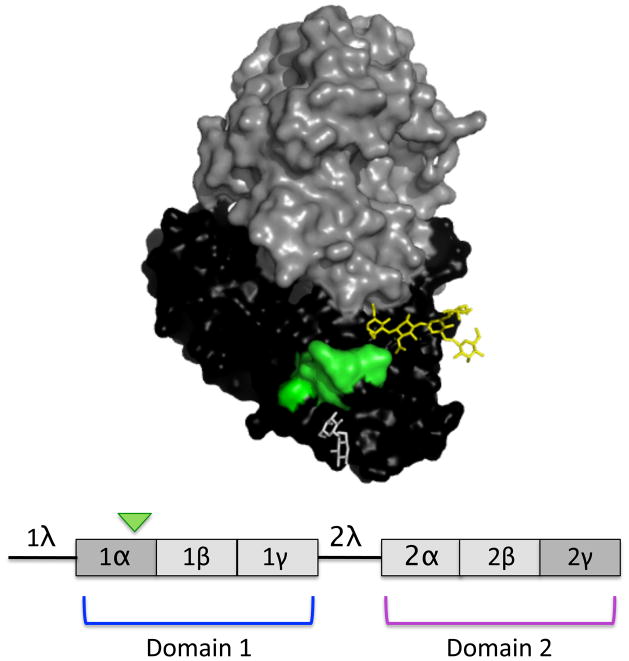
Ricin toxin, a natural by-product of the castor bean plant (Ricinus communis), is one of the most lethal toxins known [1, 2]. The toxin’s A subunit (RTA) is a 267-amino acid RNA N-glycosidase that functionally inactivates eukaryotic ribosomes by selective depurination of a highly conserved adenine residue within ribosomal RNA [3, 4]. The toxin’s B subunit (RTB), a 262-amino acid galactose- and N-acetylgalactosamine-specific lectin, is linked to RTA via a single disulfide bond and mediates RTA attachment and entry into host cells. RTB consists of two globular domains with identical folding topologies (Fig. 1) [5]. Each of the two domains (1 and 2) are themselves comprised of three homologous sub-domains (α, β, γ) that probably arose by gene duplication from a “primordial” carbohydrate recognition domain (CRD) [5]. Only sub-domains 1α and 2γ retain functional carbohydrate recognition activity [6, 7]. Sub-domain 1α binds only galactose and is considered a “low affinity” CRD, whereas sub-domain 2γ binds both galactose- and N-acetylgalactosamine and is considered a “high affinity” CRD [8–10]. The ricin-type (R-type) CRDs constitute a superfamily of lectins found in plants, animals, and toxins expressed by pathogenic bacteria, including Campylobacter jejuni, Haemophilus ducreyi, and Clostridium botulinum [11–16].
Figure 1. Structure of ricin and RTB.
(Upper panel) A 3D depiction of ricin toxin constructed using PyMOL. The subunits are highlighted: RTA (grey), RTB (black), epitope recognized by 24B11 (green), lactose within CRD (white), and mannose side chain (yellow). (Lower panel) Linear depiction of RTB showing domains (1 and 2), as well as individual sub-domains (1α, 1β, 1γ, 2α, 2β, 2γ). 1λ is a peptide linker connecting RTA to RTB in the ricin pre-protein, while 2λ connects the two RTB domains. Only subdomains 1α and 2γ retain carbohydrate recognition activity. The green arrowhead indicates the 24B11 epitope.
Ongoing efforts by public health and defense organizations in the United States and abroad to develop an effective vaccine [17, 18] and immunotherapeutic [19, 20] for ricin toxin, have focused almost exclusively on RTA, despite long-standing evidence for the existence RTB-specific antibodies that are capable of fully neutralizing ricin [21–25]. For example, in 1985, Foxwell and colleagues demonstrated that passive administration of polyclonal antibodies against RTB were as effective as antibodies against RTA in protecting mice against ricin intoxication [24]. In 1987, Colombatti and colleagues described a murine monoclonal IgG (mAb), 75/3B12 that blocked ricin binding to cell surfaces and neutralized ricin in vitro and in vivo [22, 26]. More recently, we characterized a second RTB-specific murine IgG mAb known as 24B11 that was also highly effective at inhibiting ricin attachment to host cells and at neutralizing ricin in vitro [25].
While those studies highlight the potential of antibodies directed against RTB to interfere with the earliest events in ricin intoxication, our understanding of antibody-RTB interactions is far from complete. To date, only two RTB-specific mAbs, 75/3B12 and 24B11, have been characterized in detail, and only one, 75/3B12, has been tested in vivo [22, 25, 26]. Moreover, a recent study by Maddaloni and colleagues challenged the notion that RTB-immunization is sufficient to confer immunity to ricin [27]. Additionally, we and others have reported RTB-specific mAbs that bind ricin with high affinity but lack detectable neutralizing activity, although the epitopes on RTB recognized by these mAbs remain unknown [25, 27].
Therefore, with the long-term objective of developing RTB-based vaccines and therapeutics as countermeasures against ricin toxin as a biothreat agent, the goal of this study was to better define the capacity of RTB to elicit immunity to ricin. In this study, we put forth evidence to suggest that only a very small proportion of antibodies elicited by RTB immunization are capable of neutralizing ricin and conferring protective immunity in vivo. We propose that neutralizing antibodies recognize epitopes near the CRDs within RTB sub-domains 1α and 2γ, whereas non-neutralizing antibodies bind sub-domains not involved in galactose recognition (e.g., 1β, 2α). The fact that both neutralizing and non-neutralizing mAbs bound ricin with roughly equal affinities demonstrates that epitope specificity is likely the primary determinant of antibody-mediated protection. Finally, the results of this study suggest possible strategies to engineer RTB to more efficiently elicit neutralizing antibodies and protective immunity against ricin.
2. MATERIALS AND METHODS
2.1 Chemicals, biological reagents and cell lines
Ricin, RTA, and RTB were purchased from Vector Laboratories (Burlingame, CA). Ricin toxoid (RT) was produced by treatment of holotoxin with paraformaldehyde (4% v/v), as described previously [25]. Ricin and RT were dialyzed against PBS at 4°C in 10,000 MW cutoff Slide-A-Lyzer dialysis cassettes (Pierce, Rockford, IL), prior to use in cytotoxicity studies. Paraformaldehyde (16%) was purchased from Electron Microscopy Sciences (Fort Washington, PA). GlutaMax™, fetal calf serum and goat serum were purchased from Gibco-Invitrogen (Carlsbad, CA). A ClonaCell HY™ kit for hybridoma production was purchased from STEMCELL Technologies (Vancouver, BC, Canada). Unless noted otherwise, all other chemicals were obtained from Sigma-Aldrich (St. Louis, MO). Vero cells, THP-1, and the murine myeloma cell line P3X63.Ag8.653 were purchased from the American Type Culture Collection (Manassas, VA). Cell culture media were prepared by the Wadsworth Center Media Services facility. Monoclonal antibody SylH3 was affinity-purified on a protein G column by the Wadsworth Center protein expression core. Unless otherwise noted, all cell lines and hybridomas were maintained in a humidified incubator at 37°C with 5% CO2.
2.2 Mouse strains, animal care and immunizations
Female BALB/c mice approximately 6–8 weeks of age were purchased from Taconic Labs (Hudson, NY). Animals were housed under conventional, specific pathogen-free conditions and were treated in compliance with the Wadsworth Center’s Institutional Animal Care and Use Committee (IACUC) guidelines. For serum profiling by RTB peptide array and antibody competition analysis by Biacore, female BALB/c mice were immunized by the intraperitoneal (i.p.) route with RTB or RT (50 μg per animal) without adjuvant three times at 10-day intervals. Ten days after the third immunization, blood was collected from the tail vein. For hybridoma production, female BALB/c mice were primed i.p. with RT (50 μg) on day 0, and then boosted by the same route with RT (50 μg) on days 10 and 20.
2.3 B-cell hybridoma production
Four days after the second boost with RT (50 μg), mice were euthanized, and total splenocytes were fused with the myeloma cell line P3X63.Ag8.653, using polyethylene glycol (PEG) as described previously [28]. The resulting hybridomas were seeded in methylcellulose and cloned as per the instructions in the ClonaCell -HY™ hybridoma cloning manual (STEMCELL Technologies, Vancouver, BC, Canada). Hybridomas secreting antibodies of interest were expanded and cultured in either RPMI medium containing 10% fetal calf serum, oxaloacetate, pyruvate, and insulin (OPI), 8 mM GlutaMax™, and penicillin-streptomycin, or in medium A (STEMCELL Technologies) before being transitioned to CD Hybridoma, a serum-free, protein-free, antibiotic-free medium (Gibco-Invitrogen, Carlsbad, CA).
2.4 ELISAs and RTA peptide arrays
ELISAs and peptide arrays were performed as previously described [25]. Briefly, Nunc Maxisorb F96 microtiter plates (ThermoFisher Scientific, Pittsburgh, PA) were coated overnight with ricin, RTA, RTB, BSA (0.1 μg/well) or individual peptides (1 μg/well) in PBS (pH 7.4) before being treated with primary mouse sera, hybridoma supernatants, or purified mAbs. Horseradish peroxidase (HRP)-labeled goat anti-mouse IgG-specific polyclonal antibodies (SouthernBiotech, Birmingham, AL) were used as the secondary reagent. The ELISA plates were developed using the colorimetric detection substrate 3,3′,5,5′-tetramethylbenzidine (TMB; Kirkegaard & Perry Labs, Gaithersburg, MD) and were analyzed with a SpectroMax 250 spectrophotometer, with Softmax Pro 5.2 software (Molecular Devices, Sunnyvale, CA). The RTB peptide array used in this study consisted of 37 15-mers, each overlapping its neighbors by 8 amino acids, collectively spanning the RTB sequence (Table 1). The peptides were synthesized, unbound, in 96 individual tubes, in 96-well plate format, and were provided at 2.5 μmol scale (1.8 mg per peptide, on average) at >75% purity (New England Peptide, Gardner, MA). The peptides were solubilized in DMSO, and aliquots were stored at –20°C.
Table 1.
The sequence of RTB peptides used in this study.
| Peptide | Sequence | RTB Residues |
|---|---|---|
| A1 | ADVCMDPEPIVRIVG | 1–15 |
| A2 | EPIVRIVGRNGLCVD | 8–22 |
| A3 | GRNGLCVDVRDGRFH | 15–29 |
| A4 | DVRDGRFHNGNAIQL | 22–36 |
| A5 | HNGNAIQLWPCKSNT | 29–43 |
| A6 | LWPCKSNTDANQLWT | 36–50 |
| A7 | TDANQLWTLKRDNTI | 43–57 |
| A8 | TLKRDNTIRSNGKCL | 50–64 |
| A9 | IRSNGKCLTTYGYSPG | 57–72 |
| A10 | LTTYGYSPGVYVMIY | 65–77 |
| A11 | PGVYVMIYDCNTAAT | 71–85 |
| A12 | YDCNTAATDATRWQI | 78–92 |
| B1 | TDATRWQIWDNGTII | 85–99 |
| B2 | IWDNGTIINPRSSLV | 92–106 |
| B3 | INPRSSLVLAATSGN | 99–113 |
| B4 | VLAATSGNSGTTLTV | 106–120 |
| B5 | NSGTTLTVQTNIYAV | 113–127 |
| B6 | VQTNIYAVSQGWLPT | 120–134 |
| B7 | VSQGWLPTNNTQPFV | 127–141 |
| B8 | TNNTQPFVTTIVGLY | 134–148 |
| B9 | VTTIVGLYGLCLQAN | 141–155 |
| B10 | YGLCLQANSGQVWIE | 148–162 |
| B11 | NSGQVWIEDCSSEKA | 155–169 |
| B12 | EDCSSEKAEQQWALY | 162–176 |
| C1 | AEQQWALYADGSIRPQ | 169–184 |
| C2 | YADGSIRPQQNRDNC | 176–190 |
| C3 | PQQNRDNCLTSDSNI | 183–197 |
| C4 | CLTSDSNIRETVVKI | 190–204 |
| C5 | IRETVVKILSCGPAS | 197–211 |
| C6 | ILSCGPASSGQRWMF | 204–218 |
| C7 | SSGQRWMFKNDGTIL | 211–225 |
| C8 | FKNDGTILNLYSGLV | 218–232 |
| C9 | LNLYSGLVLDVRASD | 225–239 |
| C10 | VLDVRASDPSLKQII | 232–246 |
| C11 | DPSLKQIILYPLHGD | 239–253 |
| C12 | ILYPLHGDPNQIWLPL | 246–261 |
| D1 | DPNQIWLPLF | 253–262 |
2.5 Vero cell cytotoxicity assays
Vero cell cytotoxicity assays were performed as previously described [19, 25]. Briefly, Vero cells were trypsinized, adjusted to approximately 5 ×104 cells per ml, and seeded (100 μl/well) into white 96-well plates (Corning Life Sciences, Corning, NY), and allowed to adhere overnight. Vero cells were then treated with ricin (10 ng/ml), ricin:mAb mixtures, or medium alone (negative control) for 2 hr at 37°C. The cells were washed to remove non-internalized toxin or toxin:mAb mixtures, and were then incubated for 48 hr. Cell viability was assessed using CellTiter-GLO reagent (Promega, Madison, WI). All treatments were performed in triplicate, and 100% viability was defined as the average value obtained from wells in which cells were treated with medium only.
2.6 Passive protection studies
Individual mAbs were diluted into endotoxin-free PBS and then administered in a final volume of 0.4 ml to female BALB/c mice (ages 8–10 weeks) by i.p. injection at a concentration of 20 ug/animal. Twenty-four hr later, the mice were injected by the same route with ricin (50 μg/kg) that had been diluted into PBS to a final volume of 0.4 ml. Survival was monitored over a 3-day period. In addition, hypoglycemia was used as a surrogate marker of intoxication [19, 29]. Blood (<5 μl) was collected from the tail vein of the animals at 18–24 hr intervals. Blood glucose levels were measured with an Avia ACCU-CHEK handheld blood glucose meter (Roche, Indianapolis, IN). Mice were euthanized when they became overtly moribund and/or blood glucose levels fell below 25 mg/dl. For statistical purposes, readings at or below the meter’s limit of detection of ~12 mg/dl were set to that value.
2.7 Antibody affinity measurements and binding studies
Affinity of antibodies for ricin toxin was determined by surface plasmon resonance (SPR) using a Biacore 3000 (GE Healthcare) instrument. Ricin was attached to a CM5 chip at a density of 550 to 650 RU. HEPES-buffered saline, pH 7.4, with EDTA and P20 added (HBS-EP, pH 7.4) was employed as the running buffer at a flow-rate of 30 μl/min. Serial dilutions of each antibody were made in HBS-EP, pH 7.4 from 600 nM to 18.75 nM, with each concentration series having at least one cycle of a buffer alone injection. Injection times were 3–4 minutes with dissociation times of 10 minutes. Regeneration of the chip surface was performed at a flow-rate of 50 μl/min by two 30s pulses of 10 mM glycine, pH 1.5. The regeneration was followed by a 2 min stabilization period. All kinetic experiments were run a 25°C. Kinetic constants were obtained by analysis using the BIAevaluation software.
Binding studies in the presence and absence of 1% lactose were performed under the following conditions: Running Buffer, HBS-EP, pH 7.4 ± 1% lactose; flow-rate 10 μl/min; injection time of 3 min; regeneration flow-rate of 20 μl/min; regeneration buffer, 10 mM glycine, pH 1.5, with 2 injections of 30s each. Regeneration procedures were followed by a 2 min stabilization time. All running buffers for the binding and kinetics experiments were filtered and degassed before using.
2.8 Isolation and culture of bone marrow-derived macrophages (BMMs)
BMMs were isolated from the femurs and tibias of BALB/c mice, essentially as described [30]. Using sterile technique, bone cavities were flushed using a 27-G needle attached to a 6-cc syringe with Hank’s balanced salt solution (HBSS) until the bone cavity appeared white. The cells were then washed in HBSS and pipetted through a cell strainer (100 μm). Following an additional wash, the cells were re-suspended in 10 ml of RPMI complete supplemented with antibiotics (penicillin:streptomycin at 1X) and macrophage colony stimulating factor (M-CSF, 100 ng/ml) and aliquoted at (1/4 of cells from each mouse per flask). Cells were incubated in T25 culture flasks for 24 hr at 37°C, after which the non-adherent cells were transferred to T75 flasks containing RPMI complete and M-CSF. BMMs were incubated for 4 days at 37°C. Additional 10 ml of RPMI complete + M-CSF were added and incubation was continued for another 3 days.
2.9 Ricin binding assays
For ricin binding assays to freshly isolated BMMs, BMMs were differentiated as described above for 7 days, washed with Ca+2- and Mg+2-free DPBS, and detached by incubating with 2 ml cell dissociation buffer (Invitrogen) for 5–10 minutes at 37°C. Cells were collected into a 50 ml conical tube, washed with CMF-HBSS, spun down at 1000 rpm, 4°C and re-suspended in 2–4 ml HBSS. Cells were aliquoted at ~5× 105 cells per sample into 1.5 Eppendorf tubes. Media was spun down and pre-incubated Ricin-FITC:mAb, or Ricin-FITC:galactose mixture were added to cells in a final volume of 500 μl. Mouse mannose receptor-specific Ab (MMR; CD206) (Biolegend, San Diego, CA) was pre-incubated with cells prior to addition of toxin, otherwise Ricin-FITC:mAb mixtures or Ricin-FITC:galactose were incubated with cells for 30–45 minutes on ice in the dark. Ricin-FITC was added at 2 μg/ml, mAbs were added at 20 μg/ml, galactose at 30 mg/ml, and MMR Ab was added at 10 μg/ml. Cells were washed 2x with HBSS and ricin-FITC binding to cell surface was measured using a FACS Calibur flow cytometer (BD Biosciences). A minimum of 20,000 cells were analyzed per sample.
To determine if mAbs prevent ricin binding to asialofetuin (ASF), Nunc Maxisorb F96 microtiter plates (ThermoFisher Scientific) were coated with ASF (0.4 μg/well) (EY Laboratories, San Mateo, CA) in PBS (pH 7.4) for 18 hr at 4°C. Plates were washed with PBS-Tween-20 (0.05% v/v), blocked with 2% goat serum in PBS-Tween-20, and then overlayed with biotinylated ricin (50 ng/ml) and IgG mAbs (20 μg/ml) for 1 hr. The plates were washed to remove unbound toxin, labeled with avidin-HRP (0.4 μg/ml) and developed using TMB, as described above for ELISAs.
To determine if mAbs bind the galactose binding pockets of ricin, plates were coated with ASF for 18 hr at 4°C. Plates were washed with PBS-Tween-20 (0.05% v/v), blocked with 2% goat serum in PBS-Tween-20, overlayed with ricin (10 μg/ml) for 1 hr, then with mAbs (10 μg/ml) for 1 hr. Plates were then labeled with IgG-HRP and developed using TMB, as described above for ELISAs.
2.10 Apoptosis assays
THP-1 human monocytes (1 × 105 cells per sample) were subjected to ricin (1.0 μg/ml) in the presence or absence of anti-RTB mAbs (15 μg/ml) for 4–5 hr in an incubator at 37°C and 5% CO2. After incubation, the cells were collected by centrifugation and then re-suspended in 1X binding buffer (100 μl) containing 5 μl of Annexin V-FITC and 5 μl PI, as recommended by the manufacturer (BD Pharmingen). Samples were assayed for apoptosis and necrosis using a FACS Calibur (BD Biosciences). Results were reported as % cells positive for Annexin V-FITC or PI. A minimum of 10,000 cells were analyzed per sample.
2.11 Statistical analysis and software
Statistical analysis was carried out with GraphPad Prism 5 (GraphPad Software, San Diego, CA). The open-source molecular visualization software PyMOL (DeLano Scientific LLC, Palo Alto, CA) was used for epitope modeling.
3. RESULTS
3.1 Immunization of mice with RTB confers partial protection against ricin challenge
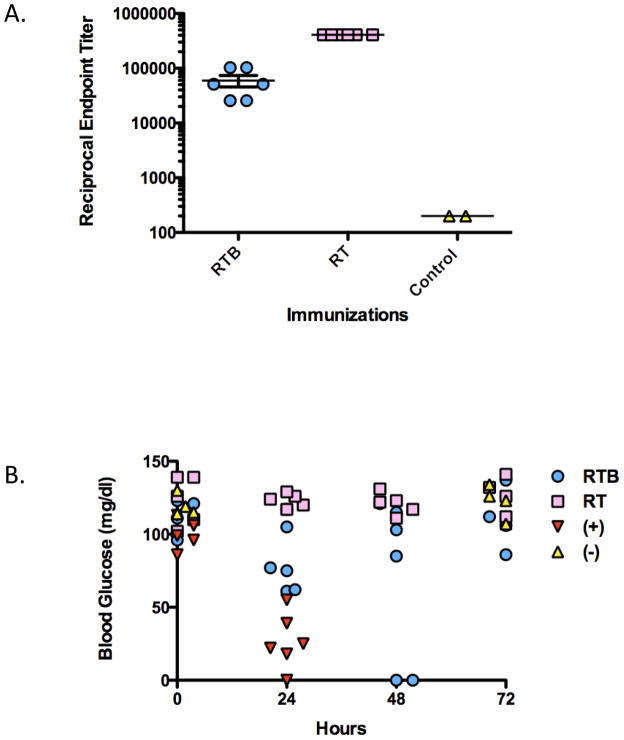
In order to determine if RTB is capable of eliciting a protective immune response against ricin toxin, we immunized BALB/c mice intraperitoneally (i.p.) with RTB (50 μg/animal) three times at 10-day intervals. For comparison, we also immunized groups of mice with formalin-inactivated holotoxin (RT) at the same concentration. Both RTB-and RT-immunization elicited high serum antibody titers against RTB and ricin holotoxin, as compared to unimmunized control animals (Fig. 2A). Antisera against RT were found to neutralize ricin in an in vitro Vero cell cytotoxicity assay, however antisera against RTB did not (data not shown). Two weeks following the third immunization with RTB or RT, mice were challenged with 5xLD50s of ricin toxin (50 μg/kg). Hypoglycemia and mean time to death were used as indicators of immunity [29]. As shown in Fig. 2B, non-immunized control mice experienced a rapid decline in blood glucose levels and expired 24 hr post ricin challenge. On the other hand, RT-immunized animals survived ricin challenge and had no demonstrable reduction in blood glucose levels. At 24 hr post toxin challenge, RTB-immunized mice demonstrated outward signs of discomfort (e.g., ruffled appearance) and had blood glucose levels that were on average 40–50% lower than pre-challenge levels. However, 4 of the 6 mice in the RTB-immunized group regained normal blood glucose levels by 72 hr and survived challenge. The remaining two animals in the RTB-immunized group expired by 48 hr. These results demonstrate that RTB elicits partial protection against ricin toxin challenge.
Figure 2. Immunity to ricin imparted by immunization with RTB.
BALB/c mice were immunized by the i.p. route with RTB or RT 3 times at 10 day intervals, and then challenged with ricin (50 μg/kg) two weeks later. (A) Ricin-specific serum IgG titers after the third immunization. Each datum point represents a single mouse and shows the mean with SEM for one representative experiment. (B) Survival following ricin challenge, as assessed by blood glucose levels. Blood glucose levels were measured at indicated times (t = 0, 24, 48 and 72 hr) post-toxin challenge. Healthy blood glucose levels were considered to be >80 mg/dl and intoxicated mice have blood glucose levels ≤40 mg/dl.
3.2 Ricin neutralizing antibodies are rare following RTB immunization
The fact that RTB immunization elicited high anti-ricin serum antibody titers, but conferred only partial protection against toxin challenge led us to hypothesize that RTB stimulates an overwhelmingly non-neutralizing antibody response. To examine this issue, we screened a panel of ricin-specific B-cell hybridoma supernatants derived from RT immunized mice for (i) reactivity with RTB and (ii) ricin neutralizing activity, as determined in a Vero cell cytotoxicity assay. Of ~1000 ricin-specific B cell hybridoma colonies that were screened, we estimated that only ~10% secreted antibodies that bound RTB, and only a single clone among these secreted a mAb with demonstrable ricin neutralizing activity. This mAb was designated SylH3 (Table 2). These data suggest that RTB, while highly immunogenic, is relatively poor at eliciting neutralizing antibodies.
Table 2.
Properties of neutralizing and non-neutralizing RTB mAbs.
| mAb | Subdomain | Peptide | Epitope | Range (aa) | KD [M]a | Neutralizing | Protection |
|---|---|---|---|---|---|---|---|
| 24B11b | 1α | A5–A6 | PCKSNT | 38–43 | 4.2 × 10−9 | Yes | Yes |
| SylH3 | ND | - | ND | ND | 3.38 × 10−9 | Yes | Yes |
| JB11 | 1β | A8 | TLKRDNTIRSNGKCL | 50–64 | ND | No | |
| CB12 | 1β | A12 | YDCNTAATDATRWQI | 78–92 | 6.74 × 10−8 | No | |
| SA3 | 1β | B1 | TDATRWQIWDNATII | 85–99 | ND | No | |
| TFTB-1 | 2α | C1 | AEQQWALYADGSIRPQ | 169–184 | 5.63 × 10−9 | No |
, as determined by Biacore using a ricin-coated chip.
, as described previously by McGuinness and Mantis [25].
3.3 Subunit specificity and affinity of SylH3 for ricin
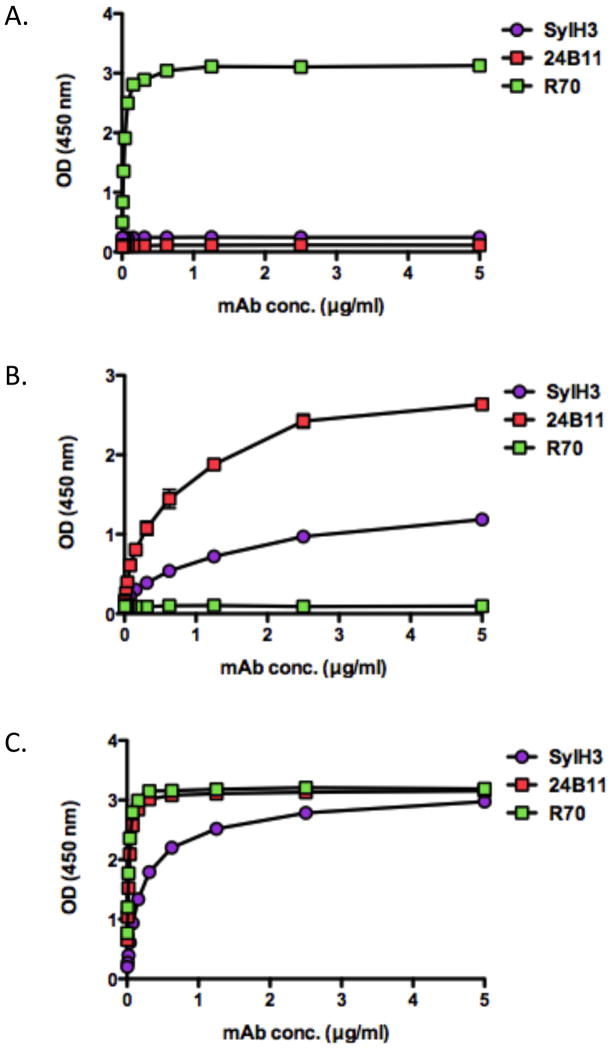
We sought to better characterize SylH3, as it is one of only three RTB-specific mAbs known to have demonstrable toxin neutralizing activity. The other two are 75/3B12 [22] and 24B11 [25], as described in the Introduction. The subunit reactivity profile of SylH3 was determined by ELISA and compared to the reactivity profiles of 24B11 and R70, the well-characterized RTA-specific neutralizing mAb [25, 26]. As expected, SylH3 bound RTB but not RTA (Fig. 3). However, SylH3 reactivity was greatest against ricin holotoxin, possibly because the mAb recognizes an epitope whose conformation is affected by RTB’s association with RTA. This profile differed from that of 24B11, which bound RTB almost as well as ricin holotoxin. The actual affinity of SylH3 for ricin holotoxin was determined by Biacore analysis, as done previously in our laboratory [19, 28]. The dissociation (KD) constant of SylH3 for ricin was 3.38 × 10−9 M (Table 2). Therefore, SylH3’s affinity for ricin is similar to that of 24B11 and R70 [25].
Figure 3. Reactivity profiles of SylH3 with RTA, RTB and ricin holotoxin.
96-well microtiter plates were coated with (A) RTA, (B) RTB or (C) ricin and then probed with mAbs SylH3, 24B11, or R70, at indicated concentration, as described in the Methods. R70 is an anti-RTA mAb that served as a control. Each symbol (shown with SEM) represents the average of at least three replicate wells. The SEM may be too small to visualize in the figure.
We next performed competitive antibody binding assays using the Biacore instrument to examine whether 24B11 and SylH3 bound similar epitopes on RTB. A CM5 chip was coated with ricin, saturated with SylH3, and then probed with 24B11. The binding of 24B11 to RTB was not precluded by the occupancy SylH3, as evidenced by the sharp increase in resonance units upon addition of 24B11 to the SylH3-saturated chip (data not shown). Conversely, 24B11 did not interfere with SylH3 binding to ricin holotoxin, thereby demonstrating conclusively that 24B11 and SylH3 recognize distinct epitopes on RTB.
3.4 SylH3 neutralizes ricin in vitro
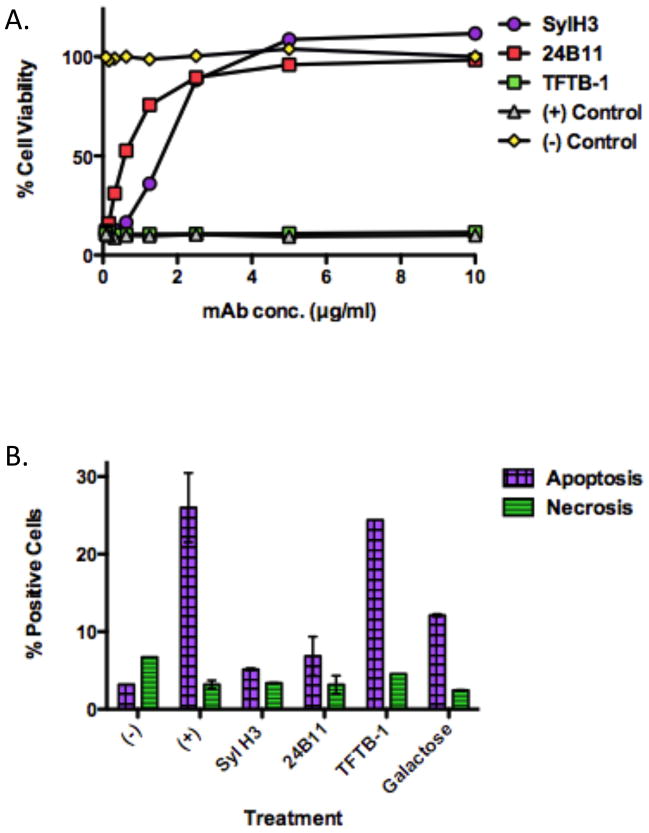
As a means to further characterize SylH3, the mAb was compared directly with 24B11 for the ability to neutralize ricin in a Vero cell cytotoxicity assay. As an additional control for these studies, TFTB-1, a known RTB-specific, non-neutralizing mAb was tested alongside 24B11 and SylH3 [25]. As shown in Fig. 4A, SylH3 was as potent as 24B11 in neutralizing ricin. TFTB-1, on the other hand, had no detectable toxin neutralizing activity, despite the fact that it binds ricin holotoxin with nanomolar affinity (see below; Table 2). Because macrophages are postulated to be a primary target of ricin intoxication in both systemic and mucosal compartments [31], we next examined whether SylH3 could protect THP-1 cells, a human monocyte cell line, from ricin-induced apoptosis. THP-1 cells were incubated with ricin holotoxin in the presence of SylH3, 24B11 or TFTB-1 for 5 hr at 37°C. The cells were then assessed for apoptosis and necrosis using Annexin V-FITC and propidium iodide (PI) staining, respectively. Compared to ricin-treated control cells, both SylH3 and 24B11 significantly reduced toxin-induced apoptosis and toxin-induced necrosis (Fig. 4B). Indeed, protection conferred by SylH3 and 24B11 was even better than that achieved by galactose (30 mg/ml), a known competitive inhibitor of ricin attachment to cell surfaces.
Figure 4. In vitro neutralizing activity of SylH3 and 24B11.
mAbs SylH3, 24B11 or TFTB-1 were assessed for their capacity to protect Vero cells or THP-1 cells from the cytotoxic effects of ricin (A) Ricin (10 ng/ml) was incubated for 1 hr with each mAb at the indicated concentrations, and then applied in triplicate to Vero cells grown in 96-well microtiter plates. Cell viability was assessed 48 hr later. Each symbol (with SEM) represents the average of at least three replicate wells. (B) mAbs were assessed for their capacity to protect THP-1 human monocytes from apoptosis in the presence of ricin. Ricin (1 μg/ml) was incubated with indicated mAbs (15 μg/ml) or galactose (30 mg/ml) for 1 hr, and then added to THP-1 cells (1×105 per sample). The cell mixtures were incubated for 5 hr at 37°C, then stained with FITC-Annexin V to assess apoptotic cells, and PI to detect for necrotic cells, before being subjected to flow cytometry. As controls, cells were not treated with ricin (-) or treated with ricin but no antibody or galactose (+). Each bar represents the average of two replicates plotted as the mean with SEM.
3.5 SylH3 and 24B11 protect mice against ricin
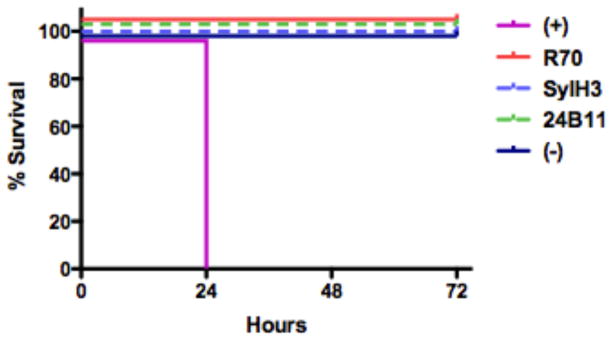
SylH3 was next compared to 24B11 and R70 in its ability to protect mice against ricin challenge. Groups of 10-week old BALB/c mice (~20 g) were administered 20 μg of SylH3, 24B11 or R70 by i.p. injection, and then challenged 24 hr later with 5xLD50s of ricin toxin by the same route. Both 24B11 and SylH3 fully protected mice against ricin-induced death, and protection was comparable to that achieved with R70 (Fig. 5). On the other hand, control mice that received ricin but no antibody expired within 24 hr. Similarly, mice treated with TFTB-1 died within 24 hr (data not shown). These data demonstrate that SylH3 and 24B11 are capable of neutralizing ricin in vitro and in vivo as effectively as mAbs against RTA.
Figure 5. SylH3 and 24B11 passively protect mice against ricin intoxication.
Groups of BALB/c mice were passively immunized with the following mAbs (20 μg mAb/animal) and then challenged 24 hr later with 5xLD50s of ricin: SyH3 (dashed light blue line), 24B11 (dashed green line) or R70 (red line). As controls, animals were not challenged with ricin (solid dark blue line) or challenged with ricin in the absence of mAb treatment (solid pink line). Negative control mice succumbed to ricin intoxication 24 hr post-ricin challenge, whereas the remaining animals survived. Each group contained n=5 mice except for the negative control group which had n=3 mice. This data is from one representative experiment.
3.6 SylH3 blocks ricin attachment to cell surface galactosides
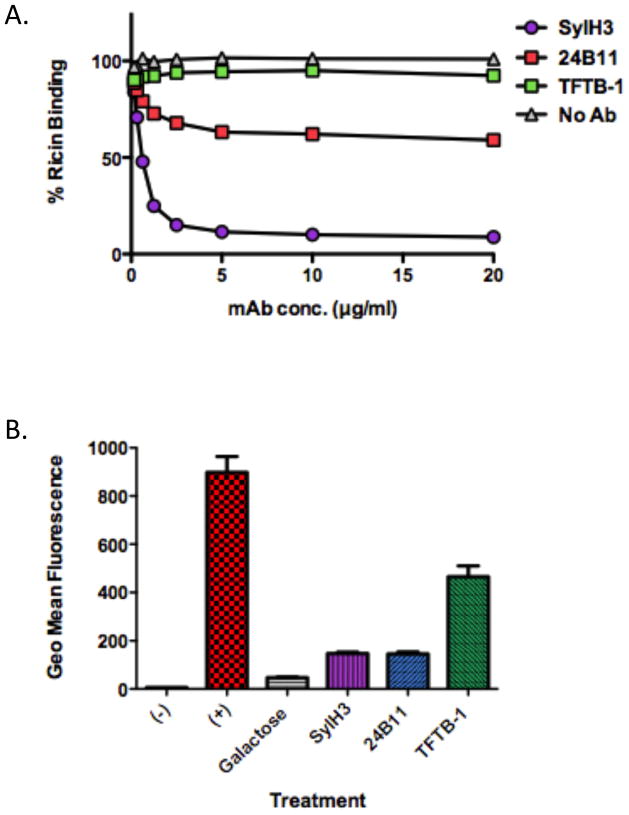
We employed a solid phase binding assay in order to determine if SylH3 interferes with the ability of RTB to associate with terminal galactose residues on cell surfaces. The serum glycoprotein, ASF served as a surrogate source of terminal galactose residues (see Materials and Methods). Biotin-labeled ricin (50 ng/ml) was incubated with a range of concentrations (0.25–20 μg/ml) of SylH3, 24B11, or TFTB-1, and then applied to 96-well microtiter plates coated with ASF. As shown in Fig. 6A, SylH3 was a potent inhibitor of ricin attachment to ASF, with an estimated IC50 of <1 μg/ml. 24B11, on the other hand, was only moderately effective at blocking ricin attachment. Indeed, even at 20 μg/ml, 24B11 only reduced ricin binding by ~40%. As expected, TFTB-1 had little to no effect on ricin binding to ASF.
Figure 6. SylH3 and 24B11 inhibit ricin binding to terminal galactosides.
(A) Biotin-labeled ricin (50 ng/ml) was mixed with indicated mAbs (20 μg/ml) and then applied to 96-well microtiter plates coated with ASF (4 μg/ml). Biotin-ricin binding to ASF was detected by incubation with avidin-HRP and TMB substrate, as for a standard ELISA. Each symbol represents the average of at least three replicate wells. (B) FITC-labeled ricin (2 μg/ml) was mixed with indicated mAbs (20 μg/ml) or galactose (30 mg/ml), and then applied to BALB/c-derived BMMs (5×105 per sample). Following a 30–45 min incubation at 4°C, the cells were subjected to analysis by flow cytometry. As controls, cells were not treated with ricin (-) or treated with ricin in the absence of mAbs or galactose (+). Each bar represents the average of two replicates plotted as the mean with SEM.
The ability of these mAbs to inhibit ricin attachment to cell surfaces was further assessed using bone marrow derived macrophages (BMMs) and flow cytometry. FITC-labeled ricin was mixed with SylH3, 24B11 or TFTB-1, and then incubated with BMMs for 30–60 min. These studies were done at 4°C to permit toxin binding but not internalization. The cells were subsequently washed to remove unbound toxin, and analyzed by flow cytometry. We observed that both SylH3 and 24B11 reduced ricin binding to BMM cell surfaces by ~90%, which was roughly equivalent to effect of galactose on toxin binding (Fig 6B). Surprisingly, TFTB-1 also impacted toxin attachment to some degree (~40%), suggesting that even the association of a non-neutralizing mAb is sufficient to interfere with ricin-host cell interactions in this assay.
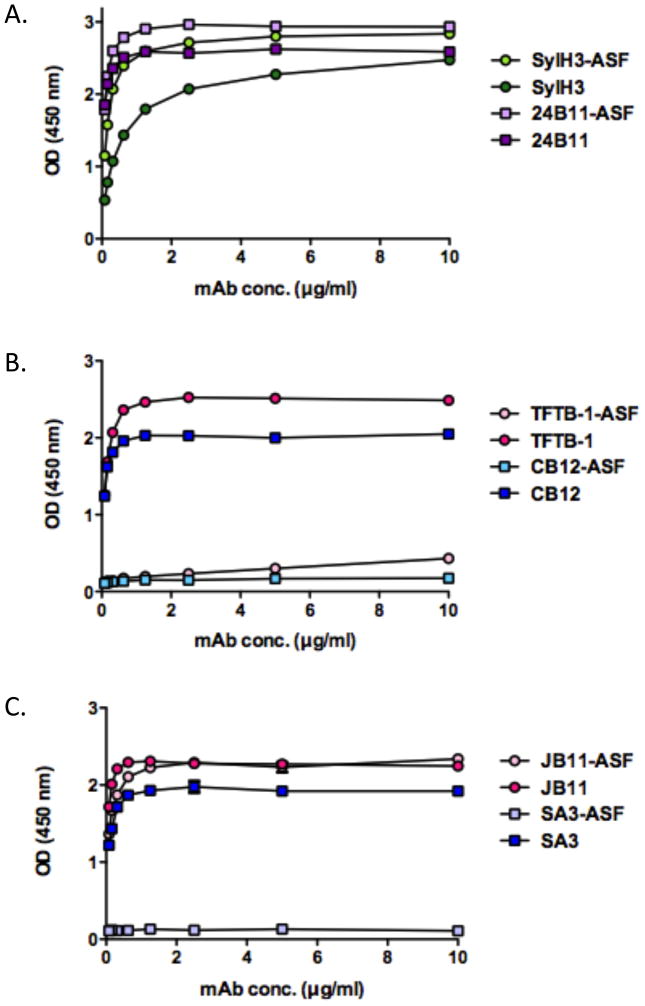
We hypothesized that SylH3 could interfere with ricin attachment to cell surfaces by physically binding an epitope within one (or both) of RTB’s two galactose binding pockets situated in sub-domains 1α or 2γ (see Fig. 1). To test this hypothesis, we performed a modified ELISA in which 96-well microtiter plates were first coated with ASF and then secondarily coated with ricin, such that the toxin only adhered to the plate by virtue of its ability to bind ASF. We found that SylH3 recognized ASF-bound ricin as well (if not slightly better than) ricin toxin directly coated on the plate (Fig. 7A), confirming that occupancy of the RTB’s galactose binding sites does not interfere with SylH3’s recognition of ricin. As expected, similar results were obtained when 24B11 was tested in this assay (Fig. 7A). These results were further confirmed with Biacore analysis by saturating ricin with lactose and then allowing each mAb to attach to the toxin (Table 3). We conclude from these data that SylH3, like 24B11, recognizes an epitope adjacent to, but not within, RTB’s galactose binding domains.
Figure 7. Binding of RTB-specific mAbs to ligand-bound ricin.
Wells of microtiter plates were coated with ricin directly (dark colored shapes) or indirectly via ASF (light colored shapes). For indirect coating, the wells were saturated with ASF (4 μg/ml) and incubated with ricin (10 μg/ml), as described in the Methods. The plates were then probed with mAbs (10 μg/ml) SylH3 and 24B11 (A), TFTB-1 and CB12 (B), SA3 and JB11 (C).
Table 3.
Inhibition of mAb attachment to ricin when bound to lactose.
| Antibody | Response RU | Response RU + Lactose |
|---|---|---|
| SylH3 | 219 | 186 |
| 24B11 | 364.2 | 322.5 |
| TFTB-1 | 112.6 | 8.8 |
3.7 Epitope-specificity of non-neutralizing RTB mAbs
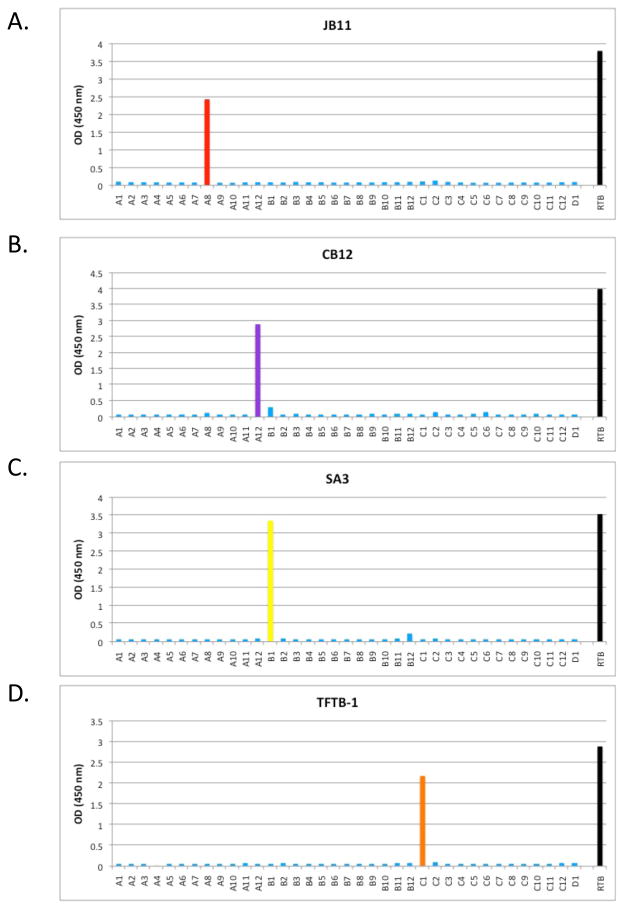
In addition to SylH3, we also characterized three non-neutralizing RTB-specific mAbs, with the expectation that these mAbs would prove informative in terms of better understanding the underlying determinants (e.g., affinity, epitope specificity) of antibody-mediated neutralization of ricin toxin. The three non-neutralizing mAbs were JB11, CB12, and SA3 (Table 2). We also included TFTB-1, which was identified as a non-neutralizing mAb more than two decades ago but has not been characterized in detail [32]. In an effort to identify the epitopes recognized by these non-neutralizing mAbs, the mAbs were subjected to pepscan analysis using a 15-mer peptide array spanning the length of RTB (Table 1). Interestingly, each of the four non-neutralizing mAbs bound to specific peptides in the peptide array (Tables 2; Fig. 8), whereas neither SylH3 nor 24B11 bound RTB peptides (data not shown). TFTB-1 bound peptide C1, located in RTB sub-domain 2α, while mAbs JB11, CB12, and SA3 recognized three distinct peptides localized within RTB sub-domain 1β. The affinities of two of the mAbs, CB12 and TFTB-1, for ricin was determined by Biacore. Each of these mAbs had a KD that was comparable to SylH3 (Table 2), ruling out affinity as the reason these antibodies failed to neutralize ricin. We also examined the ability of these non-neutralizing mAbs to recognize ricin when the toxin was bound to ASF, expecting that they would each bind to RTB irrespective of whether the galactose binding pockets were occupied by ligand. Surprisingly, this was not the case. Whereas JB11 bound RTB in the presence or absence of ASF, the binding of TFTB-1, SA3 and CB12 to RTB in the presence of ASF was completely abolished (Fig. 7B, C). These results were confirmed for TFTB-1 with Biacore analysis by saturating ricin with lactose and then allowing the mAb to attach to the toxin (Table 3). Modeling of the peptide epitopes recognized by TFTB-1, SA3 and CB12 on the surface of RTB using PyMOL revealed that TFTB-1, SA3 and CB12 bind regions of RTB that are a considerable distance from the galactose binding pockets (Fig. 9). Thus, it is unlikely that these antibodies are being displaced by ASF. Rather, we hypothesize that RTB may undergo a conformational change upon ligand binding that perturbs the epitopes recognized by these mAbs.
Figure 8. Pepscan analysis of non-neutralizing RTB mAbs.
Indicated mAbs (10 μg/ml) were examined by ELISA for the ability to bind to an RTB peptide array consisting of 37 15-mers (A1-D1, x-axis), each overlapping its neighbors by 8 amino acids. (A) JB11 reacted with peptide A8. (B) CB12 reacted peptide A12. (C) SA3 reacted with peptide B1. (D) TFTB-1 reacted with peptide C1. Each peptide array was performed at least two independent times with similar results. The data shown are from one representative experiment. The OD450 nm (y-axis) values refer to the reactivity of the mAbs with specific peptides and were obtained using peptide array ELISA.
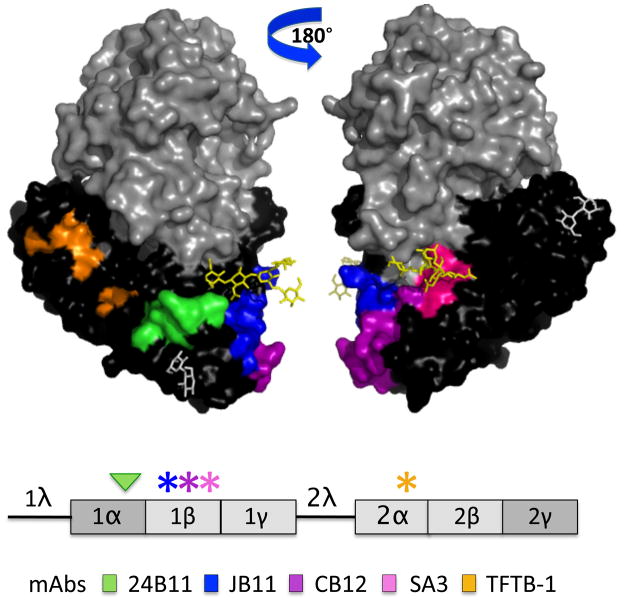
Figure 9. Modeling of neutralizing and non-neutralizing B-cell epitopes on RTB.
(Upper panel) Epitopes recognized by the RTB-specific neutralizing (24B11) and non-neutralizing mAbs (JB11, CB12, SA3 and TFTB-1) were modeled on RTB using PyMOL. The epitopes are color-coded based on the legend shown at the bottom of the figure. (Lower panel) Linear depiction of RTB sub-domains with color-coded asterisks representing the location of individual epitopes recognized by non-neutralizing mAbs, whereas the arrowhead indicates the location of the epitope recognized by 24B11.
4. DISCUSSION
RTB is essential for the toxicity of ricin; it not only promotes the attachment of the holotoxin to cell surfaces, it also mediates the retrograde transport of RTA to the ER [2, 33]. Therefore, RTB is an obvious target for antibody-based intervention therapeutics and vaccines. Despite this fact, there has been surprisingly little progress made in identifying RTB-specific neutralizing antibodies and only one definitive description of a B-cell epitope on the subunit [22, 25, 27]. In this study, we describe the identification and characterization of SylH3, a murine IgG1 mAb capable of neutralizing ricin in vitro and in vivo. SylH3 was compared to 24B11, an RTB-specific mAb previously described by our laboratory as being capable of neutralizing ricin in vitro, but never tested in vivo [25]. We also characterized a number of RTB-specific non-neutralizing antibodies, which we estimate constitute the vast majority of antibodies elicited in mice following RTB immunization. Competitive mAb binding assays and epitope identification by peptide array analysis, in conjunction with cell-based toxicity and binding assays, now provide insights into the specific sub-domains of RTB involved in eliciting neutralizing and non-neutralizing mAbs. Therefore, the results of this study have implications for the use of specific RTB sub-domains as immunogens, rather than RTB itself, as a means to more efficiently elicit protective immunity to ricin.
The potential of RTB to elicit protective immunity to ricin has been a contentious issue in the ricin research community. Foxwell and colleagues first reported that passive administration of RTB-specific rabbit antiserum to mice protected the animals against a lethal dose of ricin administered intravenously [24]. In contrast, Maddaloni and colleagues reported that immunization of mice with RTB was not sufficient to confer immunity to a systemic challenge, whereas mice immunized with RTA were protected under the same conditions [27]. In the current study, we found that RTB immunization (3 × 50 μg) conferred partial protection, as evidenced by the fact that 4/6 RTB-immunized mice survived a challenge with 5xLD50s of ricin administered by i.p. injection. The apparent discrepancy between our study and Maddaloni’s is likely due to the dose of toxin employed for the challenges. We challenged animals with 5xLD50s of ricin, whereas Maddaloni challenged animals with 25xLD50s [27]. Therefore, we propose that RTB is capable of protecting mice against relatively “low” doses (e.g., 5xLD50s) of toxin, but not more stringent challenges. We postulate that RTB’s failure as a vaccine reflects the inherent inability of RTB to give rise to neutralizing antibodies, not RTB’s immunogenicity. Indeed, RTB stimulated relatively high-titer ricin-specific serum IgG antibodies when administered to mice i.p., even in the absence of adjuvant.
We observed that a number of RTB-immunized mice succumbed to ricin challenge despite high, RTB-specific serum antibody titers. While this represents an observation in a very limited number of animals, it is intriguing to speculate that the RTB may elicit an antibody response largely consisting of non-neutralizing antibodies. This would be consistent with our observation that sera from RTB-immunized mice had no detectable toxin-neutralizing activity when examined in a cell-based cytotoxicity assay. In contrast, RT-immunized mice had readily detectable neutralizing activity in their serum. Second, from a panel of ~100 RTB-specific B-cell hybridomas, only a single mAb capable of neutralizing ricin was identified, suggesting that neutralizing mAbs are relatively rare. Others have made this same observation [23, 27]. While it remains unclear at the present time whether the failure of RTB to elicit high-titer ricin neutralizing antibodies is due to the scarcity of neutralizing B-cell epitopes on RTB or an overwhelming bias of the immune response to recognize regions (or epitopes) on RTB that do not result in protective immunity, the results of our study would favor the former over the latter. Nonetheless, further studies with larger experimental numbers, as well as a different animals model are needed to validate this hypothesis.
SylH3 represents only the third RTB-specific mAb with ricin neutralizing activity to be characterized in detail: the other two mAbs are 75/3B12 [22] and 24B11 [25]. Several lines of evidence indicate that SylH3 is distinct from those previously described mAbs. While we were unable to directly compare SylH3 to 75/3B12 because 75/3B12 is no longer available to the research community, the two mAbs are distinct from each other in that the binding of SylH3 to RTB was not abrogated by lactose or ASF. In contrast, Colombatti and colleagues reported that saturation of RTB with galactose completely inhibited 75/3B12 from recognizing ricin [22]. SylH3 and 24B11 are also distinct from each other. The two mAbs bind different epitopes on RTB, based on our observation that 24B11 did not competitively inhibit SylH3 (and vice versa) from binding ricin holotoxin by SPR. 24B11 also bound RTB better than SylH3, even though the two mAbs have similar affinities for the holotoxin. Nonetheless, despite the differences between SylH3 and 24B11, the two mAbs were equally effective at conferring passive immunity to ricin in a mouse model. However, that conclusion is subject to the caveat that the mAbs were compared at only a single concentration. Therefore, we cannot exclude the possibility that SylH3, for example, may be more effective than 24B11 at lower concentrations. These types of experiments, although beyond the scope of the current study, are ongoing in our laboratory.
Although we have not identified the epitope recognized by SylH3, we propose that the mAb likely binds within RTB’s sub-domain 2γ. Sub-domain 2γ constitutes the high-affinity galactose and N-acetylgalactosamine recognition sub-domain of RTB, whereas sub-domain 1α represents the low-affinity galactose-specific binding subdomain [6]. The two sub-domains, separated by more than 70Å, are located on opposite sides of RTB and have been shown to bind galactosides non-cooperatively [5, 8, 34]. We speculate that 2γ is the target of SylH3 for two reasons. First, SylH3 was highly effective at blocking ricin attachment to galactosides, either displayed on cell surfaces or immobilized on plastic. Second, the binding of SylH3 to RTB was not competitively inhibited by 24B11, which we have shown recognizes an epitope within sub-domain 1α [25]. Moreover, the ASF binding assays revealed that SylH3 was significantly more effective than 24B11 in blocking ricin attachment to terminal galactose residues, which is consistent with SylH3 binding to the high-affinity galactose recognition sub-domain of RTB. Definitive identification of the SylH3 epitope will require screening a library of RTB point and deletion mutants and/or solving the structure of SylH3 in complex with ricin holotoxin.
In addition to SylH3, we also characterized three non-neutralizing mAbs in this study that were originally identified from our collection of RTB-specific B-cell hybridomas as being capable of recognizing 15-mer peptides by RTB pepscan analysis. Each of the mAbs, JB11, CB12, SA3, and TFTB-1 (previously identified) bound a single RTB-derived peptide, which enabled us to model four distinct non-neutralizing epitopes on the surface of RTB (Fig. 9). Interestingly, all four of the non-neutralizing epitopes localized within sub-domains 1β and 2α, neither of which is directly involved in galactoside recognition. Moreover, because these mAbs had affinities for ricin that were similar to those exhibited by SylH3 and 24B11, we conclude that the failure of JB11, CB12, SA3, and TFTB-1 to neutralize ricin is likely dictated by epitope specificity. We speculate that these mAbs bind epitopes on RTB that are sufficiently distant from either of the two galactose binding pockets so as to not enable them to sterically interfere with ligand recognition. However, the observation that TFTB-1, SA3 and CB12 were unable to bind to RTB in the presence of ASF suggests that these two mAbs may fail to neutralize ricin because they are unable to recognize ricin when RTB is associated with its ligands. There is no evidence at present to indicate that RTB undergoes a conformational change upon ligand recognition (J. Robertus, personal communication), so it remains unclear whether this model is in fact plausible.
The results of this study have implications for the development of an RTB-based vaccine for ricin, as well as an RTB-based carrier for heterologous vaccine antigens. We postulate that the failure of RTB immunization to elicit complete protection against ricin toxin is due to a disproportionate antibody response directed against sub-domains of RTB, namely 1β and 2α that are not involved in mediating toxin attachment or uptake into cells. Therefore, we would predict that elimination of one or more of these deleterious sub-domains would result in an antigen with greater capacity to elicit neutralizing antibodies. Indeed, it is well-documented that domains 1 and 2 when separated and expressed as recombinant proteins each retain the ability to bind galactose [7, 8]. Based on that information, we would predict that sub-domains 1α and 2γ expressed individually or in combination as recombinant proteins could serve as a vaccine antigen. Moreover, the same sub-domains if fused to heterologous antigens could serve as a highly efficient vaccine delivery vehicle by systemic or mucosal routes [35]. We are currently expressing full-length and truncated derivates of RTB to test these hypotheses.
Highlights (JVAC-D-11-00734).
We have produced and characterized a collection of RTB-specific murine monoclonal antibodies (mAbs)
We have demonstrated that two RTB-specific mAbs are capable of protecting mice against ricin challenge.
We have identified for the first time the epitopes on RTB recognized by non-neutralizing antibodies.
The results of this study provide for the first time an immunologic basis for RTB-based vaccine and therapeutic design.
Acknowledgments
We gratefully acknowledge Dr. Jane Kasten-Jolly of the Wadsworth Center’s Immunology Core for performing the affinity determinations and antibody competition assays by Biacore. We thank Mr. Renjie Song, also of the Immunology core, for assistance with the flow cytometry and Dr. Karen Chave and Ms. Marcella Powell of the Wadsworth Center Protein Expression core for monoclonal antibody purification. Finally, we extend a special thanks to Joanne O’Hara for assistance in B-cell hybridoma production, Emily Gage for help with macrophage cytotoxicity assays, and Jennifer Yates for assistance with isolation and purification of murine BMMs. This work was supported in part through National Institutes of Health grant AI081053 to NJM. A.Y. was the recipient of a pre-doctoral fellowship from the Wadsworth Center’s Biodefense and Emerging Infectious Diseases program (T32AI055429; PI-McDonough).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Audi J, Belson M, Patel M, Schier J, Osterloh J. Ricin poisoning: a comprehensive review. JAMA. 2005 Nov 9;294(18):2342–51. doi: 10.1001/jama.294.18.2342. [DOI] [PubMed] [Google Scholar]
- 2.Olsnes S. The history of ricin, abrin and related toxins. Toxicon. 2004 Sep 15;44(4):361–70. doi: 10.1016/j.toxicon.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Endo Y, Mitsui K, Motizuki M, Tsurugi K. The mechanism of action of ricin and related toxins on eukaryotic ribosomes. J Biol Chem. 1987;262:5908–12. [PubMed] [Google Scholar]
- 4.Ready MP, Kim Y, Robertus JD. Site-directed mutagenesis of ricin A-chain and implications for the mechanism of action. Proteins. 1991;10(3):270–8. doi: 10.1002/prot.340100311. [DOI] [PubMed] [Google Scholar]
- 5.Montfort W, Villafranca JE, Monzingo AF, Ernst SR, Katzin B, Rutenber E, et al. The three-dimensional structure of ricin at 2.8 A. Journal of Biological Chemistry. 1987;262(11):5398–403. [PubMed] [Google Scholar]
- 6.Rutenber E, Ready M, Robertus JD. Structure and evolution of ricin B chain. Nature. 1987;326(6113):624–6. doi: 10.1038/326624a0. [DOI] [PubMed] [Google Scholar]
- 7.Swimmer C, Lehar SM, McCafferty J, Chiswell DJ, Blattler WA, Guild BC. Phage display of ricin B chain and its single binding domains: system for screening galactose-binding mutants. ProcNatlAcad Sci U S A. 1992 Jun 1;89(9):3756–60. doi: 10.1073/pnas.89.9.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newton DL, Wales R, Richardson PT, Walbridge S, Saxena SK, Ackerman EJ, et al. Cell surface and intracellular functions for ricin galactose binding. J Biol Chem. 1992 Jun 15;267(17):11917–22. [PubMed] [Google Scholar]
- 9.Rutenber E, Katzin BJ, Ernst S, Collins EJ, Mlsna D, Ready MP, et al. Crystallographic refinement of ricin to 2.5 A. Proteins. 1991;10(3):240–50. doi: 10.1002/prot.340100308. [DOI] [PubMed] [Google Scholar]
- 10.Zentz C, Frenoy JP, Bourrillon R. Binding of galactose and lactose to ricin. Equilibrium studies. Biochim Biophys Acta. 1978 Sep 26;536(1):18–26. doi: 10.1016/0005-2795(78)90047-8. [DOI] [PubMed] [Google Scholar]
- 11.Cao L, Volgina A, Korostoff J, DiRienzo JM. Role of intrachain disulfides in the activities of the CdtA and CdtC subunits of the cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Infect Immun. 2006 Sep;74(9):4990–5002. doi: 10.1128/IAI.00697-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cummings R, Etzler M. R-type Lectins. In: Varki A, Cummings R, Esko J, Freeze H, Stanley P, Bertozzi C, et al., editors. Essentials of Glycobiology. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2009. [PubMed] [Google Scholar]
- 13.Inoue K, Sobhany M, Transue TR, Oguma K, Pedersen LC, Negishi M. Structural analysis by X-ray crystallography and calorimetry of a haemagglutinin component (HA1) of the progenitor toxin from Clostridium botulinum. Microbiology. 2003 Dec;149(Pt 12):3361–70. doi: 10.1099/mic.0.26586-0. [DOI] [PubMed] [Google Scholar]
- 14.Lara-Tejero M, Galan JE. CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infect Immun. 2001 Jul;69(7):4358–65. doi: 10.1128/IAI.69.7.4358-4365.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nesic D, Hsu Y, Stebbins CE. Assembly and function of a bacterial genotoxin. Nature. 2004 May 27;429(6990):429–33. doi: 10.1038/nature02532. [DOI] [PubMed] [Google Scholar]
- 16.Nesic D, Stebbins CE. Mechanisms of assembly and cellular interactions for the bacterial genotoxin CDT. PLoS Pathog. 2005 Nov;1(3):e28. doi: 10.1371/journal.ppat.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brey RN, Mantis NJ. Vaccines for Ricin – a Type II Ribosome Inactivating Protein. In: Barrett ADT, Stanberry LR, editors. Vaccines for Biodefense and Neglected Diseases. New York: Elsevier Inc; 2009. [Google Scholar]
- 18.Smallshaw JE, Richardson JA, Vitetta ES. RiVax, a recombinant ricin subunit vaccine, protects mice against ricin delivered by gavage or aerosol. Vaccine. 2007 Oct 16;25(42):7459–69. doi: 10.1016/j.vaccine.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neal LM, O’Hara J, Brey RN, 3rd, Mantis NJ. A monoclonal immunoglobulin G antibody directed against an immunodominant linear epitope on the ricin A chain confers systemic and mucosal immunity to ricin. Infect Immun. 2010 Jan;78(1):552–61. doi: 10.1128/IAI.00796-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roche JK, Stone MK, Gross LK, Lindner M, Seaner R, Pincus SH, et al. Post-exposure targeting of specific epitopes on ricin toxin abrogates toxin-induced hypoglycemia, hepatic injury, and lethality in a mouse model. Lab Invest. 2008 Nov;88(11):1178–91. doi: 10.1038/labinvest.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chanh TC, Romanowski MJ, Hewetson JF. Monoclonal antibody prophylaxis against the in vivo toxicity of ricin in mice. Immunol Invest. 1993;22(1):63–72. doi: 10.3109/08820139309066194. [DOI] [PubMed] [Google Scholar]
- 22.Colombatti M, Johnson VG, Skopicki HA, Fendley B, Lewis MS, Youle RJ. Identification and characterization of a monoclonal antibody recognizing a galactose-binding domain of the toxin ricin. JImmunol. 1987;138(10):3339–44. [PubMed] [Google Scholar]
- 23.Colombatti M, Pezzini A, Colombatti A. Monoclonal antibodies against ricin: effects on toxin function. Hybridoma. 1986;5(1):9–19. doi: 10.1089/hyb.1986.5.9. [DOI] [PubMed] [Google Scholar]
- 24.Foxwell BM, Detre SI, Donovan TA, Thorpe PE. The use of anti-ricin antibodies to protect mice intoxicated with ricin. Toxicology. 1985;34(1):79–88. doi: 10.1016/0300-483x(85)90080-0. [DOI] [PubMed] [Google Scholar]
- 25.McGuinness CR, Mantis NJ. Characterization of a novel high-affinity monoclonal immunoglobulin G antibody against the ricin B subunit. Infect Immun. 2006 Jun;74(6):3463–70. doi: 10.1128/IAI.00324-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemley PV, Amanatides P, Wright DC. Identification and characterization of a monoclonal antibody that neutralizes ricin toxicity in vitro and in vivo. Hybridoma. 1994;13(5):417–21. doi: 10.1089/hyb.1994.13.417. [DOI] [PubMed] [Google Scholar]
- 27.Maddaloni M, Cooke C, Wilkinson R, Stout AV, Eng L, Pincus SH. Immunological characteristics associated with the protective efficacy of antibodies to ricin. J Immunol. 2004 May 15;172(10):6221–8. doi: 10.4049/jimmunol.172.10.6221. [DOI] [PubMed] [Google Scholar]
- 28.O’Hara JM, Neal LM, McCarthy EA, Kasten-Jolly JA, Brey RN, 3rd, Mantis NJ. Folding domains within the ricin toxin A subunit as targets of protective antibodies. Vaccine. 2010 Aug 18; doi: 10.1016/j.vaccine.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pincus SH, Eng L, Cooke CL, Maddaloni M. Identification of hypoglycemia in mice as a surrogate marker of ricin toxicosis. Comp Med. 2002 Dec;52(6):530–3. [PubMed] [Google Scholar]
- 30.Weischenfeldt J, Porse B. Cold Spring Harb Protoc. 12. Dec 1, 2008. Bone Marrow-Derived Macrophages (BMM): Isolation and Applications. pdb.prot5080- [DOI] [PubMed] [Google Scholar]
- 31.Lindauer ML, Wong J, Iwakura Y, Magun BE. Pulmonary inflammation triggered by ricin toxin requires macrophages and IL-1 signaling. J Immunol. 2009 Jul 15;183(2):1419–26. doi: 10.4049/jimmunol.0901119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fulton RJ, Uhr JW, Vitetta ES. The effect of antibody valency and lysosomotropic amines on the synergy between ricin A chain- and ricin B chain-containing immunotoxins. JImmunol. 1986 Apr 15;136(8):3103–9. [PubMed] [Google Scholar]
- 33.Sandvig K, Torgersen ML, Engedal N, Skotland T, Iversen TG. Protein toxins from plants and bacteria: probes for intracellular transport and tools in medicine. FEBS Lett. 2010 Jun 18;584(12):2626–34. doi: 10.1016/j.febslet.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Hatakeyama T, Yamasaki N, Funatsu G. Evidence for involvement of tryptophan residue in the low-affinity saccharide binding site of ricin D. Journal of Biochemistry. 1986;99(4):1049–56. doi: 10.1093/oxfordjournals.jbchem.a135568. [DOI] [PubMed] [Google Scholar]
- 35.Mantis NJ. Vaccines against the category B toxins: Staphylococcal enterotoxin B, epsilon toxin and ricin. Adv Drug Deliv Rev. 2005 Jun 17;57(9):1424–39. doi: 10.1016/j.addr.2005.01.017. [DOI] [PubMed] [Google Scholar]